Abstract
Objectives To assess the diagnostic accuracy and clinical utility of a simplified low cost method for measuring absolute and percentage CD4 counts with flow cytometry.
Design A CD4 counting method (Blantyre count) using a CD4 and CD45 antibody combination with reduced blood and reagent volumes. Diagnostic accuracy was assessed by measuring agreement of the index test with two other assays (TruCount and FACSCount). Clinical utility was investigated by comparing CD4 counts with the new assay with WHO clinical staging in patients with HIV.
Setting Research laboratories and antiretroviral therapy clinic at a medical school and large government hospital in southern Malawi.
Participants Assay comparisons were performed on consecutive blood samples sent for CD4 counting from 129 patients with HIV. Comparison of CD4 count with staging was conducted on 253 consecutive new patients attending the antiretroviral therapy clinic.
Main outcome measures Limits of agreement with 95% confidence intervals between index test and reference standards.
Results The limits of agreement for Blantyre count and TruCount were excellent (cell count −48.9 to 27.0 ×109/l for absolute counts in the CD4 range <400×109/l and −2.42% to 2.37% for CD4 percentage). The assay was affordable with reagent costs per test of $0.44 (£0.22, €0.33) for both absolute count and CD4 percentage, and $0.11 for CD4 percentage alone. Of 193 patients with clinical stage I or II disease, who were ineligible for antiretroviral therapy by clinical staging criteria, 73 (38%) had CD4 counts <200×109/l. By contrast, 12 (20%) of 60 patients with stage III or IV disease had CD4 counts >350×109/l.
Conclusions This simplified method of counting CD4 cells with flow cytometry has good agreement with established commercial assays, is affordable for routine clinical use in Africa, and could improve clinical decision making in patients with HIV.
Introduction
In Malawi, a subSaharan African country with a population of 12 million, an estimated million people are infected with HIV.1 In 2004 the Ministry of Health embarked on an ambitious antiretroviral therapy programme. By the end of March 2007, 95 674 patients had started free antiretroviral therapy in public sector clinics,2 largely on the basis of a clinical diagnosis of WHO stage III or stage IV HIV/AIDS.3 Clinical events, however, do not fully predict immunological status.4 When clinical criteria alone are used, some patients with stage I and stage II disease and severe immune suppression will not receive the treatment they need, while others with stage III and IV disease may still have high CD4 T cell counts and the start of antiretroviral therapy might be delayed.5
CD4 counting could therefore improve appropriate allocation of antiretroviral therapy.6 Despite initiatives by the Clinton Foundation and others to reduce the price of the necessary reagents for developing nations to $3-6 (£1.5-3.0; €2.2-4.4) per test,7 this cost is still high for Africa.8 CD4 counting with flow cytometry is perceived by many to be too complex for use in Africa. For these reasons, CD4 counts are not routinely performed in Malawi.9 WHO guidelines state that where CD4 counting is available, adults and children over 5 years with HIV should start antiretroviral therapy as soon as their CD4 counts drop below 200×109/l, regardless of clinical staging.3 In children under 5 years, CD4 percentage of total lymphocyte count (CD4 percentage) varies less than absolute counts with age10 and so the percentage value is recommended to help decide on initiation of antiretroviral therapy.11
There are two main approaches for making CD4 counting more widely available in Africa: firstly, to reduce the cost of and simplify flow cytometric CD4 counting, and, secondly, to develop alternative counting methods. Flow cytometry, however, is the ideal method and has high accuracy.6 12 High throughput is possible as about 250 samples a day can be processed.8 Effective external quality assurance schemes are available in Africa with NEQAS (United Kingdom national external quality assessment scheme)13 and WHO CD4 REQAS/QASI (regional external quality assurance scheme/quality assessment and standardisation for immunological measures relevant to HIV/AIDS programme).14 Finally, flow cytometers can measure CD4 percentage as well as absolute counts. The main disadvantages are that flow cytometers are expensive and complex, reagent costs are high, and skilled laboratory staff are required.
Alternative counting methods include enzyme linked immunosorbent assays (ELISA),15 dried whole blood spots,16 lymphocyte rosetting,17 and magnetic beads.18 Such methods do not require complex equipment or the same level of staff training. The major disadvantage of such methods is poor ability to discriminate between CD4 T cells and monocytes, which also express CD4,19 low throughput, and poor ability to determine CD4 percentage. Reagent costs are similar to those of flow cytometric methods.8
Over recent years several technological developments have shown that flow cytometric CD4 counting could be more straightforward. “Primary CD4 gating” uses just one antibody against CD4 and side scattered light to discriminate between lymphocytes and monocytes.20 21 Gating of lymphocytes by using CD45 expression and side scattered light is more accurate and reproducible than using light scatter characteristics alone.22 23 Recently, CD45 has been used to gate all leucocytes on dual platforms, using a flow cytometer for CD4 percentage and a haematological analyser for absolute leucocyte counts.24 This “panleucogating” strategy has been modified for single platforms (flow cytometer alone) but remains primarily focused on total leucocytes rather than on lymphocytes.
We investigated whether these technologies could be miniaturised to reduce costs and applied them to the FACSCalibur flow cytometer. We developed a single platform method (the Blantyre count) that could be performed with reduced reagent costs and could accurately determine both absolute and percentage CD4 with increased simplicity compared with existing flow cytometric methods. We compared our method with the existing TruCount and FACSCount CD4 counting assays for diagnostic accuracy and assessed the potential impact on clinical decision making.
Methods
Setting
The study was conducted at the Malawi-Liverpool-Wellcome Trust Research Programme and Queen Elizabeth Central Hospital in Blantyre, the largest city in the southern region of Malawi. The estimated prevalence of HIV infection among adults in Blantyre district is 22%.25 All participants gave informed consent for CD4 counting.
Instrumentation
We used a FACSCalibur flow cytometer, a FACSCount instrument (both Becton Dickinson, CA, USA), and HmX haematological analyser (Beckman Coulter, CA, USA). We analysed flow cytometric data with CellQuest and MultiSet software.
Reference standards (TruCount and FACSCount assays)
We used TruCount26 and FACSCount27 assays for CD4 counts using Multitest CD3/8/45/4 kits with TruCount tubes and FACSCount reagent kits (Becton Dickinson) according to the manufacturer's instructions. TruCount assays use four antibodies, a complex subgating strategy, and tubes containing pre-pipetted beads. We chose TruCount as the reference standard because it is a commercial CD4 counting method that was developed to be used on the same instrument as the index test (Becton Dickinson FACSCalibur flow cytometer) and generates both absolute and percentage CD4. We used FACSCount as a second reference standard because it is one of the most widely used CD4 counting technologies in Africa. Both TruCount and FACSCount generate CD4 counts on a single platform, although FACSCount requires a lymphocyte count from a haematological analyser to generate CD4 percentage. Both assays are used by clinical laboratories throughout the world and have been validated by consistently high performance in external quality assurance schemes such as UK NEQAS13 over a period of years.
Index test (Blantyre count assay)
We used venous blood from healthy adults anticoagulated with EDTA to develop our assay. We used antihuman CD4 antibody conjugated with phycoerythrin (CD4-PE) and antihuman CD45 antibody conjugated with fluorescein isothiocyanate (CD45-FITC), FACS lysing solution (all Becton Dickinson), and CytoCount fluorescent microbeads (Dako, Denmark). Adjustable air-diplacement pipettes (Pipetman; Gilson, France) were used for all pipetting steps. The same pipette was used for reverse pipetting of blood and counting beads. CD4 T cell counts and total lymphocyte populations were determined by using staining for CD4 and CD45 with no attempt to gate total leucocytes or total T cells. We mixed 20 µl whole blood with 0.5 µl CD4-PE and 0.5 µl CD45-FITC antibodies and incubated samples for 15 minutes in the dark at room temperature. Red cells were lysed with 180 µl of 1× FACS lysing solution and incubated for a further 10 minutes. We added 10 µl of CytoCount beads by reverse pipetting before we ran samples through the cytometer. We used reverse pipetting for pipetting blood and counting beads as precise volumes are critical and this method is more accurate than conventional pipetting.12 Pipette calibration and pipetting accuracy were assessed by dispensing 10 µl and 20 µl aliquots of water on to a scientific balance.
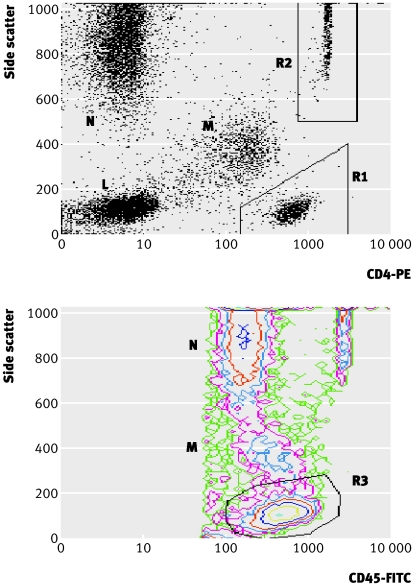
Bead events, 2000 per sample over about 60 seconds, were acquired by using a live gate with acquisition threshold set on the FL1 (FITC) channel. Analysis was performed with a CD4-PE against side scatter dot plot with manual gating of the CD4 T cell population (R1) and counting beads (R2) (fig 1 top) and a separate side scatter against CD45-FITC contour plot for manual gating of the total lymphocyte population (R3) (fig 1 bottom).
Fig 1 Gating strategies for determining absolute and percentage CD4 counts from flow cytometric data acquired with Blantyre count. R1=CD4 positive T lymphocytes, R2=CytoCount fluorescent microbeads, R3=total lymphocytes, M=monocytes, N=neutrophils, L=CD4 negative lymphocytes. Plots are for data acquired from same blood sample with CD4 count=199×109/l and CD4 percentage 17.6% with Blantyre count assay. Top: dot plot of side scattered light against CD4-PE (phycoerythrin). Absolute CD4 count=(R1/R2)×([beads] (beads/µl)/2); bottom: contour plot of side scattered light against CD45-FITC (fluorescein isothiocyanate). CD4 percentage=(R1 (from top)/R3)×100
We calculated absolute CD4 counts (×109/l) with the formula: (CD4 T cell events (R1)/bead events (R2))×([bead solution] (beads/µl)/2).
We calculated CD4 percentage with the formula: (CD4 T cell events (R1)/lymphocyte events (R3))×100.
We assessed repeatability of our assay by performing five repeats of the assay on four blood samples and examined stability of results with time by leaving a blood sample in the laboratory at room temperature and performing five repeats of the assay daily on the sample over five days.
Modified Blantyre count assays
We modified our assay to reduce costs further when only an absolute or percentage CD4 is required. The absolute CD4 count alone variant used CD4-PE antibody plus beads but without CD45-FITC antibody (Blantyre count (absolute)). The variant giving the CD4 percentage alone used CD4 and CD45 antibodies without counting beads (Blantyre count (percentage)).
CD4 counting comparison studies
In the main CD4 counting comparison study we included consecutive blood samples from patients with HIV sent to our laboratory for full blood count and CD4 count determination from 27 January to 17 February and from 18 April to 9 May 2006 (n=134). We measured CD4 and CD4 percentage for each sample using Blantyre count, Blantyre count (absolute), TruCount, and FACSCount assays.
We carried out a subsequent smaller study on consecutive blood samples from patients with HIV sent to the laboratory in June 2006 to compare CD4 percentages generated by Blantyre count and Blantyre count (percentage) assays (n=28). Samples were not used if they exhibited clots, were sent from outside Queen Elizabeth Central Hospital, or were received after the day of blood collection or if insufficient blood was available to complete all tests. There were no other selection criteria. Blood samples were analysed on the day that they were taken unless they were received after 4 pm in which case they were processed the next morning. Data collection was planned before the index tests and reference standards were performed. All blood samples from all participants meeting the inclusion criteria underwent the index and reference standard tests. No adverse events occurred from performing these tests.
Two authors (FS and JB) performed and read the FACSCount assay and full blood count. Two other authors (MKPL and CAM), both of whom had previous experience of flow cytometry, performed and read TruCount and Blantyre count assays together within six hours of the FACSCount assay. We have subsequently trained local laboratory technicians over two to three days to perform the Blantyre count method. Manual gating of events acquired with Blantyre count was performed blind to other results. As the FACSCount does not give CD4 percentages, we calculated this from the FACSCount absolute CD4 count and total lymphocyte count from the haematological analyser using the formula (CD4 (cells×109/l)/total lymphocyte count (cells×109/l))×100. This procedure is prone to error because CD4 percentage is generated on a dual platform setting, which is inherently more variable than single platform operations. For absolute CD4 counts, we assessed agreement only for samples with a TruCount CD4 count below 400×109/l as this is the relevant range for clinical decision making. For comparisons of CD4 percentage we used all samples.
Clinical utility study
We tested a further 253 EDTA anticoagulated venous blood samples from new patients attending the adult antiretroviral therapy clinic from May to July and from September to October 2006 for CD4 count using Blantyre count. CD4 counts and clinical staging were compared for each patient. Clinical staff in the antiretroviral therapy clinic performed staging blind to CD4 count results.
External quality assurance
To determine the accuracy of Blantyre count, we enrolled the assay for external quality assurance with the NEQAS immune monitoring scheme.13 CD4 results were determined on six NEQAS stabilised blood samples from the UK between January and May 2006.
Statistical analysis
We examined agreement between each pair of methods using Stata 9 by estimating bias and limits of agreement (=bias plus or minus 1.96×SD) with 95% confidence intervals as described by Bland and Altman.28 Repeatability was assessed with coefficients of variation obtained from five repeats of assays.
Results
Refinement of Blantyre count
With 20, 10, 5, 2, and 1 µl of control blood per Blantyre count assay, coefficients of variation were 4.2%, 4.2%, 6.1%, 8.1%, and 10.7%, respectively, showing a progressive increase in coefficients of variation below 10 µl of blood. There was a decrease in mean absolute CD4 count as blood volume was reduced (712, 681, 645, 539, and 448×109/l, respectively). CD4 percentages showed better overall repeatability (coefficients of variation 2.8% with 20 µl blood) and the coefficient of variation did not noticeably increase until blood volume was reduced to 2 µl (4.8%). We used 20 µl blood for our assay as twice the lowest volume at which optimal assay repeatability was maintained.
We used a similar process to determine optimal counting bead volume. With 10 µl counting beads the coefficient of variation was 4.2%, and similarly with 5 µl beads (4.3%), but increased to 8.9% and 5.7% with 2 and 1 µl beads, respectively. Mean CD4 counts were not affected by bead volume. We used 10 µl counting beads for our assay. Finally, we titrated down the quantities of antibody per assay. Discrimination of CD4 T cell and total lymphocytes as discrete populations was still possible down to 0.25 µl of CD4-PE and 0.25 µl CD45-FITC. We chose 0.5 µl of each antibody for use in our assay. Using these quantities, the costs of reagents per assay were $0.44 (£0.22, €0.33) for both absolute and percentage counts, $0.40 for an absolute CD4 count, and $0.11 for CD4 percentage alone (table 1).
Table 1.
Reagent costs per assay with flow cytometry for counting CD4 cells. Costs calculated with prices available to us in $ for all reagents
| CD4 assay | CD4-PE antibody | CD45-FITC antibody | Red cell lysing solution | Fluorescent beads* | Kit | Total |
|---|---|---|---|---|---|---|
| Blantyre count: | ||||||
| Absolute and percentage | 0.056 | 0.042 | 0.014 | 0.329 | 0.44 | |
| Absolute | 0.056 | — | 0.014 | 0.329 | — | 0.40 |
| Percentage | 0.056 | 0.042 | 0.014 | — | — | 0.11 |
| TruCount (Multitest) (absolute and percentage) | — | — | 0.036† | — | 5.00 | 5.04 |
| FACSCount (absolute) | — | — | — | — | 6.00 | 6.00 |
CD4-PE=phycoerythrin conjugated antihuman CD4 antibody; CD4-FITC=fluorescein isothiocyanate conjugated antihuman CD45 antibody.
*Allowing for 12 µl/assay with reverse pipetting.
†Not provided with kit; 450 µl of 1× lysing solution required/assay.
Characteristics of patients
In agreement studies we tested blood samples from 130 patients with HIV. One sample gave an absolute CD4 count >2000×109/l with FACSCount and was excluded from subsequent analyses. There were no indeterminate or missing results. The median age of patients was 33 years (range 2-75 years); 79 were females; and 38 (29%) were taking antiretroviral therapy at the time of testing. Of the 253 new patients for whom CD4 count was compared with clinical staging in the antiretroviral therapy clinic, the median age was 33 (range 14-71), and 173 were female. None was taking antiretroviral therapy. We used a subset of 28 of these patients for the separate comparison of Blantyre count with the Blantyre count (percentage) variant. The median age in this subset was 35 years (range 24-64 years), and 18 were female.
Absolute CD4 counts in agreement studies
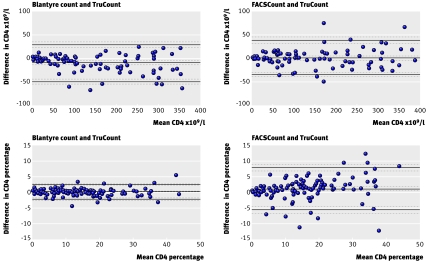
The median CD4 count was 193×109/l (range 0 to 1884×109/l) with TruCount. The mean bias when we used Blantyre count rather than TruCount for samples with a CD4 count of <400×109/l was −11.0×109/l for Blantyre count. Similarly, low biases were found for other assay comparisons: 1.2×109/l for FACSCount compared with TruCount, and 5.8×109/l for Blantyre count (absolute) compared with Blantyre count (table 2). Limits of agreement were −48.9 to 27.0×109/l for Blantyre count and TruCount and were within the range −55 to 40×109/l for all other assay comparisons (table 2, fig 2).
Table 2.
Estimated bias and limits of agreement, with 95% confidence intervals for pairs of flow cytometric methods used to measure absolute and percentage CD4 cell counts
| Assay comparison | Bias (95% CI) | Limits of agreement | |
|---|---|---|---|
| Lower limit (95% CI) | Upper limit (95% CI) | ||
| Absolute CD4 | |||
| Blantyre count and TruCount | −11.0 (−14.9 to −7.1) | −48.9 (−55.7 to −42.1) | 27.0 (20.2 to 33.8) |
| FACSCount and TruCount | 1.2 (−2.6 to 4.9) | −35.4 (−41.9 to −28.8) | 37.7 (31.2 to 44.3) |
| Blantyre count and FACSCount | −12.1 (−16.6 to −7.7) | −54.8 (−62.4 to −47.1) | 30.5 (22.8 to 38.1) |
| Blantyre count and Blantyre count (absolute) | −5.8 (−9.3 to −2.3) | −39.3 (−45.3 to −33.3) | 27.7 (21.7 to 33.7) |
| CD4 percentage | |||
| Blantyre count and TruCount | −0.03 (−0.24 to 0.19) | −2.42 (−2.78 to −2.05) | 2.37 (2.00 to 2.73) |
| FACSCount* and TruCount | 0.92 (0.32 to 1.52) | −5.83 (−6.87 to −4.79) | 7.66 (6.62 to 8.70) |
| Blantyre count and FACSCount* | −0.94 (−1.53 to −0.35) | −7.56 (−8.57 to −6.54) | 5.67 (4.66 to 6.69) |
| Blantyre count and Blantyre count (percentage) | 0.01 (−0.26 to 0.28) | −1.35 (−1.82 to −0.88) | 1.37 (0.90 to 1.84) |
*CD4 percentages with FACSCount calculated from absolute CD4 count by FACSCount and total lymphocyte count from haematological analyser.
Fig 2 Comparison of CD4 counts determined with three flow cytometric methods using 96 blood samples from patients with HIV with CD4 counts <400×109/l for absolute CD4 cell counts (×109/l) and 129 blood samples for CD4 cell counts as a percentage of total lymphocyte count (CD4 percentage). FACSCount CD4 percentage was obtained from FACSCount absolute CD4 counts and total lymphocyte counts from a haematological analyser. Black lines depict bias and upper and lower limits of agreement. Grey broken lines denote 95% confidence intervals for these values
CD4 percentage in agreement studies
The median CD4 percentage was 13.0% (range 0.0-44.0%) using TruCount. Agreement between CD4 percentage generated by Blantyre count and TruCount was excellent over the full range of values, with a bias of −0.03% and limits of agreement −2.42% to 2.37%. Comparison of either Blantyre count or TruCount CD4 percentage with values generated using FACSCount showed poorer agreement, with a bias of 0.92% and limits of agreement −5.83% to 7.66% for FACSCount and TruCount and a bias of −0.94% and limits of agreement −7.56% to 5.67% for Blantyre count and FACSCount. Blantyre count and the Blantyre count (percentage) variant could be used interchangeably for CD4 percentage with excellent limits of agreements (table 2, fig 2).
Repeatability of Blantyre count
We calculated coefficients of variation on five repeats of our assay on four blood samples with mean CD4 values 718, 712, 260, and 191×109/l and mean CD4 percentage 40.3%, 42.9%, 15.0%, and 13.8%. Mean (SD) coefficients of variation were 5.2% (2.7%) for absolute CD4 count and 2.5% (0.8%) for CD4 percentage.
Accuracy of Blantyre count
In tests on six stabilised blood samples (CD4 count 117-1269×109/l and percentage 7.28%-60.73%) from NEQAS with our assay, five of six absolute CD4 counts and five of six CD4 percentages were within 1 SD of the NEQAS value, with one result of six between 1 and 2 SD of this value for each test. Blantyre count values were on average 95% of the absolute NEQAS CD4 count and 97% of the CD4 percentage.
Stability of aged samples
CD4 T cell and lymphocyte populations could be clearly distinguished and gated over the five days of the stability study, with a blood sample with day 1 CD4 count of 487×109/l and CD4 percentage of 36.1%. Daily coefficients of variation for absolute counts remained below 6% and for CD4 percentage below 2.5%. The mean absolute CD4 count stayed within 10% and the CD4 percentage within 5% of the day 1 values.
Clinical staging and CD4 counts for new patients attending antiretroviral therapy clinic
Of the new patients attending the antiretroviral therapy clinic, 76% (193/253) were clinical stage I (n=77) or stage II (n=116), while 24% (60/253) had stage III (n=51) or stage IV (n=9) HIV/AIDS. The range of CD4 counts in each group was wide with a progressive fall in median CD4 counts from stage I (286×109/l) to stage IV groups (110×109/l). Twenty five (32%) patients with stage I disease and 48 (42%) with stage II disease had a CD4 count <200×109/l. Eleven (22%) patients with stage III and one (11%) patient with stage IV HIV/AIDS had a CD4 count >350×109/l (table 3).
Table 3.
Comparison of clinical staging with absolute CD4 count with Blantyre count for new patients seen at antiretroviral clinic
| Clinical stage | No (%) of patients | Median (range) CD4×109/l | No (%) with CD4×109/l | |||
|---|---|---|---|---|---|---|
| <200 | 200-350 | >350 | ||||
| I | 77 (30) | 286 (20-1020) | 25 (32) | 25 (32) | 27 (35) | |
| II | 116 (46) | 249 (4-1261) | 48 (42) | 41 (35) | 27 (23) | |
| III | 51 (20) | 149 (11-826) | 29 (57) | 11 (22) | 11 (22) | |
| IV | 9 (4) | 110 (16-552) | 7 (78) | 1 (11) | 1 (11) | |
| All | 253 | 239 (4-1261) | 110 (43) | 78 (31) | 65 (26) | |
Discussion
Within Malawi, we have developed an affordable accurate method of counting CD4 cells with flow cytometry by refining and miniaturising existing technology. Increasing affordability by reducing reagent costs is a critical step in making this available in countries with limited resources. Currently the reagent cost of a comparable commercially available flow cytometric assay in Africa is $5.04 (£2.52, €3.74). As we were able to reduce costs of reagents to $0.44 (£0.22, €0.33) per assay, there is the potential for 91% cost savings. This would increase to 98% if only CD4 percentage is required but would decrease if the costs of competing tests are reduced.
Cost reduction was not achieved at the expense of accuracy. Over the CD4 count range of 0-400×109/l, our assay showed minimal bias and excellent agreement compared with established CD4 counting methods (TruCount and FACSCount). Determination of CD4 percentage by Blantyre count and TruCount methods showed excellent agreement over the full range of CD4 percentages. The good performance of Blantyre count in the NEQAS immunophenotyping scheme further shows the accuracy of this method.
As well as reducing the assay price, the modifications in our assay have simplified CD4 counting with flow cytometry. Use of a primary CD4 gating strategy avoids extra sub-gating steps involving the CD3 or CD45 cell populations as performed by other established methods, including TruCount/Multitest and Panleucogating technologies.24 26 It has proved straightforward to train technicians to gate the CD4 T cells (R1), counting beads (R2), and total lymphocyte populations (R3) using CD4/side scatter and CD45/side scatter dot plots (fig 1).
Strengths and weakness of study
Our study validates the use of a simplified, affordable, and accurate method of CD4 counting with flow cytometry. Unlike many previous studies of affordable flow cytometry,20 21 22 24 we carried out this work in a country where affordability is of chief importance. We looked at both absolute and percentage CD4, which have previously been neglected. The limits of agreement are similar to those of previous comparison studies of flow cytometry and narrower than studies using other methods,15 16 17 18 which are inherently less accurate. By miniaturising the present assay, we managed to reduce reagent costs further compared with previous studies.
Even with the simplifications introduced, however, CD4 counting with flow cytometry requires a level of technical skill not always present in resource poor settings,9 a reliable power supply, and a cold chain for reagent supplies. A flow cytometer represents a large capital outlay, which is not always feasible, although donor funding is sometimes available to help provide such instruments.
Users of FACSCount and other methods that provide only the absolute CD4 count have needed to use total lymphocyte counts (usually from haematological analysers) to obtain the CD4 percentage. The poor agreement between CD4 percentage obtained by combined FACSCount plus haematological analysis when compared with CD4 percentage produced by either Blantyre count or TruCount (table 2, fig 2) shows the inaccuracy of this laborious “reversed dual platform” approach. It is well recognised that flow cytometers and haematological analysers can produce significantly different total lymphocyte counts for the same blood sample.12 24
The excellent agreement between Blantyre count and TruCount assays indicates that use of CD3 antibody by TruCount is redundant in CD4 counting with flow cytometry. This means that Blantyre count technology could be operated on less complex instruments than the FACSCalibur, deploying only one laser and three photomultiplier tubes to detect side scattered light and fluorescence emitted from FITC and PE fluorochromes. Such an instrument could be manufactured at lower cost compared with the FACSCalibur and would be simpler and less expensive to maintain. Even on five day old blood, our gating strategy enabled both CD4 T cells and lymphocytes to be easily discriminated from monocytes, thereby maintaining good repeatability with little variability from day one counts.
Blantyre count could make the greatest impact on the care of children under 5 with HIV. Appropriate determination of CD4 percentage has often been neglected by investigators seeking to produce affordable CD4 counting.6 8 Determination of CD4 percentage alone by the Blantyre count (percentage) variant is not only much cheaper than determining absolute CD4 counts but also technically easier, as accurate volumetric pipetting and counting beads are not required. CD4 percentages were also more stable than absolute counts over five days in the same sample.
The determination of CD4 counts with Blantyre count in the antiretroviral therapy clinic confirms that use of WHO clinical staging criteria alone for deciding who should start antiretroviral therapy is suboptimal. About a third of patients with clinical stage I or II disease who would not be eligible for antiretroviral therapy on clinical grounds were severely immunosuppressed with a CD4 count of <200×109/l. Conversely, two fifths of patients with stage III and IV disease who were eligible to start antiretroviral therapy had CD4 counts >200×109/l and a fifth had counts >350×109/l. Clinical staging alone is missing many patients who urgently need to start antiretroviral therapy, while some stage III and IV patients are started on antiretroviral therapy when treatment could possibly be postponed.
What would it cost?
Consideration of the economic feasibility of using the Blantyre count in Malawi has to include the capital cost of the flow cytometer (about $100 000), the annual service contract (about $10 000), and the salary of a laboratory technician (typical monthly salary $500) as well as reagent prices. The contribution of these non-reagent costs to the total cost per assay is inversely proportional to assay throughput. With the cost of such an instrument spread over 10 years, CD4 counting with flow cytometry would not be viable if only 200 samples were run on an instrument each month, as “non-reagent costs” per sample would be $10.83. If, however, 200 samples are run on a flow cytometer each day, which is well within the capacity of the instrument (over 250 days this would be 50 000 samples a year), non-reagent costs are $0.52 per sample, giving a total assay cost of $0.96. Therefore, use of the Blantyre count method would be most cost effective with a limited number of flow cytometers operating at high sample throughput in regional centres and a coordinated system for transporting samples to these centres from peripheral clinics.
We have described an affordable accurate method of CD4 counting that has the potential to improve clinical decision making in the treatment of patients with HIV and service the whole of a country the size of Malawi using a limited number of instruments in regional centres. This arrangement could be facilitated by the use of blood stabilising agents such as Transfix, permitting delays in sending samples to regional centres.29 It remains to be seen whether such a service could be successfully implemented in such a resource poor setting.
What is already known on this topic
CD4 counting is the main laboratory investigation for monitoring people with HIV but is often deemed too expensive and too complex to perform in resource poor settings
CD4 counting with flow cytometry can be made more affordable by the use of simple technical modifications, but CD4 percentages required in children under 5 years and miniaturisation of blood and reagent volumes have received little attention
What this study adds
Technical modifications of flow cytometry with miniaturisation can simplify and reduce the cost of absolute and percentage CD4 counts while maintaining diagnostic accuracy
This CD4 counting method could improve clinical decision making in patients with HIV disease in settings with limited resources
We thank Emily Lifa for blood sampling in the antiretroviral therapy clinic at Queen Elizabeth Central Hospital and all staff at the antiretroviral therapy clinic for their assistance with this study.
Contributors: CAMacL was responsible for the study conception, coordinated the study, drafted the initial manuscript, and is guarantor. CAMacL, MKPL, MEM, MTD, JJGvO, MJM, and EEZ contributed to the study design. MKPL and CAMacL optimised the Blantyre count method. CAMacL, MKPL, FS, and JB did flow cytometric assays. CAMacL, SAW, and MKPL analysed the data. All authors contributed to the writing of the manuscript and approved the final version.
Funding: CAMacL holds a training fellowship in clinical tropical medicine from the Wellcome Trust (No 067902/Z/02/Z). MKPL received a travel award from the British Society for Immunology. MEM holds a programme grant from the Wellcome Trust (No 074124/Z/04/Z).
Competing interests: None declared.
Ethical approval: College of Medicine research and ethics committee.
Peer review and provenance: Non-commissioned, externally peer reviewed.
References
- 1.National AIDS Commission. National estimates of HIV/AIDS in Malawi Lilongwe, Malawi: National AIDS Commission, 2003. www.aidsmalawi.org.mw/resources/Summary%20of%20national%20estimate%202003.pdf
- 2.Ministry of Health. HIV Unit, ART quarterly report for March 2007 Lilongwe, Malawi: Ministry of Health, 2007
- 3.WHO. Interim WHO clinical staging of HIV/AIDS and HIV/AIDS case definitions for surveillance. African region Geneva: World Health Organization, 2005. www.who.int/hiv/pub/guidelines/clinicalstaging.pdf
- 4.Kassa E, Rinke de Wit TF, Hailu E, Girma M, Messele T, Mariam HG, et al. Evaluation of the World Health Organization staging system for HIV infection and disease in Ethiopia: association between clinical stages and laboratory markers. AIDS 1999;13:381-9. [DOI] [PubMed] [Google Scholar]
- 5.Zachariah R, Teck R, Ascurra O, Humblet P, Harries AD. Targeting CD4 testing to a clinical subgroup of patients could limit unnecessary CD4 measurements, premature antiretroviral treatment and costs in Thyolo District, Malawi. Trans R Soc Trop Med Hyg 2006;100:24-31. [DOI] [PubMed] [Google Scholar]
- 6.WHO. CD4+ T-cell enumeration technologies. Technical information Geneva: World Health Organization, 2005. www.who.int/diagnostics_laboratory/CD4_Technical_Advice_ENG.pdf
- 7.Schoof M. HIV-test makers agree to discounts for poorer nations. Wall Street Journal 14 Jan 2004. www.aegis.com/news/wsj/2004/WJ040104.html
- 8.UNICEF, UNAIDS, MSF, WHO. Sources and prices of selected medicines and diagnostics for people living with HIV/AIDS. June 2005 report and December 2006 data http://www.unicef.org/supply/index_8362.html
- 9.Harries AD, Schouten EJ, Libamba E. Scaling up antiretroviral treatment in resource-poor settings. Lancet 2006;367:1870-2. [DOI] [PubMed] [Google Scholar]
- 10.Comans-Bitter WM, de Groot R, van den Beemd R, Neijens HJ, Hop WC, Groeneveld K, et al. Immunophenotyping of blood lymphocytes in childhood. Reference values for lymphocyte subpopulations. J Paediatr 1997;130:388-93. [DOI] [PubMed] [Google Scholar]
- 11.WHO. Antiretroviral therapy of HIV infection in infants and children in resource-limited settings: towards universal access Geneva: World Health Organization, 2006. www.who.int/hiv/pub/guidelines/WHOpaediatric.pdf
- 12.Brando B, Barnett D, Janossy G, Mandy F, Autran B, Rothe G, et al. Cytofluorometric methods for assessing absolute numbers of cell subsets in blood. European Working Group on Clinical Cell Analysis. Cytometry 2000;42:327-46. [DOI] [PubMed] [Google Scholar]
- 13.Whitby L, Granger V, Storie I, Goodfellow K, Sawle A, Reilly JT, et al. Quality control of CD4+ T-lymphocyte enumeration: results from the last 9 years of the United Kingdom national external quality assessment scheme for immune monitoring (1993-2001). Cytometry 2002;50:102-10. [DOI] [PubMed] [Google Scholar]
- 14.Mandy F, Bergeron M, Houle G, Bradley J, Fahey J. Impact of the international program for quality assessment and standardization for immunological measures relevant to HIV/AIDS: QASI. Cytometry 2002;50:111-6. [DOI] [PubMed] [Google Scholar]
- 15.Loua A, Kestens L, Vanham G, Boel L, Colebunders R, Gigase P, et al. Validity of an ELISA test for CD4+ T lymphocyte count and validity of total lymphocyte count in the assessment of immunodeficiency status in HIV infection. Ann Soc Belg Med Trop 1994;74:61-8. [PubMed] [Google Scholar]
- 16.Mwaba P, Cassol S, Pilon R, Chintu C, Janes M, Nunn A, et al. Use of dried whole blood spots to measure CD4+ lymphocyte counts in HIV-1-infected patients. Lancet 2003;362:1459-60. [DOI] [PubMed] [Google Scholar]
- 17.Nouanthong P, Pata S, Sirisanthana T, Kasinrerk W. A simple manual rosetting method for absolute CD4+ lymphocyte counting in resource-limited countries. Clin Vaccine Immunol 2006;13:598-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diagbouga S, Chazallon C, Kazatchkine MD, Van de Perre P, Inwoley A, M'Boup S, et al. Successful implementation of a low-cost method for enumerating CD4+ T lymphocytes in resource-limited settings: the ANRS 12-26 study. AIDS 2003;17:2201-8. [DOI] [PubMed] [Google Scholar]
- 19.Janossy G. Dried blood spot technology for CD4+ T-cell counting. Lancet 2004;363:1074. [DOI] [PubMed] [Google Scholar]
- 20.Sherman GG, Galpin JS, Patel JM, Mendelow BV, Glencross DK. CD4+ T cell enumeration in HIV infection with limited resources. J Immunol Methods 1999;222:209-17. [DOI] [PubMed] [Google Scholar]
- 21.Janossy G, Jani I, Göhde W. Affordable CD4+ T-cell counts on ‘single-platform' flow cytometers I. Primary CD4 gating. Br J Haematol 2000;111:1198-208. [DOI] [PubMed] [Google Scholar]
- 22.Gelman R, Wilkening C. Analyses of quality assessment studies using CD45 for gating lymphocytes for CD3+CD4+%. Cytometry 2000;42:1-4. [DOI] [PubMed] [Google Scholar]
- 23.Schnizlein-Bick CT, Mandy FF, O'Gorman MRG, Paxton H, Nicholson JKA, Hultin LE, et al. Use of CD45 gating in three and four-color flow cytometric immunophenotyping: guideline from the National Institute of Allergy and Infectious Diseases, Division of AIDS. Cytometry 2002;50:46-52. [DOI] [PubMed] [Google Scholar]
- 24.Glencross D, Scott LE, Jani IV, Barnett D, Janossy G. CD45-assisted PanLeucogating for accurate, cost-effective dual-platform CD4+ T-cell enumeration. Cytometry 2002;50:69-77. [DOI] [PubMed] [Google Scholar]
- 25.National Statistical Office (NSO) [Malawi], and ORC Macro. Malawi demographic and health survey 2004. HIV/AIDS prevalence Calverton, Maryland: NSO and ORC Macro, 2005
- 26.Schnizlein-Bick CT, Spritzler J, Wilkening CL, Nicholson JKA, O'Gorman MRG. Evaluation of TruCount Absolute-Count tubes for determining CD4 and CD4 numbers in human immunodeficiency virus-positive adults. Clin Diagn Lab Immunol 2000;7:336-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopez A, Caragol I, Candeias J, Villamor N, Echaniz P, Ortuño F, et al. Enumeration of CD4+ T-cells in the peripheral blood of HIV-infected patients: an interlaboratory study of the FACSCount system. Cytometry 1999;38:231-7. [DOI] [PubMed] [Google Scholar]
- 28.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;i:307-31. [PubMed]
- 29.Jani IV, Janossy G, Iqbal A, Mhalu FS, Lyamuya EF, Biberfeld G, et al. Affordable CD4+ T cell counts by flow cytometry II. The use of fixed whole blood in resource-poor settings. J Immunol Methods 2001;257:145-54. [DOI] [PubMed] [Google Scholar]