Abstract
The cancer bioassay for 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) conducted by the Dow Chemical company in the mid 70s been used extensively for conducting quantitative cancer risk assessments for human exposure to TCDD. More recently the National Toxicology Program (NTP) conducted a cancer bioassay of similar design as part of its evaluation of the dioxin TEF methodology. This report compares the design and the results of these two cancer bioassays. This comparison confirms, in most cases, previously published and widely used carcinogenic response characteristics with respect to dose, time course, organ selectivity, tumor type and maximum intensity of TCDD-induced carcinogenicity and toxicity in the Sprague-Dawley rat. Specifically increased in the incidences of neoplasms were seen in both studies in the liver, lung and oral mucosa. The most notable difference was the significant increase in the incidence of cholangiocarcinoma of the liver seen in the NTP study but not in the Dow study. The experimental designs for the two studies are similar but some protocol parameters differed such as vehicle, dosing schedule, diet and rat sub-strain utilized. Differences in the shapes of the dose response curves for several neoplasms were noted between the studies, with the NTP study showing non-linearity for all neoplasms. This may result from differences in the experimental protocols as well as divergence in the biological behavior of the different stocks of Sprague-Dawley rat strains used.
Keywords: carcinogenicity, TEF, PCBs, risk assessment, mixtures
Introduction
TCDD is a known human carcinogen as determined by the National Toxicology Program and the International Agency for Research on Cancer [1].The primary data in support of these listings are from animal cancer studies, an extensive literature on mechanism of action of TCDD and human epidemiology findings providing additional support. There is an extensive body of research data that provides compelling evidence of basic similarities in the mechanisms by which laboratory animals and humans respond to TCDD exposure [2]. These mechanistic considerations are primarily based on Ah receptor (AhR) activation and consequential downstream events as important components of the carcinogenic response [3]. Many other biopersistent chemicals that bind to and activate the AhR are considered “dioxin-like”, and the carcinogenic potency of this class of materials, when given alone or as mixtures, is an active area of investigation. Contributing to this effort, the NTP has completed a series of two-year cancer bioassays in female rats using individual dioxin-like compounds (including 2,3,7,8-TCDD) given alone and also as mixtures [4-8].
Although many TCDD cancer studies using laboratory animals have been performed, the protocol and results provided by Kociba et al., [9] continue to provide a critical foundation for risk assessment and regulation in this country [10]. For this reason, the study design of the NTP “dioxin-like” chemical carcinogenicity assessment program [8, 11] was based on a 2-year carcinogenicity study of TCDD conducted by Dow Chemical in the early 1970s and published in 1978 [9]. The NTP series of studies, in addition to investigating prepared mixtures containing selected, TCDD, PCDF and PCB congeners, provided an opportunity to reevaluate the carcinogenicity of 2,3,7,8-TCDD given to female Sprague-Dawley rats. Male rats were not used in the current NTP study because they proved to be less sensitive than females to the carcinogenic effects of TCDD [9].
This report presents a review of the results of the recent NTP cancer bioassay of TCDD [6] and compares them with prior observations made in the Dow study [9]. Since dose selection for the NTP studies of TCDD and related chemicals was based on the Dow study [9] a comparison of the studies provides insights into the reproducibility of the site specific effects of chronic exposure to TCDD in the rat and comparisons of specific tumor incidences.
This comparison largely confirms, in most cases, previously published and widely used TCDD response characteristics with respect to dose, time course, organ selectivity, tumor type and maximum intensity of TCDD-induced carcinogenicity and toxicity in the Sprague-Dawley rat. The two sets of data used in the comparison are derived from experimental designs that are similar but some protocol parameters differed such as vehicle, dosing schedule, diet and rat sub-strain utilized.
Materials and methods
The methods presented here are a brief summary of specific methods outlined in technical report TR521 for the NTP's study of TCDD[6]. For a comparable overview of the Dow study refer to Kociba et al. [9]. Comparison of the present design with that of the Dow study is presented in the results section.
Chemical
For the NTP study, TCDD was obtained from IIT Research Institute (Chicago, IL) in one lot (CR82-2-2). Identity and purity analyses were conducted by Research Triangle Institute (Research Triangle Park, NC). The chemical, a white crystalline powder was identified as TCDD by infrared spectroscopy, proton nuclear magnetic resonance (NMR) spectroscopy, direct probe mass spectroscopy (MS), low-resolution gas chromatography (GC) coupled with MS. The purity determined by GC was >98%. Three impurities were detected and the major impurity (∼ 1.5%) was identified as 1,2,4-trichlorodibenzo-p-dioxin. A small peak eluting immediately after TCDD was tentatively identified as a dimethyl isomer of trichloro-p-dioxin with the positional substitution unknown). A trace of a higher molecular weight tetrachlorinated dioxin was also detected, but could not be identified further. TCDD was dissolved and stored in acetone. Dose formulations were prepared by mixing the TCDD acetone solutions with corn oil such that the final corn oil solutions contained 1% acetone. Periodic analyses of dose formulations by GC/MS showed that 56 of 58 formulations were within 10% of target concentrations (NTP, 2004).
Study design
For the NTP study, groups of 81 or 82 female Sprague-Dawley rats, 8 weeks old at study start, were obtained from Harlan Sprague-Dawley, Inc. (Indianapolis, IN) and received TCDD in corn oil:acetone (99:1), by gavage at doses of 3, 10, 22, 46, or 100 ng/kg, 5 days per week for up to 105 weeks. Up to 10 rats per group were evaluated at 14, 31, or 53 weeks for histopathology, serum thyroid hormones, lung and liver cytochromes P450 1A1 and 1A2 activity and liver cell turnover and the remaining 50-54 animals per dose group continued on the study for up 2 years (Table 1). Additionally a “stop-exposure” group of 50 rats received 100 ng/kg TCDD for 30 weeks and then was given vehicle for the remainder of the study. A complete necropsy and microscopic examination was performed on all rats. Complete study details are outlined in full NTP report [6]. The study was conducted at Battelle Columbus Labs, Columbus, OH in compliance with Good Laboratory Practice Regulations (21 CFR, Part 58).
Table 1.
Comparison of study details
| Study element | NTP Study | Dow Study |
|---|---|---|
| Chemical Purity (methods) | >98% GC & GC/MS | >99% (EC/GC &GC/MS |
| Compound Source | IIT Research Institute | Dow Chemical |
| Experiment Location | Battelle, Columbus OH | Dow Chemical, Midland MI |
| Rat Strain | Harlan Sprague Dawley (Indianapolis, IN) | Spartan Sprague Dawley (Haslett, MI) |
| Animals/Dose Group | 50-54 females | 50 females |
| Duration of Treatment | 2 years (Interim groups 14, 31 and 53 weeks) | 2 years (Interim groups at 12, 48 and 92 weeks) |
| Treatment (days/week) | 5 | 7 |
| Doses (ng/kg) | 0, 3, 10, 22, 46, 100 | 0, 1, 10, 100 |
| Administration | Gavage in corn oil:acetone (99:1) vehicle | Ingested in food |
| Daily averaged dose (ng/kg/d) | 0, 2.1, 7.1, 15.7, 32.9, 71.4 | 0, 1, 10, 100 |
| Diet | NTP Pelleted Diet (Ziegler Bros. Gardners, PA) | Purina Lab Chow |
| Stop Treatment Group | Stop treatment at 30 weeks | none |
| Animals per Cage | 5 | 2 |
| Cage type | Polycarbonate, solid with hardwood chips and cover | Hanging stainless wire |
| Sacrifice Method | CO2 asphyxiation | Decapitation |
| Blood | Not measured | Hematology and enzymes, 8 animals at 3,12 and 23 weeks |
| Urine | Not measured | Metabolites, porphyrins, etc. 8 animals 3, 12 and 23 months. |
| Tissues | Termination histology (>40 tissues), interim at 14 ,31 and 53 weeks (liver) |
Termination histology, interim at 13-24 months, (>40 tissues), |
| Body Weight | All animals, weekly for 13 weeks then monthly | 20 animals, weekly |
| Food Consumption | Not measured | 20 animals, weekly |
| Thyroid Hormone Analysis | Weeks 14, 31 and 53 | Not measured |
| Cytochrome P450 Activities | CYP1A1 and 1A2 in liver and lung (CYP1A1 only) at 14, 31 and 53 weeks |
Not measured |
| Cell Proliferation Analysis | Liver at 14, 31 and 53 weeks (10 animals) | Not measured |
| Tissue dosimetry | Liver, adipose, blood, lung (all time points) | Liver and adipose at 2 years |
Pathology
For the NTP study, at necropsy all tissues were examined grossly, any lesions observed were recorded, and a full complement of tissues was removed and fixed in 10% neutral buffered formalin for microscopic evaluation. After fixation, the tissues were trimmed, processed, embedded in paraffin, sectioned at a thickness of 5μm, stained with hematoxylin and eosin (H&E), and examined microscopically. The severity of lesions was graded on a four-point scale of 1=minimal, 2=mild, 3=moderate, and 4=marked. The pathological findings from all studies were subjected to a full NTP pathology peer review [12]. For assuring the consistency of the histopathological diagnoses among the TEF dioxin projects, the same study pathologist, Quality Assurance pathologist, Pathology Working Group (PWG) chairperson, NTP pathologist, and members of the PWG served in all studies. In addition to the routine peer-review process, a different group of pathologists, experts in rodent liver carcinogenicity, was convened to provide additional guidance on the most appropriate classification of the hepatocellular proliferative lesions from the TEF studies. Final diagnoses for hepatocellular proliferative lesions reflect the consensus of this complete review process.
Statistical Analyses and Dose response modeling
The probability of survival was estimated for the NTP study, by the product-limit procedure of Kaplan and Meier [13]. Animals found dead of other than natural causes or missing were censored from the survival analyses; animals dying from natural causes were not censored. Statistical analyses for possible dose related effects on survival used Cox's method [14] for testing two groups for equality and Tarone's life table test [15] to identify dose-related trends. All reported P values for the survival analyses are two sided. The Poly-k test [16, 17] was used to assess neoplasm and nonneoplastic lesion prevalence. This survival-adjusted rate was based on the Poly-3 method. Survival adjusted incidences for the Dow study were from Portier and Kohn [18] and based on the pathology reevaluation of Goodman and Sauer [19].
Representative dose response plots were made using Prism 4.0 for Macintosh (Graph Pad software). Administered average daily dose (ng/kg) was selected as the dose metric to compare the TCDD-induced neoplastic response between the Dow study and the NTP study. For the NTP study the 5-day total weekly dose was averaged over 7 days to provide the 7-day daily averaged dose.
Results
A comparison of the procedures and outcomes of two cancer bioassays of TCDD was made using previously published information. The assays were conducted in a similar manner but differences existed. Table I provides an overview of similarities and differences in the experimental procedures used in the NTP and Dow studies. The method by which the chemical used in each of the studies was produced was not reported but the source of material was different as indicated in the Table. The purity of the chemicals was reported to be > 98% in each case and similar methods of analysis were used. Of potential importance is the fact that a different stocks of Sprague-Dawley rats from different suppliers was used in each study. Male and female animals were exposed to the chemical in the Dow study study whereas the NTP study only female rats were exposed to TCDD. However, in the latter study a sufficient number of male animals were housed with the females to ensure the continuation of estrus.
Administration of TCDD was different in each study with gavage occurring 5 days of each week in the NTP study whereas in the Dow study the chemical was mixed in lab chow to provide 7 days of exposure. The range of daily administered TCDD dose levels (estimated in Kociba et al., actual in NTP) used in each study was similar (1 to 100 ng/kg of body weight) with the 10 and 100 ng/kg dose levels being used in both studies. Given that the aim of the NTP study was to compare the tumor responses, the study was designed in an attempt to maximize the number of doses in the range where tumors were expected, and therefore, given that 1ng/kg was NOEL in the Dow study, this dose was omitted in the NTP study in favor of additional dose groups at higher doses. Its is of note that while the daily administered doses were similar, the weekly averaged daily exposures in the NTP study are lower since animals were treated with only 5 daily gavage doses (Monday to Friday) within each week rather than exposed 7 days per week via dosed feed (Table 1). The dosing media, corn oil acetone in the NTP and lab chow for the Dow study were routinely analyzed to confirm the exposure levels in each study.
Some differences in caging of animals, method of sacrifice, and body weight measurement protocol are noted in Table 1. Several categories of measurements were made in one study but not in the other and selected examples are shown in the Table 1. Measurements of endogenous components of blood and urine were not measured in the NTP study but results of these tests were reported by Kociba et al. Urinary components did not change with treatment but changes in some hematology measurements occurred particularly at the highest dose level. Thyroid hormones, cytochrome P-450 enzymes [20] and cell proliferation were evaluated in selected animals in the NTP study but were not made in the Dow study [9] .
A comparison of results in Table 2 indicate that cumulative survival of animals were not altered in any treatment group in the NTP study whereas Kociba et al. reported a decrease in survival in the highest dose group during the latter half of the study. Both studies reported lower body weight gain at less than the highest dose (100 ng/kg). Measurement of TCDD in fat and liver at termination was conducted in both studies (Table 2 and Figure 1). The results shown indicate that tissue levels in the Dow study were approximately twice those observed in the NTP study, indicative of a higher internal exposure at equivalent daily averaged doses (Figure 1).
Table 2.
Comparison of study results
| RESULTS | NTP Study | Dow Study |
|---|---|---|
| Cumulative Survival | No difference from control | Lower than control at highest dose (100 ng/kg) |
| Body Weight at Termination | Lower than control at doses of 22, 46 and 100 ng/kg | Lower than control at 10 ng/kg and 100 ng/kg |
| Mean TCDD Concentrations in Tissues at Termination (pg/g wet weight) | ||
| Daily averaged dose (ng/kg/day) | ||
| 1 | 540 pg/g in fat | |
| 540 pg/g in liver | ||
| 2.1 | 505 ± 92 pg/g in fat | |
| 681 ± 58 pg/g in liver | ||
| 7.1 | 753 ± 68 pg/g in fat | |
| 2213 ± 163 pg/g in liver | ||
| 10 | 1700 pg/g in fat | |
| 5100 pg/g in liver | ||
| 15.7 | 1404 ± 117 pg/g in fat | |
| 4364 ± 268 pg/g in liver | ||
| 32.9 | 1996 ± 123 pg/g in fat | |
| 6413 ± 242 pg/g in liver | ||
| 71.4 | 3177 ± 506 pg/g in fat | |
| 9325 ± 1163 pg/g in liver | ||
| 100 | 8100 pg/g in fat | |
| 24000 pg/g in liver | ||
Figure 1.
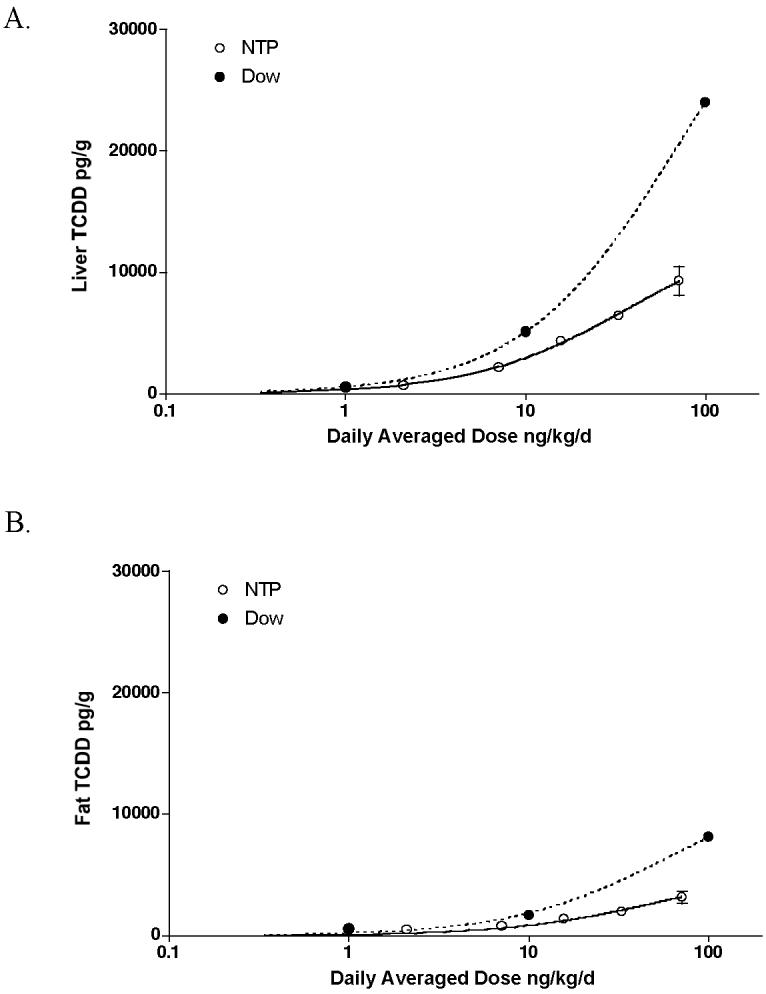
Comparison of liver TCDD concentrations at 2 years in the NTP study (solid line) and the Dow study [9] (dotted line). Data are plotted as daily averaged doses relative to the wet weight liver concentration.
The incidences of neoplastic and non neoplastic lesions induced by TCDD in the NTP study, with comparison to the incidences noted in the Dow study are presented in Tables 3 and 4. Treatment-related neoplasms were seen in the two studies in the liver, lungs, and oral cavity, arising in each organ from the same cell type. However, a striking difference between the two studies was the lack of cholangiocarcinoma and /or cholangiofibrosis in the Dow study. Proliferative lesions of the bile duct (e.g. bile duct hyperplasia, cholangiofibrosis and cholangiocarcinoma) were not specifically diagnosed in the Dow study study, although oval cell proliferation, which is suggested to be of bile duct origin, was noted. Two cases of bile duct adenoma were noted only in females treated with the high dose in the Dow study. According to Goodman and Sauer [19], who peer-reviewed the liver slides from Dow 2-year study, regenerative hyperplasia of the liver was part of the process of “hepatotoxicity” in the Dow study, in contrast to the NTP study, where the term “nodular hyperplasia” was used, and was distinguished and reported separately from “toxic hepatopathy”, a term used to indicate a distinct spectrum of lesions that was seen in the livers of rats treated with TCDD and with other “dioxin-like” chemicals in the NTP study series [21]. Nodular hyperplasia was generally composed of larger than normal hepatocytes (hepatocytic hypertrophy) sometimes mixed with areas of increased numbers of small hepatocytes (hepatocytic hyperplasia). Areas of nodular hyperplasia blended with the surrounding parenchyma, although often they had a distinct border. Large, focal to multifocal areas of nodular hyperplasia were sometimes seen that caused compression of surrounding tissue, and/or bulging of the capsular surface. Bile duct hyperplasia and portal areas were usually present within nodular hyperplasia. The nodular hyperplasia was considered to be the result of the presence of a proliferative stimulus. Evaluation of the lower doses of the present study as well as livers from other NTP TEF studies indicated that the nodular hyperplasia was sometimes seen in animals where the toxic hepatopathy was minimal or non-existent. According to Goodman and Sauer [19], there was a “distinct correlation between the presence of overt hepatotoxicity and development of hepatocellular neoplasms”. These authors used “hepatotoxicity” as a comprehensive terminology to include similar range of non-neoplastic changes, except of the above-mentioned bile duct proliferative lesions, as described in the NTP studies under the term “toxic hepatopathy”.
Table 3.
Incidence of treatment-related neoplastic lesions in the NTP and Dow studies
| NTP Study | Dow Study a | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose ng/kg | 0 | 3 | 10 | 22 | 46 | 100 | Stop | 0 | 1 | 10 | 100 | |
| # animals examined | 54 | 54 | 54 | 54 | 54 | 54 | 50 | 86 | 50 | 50 | 49 | |
| Liver | Hepatocellular adenoma | 0** | 0 | 0 | 0 | 1 | 13** | 2 | 2* | 1* | 9* | 14* |
| (2.6) | (29.9) | (5.2) | (3.5) | (2.9) | (33.3) | (45.2) | ||||||
| Hepatocellular carcinoma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | |
| Hepatocellular adenoma/carcinoma | 2 | 1 | 9 | 18* | ||||||||
| (3.5) | (2.9) | (33.3) | (58.1) | |||||||||
| Cholangioma | 0 | 0 | 0 | 0 | 0 | 0 | 1 | NR | ||||
| Hepatocholangioma | 0 | 0 | 0 | 0 | 0 | 2 | 0 | NR | ||||
| Cholangiocarcinoma | 0 | 0 | 0 | 1 | 4 | 25** | 2 | NR | ||||
| (2.9) | (10.3) | (54.9) | (5.2) | |||||||||
| Bile duct adenoma | NR | 0 | 0 | 0 | 2 | |||||||
| Lungs | Cystic keratinizing epithelioma | 0** | 0 | 0 | 0 | 0 | 9** | 0 | NR | |||
| Keratinizing squamous cell carcinoma | NR | 0 | 0 | 0 | 7* | |||||||
| Oral mucosa | Gingival squamous cell carcinoma b | 1** | 2 | 1 | 0 | 4 | 10 | 5 | 0 | 0 | 1 | 4 |
| (2.5) | (5.7) | (2.6) | (10.2) | (22.0) | (12.4) | |||||||
| Tongue squamous cell carcinoma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | |
| Pancreas | acinar cell adenoma and carcinoma | 0** | 0 | 0 | 0 | 0 | 3 | 1 | 0 | 1 | 0 | 1 |
| Thyroid | C-cell adenoma and carcinoma | 21 | 15 | 17 | 16 | 14 | 11 | 14 | 16 | 3 | 2 | 6 |
| Uterus | Endometrial polyp | 11 | 7 | 4 | 11 | 10 | 8 | 4 | 28 | 12 | 11 | 7 |
| Squamous cell carcinoma | 0 | 0 | 0 | 0 | 5 | 0 | 2 | 0 | 0 | 0 | 0 | |
| Mammary gland | Benign and/or malignant neoplasms | 39 | 48 | 39 | 31 | 38 | 25 | 35 | 73 | 39 | 40 | 24 |
| Pituitary | Adenoma (all lobes) | 27 | 20 | 26 | 15 | 20 | 11 | 19 | 43 | 12 | 12 | 18 |
Data for hepatocellular neoplasms is from the liver pathology reevaluation of Goodman and Sauer (1992). Survival adjusted incidence rates (shown in parentheses) for adenoma /carcinoma combined is from Portier and Kohn (1996). Survival adjusted incidence rates for liver adenoma is based on the survival adjusted group size based on adenoma and carcinoma combined. Other data as reported by Kociba et al (1978). Data shown is number of animals exhibiting a specific neoplasm.
Described in the Kociba study as hard palate/nasal turbinates
p<0.05
p<0.01 For NTP dataset based, statistical analyses were based on poly 3 test of survival adjusted incidence (Survival adjusted incidences shown in parentheses).
For the Kociba et al dataset, statistical analyses were based on the Fishers exact test, as reported.
NR Not specifically reported or described
Table 4.
Incidence of treatment-related non-neoplastic lesions in the NTP and Dow studies
| NTP | Dow Study | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Dose ng/kg | 0 | 3 | 10 | 22 | 46 | 100 | Stop | 0, 1, 10, 100 | |
| # animals examined | 54 | 54 | 54 | 54 | 54 | 54 | 50 | ||
| Liver | Hepatocytic hypertrophy | 0 | 19 | 19 | 42 | 41 | 52 | 22 | Observed as part of increased hepatotoxicity |
| Multinucleated hepatocytes | 0 | 0 | 16 | 26 | 36 | 51 | 32 | Observed as part of increased hepatotoxicity | |
| Eosinophilic focus | 11 | 14 | 21 | 27 | 27 | 44 | 27 | Observed as part of increased hepatotoxicity | |
| Inflammation | 33 | 46 | 47 | 50 | 52 | 49 | 43 | Observed as part of increased hepatotoxicity | |
| Pigmentation | 4 | 9 | 34 | 48 | 52 | 53 | 45 | Observed as part of increased hepatotoxicity | |
| Diffuse fatty change | 0 | 2 | 12 | 17 | 30 | 48 | 10 | Observed as part of increased hepatotoxicity | |
| Necrosis | 1 | 4 | 4 | 8 | 10 | 17 | 8 | Observed as part of increased hepatotoxicity | |
| Oval cell hyperplasia | 0 | 4 | 3 | 20 | 38 | 53 | 1 | Observed as part of increased hepatotoxicity | |
| Bile duct hyperplasia | 5 | 4 | 7 | 22 | 40 | 53 | 7 | Observed as part of increased hepatotoxicity | |
| Bile duct cyst | 3 | 1 | 2 | 2 | 0 | 21 | 6 | NR | |
| Nodular hyperplasia | 0 | 0 | 0 | 3 | 7 | 36 | 0 | Observed as part of increased hepatotoxicity | |
| Portal fibrosis | 0 | 0 | 0 | 0 | 5 | 27 | 1 | NR | |
| Toxic hepatopathy | 0 | 2 | 8 | 30 | 45 | 53 | 16 | Observed as part of increased hepatotoxicity | |
| Cholangiofibrosis | 1 | 1 | 2 | 1 | 11 | 31 | 1 | NR | |
| Lungs | Alveolar epithelium, metaplasia bronchiolar |
2 | 19 | 33 | 35 | 45 | 46 | 31 | NR |
| Oral mucosa | Gingival squamous hyperplasia |
1 | 7 | 14 | 13 | 15 | 16 | 8 | NR |
| Pancreas | Acinar cytoplasmic vacuolation |
1 | 0 | 0 | 1 | 15 | 42 | 0 | Increased incidence of atrophy and fibrosis were noted in the 100 ng/kg dosed group |
| Chronic active inflammation | 0 | 0 | 2 | 2 | 3 | 6 | 4 | ||
| Acinar atrophy | 1 | 2 | 4 | 4 | 4 | 9 | 4 | ||
| Arterial chronic active inflammation |
0 | 1 | 1 | 2 | 2 | 7 | 2 | Increased incidence of hemorrhage in the brain and spinal cord, periarteritis in the mesenteric and thoracic blood vessels of animals treated with 100 ng/kg |
|
| Thyroid | Follicular cell hypertrophy | 3 | 4 | 4 | 7 | 10 | 17 | 6 | NR |
| Thymus | Atrophy (severity) | 36 (2.6) | 1 (2.7) | 44(3.0) | 1(3.1) | 44(3.6) | 2 (3.9) | 45(3.3) | Increased incidence of atrophy in the 100 ng/kg dosed group |
| Adrenal gland |
Atrophy | 2 | 0 | 4 | 5 | 5 | 27 | 4 | Increased incidence of cortical necrosis and hemorrhage |
| Hyperplasia | 16 | 16 | 18 | 25 | 29 | 30 | 20 | ||
| Heart | Cardiomyopathy | 10 | 12 | 22 | 35 | 32 | 36 | 22 | NR |
| Clitoral gland |
Cystic duct | 34 | 37 | 41 | 42 | 41 | 48 | 35 | NR |
| Kidney | Nephropathy(severity) | 34(1.2) | 26(1.1) | 32(1.3) | 36(1.4) | 39(1.4) | 52(2.2) | 41(1.4) | NR |
| Forestomach | Squamous hyperplasia | 3 | 4 | 4 | 2 | 7 | 11 | 5 | NR |
Data shown is the number of animals exhibiting a specific lesion. NR Not specifically reported or described
Although the lung responded in both studies with proliferative squamous cell lesions, the NTP reported that all squamous cell tumors were benign cystic keratinizing epithelioma (CKE), while in the Dow study the squamous tumors were interpreted as malignant squamous cell carcinomas (SCC) (Tables 3 and 4). In addition the NTP reported increased acinar pancreatic tumors and uterine SCC, in contrast to no change noted in the incidence of these tumors in the Dow study. Both studies reported significant decreased incidences of thyroidal C-cell tumors, pituitary and mammary tumors. Kociba et al. reported decreased benign tumors of the uterus[9], in contrast to no change noted in the incidence of these tumors in the NTP study.
The NTP study reported additional changes in other organs (Table 4), but due to insufficient details included in the report of the Dow study [9], more complete comparison was not possible for some of the findings.
The incidence of pulmonary bronchiolar metaplasia of alveolar epithelium increased in all 2-year NTP study treated groups. This change consisted of replacement of the normal alveolar epithelium by cuboidal to columnar, sometimes ciliated cells, and was often accompanied by abundant mucus production in the affected area [22]. The lesion generally diffusely affected the epithelium located at the bronchiolar-alveolar junction and adjacent alveoli. The incidence of squamous metaplasia of alveolar epithelium increased in the 46, 100 and 100 ng/kg stop groups. The incidence of histiocytic infiltration increased in the 22, 46, 100 ng/kg treated groups. Alveolar hyperplasia was seen only in the control group. For the Dow study, Kociba et al. reported treatment-related hyperplastic changes only in the 100 ng/kg-dosed groups.
Increased incidences of minimal to mild multifocal cardiomyopathy were seen in rats of the NTP 2-year study, administered 10 ng/kg or greater[23]. The incidence of cardiomyopathy was lower in the 100-ng/kg stop-exposure groups compared to the 100-ng/kg core study group, but was greater than in the vehicle controls. Cardiomyopathy had the typical microscopic appearance of spontaneous cardiomyopathy as seen in aging F344/N rats [23]. The heart was also considered a target organ in the Dow study, reporting an increase above the background incidence of myocardial degenerative changes.
Increased incidence of chronic arteriopathy was noted in all treated groups in the pancreas and mesenteric arteries of the NTP 2-year study. Kociba et al. indicated increased incidence of hemorrhage in the brain and spinal cord, as well as increased incidence of periarteritis in the mesenteric and thoracic blood vessels of animals treated with the 100 ng/kg.
The incidence and/or severity of thymic atrophy were significantly increased in the 46 and 100 ng/kg treated groups in the NTP 14, 31, and 53 weeks interim sacrifices, and in the two-year 22 ng/kg treated group and higher. The incidence in the 100ng/kg stop-exposure group was greater than those in the vehicle controls. Atrophy consisted varying degrees of loss of lymphoid cells from the cortex. The thymus was also a target organ in the 10 ng/kg dosed group of the Dow study.
Increased incidence of minimal thyroid follicular cell hypertrophy was noted in the NTP 2-year rats administered 22 ng/kg or greater [24]. The incidence of this lesion was lower in the 100 ng/kg stop-exposure group compared to the 100 ng/kg core study group. Follicular cell hypertrophy was a localized to diffuse change, characterized by follicles that were decreased in size and contained decreased amounts of colloid in which aggregates of amphophilic, flocculant appearing material were often present.
In the uterus, the incidence of SCC in the 46 ng/kg treated group of the NTP study was greater than that in the vehicle controls, and there were two SCCs in the 100 ng/kg stop-exposure group. The tumor was characterized by irregular cords and clusters of atypical stratified squamous epithelial cells that invaded the underlying myometrium. The incidence of squamous metaplasia was lower in the 100 ng/kg core study group compared to vehicle controls and was less than that in the 100 ng/kg stop-exposure group. Squamous metaplasia was generally a minimal to mild, multifocal change, consisting of tubular structures within the endometrium that were lined by stratified squamous epithelium.
The incidence of gingival SCC increased in the NTP 2-year 46, 100 and 100 ng/kg stop groups[25]. The incidence of gingival squamous hyperplasia was increased in all dosed groups, including the stop-exposure group. The SCC occurred within the oral mucosa of the palate and was located adjacent to the molars teeth in nasal section III. It was characterized by invading cords and clusters of stratified epithelium. The squamous hyperplasia was seen in the same location as the tumors, and consisted of varying degrees of thickened epithelium. Kociba et al. also reported increased incidence of SCC of the hard palate/nasal turbinate region in the group treated with 100 ng/kg [9]. They also reported increased incidence of SCC of the tongue noted in the 100 ng/kg treated group, which was not a target organ in the NTP study.
In the NTP study in the pancreas, the incidence of acinar cytoplasmic vacuolation were increased in the 100 ng/kg treated groups of the 31-, and 53-week interim sacrifices, and chronic active inflammation and acinar atrophy were present in 100 ng/kg at the 14 and 53 weeks [26, 27]. In the 2 year treated groups of the same study acinar adenoma and/or carcinomas were seen only in the 100 ng/kg and 100 ng/kg stop study treated groups. The incidence of acinar cytoplasmic vacuolation was increased in the 46 and 100 ng/kg core study groups, and the incidence of chronic inflammation, and acinar atrophy were increased in the 100 ng/kg core study group [26]. Kociba et al. did not report treatment-related change in the acinar cell tumors among the females in the Dow study, but indicated increased incidence of atrophy and fibrosis of the acinar tissue of animals treated with 100 ng/kg, which was or was not associated with increased incidence of periarteritis.
The incidence of adrenal cortical atrophy and hyperplasia increased in the NTP 2-year in groups treated with 10 ng/kg and higher. The incidence of cytoplasmic vacuolation was increased in the 22 ng/kg or greater groups. The incidence of cortical cystic degeneration increased in the 10 and 22 ng/kg groups when compared to the concurrent controls. Kociba et al. reported decreased incidence of medullary hyperplasia (which was not seen in the NTP study), and increased incidence of cortical necrosis and hemorrhage, changes which may be consistent with the cortical atrophy and cystic degeneration observed in the NTP study.
In contrast to the Dow study, the NTP investigation included a 100 ng/kg stop treatment group following 30 weeks of TCDD treatment. The majority of the treatment-related neoplasms were not seen in the stop treatment group, except for the oral SCC. These data suggest that there is need for a longer period than 6 months of exposure in order that the TCDD-related neoplasms will develop and/or acquire potential of autonomous growth, except for the gingival tumors, that apparently were initiated at early stage of exposure, and did not regress even after relatively long period of withdrawal of treatment.
Dose response comparison of the two studies indicated marked differences. The data from the Dow study has been shown to be essentially low dose linear, the Hill shape parameter for hepatocellular neoplasms essentially being close to 1 [10]. In contrast, modeling of the tumor data from the NTP study in general gave higher estimated Hill shape parameters greater than 2.0 [8] and as such were more low-dose non-linear in nature.
Discussion
The 2-year study of the chronic toxicity and carcinogenicity of TCDD in female Harlan Sprague-Dawley rats described in this paper is one in a series of studies carried out as part of a multi-study NTP initiative examining the relative chronic toxicity and carcinogenicity of dioxin-like compounds (DLCs) and structurally related polychlorinated biphenyls (PCBs). Detailed reports on data from these studies can be found elsewhere [4-7]. A quantitative analysis of the effects observed in this study of TCDD to responses observed with other compounds studied as part of the Dioxin TEF Evaluation has also been reported elsewhere [8].
The principal findings of the NTP TCDD study were increased incidences of benign and malignant neoplasms in several organs, with specific increased incidences of neoplasms in the lung (CKE), liver (cholangiocarcinoma, and hepatocellular adenoma) and oral mucosa (gingival SCC). In addition there was an increase in the incidence of SCC of the uterus in the 46ng/kg group that was considered to be related to treatment. A significant trend for increased incidence of pancreatic adenoma or carcinoma (combined) and occurrences of hepatocholangioma and cholangioma of the liver may also have been related to TCDD treatment. The principal nonneoplastic finding in the NTP study of TCDD was a significant increase in incidence and severity of hepatotoxicity as well as increased incidences of nonneoplastic lesions in numerous other organs; notably the lung, oral mucosa, pancreas, thymus, adrenal cortex, mesenteric artery, heart, clitoral gland, kidney, forestomach, and thyroid gland.
Overall there was remarkable similarity in the responses seen in the NTP and Dow studies of TCDD, confirming the suitability of the Dow study as the basis for the design of the NTPs studies. The site specificities for the highest increased incidences of neoplasms were the same; the liver, lung and oral mucosa. In the liver, both studies reported an increased incidence of hepatocellular adenoma. In the lung, both studies revealed an increased incidence of squamous cell neoplasms. Moreover, given the similarity in pathological description, keratinizing SCC of the lung observed in the Dow study was likely the same lesion diagnosed as cystic keratinizing epithelioma (CKE) in the NTP study, especially since at the time of the evaluation of the Dow study, CKE was not a consistently used diagnostic term. Diagnostic criteria for CKE as a lesion distinct from SCC were later developed at workshop held in the mid 1990's[28]. In addition to similar liver and lung effects, an increased incidence of oral mucosal neoplasms was observed in both studies. The location of the SCCs of the hard palate/nasal cavity in the Dow study was the same as that observed in the NTP study diagnosed in the gingival region. In addition to the neoplastic effects, many nonneoplastic effects in other organs were seen in common between the studies. The similarity in target organ specificity addresses an uncertainty that has lingered regarding the respiratory tract and oral tumors seen in the Dow study. It was suggested that these might have been due to prolonged direct contact to the TCDD containing feed or accidental aspiration of the dosed feed in that study. The observations of the same site specificity in a controlled gavage dosing regimen used in the NTP study would suggest that the respiratory tract and oral mucosal tumors seen in both studies were likely the result of systemic exposure.
The most notable qualitative difference between the studies was the increased incidence of cholangiocarcinoma and cholangiofibrosis seen in the NTP study of TCDD and other dioxin-like compounds [4-7] but not in the Dow study [9]. While cholangiofibrosis and cholangiocarcinoma may be a morphological continuum, there is limited biological information relative to the pathogenesis or progression of those lesions. Spontaneous cholangioma and cholangiocarcinoma are rare in the Harlan Sprague-Dawley rat, and were not observed in any vehicle control animals from this study or the other NTP studies using this strain. It is not clear why these lesions were observed in the present study and not in the Dow study given that there was clearly an effect on bile duct proliferation in both studies. These lesions may be an indirect response to the toxicity observed as a result of the TCDD related effects on the hepatocytes or due to a direct effect on the biliary cells themselves. The observed bile duct proliferation, may represent a process of excessive and long term repair, following specific damage to hepatocytes, leading to the death of hepatocytes and perhaps also of the bile duct epithelium. The proliferative response may be a reparative response of proliferating hepatocytes, bile duct cells, and scar tissue (cholangiofibrosis). The inflammation also observed can produce oxidative stress that may also result in promotion of DNA damage[29-31]. Consequently, the oxidative stress may be only a secondary phenomenon due to the ongoing response to the toxic hepatopathy. In addition there may also be a direct stimulatory effect on the oval cells themselves, which is supported by the observed increases in oval cell hyperplasia. Since the oval cells may differentiate into both hepatocytes or biliary cells this may explain why both hepatocellular proliferative and biliary lesions are associated with exposure [32].
Given the commonality of these nonneoplastic effects of TCDD in the liver, there are possibly several reasons for the difference in the incidence of cholangiocarcinoma between the two studies. Firstly, the Dow study, in Spartan Sprague-Dawley rats, used dosed-feed compared to the NTP study in which animals were treated by gavage with TCDD in corn oil:acetone (99:1), 5 days per week. Gavage dosing with TCDD in corn oil compared to dosed feed may lead to higher transient doses of TCDD in the liver.. This could have occurred despite the observation of higher measured steady state liver levels of TCDD in the Dow dosedfeed study compared to the NTP gavage study (Figure 2). This could then lead to higher transient increases in AhR signaling pathways and/or other biological processed that impact on the development of the biliary lesions. Secondly, the increased incidence may be due to an interaction between the corn oil vehicle and TCDD in the toxicodynamics of TCDD action on the bile duct. However an NTP study of TCDD in Osborne Mendel rats that also used gavage with a corn oil: acetone vehicle with higher, twice weekly administered doses, did not exhibit an increased incidence of cholangiocarcinoma or cholangiofibrosis. Thirdly, and more plausibly, the current NTP study and the Dow study used different stocks of rat. While both were the Sprague-Dawley rat strain, the Dow study used the Spartan stock compared to the Harlan stock used in the present study. The Sprague-Dawley rat is an outbred stock. Moreover, the Spartan and Harlan stocks represent colonies established independently in the 1950s. In addition, even within the Harlan Sprague-Dawley stock, it has been documented that genetic drift from the same source colony can occur within a decade leading to differences in biological responses[33, 34]. Consequently, it seems most plausible that the minor differences between the NTP and the Dow study may be due to biological variation and differences in response between these different stocks of rats.
Figure 2.
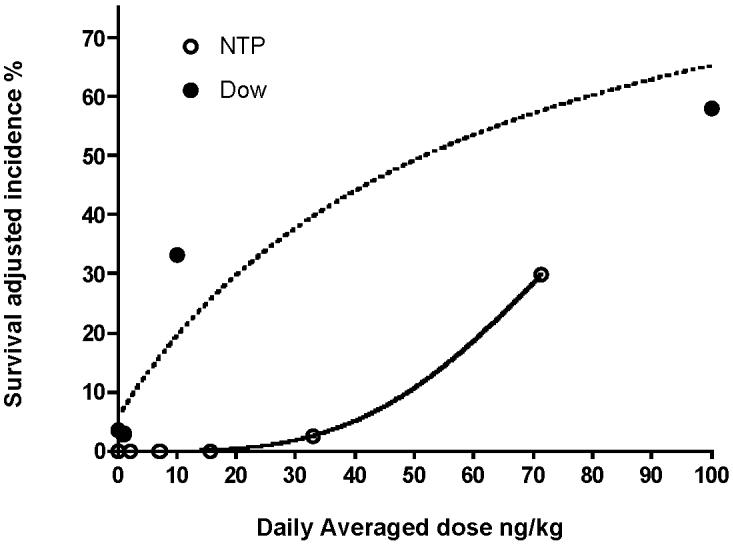
Comparison of representative dose responses of the incidences of hepatocellular adenoma/carcinoma in the NTP and Dow studies. Survival adjusted incidences for the Dow study were from Portier and Kohn [18] and based on the pathology reevaluation of Goodman and Sauer [19]. Dose is the daily averaged dose; in the case of the NTP study this is the 5 day dose administered dose averaged over the 7 days per week.
Comparing liver responses, the highest incidence of hepatocellular adenoma in the present study was lower than that seen in a reevaluation of the slides from the Dow study (Goodman and Sauer, 1992). In addition, no hepatocellular carcinomas were observed in the present NTP study whereas hepatocellular carcinomas were observed at the highest dose in the Dow study (9% incidence at 100 ng/kg). The terminal liver levels of TCDD in the present study are over 2-fold lower than that reported by Kociba et al. [9] in the Dow dosed feed study, which is most likely due to the different dosing regimens used and the consequential lower daily averaged administered dose used in the NTP study. Consequently the lower incidence of these neoplasms may primarily be a dose-response issue.
When comparing these studies it is important to compare incidences under similar pathology evaluations, especially for the liver. The initial report of data from the Dow indicated a 47% incidence of “hepatocellular hyperplastic nodule” in the 100 ng TCDD/kg group compared to a 9% incidence in control animals. Subsequent to that study there was an evolution of nomenclature for hepatocellular proliferative lesions, and a reevaluation of the slides from the study [19] using NTP guidelines and criteria for diagnosis of hepatocellular adenoma compared to altered hepatic foci. In that evaluation, neoplastic lesions were classified as adenoma or carcinoma. Using the newer nomenclature, the incidence of hepatocellular adenoma was 31% at the highest dose of 100 ng TCDD/kg. It is clear from the pathology reevaluations that some of the neoplastic nodules originally seen in the evaluation of the Dow study by Kociba et al. were hyperplastic in nature and subsequently considered indeed non-neoplastic. Considerable effort was made in the NTP TCDD study during the examination of the proliferative hepatocellular lesions and notably the diagnostic criteria for hepatocellular adenoma and nodular hyperplasia [35]. In some cases, the presence of hepatotoxicity made diagnosis of the incidence of hyperplasia and adenomas a challenge. A specific pathology review group of experts in the pathology of hepatocarcinogenicity was convened to examine these lesions and to provide guidance on the most appropriate classification and diagnosis. Given the nature of pathology evaluations, it is possible that there may have been some lesions in the Dow study that might have been interpreted differently by the group of pathologists that conducted the NTP TCDD study peer review.
One of the principal reasons for conducting the present comparison was that the data from the Dow study has in the past been used extensively for conducting quantitative cancer risk assessments for human exposure to TCDD. Moreover, the NTP TCDD study is the foundation for comparison to companion NTP bioassays of dioxin-like compounds that have recently been studied. While more recently human epidemiological data has been considered in developing cancer risk assessments, there is still debate on the appropriate weight to be given to the human studies. As such the Dow study dataset continues to serve as a suitable foundation for these assessments. Dose response comparison of the two studies showed that the shape of the dose response curve for increased incidence of adenomas was clearly different between the two studies with the increased incidence at 10 ng/kg in the Dow study being most notable. Specifically the data from the NTP appeared to be non linear whereas the data from the Dow study appeared to show linear behavior. At the 10ng/kg dose the incidence of adenomas was 18% in the Dow study, whereas at the comparable administered dose in the NTP study there were no observed neoplasms. Understanding the reason for these differences would provide insight into the extrapolation of this to the human scenario. Given that the dose of TCDD to the liver was similar in both studies at these lower doses, it is unlikely that this difference in liver neoplasm incidence was due to pharmacokinetic differences and is more likely related to the rat stock issues discussed above.
The differences in hepatic neoplastic response at the 10 ng/kg dose were also reflected in differences in the degree of hepatotoxicity. Toxic hepatopathy in the NTP study was similar to hepatotoxicity in the Dow study at the highest dose [9], but was markedly more severe in the Dow study at the 10 ng/kg dose. Comparison of the current NTP interim 14- week study to a 13-week subchronic gavage study of TCDD reported by Kociba et al. [36] indicates similar minor effects on increased liver weight, hepatic pathology and thymic atrophy suggesting that under a similar dosing regimen at a similar dose after only 13 weeks of exposure, there is not a significant difference between the Harlan and Spartan stocks. Indeed the livers in the 1 ng/kg and 10 ng/kg group were indistinguishable from controls whereas there were observable changes in the NTP study at 14 weeks indicating that if anything, there was a more pronounced effect of TCDD in the earlier stages of the NTP study. While there is no directly comparable data on AhR binding or another clearly AhR mediated response, these data do suggest that there appears to be no gross difference in susceptibility to the early subchronic effects of TCDD in these two strains of rat used in the two studies that would explain the differences in shape of the dose response for carcinogenesis. This would suggest that the differences in dose response behavior between the two studies is more likely due to biologically based differences in the more chronic preneoplastic and early neoplastic stages of carcinogenesis in the different rat stocks used.
Acknowledgements
The authors would like to thank all those involved in the conduct of these studies, with special thanks to Denise Orzech, Milton Hejtmancik, Cynthia Smith, Steve Graves, Rick Hailey, Amy Brix, Don Sells and Mike Jokinen. “We also thank Drs Raj Chhabra and Gary Boorman for critical review of this manuscript. This research was supported by the Intramural Research Program of the NIH, and NIEHS. The content of this paper reflect the opinions and views of the authors and do not represent the official views or policies of NIEHS, NIH or NTP.
Abbreviations used
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- PCB
Polychlorinated biphenyl
- PCDF
Polychlorinated dibenzofuran
- PeCDF
2,3,4,7,8-pentachlorodibenzofuran
- DLC
Dioxin like compound
- TEF
Toxic Equivalency factor
- CKE
Cystic keratinizing epithelioma
- SCC
Squamous cell carcinoma
- AhR
Aryl hydrocarbon receptor
- NTP
National Toxicology Program
- PWG
Pathology Working Group
References
- 1.IARC . IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 69. Polychlorinated dibenzo-para-dioxins and polychlorinated dibenzofurans. Vol. 69. IARC (International Agency for Research on Cancer); Lyon, France: 1997. [PMC free article] [PubMed] [Google Scholar]
- 2.Schmidt JV, Bradfield CA. Ann. Rev. Cell. Dev. Biol. 1996;12:55. doi: 10.1146/annurev.cellbio.12.1.55. [DOI] [PubMed] [Google Scholar]
- 3.Birnbaum LS. Prog Clin Biol Res. 1994;387:139. [PubMed] [Google Scholar]
- 4.NTP Natl Toxicol Program Tech Rep Ser. 2004;526 [Google Scholar]
- 5.NTP Natl Toxicol Program Tech Rep Ser. 2004;525 [Google Scholar]
- 6.NTP Natl Toxicol Program Tech Rep Ser. 2004;521 [Google Scholar]
- 7.NTP Natl Toxicol Program Tech Rep Ser. 2004;520 [Google Scholar]
- 8.Walker NJ, Crockett PW, Nyska A, Brix AE, Jokinen MP, Sells DM, Hailey JR, Easterling M, Haseman JK, Yin M, Wyde ME, Bucher JR, Portier CJ. Environ Health Perspect. 2005;113:43. doi: 10.1289/ehp.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kociba RJ, Keyes DG, Beyer JE, Carreon RM, Wade CE, Dittenber DA, Kalnins RP, Frauson LE, Park CN, Barnard SD, Hummel RA, Humiston CG. Toxicol. Appl. Pharmacol. 1978;46:279. doi: 10.1016/0041-008x(78)90075-3. [DOI] [PubMed] [Google Scholar]
- 10.USEPA . U.S. Environmental Protection Agency; Washington, DC: 2000. National Center for Environmental Assessment, Office of Research and Development. [Google Scholar]
- 11.Van Birgelen APJM, DeVito MJ, Orzech D, Walker N, Birnbaum LS, Bucher J, Lucier G. Dioxin ′97: 17th International Symposium on Chlorinated Dioxins and Related Compounds; Indianapolis, USA. 1997. p. 154. [Google Scholar]
- 12.Boorman GA, Haseman JK, Waters MD, Hardisty JF, Sills RC. Toxicol Pathol. 2002;30:88. doi: 10.1080/01926230252824752. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan E, Meier P. J. Am. Stat. Assoc. 1958;53:457. [Google Scholar]
- 14.Cox D. J.R. Stat. Soc. 1972;B34:187. [Google Scholar]
- 15.Tarone R. Biometrika. 1975;62:679. [Google Scholar]
- 16.Bailer AJ, Portier CJ. Biometrics. 1988;44:417. [PubMed] [Google Scholar]
- 17.Portier CJ, Bailer AJ. Fundam Appl Toxicol. 1989;12:731. doi: 10.1016/0272-0590(89)90004-3. [DOI] [PubMed] [Google Scholar]
- 18.Portier C, Kohn M. Organohalogen Compounds. 1996;29:222. [Google Scholar]
- 19.Goodman DG, Sauer RM. Regul. Toxicol. Pharmacol. 1992;15:245. doi: 10.1016/0273-2300(92)90036-9. [DOI] [PubMed] [Google Scholar]
- 20.Toyoshiba H, Walker NJ, Bailer AJ, Portier CJ. Toxicol Appl Pharmacol. 2004;194:156. doi: 10.1016/j.taap.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 21.Hailey JR, Walker NJ, Sells DM, Brix AE, Jokinen MP, Nyska A. Toxicol Pathol. 2005;33:165. doi: 10.1080/01926230590888324. [DOI] [PubMed] [Google Scholar]
- 22.Brix AE, Jokinen MP, Walker NJ, Sells DM, Nyska A. Toxiocologic Pathol. 2004;32:333. doi: 10.1080/01926230490431817. [DOI] [PubMed] [Google Scholar]
- 23.Jokinen MP, Walker NJ, Brix AE, Sells DM, Haseman JK, Nyska A. Cardiovascular Toxicol. 2003;3:299. doi: 10.1385/CT:3:4:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tani Y, Maronpot RR, Foley JF, Haseman JK, Walker NJ, Nyska A. Toxicol Pathol. 2004;32:41. doi: 10.1080/01926230490260952. [DOI] [PubMed] [Google Scholar]
- 25.Yoshizawa K, Walker NJ, Jokinen MP, Brix AE, Sells DM, Marsh T, Wyde ME, Orzech D, Haseman JK, Nyska A. Toxicol Sci. 2005;83:64. doi: 10.1093/toxsci/kfi016. [DOI] [PubMed] [Google Scholar]
- 26.Nyska A, Jokinen MP, Brix AE, Sells DM, Wyde ME, Orzech D, Haseman JK, Flake G, Walker NJ. Environ Health Perspect. 2004;112:903. doi: 10.1289/ehp.6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshizawa K, Marsh T, Foley JF, Cai B, Peddada S, Walker NJ, Nyska A. Toxicol Sci. 2005;85:594. doi: 10.1093/toxsci/kfi121. [DOI] [PubMed] [Google Scholar]
- 28.Boorman GA, Brockmann M, Carlton WW, Davis JM, Dungworth DL, Hahn FF, Mohr U, Reichhelm HB, Turusov VS, Wagner BM. Toxicol Pathol. 1996;24:564. doi: 10.1177/019262339602400505. [DOI] [PubMed] [Google Scholar]
- 29.Hassoun EA, Li F, Abushaban A, Stohs SJ. Toxicology. 2000;145:103. doi: 10.1016/s0300-483x(99)00221-8. [DOI] [PubMed] [Google Scholar]
- 30.Hassoun EA, Li F, Abushaban A, Stohs SJ. J Appl Toxicol. 2001;21:211. doi: 10.1002/jat.744. [DOI] [PubMed] [Google Scholar]
- 31.Hassoun EA, Wang H, Abushaban A, Stohs SJ. J Toxicol Environ Health A. 2002;65:825. doi: 10.1080/00984100290071054. [DOI] [PubMed] [Google Scholar]
- 32.Vessey CJ, de la Hall PM. Pathology. 2001;33:130. [PubMed] [Google Scholar]
- 33.Swerdlow NR, Platten A, Kim YK, Gaudet I, Shoemaker J, Pitcher L, Auerbach P. Pharmacol Biochem Behav. 2001;70:219. doi: 10.1016/s0091-3057(01)00598-6. [DOI] [PubMed] [Google Scholar]
- 34.Lawson DM, Haisenleder DJ, Gala RR, Moy JA. J Endocrinol. 1987;113:71. doi: 10.1677/joe.0.1130071. [DOI] [PubMed] [Google Scholar]
- 35.Hailey J, Walker N, Sells D, Brix A, Jokinen M, Nyska A. Toxicol Pathol. 2005;33:165. doi: 10.1080/01926230590888324. [DOI] [PubMed] [Google Scholar]
- 36.Kociba RJ, Keeler PA, Park CN, Gehring PJ. Toxicol Appl Pharmacol. 1976;35:553. doi: 10.1016/0041-008x(76)90078-8. [DOI] [PubMed] [Google Scholar]


