Abstract
Doppel (Dpl) is a prion protein paralog that causes neurodegeneration when expressed ectopically in the brain. To investigate the cellular mechanism underlying this effect, we analyzed Dpl-expressing transgenic mice in which the gene for the proapoptotic protein Bax had been deleted. We found that Bax deletion does not alter either clinical symptoms or Purkinje cell degeneration in Dpl transgenic mice. In addition, we observed that degenerating Purkinje cells in these animals do not display DNA fragmentation or caspase-3 activation. Our results suggest that non-Bax-dependent pathways mediate the toxic effects of Dpl in Purkinje cells, highlighting a possible role for nonapoptotic mechanisms in the death of these neurons.
Transmissible spongiform encephalopathies are fatal neurodegenerative disorders of humans and animals caused by infectious proteins called prions.1,2 Mammalian prions are composed of PrPSc, a conformationally altered isoform of a normal cellular glycoprotein called PrPC. Prions propagate by the templated autocatalytic conversion of PrPC into PrPSc. Although a great deal is known about the role of PrPSc in the infectious transmission of prion diseases, much less is understood about PrPC.3 The PrPC molecule of ∼250 amino acids consists of a flexible, N-terminal tail and a structured C-terminal half composed of three α-helices and two β-strands flanking the first helix.4 The N-terminal tail contains a series of five octapeptide repeats and a highly conserved hydrophobic segment that serves as a transmembrane anchor under some circumstances.5,6 The C terminus of PrPC is attached to the cell membrane via a glycosyl-phosphatidylinositol anchor.7,8
Analysis of the region surrounding the PrP gene (Prn-p) uncovered a second gene (Prn-d) that encodes a PrP-like protein called Doppel (Dpl, German for “double”).9 Dpl is a protein of 179 amino acids that is homologous to the structured C-terminal half of PrP and lacks the flexible N-terminal tail. Dpl is normally expressed primarily in testis, where it seems to play a role in spermatogenesis.10,11 However, several lines of PrP knockout (Prn-p0/0) mice display ectopic expression of Dpl in the central nervous system (CNS) because of aberrant mRNA splicing between the adjacent Prn-p and Prn-d genes.9,12,13,14 Surprisingly, these mice, but not those lines of Prn-p0/0 mice without up-regulation of Dpl, develop a severe neurodegenerative disorder characterized by ataxia and loss of cerebellar Purkinje cells. Interestingly, this phenotype is completely suppressed by the presence of the Prn-p gene. Subsequent studies of Dpl transgenic mice and Prn-d0/0 mice confirmed that expression of Dpl in the CNS is sufficient to produce neurodegeneration and that the neurotoxic effect of Dpl is antagonized in a dose-dependent fashion by coexpression of PrP.12,15,16,17,18 Dpl causes loss of both Purkinje and granule cells in the cerebellum, depending on the cell type in which the protein is expressed.15,16 Dpl also produces a leukoencephalopathy characterized by axon loss and myelin degeneration.19
Although it is not essential for propagation of prions,20,21 Dpl is likely to provide important clues to the normal physiological function of PrPC. Interestingly, transgenic mice expressing PrP forms deleted for portions of the N-terminal tail (Δ32–121, Δ32–134, Δ94–134, and Δ105–125) display neurodegenerative phenotypes that are suppressible by coexpression of wild-type PrP, as is the case for mice expressing Dpl in the CNS.22,23,24 These observations suggest that Dpl and the deleted forms of PrP act via a similar neurotoxic mechanism and that the N-terminal tail of PrP (in particular, residues 105 to 125) is capable of suppressing a potent neurotoxic activity that resides in the C-terminal half of both PrPC and Dpl.
What are the neurotoxic pathways activated by Dpl? To begin to address this question, we have analyzed how the phenotype of Tg(Dpl) mice is affected by deleting the gene that encodes Bax. Bax is a proapoptotic member of the Bcl-2 family that plays a major role in regulating cell death in the CNS, both during development and following injury.25,26 Bax is a cytoplasmic protein that translocates to mitochondria in response to extrinsic or intrinsic signals, thereby causing release of cytochrome c and subsequent activation of caspases.27,28 In this study, we find that Bax deletion does not alter clinical symptoms or Purkinje cell degeneration in Tg(Dpl) mice, suggesting that the neurotoxic effects of Dpl occur independently of Bax, possibly via nonapoptotic processes.
Materials and Methods
Mice
Tg(N-Dpl) mice (line 32)29 and Bax0/0 mice30 have been previously described. Prn-p0/0 mice,31 which do not spontaneously develop ataxia, were obtained from Charles Weissmann (The Scripps Research Institute, Jupiter, FL) and Tg(F35) mice22 from Adriano Aguzzi (University of Zurich, Zurich, Switzerland). All of these mice were maintained on a C57BL/6J × CBA/J hybrid background. Lurcher mice were purchased from the Jackson Laboratory (Bar Harbor, ME).
To generate mice for this study, we first crossed Dpl+/0 Prn-p+/0 Bax+/+ males with Prn-p+/+ Bax0/0 females and recovered Dpl+/0 Prn-p+/0 Bax+/0 offspring. Males of the latter genotype were then mated to Prn-p0/0 Bax0/0 females to produce littermate offspring in groups 1 to 4 (see Results). Prn-p0/0 Bax0/0 mice were produced by mating Prn-p+/+ Bax0/0 females to Prn-p0/0 Bax+/+ males and then intercrossing the resulting Prn-p+/0 Bax+/0 offspring. Prn-p0/0 Bax+/0 mice were generated by crossing Prn-p0/0 Bax0/0 females to Prn-p0/0 Bax+/+ males.
Mice were genotyped by polymerase chain reaction analysis of tail DNA prepared using the Puregene DNA Isolation Kit (Gentra Systems, Inc., Minneapolis, MN). Primer pairs for Prn-p,32 Dpl,29 and Bax30 have been previously been reported. Ataxia was assessed using a set of objective criteria as described previously.32
Histology
Mice were anesthetized and perfused transcardially with 40 ml of 0.9% (w/v) NaCl and then with 40 ml of 4% paraformaldehyde in 0.1 mol/L phosphate-buffered saline (PBS), pH 7.35. Brains were removed and postfixed in 4% paraformaldehyde for 1 hour and then transferred to PBS for 24 hours at 4°C. After freezing, sagittal sections (14-μm thickness) were cut on a cryostat starting from the midline of each bisected brain. Sections were floated in PBS and stored at 4°C before staining.
Staining was performed on free-floating cryostat sections. Sections were permeabilized in PBS containing 0.1% Triton X-100 for 15 to 30 minutes, followed by blocking with 1% bovine serum albumin-PBS for 1 hour, all at room temperature. Sections were then incubated overnight at 4°C with antibodies directed against the following antigens: calbindin (1:1000 dilution; Sigma Chemical Co., St. Louis, MO); glial fibrillary acidic protein (1:1000 dilution; Dako, Carpinteria, CA); activated caspase-3 (1:1000 dilution; Cell Signaling Technology, Danvers, MA). Antibodies were diluted in PBS containing 0.1% bovine serum albumin and 0.1% Triton X-100. Sections were washed in PBS and then incubated with Alexa Fluor 488- or Alexa Fluor 594-labeled anti-IgG antibody (1:100 dilution; Invitrogen, Carlsbad, CA) for 45 minutes at room temperature. After further washing in PBS, sections were mounted on slides and imaged with a Zeiss LSM 510 confocal fluorescence microscope. Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) was performed on calbindin-stained cryostat sections using the In Situ Cell Death Detection Kit according to the manufacturer’s directions (Roche Diagnostics, Indianapolis, IN).
Purkinje Cell Counts
Counts were performed in cerebellar lobule IV, which displayed consistent Purkinje cell loss in all sections analyzed. Serial cryostat sections (14-μm thickness) were cut from each half brain beginning at the midline. The first section to show all of the cerebellar lobules was identified, and then every third consecutive section after that one was collected for a total of six sections. Sections were stained for calbindin as described above, and the total number of Purkinje cells residing in lobule IV of all six sections was counted. The position of the first section used for counting varied by <28 μm (plus or minus one section thickness). The six sections used for counting spanned a total thickness of 224 μm in each half cerebellum.
Western Blotting
Brain homogenates 10% (w/v) in PBS were centrifuged at 1000 × g for 10 minutes, and the supernatant was collected. Total protein concentration was determined using BCA protein assay kit (Pierce Biotechnology, Rockford, IL). Two hundred micrograms of protein was analyzed in each lane of 12% sodium dodecyl sulfate-polyacrylamide electrophoresis gels. After transfer, the blot was blocked in 5% nonfat dried milk-PBS and then probed with primary antibodies to PrP (8H433), Dpl,34 Bax (Santa Cruz Biotechnology, Santa Cruz, CA), Bak (Sigma Chemical Co.), or β-actin (Chemicon, Temecula, CA). Blots were developed using an enhanced chemiluminescence system (Amersham Biosciences, Piscataway, NJ).
Results
Bax Deletion Does Not Affect the Clinical Phenotype of Tg(Dpl) Mice
The Tg(Dpl) mice used in this study (N-Dpl, line 32) express Dpl in the CNS driven by the neural-specific enolase (NSE) promoter.29 On the Prn-p0/0 background, these mice develop ataxia at ∼60 days of age, accompanied by massive degeneration of cerebellar Purkinje cells. This phenotype is rescued in a dose-dependent fashion by coexpression of wild-type PrP encoded by the endogenous Prn-p allele.
To determine whether Bax inactivation affects the neurodegenerative phenotype of Tg(Dpl) mice, we generated four groups of mice with the following genotypes, as described in Materials and Methods: Dpl+/0 Prn-p0/0 Bax+/0 (group 1); Dpl+/0 Prn-p0/0 Bax0/0 (group 2); Dpl+/0 Prn-p+/0 Bax+/0 (group 3); and Dpl+/0 Prn-p+/0 Bax0/0 (group 4). We did not observe any clinical or histological differences between mice from groups 1 and 3 (which are Bax+/0) and mice with the same genotypes but carrying two Bax alleles (Bax+/+) (data not shown). Thus, one intact Bax allele is sufficient for production of the Tg(Dpl) phenotype. In the experiments described below, we therefore used Bax+/0 mice (groups 1 and 3) as littermate controls to compare with Bax0/0 mice (groups 2 and 4).
Group 1 mice, which carry a single Bax gene, developed ataxia beginning at 56 ± 5 days of age and became terminally ill at 157 ± 35 days (Table 1), similar to the values reported previously.29 Group 2 mice, which lack Bax expression, showed almost identical times of symptom onset and terminal illness (55 ± 6 and 154 ± 30 days, respectively). Introduction of a single endogenous Prn-p allele significantly delayed the onset of ataxia and development of terminal illness to ∼260 and >365 days, respectively, regardless of the presence of a copy of the Bax gene. Thus, deletion of the Bax gene had no significant effect on the clinical phenotype of Tg(Dpl) mice.
Table 1.
Bax Deletion Does Not Affect the Clinical Phenotype of Tg(Dpl) Mice
| Genotype | Symptom onset | Death |
|---|---|---|
| Group 1 (Dpl+/0Prn-p0/0 Bax+/0) | 56 ± 5 (46) | 157 ± 35 (9) |
| Group 2 (Dpl+/0Prn-p0/0 Bax0/0) | 55 ± 6 (31) | 154 ± 30 (11) |
| Group 3 (Dpl+/0Prn-p+/0 Bax+/0) | 266 ± 40 (6) | >365 (12) |
| Group 4 (Dpl+/0Prn-p+/0 Bax0/0) | 261 ± 52 (5) | >365 (10) |
Ages at symptom onset and death are given as mean number of days ± SD, with the number of animals in each group in parentheses. Mice in groups 3 and 4 were still alive at the time of writing.
Bax Deletion Does Not Prevent Purkinje Cell Degeneration in Tg(Dpl) Mice
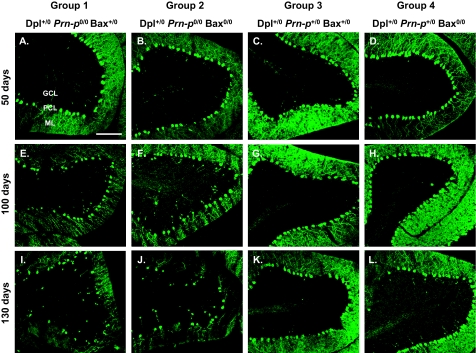
To determine whether Bax deletion had any effect on the neuropathology of Tg(Dpl) mice, we stained brain sections with an antibody to calbindin to visualize Purkinje cells, the major neuronal type that undergoes degeneration in these animals. Mice were analyzed during the presymptomatic, symptomatic, and terminal phases of illness (50, 100, and 130 days of age, respectively). In group 1 animals, Purkinje cell numbers decreased progressively from 50 to 130 days (Figure 1, A, E, and I). Calbindin-positive deposits in the granule cell layer and underlying white matter could also be observed (Figure 1, E and I). These most likely represent swellings or torpedoes in the axons of degenerating Purkinje cells, a feature common in other cerebellar mutant mice with Purkinje cell loss.35 There was also a decrease in the staining of Purkinje cell dendrites in the molecular layer as the illness progressed (Figure 1, A, E, and I). Deletion of Bax (group 2) did not have any observably effect on the development of these pathological features (Figure 1, B, F, and J). The presence of a single Prn-p allele suppressed Purkinje cell degeneration, regardless of whether or not Bax was present (Figure 1, C, D, G, H, K, and L). We did not observe any loss of granule neurons in Tg(Dpl) mice from any of the four experimental groups (data not shown).
Figure 1.
Bax deletion does not prevent Purkinje cell degeneration in Tg(Dpl) mice. Mice from group 1 (A, E, and I), group 2 (B, F, and J), group 3 (C, G, and K), and group 4 (D, H, and L) were sacrificed at 50 days (A–D), 100 days (E–H), and 130 days (I–L) of age. Brain sections were stained with anti-calbindin antibody to visualize cerebellar Purkinje cells. A representative area from lobule IV is shown for each brain. Progressive loss of Purkinje cells occurs between 50 and 130 days of age in Dpl+/0 Prn-p0/0 mice and is not affected by Bax deletion (compare groups 1 and 2). GCL, granule cell layer; PCL, Purkinje cell layer; ML, molecular layer. Scale bar = 100 μm.
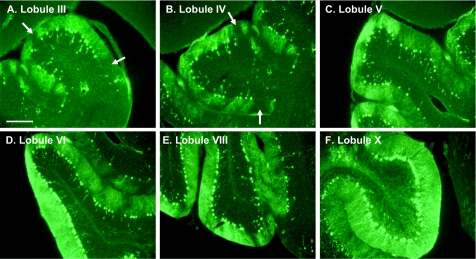
We observed that loss of Purkinje cells in Tg(Dpl) mice was not uniform in all parts of the cerebellum. Purkinje cell loss was most severe in lobules III to V, with lobules VI to VIII showing moderate loss, and lobules IX and X remaining relatively unaffected (Figure 2). This gradient of Purkinje cell degeneration was apparent at all stages of the disease but was most obvious during the middle of the clinical phase (100 days of age). A similar sparing of Purkinje cells in lobules IX and X has been observed in other mice expressing Dpl in the CNS.12,16 Even within a single lobule, Purkinje cell loss was often patchy, with some areas being completely devoid of Purkinje cells, other areas showing calbindin-positive torpedoes of degenerating Purkinje cell axons, and still other areas displaying a relatively intact Purkinje cell layer. Areas with fewer Purkinje cells showed diminished calbindin staining of the molecular layer, due to the absence of Purkinje cell dendrites (Figure 2, A and B, arrows). Nonuniform loss of Purkinje cells in a parasagittally oriented stripe-like pattern has been observed in several cerebellar mouse mutants and is thought to reflect differential sensitivity of cells residing in different anatomical compartments.36
Figure 2.
Degeneration of Purkinje cells in Tg(Dpl) mice is asymmetric. Brain sections from a group 2 mouse at 100 days of age were stained with anti-calbindin antibody to visualize cerebellar Purkinje cells. Representative images from lobules III (A), IV (B), V (C), VI (D), VIII (E), and X (F) are shown. At this stage, lobules III to V show severe Purkinje cell loss (A–C), lobules VI and VIII are less affected (D and E), and lobule X is spared (F). Purkinje cell loss was not uniform within a lobule; Purkinje cell-free regions showed reduced calbindin staining of the adjacent molecular layer due to the absence of Purkinje cell dendrites (arrows in A and B). Scale bar = 150 μm.
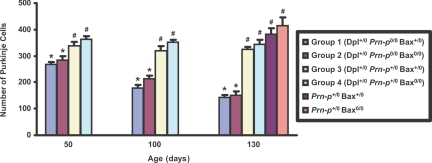
To quantitate Purkinje cell loss, the number of Purkinje cells in lobule IV of the cerebellum was counted in six regularly spaced serial sections from three mice of each genotype (Figure 3). Group 1 mice (Bax+/0) showed a progressive loss of Purkinje cells beginning as early as 50 days of age (before development of symptoms), with about 50% of the cells being lost by 130 days (in the terminal phase). Purkinje cell loss in group 2 mice (Bax0/0) was almost the same as in group 1 mice at 50, 100, and 130 days of age. The presence of a single Prn-p allele (groups 3 and 4) effectively preserved Purkinje cells, so that at 130 days their numbers were almost the same as those in mice lacking the Dpl transgene (Figure 3, bars labeled Prn-p+/0 Bax+/0 and Prn-p+/0 Bax0/0). We noted that Purkinje cell number was consistently 5 to 10% higher in Bax0/0 mice compared with Bax+/0 mice. This difference was observable in Dpl+/0 mice whether or not the Prn-p gene was present (compare group 1 versus group 2, group 3 versus group 4), and was also seen in the absence of the Dpl transgene (compare Prn-p+/0 Bax+/0 versus Prn-p+/0 Bax0/0 at 130 days). This difference is probably due to a rescuing effect of Bax deletion on developmental death of Purkinje cells, a phenomenon described previously.37 Taken together, the quantitative results confirm the lack of effect of Bax deletion on Dpl-induced Purkinje cell loss.
Figure 3.
Quantitation of Purkinje cell loss in Tg(Dpl) mice. Purkinje cells were counted in six serial sections from lobule IV of the cerebellum from three mice of each of the indicated genotypes, as described in Materials and Methods. Bars represent the mean ± SD. Means for bars marked with an asterisk are statistically different (P < 0.01, Student’s t-test) from means for bars marked with a number sign. Means for groups 1 and 2 are not statistically different from each other at 50, 100, or 130 days.
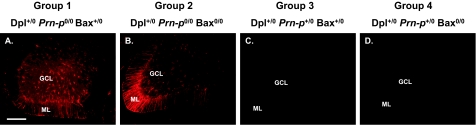
Purkinje cell degeneration in Tg(Dpl) mice is accompanied by a marked astrocytic reaction in the molecular and granule cell layers of the cerebellum, including prominent hypertrophy of radial Bergmann glial fibers, as revealed by staining for glial fibrillary acidic protein (Figure 4A). This pathology is not altered by deletion of Bax (Figure 4B). As expected, introduction of a Prn-p allele prevented the astrocytic reaction (Figure 4, C and D).
Figure 4.
Bax deletion does not affect astrocytosis in the cerebellum of Tg(Dpl) mice. A–D:Brain sections from mice of the indicated genotypes at 130 days of age were stained with an antibody to glial fibrillary acidic protein. GCL, granule cell layer; ML, molecular layer. Scale bar = 100 μm.
Purkinje Cell Death in Tg(Dpl) Mice Occurs Independently of Detectable DNA Cleavage and Caspase-3 Activation
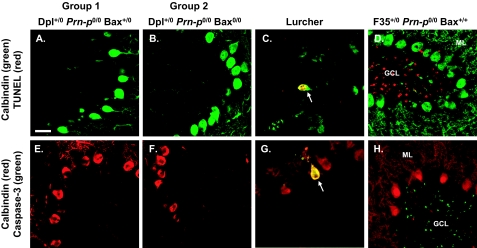
Bax-mediated apoptosis is typically accompanied by internucleosomal cleavage of DNA and activation of caspase-3.27,28 To test for these changes in the cerebella of Tg(Dpl) mice, we stained brain sections using the TUNEL method and using an antibody that selectively recognizes the cleaved form of caspase-3. We failed to observe any Purkinje or granule cells that were positive for TUNEL or activated caspase-3 in cerebellar lobule IV of Tg(Dpl) mice from groups 1 or 2 at 100 days of age (Figure 5, A, B, E, and F). Purkinje cells are undergoing extensive degeneration in lobule IV at this stage (Figure 1). To be sure that we had not missed positively stained cells at other ages or in other lobules, we analyzed the entire cerebellar cortex (lobules II to X) of groups 1 and 2 mice at 30, 100 and 180 days of age, corresponding to the presymptomatic, symptomatic, and terminal phases of illness. We again failed to detect any TUNEL-positive or activated caspase-3-positive cells (data not shown). Because the loss of Purkinje cells occurs in a gradient from lobules III to X (see above), it is likely that our analysis would have captured cells in several different stages of degeneration. As controls for the staining procedures, we analyzed cerebellar sections from Lurcher mice, which display apoptosis of Purkinje cells due to a mutation in the δ2 glutamate receptor,38 and from Tg(F35) mice, which show apoptosis of granule cells due to expression of an N-terminally truncated form of PrP.22,39 Positive staining for TUNEL and activated caspase-3 was evident in degenerating Purkinje cells (0.1 to 1 per ×20 field) in Lurcher mice (Figure 5, C and G) and in degenerating granule cells (20 to 50 per ×20 field) in Tg(F35) mice (Figure 5, D and H).
Figure 5.
Purkinje cell death in Tg(Dpl) mice occurs independently of detectable DNA cleavage and caspase-3 activation. Brain sections were prepared from the following mice at 100 days of age: group 1 (A and E), group 2 (B and F), Lurcher (C and G), and Tg(F35) (D and H). Sections were stained for calbindin (green) and TUNEL (red) (A–D) or for calbindin (red) and activated caspase-3 (green) (E–H). A representative area from cerebellar lobule IV is shown for each brain. Arrows in C and G indicate Purkinje cells in Lurcher mice that are positive for TUNEL and activated caspase-3, respectively. In contrast, no Purkinje cells positive for these markers are seen in groups 1 or 2 mice (A, B, E, and F). Numerous granule cells stained by TUNEL and for activated caspase-3 are apparent in the granule cell layer of Tg(F35) mice (D and H). GCL, granule cell layer; ML, molecular layer. Scale bar = 20 μm.
Bax Deletion Does Not Alter Expression Levels of Dpl or Bak
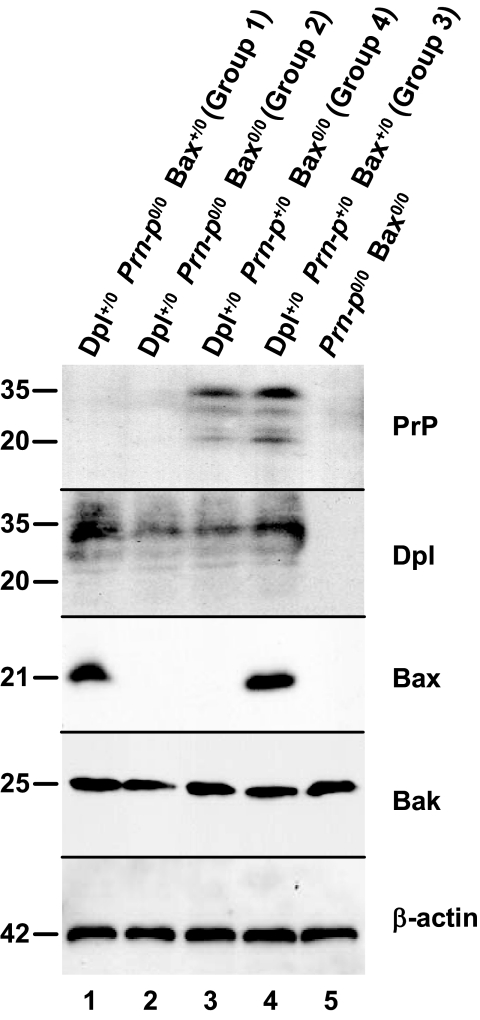
Western blotting was used to analyze the levels of PrP, Dpl, Bax, and Bak in brain homogenates prepared from Tg(Dpl) mice (Figure 6). Bak, a proapoptotic member of the Bcl-2 family, is expressed as an alternatively spliced BH3-only isoform in postnatal neurons.40 β-Actin was used as a loading control. Groups 1 to 4 mice all expressed Dpl at similar levels (lanes 1 to 4), but only groups 3 and 4 mice expressed PrP (lanes 3 and 4). Bax was present only in groups 1 and 3 mice (lanes 1 and 4), whereas Bak was present at similar levels in all mice (lanes 1 to 5). Prn-p0/0 Bax0/0 mice lacking the Dpl transgene did not express PrP, Dpl, or Bax (lane 5). Thus, the lack of effect of Bax deletion on the Tg(Dpl) phenotype is not because of compensatory up-regulation of Bak or Dpl. We also found that levels of Bcl-2 were similar in mice from groups 1 to 4 (not shown), suggesting that expression of antiapoptotic family members are not altered by Bax deletion.
Figure 6.
Bax deletion does not alter expression levels of Dpl or Bak. Brain homogenates from mice of the indicated genotypes were analyzed by Western blotting using antibodies directed against PrP, Dpl, Bax, Bak, or β-actin (as a loading control). Molecular size markers are given in kilodaltons.
Discussion
In this study, we have tested whether Bax plays a role in the neurodegenerative phenotype produced in transgenic mice by neuronal expression of the PrP paralog Dpl. Bax is a multidomain, proapoptotic member of the Bcl-2 family, which is known to play a major role in mitochondrially mediated apoptosis in the CNS, including in the cerebellum.25,26,41 We found that deletion of both copies of the Bax gene had no effect on the development of clinical symptoms in Tg(Dpl) mice or on the characteristics of Purkinje cell degeneration seen in these animals.
Bax-Independent Purkinje Cell Death in Tg(Dpl) Mice
Why does Purkinje cell degeneration in Tg(Dpl) mice occur independently of Bax? Purkinje cells have been shown to express Bax,42 which appears to be the only multidomain, proapoptotic regulator present in neurons.40 Moreover, Bax has been demonstrated to play a role in developmental death of Purkinje cells. Thus, elimination of Bax partially rescues the apoptotic death of Purkinje cells that is thought to occur naturally during the embryonic and postnatal periods, causing as much as a 30% increase in total Purkinje cell number.37 Therefore, Purkinje cells possess the molecular machinery required for execution of a Bax-dependent, mitochondrially mediated pathway of apoptosis.
Presumably, Dpl must be activating alternative non-Bax-dependent pathways in Purkinje cells. Current thinking suggests that cells die via three kinds of processes: apoptosis, autophagy, and necrosis.27,43 Apoptosis can involve the intrinsic (mitochondrial) pathway, which is dependent on Bcl-2 family members such as Bax, or the extrinsic pathway, which is initiated by cell surface death receptors.27 Thus, Dpl may be acting via an extrinsic apoptotic pathway, which in some cell types can induce death without amplification by the mitochondrial pathway.44 Alternatively, Dpl may activate autophagic or necrotic mechanisms.
Evidence from mouse cerebellar mutants indicates that Purkinje cells possess several non-Bax-dependent pathways for neuronal death. For example, elimination of Bax does not prevent postnatal degeneration of Purkinje cells in Lurcher mice, which express a mutant form of the δ2 glutamate receptor.45,46 Recent work has demonstrated that Purkinje cell death in Lurcher mice occurs via an autophagic process mediated by the protein Beclin-1.47,48 Autophagy is an organelle engulfment process that is usually considered to be distinct from apoptosis.43 Interestingly, however, dying Purkinje cells in Lurcher animals display caspase activation and DNA fragmentation, characteristics of apoptosis.38,49 Elimination of Bax prevented caspase activation and DNA fragmentation, even though Purkinje cell death was not blocked.46,50 Thus, it has been suggested that both autophagic and apoptotic processes play a role in Purkinje cell degeneration in Lurcher mice, as well as in other cerebellar mutants.51
Possible Nonapoptotic Neuronal Death in Tg(Dpl) Mice
Interestingly, we did not observe DNA cleavage or caspase-3 activation in Purkinje cells of Tg(Dpl) mice, suggesting the absence of apoptosis in these cells. Purkinje cells are known to contain pro-caspase-3.38 In addition, caspase-3 activation and DNA fragmentation have been documented in these cells during developmentally regulated death, as well as in many cerebellar mouse mutants including Lurcher, Purkinje cell degeneration (pcd), Toppler, Woozy, Tambaleante, and Sticky.51,52 Thus, Purkinje cells are capable of undergoing caspase-mediated apoptotic death. Of course, it is possible that our failure to detect DNA cleavage and caspase-3 activation in Tg(Dpl) mice is attributable to the relatively small number of Purkinje cells present in the cerebellum (∼220,000),37 the extended time course over which cell loss occurred (>50 days), or the rapid engulfment and elimination of apoptotic cells. Apoptosis is much easier to appreciate in the granule cell layer, which contains a much larger number of cells (3 × 107).37 Arguing against these possibilities, however, we monitored staining for TUNEL and activated caspase-3 throughout the whole cerebellum from 30 to 180 days, corresponding to the entire course of Purkinje cell degeneration. Because the loss of Purkinje cells occurs asymmetrically in different lobules of the cerebellum (Figure 2), our analysis is likely to have captured cells in several different stages of degeneration. As a positive control, we were able to capture small numbers of apoptotic Purkinje cells in the brains of Lurcher mice. If Purkinje cells in Tg(Dpl) mice are dying in the absence of caspase activation and DNA fragmentation, this would suggest the involvement of nonapoptotic processes, perhaps autophagic mechanisms as in Lurcher mice. Analysis of Tg(Dpl) brain tissue using markers for these alternative death pathways will help shed light on this possibility.
Comparison of Neuronal Degeneration Induced by Dpl and N-Terminally Deleted PrP
In a recently published study, we analyzed the effect of Bax elimination on the neurodegenerative phenotypes produced in Tg mice by expression of either of two N-terminally deleted forms of PrP, Δ32–134 or Δ105–125 (collectively referred to here as ΔPrP).39 We found that deletion of Bax slowed but did not prevent clinical illness and development of neuropathology induced by PrP Δ32–134 and had no effect on neurodegeneration induced by PrP Δ105–125. We thus concluded that ΔPrP activates both Bax-dependent and Bax-independent neurotoxic pathways, with the latter assuming a dominant role in the terminal stage of the disease. Because Dpl structurally resembles ΔPrP and because each of these proteins induces neurodegeneration that is suppressible by coexpression of wild-type PrP, it is likely that they activate related neurotoxic pathways. Indeed, we have postulated that these proteins interact with a common receptor that serves to transduce the toxic signal, with PrP Δ105–125 having the highest affinity for the receptor.23 The Bax-independent mechanisms engaged by both Dpl and ΔPrP may represent the downstream elements of such a common cellular program.
In contrast to the Tg(NSE-Dpl) mice analyzed here, mice expressing PrP Δ32–134 and PrP Δ105–125 display neuronal degeneration with prominent apoptotic features, including caspase-3 activation and DNA fragmentation.22,23,39 This discrepancy between the effects of Dpl and of ΔPrP may reflect the different neuronal populations that are impacted by the two kinds of proteins. Neuronal loss in Tg(ΔPrP) mice involves primarily cerebellar granule cells with sparing of Purkinje cells, whereas Tg(NSE-Dpl) mice show Purkinje cell loss with sparing of granule cells. It is thus possible that granule cells and Purkinje cells differ in how they read out the death signals initiated by Dpl and ΔPrP. The fact that granule cell death induced by PrP Δ32–134 includes a Bax-dependent component, whereas Dpl-induced death of Purkinje cells is entirely Bax-independent, may also reflect the presence of different cell death pathways in these two neuronal cell types. Indeed, there are prominent differences between granule and Purkinje cells in how they respond to several other kinds of death-inducing stimuli.41
The different pathologies observed in Tg(NSE-Dpl) and Tg(ΔPrP) mice are probably attributable to expression in different neuronal cell types, rather than to intrinsic differences in the neurotoxic activities of the two kinds of proteins. Dpl mRNA expression in Tg(NSE-Dpl) mice is detectable in Purkinje cells but not in granule cells,29 consistent with other evidence that the NSE promoter is more active in Purkinje than in granule cells.53,54 This restricted expression pattern presumably explains the Purkinje cell-specific pathology seen in Tg(NSE-Dpl) mice. However, when expression is driven by a Prn-p cosmid, Dpl causes TUNEL-positive apoptosis of granule cells as well as degeneration of Purkinje cells.15 Conversely, expression of PrP Δ32–134 using the Purkinje cell-specific L7 promoter causes Purkinje cell degeneration.55 It would be of interest to determine whether neuronal loss in the latter two lines of mice is Bax-independent, as it is in Tg(NSE-Dpl) animals, or whether it also includes a Bax-dependent component as in Tg(PrP Δ32–134) mice.
Conclusion
Taken together, our results highlight an important role for Bax-independent pathways in the neurotoxicity of Dpl and raise the possibility that Dpl activates nonapoptotic mechanisms of neuronal death in Purkinje cells. It will be important now to define further the relevant neurotoxic pathways, to identify the commonalities between the actions of Dpl and ΔPrP, and to determine how the activities of these proteins manifest themselves in different neuronal and non-neuronal cell types. It is likely that this information will provide important clues to the normal functions of Dpl and PrPC and how subversion of PrPC activity might contribute to prion-induced neurodegeneration.
Acknowledgments
We thank Charles Weissmann for Prn-p0/0 mice and Adriano Aguzzi for Tg(F35) mice. 8H4 antibody was kindly supplied by Man-Sun Sy. We are grateful to Cheryl Adles and Su Deng for mouse colony maintenance and genotyping and to Maolei Xiao for help with cryostat sectioning.
Footnotes
Address reprint requests to David A. Harris, Department of Cell Biology and Physiology, Washington University School of Medicine, 660 South Euclid Ave., St. Louis, MO 63110. E-mail: dharris@wustl.edu.
Supported by National Institutes of Health grant NS040975 (to D.A.H.) and a Research on Specific Diseases Grant from the Japanese Ministry of Health, Labor, and Welfare (to S.S.).
References
- Aguzzi A, Polymenidou M. Mammalian prion biology: one century of evolving concepts. Cell. 2004;116:313–327. doi: 10.1016/s0092-8674(03)01031-6. [DOI] [PubMed] [Google Scholar]
- Prusiner SB. Prions. Proc Natl Acad Sci USA. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergard L, Christensen HM, Harris DA: The cellular prion protein (PrPC): its physiological function and role in disease. Biochim Biophys Acta 2007, [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn R, Liu A, Luhrs T, Riek R, von Schroetter C, Lopez Garcia F, Billeter M, Calzolai L, Wider G, Wüthrich K. NMR solution structure of the human prion protein. Proc Natl Acad Sci USA. 2000;97:145–150. doi: 10.1073/pnas.97.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart RS, Drisaldi B, Harris DA. A transmembrane form of the prion protein contains an uncleaved signal peptide and is retained in the endoplasmic reticulum. Mol Biol Cell. 2001;12:881–889. doi: 10.1091/mbc.12.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde RS, Mastrianni JA, Scott MR, Defea KA, Tremblay P, Torchia M, DeArmond SJ, Prusiner SB, Lingappa VR. A transmembrane form of the prion protein in neurodegenerative disease. Science. 1998;279:827–834. doi: 10.1126/science.279.5352.827. [DOI] [PubMed] [Google Scholar]
- Narwa R, Harris DA. Prion proteins carrying pathogenic mutations are resistant to phospholipase cleavage of their glycolipid anchors. Biochemistry. 1999;38:8770–8777. doi: 10.1021/bi990736c. [DOI] [PubMed] [Google Scholar]
- Stahl N, Baldwin MA, Hecker R, Pan K-M, Burlingame AL, Prusiner SB. Glycosylinositol phospholipid anchors of the scrapie and cellular prion proteins contain sialic acid. Biochemistry. 1992;31:5043–5053. doi: 10.1021/bi00136a600. [DOI] [PubMed] [Google Scholar]
- Moore RC, Lee IY, Silverman GL, Harrison PM, Strome R, Heinrich C, Karunaratne A, Pasternak SH, Chishti MA, Liang Y, Mastrangelo P, Wang K, Smit AF, Katamine S, Carlson GA, Cohen FE, Prusiner SB, Melton DW, Tremblay P, Hood LE, Westaway D. Ataxia in prion protein (PrP)-deficient mice is associated with upregulation of the novel PrP-like protein doppel. J Mol Biol. 1999;292:797–817. doi: 10.1006/jmbi.1999.3108. [DOI] [PubMed] [Google Scholar]
- Behrens A, Genoud N, Naumann H, Rulicke T, Janett F, Heppner FL, Ledermann B, Aguzzi A. Absence of the prion protein homologue Doppel causes male sterility. EMBO J. 2002;21:3652–3658. doi: 10.1093/emboj/cdf386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paisley D, Banks S, Selfridge J, McLennan NF, Ritchie AM, McEwan C, Irvine DS, Saunders PT, Manson JC, Melton DW. Male infertility and DNA damage in Doppel knockout and prion protein/Doppel double-knockout mice. Am J Pathol. 2004;164:2279–2288. doi: 10.1016/S0002-9440(10)63784-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi D, Cozzio A, Flechsig E, Klein MA, Rülicke T, Aguzzi A, Weissmann C. Onset of ataxia and Purkinje cell loss in PrP null mice inversely correlated with Dpl level in brain. EMBO J. 2001;20:694–702. doi: 10.1093/emboj/20.4.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S, Katamine S, Nishida N, Moriuchi R, Shigematsu K, Sugimoto T, Nakatani A, Kataoka Y, Houtani T, Shirabe S, Okada H, Hasegawa S, Miyamoto T, Noda T. Loss of cerebellar Purkinje cells in aged mice homozygous for a disrupted PrP gene. Nature. 1996;380:528–531. doi: 10.1038/380528a0. [DOI] [PubMed] [Google Scholar]
- Li A, Sakaguchi S, Atarashi R, Roy BC, Nakaoke R, Arima K, Okimura N, Kopacek J, Shigematsu K. Identification of a novel gene encoding a PrP-like protein expressed as chimeric transcripts fused to PrP exon 1/2 in ataxic mouse line with a disrupted PrP gene. Cell Mol Neurobiol. 2000;20:553–567. doi: 10.1023/a:1007059827541. [DOI] [PubMed] [Google Scholar]
- Moore RC, Mastrangelo P, Bouzamondo E, Heinrich C, Legname G, Prusiner SB, Hood L, Westaway D, DeArmond SJ, Tremblay P. Doppel-induced cerebellar degeneration in transgenic mice. Proc Natl Acad Sci USA. 2001;98:15288–15293. doi: 10.1073/pnas.251550798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L, Rossi D, Linehan J, Brandner S, Weissmann C. Transgene-driven expression of the Doppel protein in Purkinje cells causes Purkinje cell degeneration and motor impairment. Proc Natl Acad Sci USA. 2004;101:3644–3649. doi: 10.1073/pnas.0308681101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida N, Tremblay P, Sugimoto T, Shigematsu K, Shirabe S, Petromilli C, Erpel SP, Nakaoke R, Atarashi R, Houtani T, Torchia M, Sakaguchi S, DeArmond SJ, Prusiner SB, Katamine S. A mouse prion protein transgene rescues mice deficient for the prion protein gene from Purkinje cell degeneration and demyelination. Lab Invest. 1999;79:689–697. [PubMed] [Google Scholar]
- Genoud N, Behrens A, Miele G, Robay D, Heppner FL, Freigang S, Aguzzi A. Disruption of Doppel prevents neurodegeneration in mice with extensive Prnp deletions. Proc Natl Acad Sci USA. 2004;101:4198–4203. doi: 10.1073/pnas.0400131101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radovanovic I, Braun N, Giger OT, Mertz K, Miele G, Prinz M, Navarro B, Aguzzi A. Truncated prion protein and Doppel are myelinotoxic in the absence of oligodendrocytic PrPC. J Neurosci. 2005;25:4879–4888. doi: 10.1523/JNEUROSCI.0328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens A, Brandner S, Genoud N, Aguzzi A. Normal neurogenesis and scrapie pathogenesis in neural grafts lacking the prion protein homologue Doppel. EMBO Rep. 2001;2:347–352. doi: 10.1093/embo-reports/kve088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuzi NL, Gall E, Melton D, Manson JC. Expression of doppel in the CNS of mice does not modulate transmissible spongiform encephalopathy disease. J Gen Virol. 2002;83:705–711. doi: 10.1099/0022-1317-83-3-705. [DOI] [PubMed] [Google Scholar]
- Shmerling D, Hegyi I, Fischer M, Blättler T, Brandner S, Götz J, Rülicke T, Flechsig E, Cozzio A, von Mering C, Hangartner C, Aguzzi A, Weissmann C. Expression of amino-terminally truncated PrP in the mouse leading to ataxia and specific cerebellar lesions. Cell. 1998;93:203–214. doi: 10.1016/s0092-8674(00)81572-x. [DOI] [PubMed] [Google Scholar]
- Li A, Christensen HM, Stewart LR, Roth KA, Chiesa R, Harris DA. Neonatal lethality in transgenic mice expressing prion protein with a deletion of residues 105–125. EMBO J. 2007;26:548–558. doi: 10.1038/sj.emboj.7601507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann F, Tolnay M, Brabeck C, Pahnke J, Kloz U, Niemann HH, Heikenwalder M, Rülicke T, Bürkle A, Aguzzi A. Lethal recessive myelin toxicity of prion protein lacking its central domain. EMBO J. 2007;26:538–547. doi: 10.1038/sj.emboj.7601510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407:802–809. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]
- Akhtar RS, Ness JM, Roth KA. Bcl-2 family regulation of neuronal development and neurodegeneration. Biochim Biophys Acta. 2004;1644:189–203. doi: 10.1016/j.bbamcr.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- van Delft MF, Huang DC. How the Bcl-2 family of proteins interact to regulate apoptosis. Cell Res. 2006;16:203–213. doi: 10.1038/sj.cr.7310028. [DOI] [PubMed] [Google Scholar]
- Yamaguchi N, Sakaguchi S, Shigematsu K, Okimura N, Katamine S. Doppel-induced Purkinje cell death is stoichiometrically abrogated by prion protein. Biochem Biophys Res Commun. 2004;319:1247–1252. doi: 10.1016/j.bbrc.2004.05.115. [DOI] [PubMed] [Google Scholar]
- Shindler KS, Latham CB, Roth KA. bax deficiency prevents the increased cell death of immature neurons in bcl-x-deficient mice. J Neurosci. 1997;17:3112–3119. doi: 10.1523/JNEUROSCI.17-09-03112.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büeler H, Fischer M, Lang Y, Fluethmann H, Lipp H-P, DeArmond SJ, Prusiner SB, Aguet M, Weissmann C. Normal development and behavior of mice lacking the neuronal cell-surface PrP protein. Nature. 1992;356:577–582. doi: 10.1038/356577a0. [DOI] [PubMed] [Google Scholar]
- Chiesa R, Piccardo P, Ghetti B, Harris DA. Neurological illness in transgenic mice expressing a prion protein with an insertional mutation. Neuron. 1998;21:1339–1351. doi: 10.1016/s0896-6273(00)80653-4. [DOI] [PubMed] [Google Scholar]
- Zanusso G, Liu D, Ferrari S, Hegyi I, Yin X, Aguzzi A, Hornemann S, Liemann S, Glockshuber R, Manson JC, Brown P, Petersen RB, Gambetti P, Sy MS. Prion protein expression in different species: analysis with a panel of new mAbs. Proc Natl Acad Sci USA. 1998;95:8812–8816. doi: 10.1073/pnas.95.15.8812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaglio E, Chiesa R, Harris DA. Copper converts the cellular prion protein into a protease-resistant species that is distinct from the scrapie isoform. J Biol Chem. 2001;276:11432–11438. doi: 10.1074/jbc.M009666200. [DOI] [PubMed] [Google Scholar]
- Rossi F, Jankovski A, Sotelo C. Target neuron controls the integrity of afferent axon phenotype: a study on the Purkinje cell-climbing fiber system in cerebellar mutant mice. J Neurosci. 1995;15:2040–2056. doi: 10.1523/JNEUROSCI.15-03-02040.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassef M, Sotelo C, Cholley B, Brehier A, Thomasset M. Cerebellar mutations affecting the postnatal survival of Purkinje cells in the mouse disclose a longitudinal pattern of differentially sensitive cells. Dev Biol. 1987;124:379–389. doi: 10.1016/0012-1606(87)90490-8. [DOI] [PubMed] [Google Scholar]
- Fan H, Favero M, Vogel MW. Elimination of Bax expression in mice increases cerebellar Purkinje cell numbers but not the number of granule cells. J Comp Neurol. 2001;436:82–91. [PubMed] [Google Scholar]
- Selimi F, Doughty M, Delhaye-Bouchaud N, Mariani J. Target-related and intrinsic neuronal death in Lurcher mutant mice are both mediated by caspase-3 activation. J Neurosci. 2000;20:992–1000. doi: 10.1523/JNEUROSCI.20-03-00992.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Barmada SJ, Roth KA, Harris DA. N-terminally deleted forms of the prion protein activate both Bax-dependent and Bax-independent neurotoxic pathways. J Neurosci. 2007;27:852–859. doi: 10.1523/JNEUROSCI.4244-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uo T, Kinoshita Y, Morrison RS. Neurons exclusively express N-Bak, a BH3 domain-only Bak isoform that promotes neuronal apoptosis. J Biol Chem. 2005;280:9065–9073. doi: 10.1074/jbc.M413030200. [DOI] [PubMed] [Google Scholar]
- Vogel MW. Cell death, Bcl-2, Bax, and the cerebellum. Cerebellum. 2002;1:277–287. doi: 10.1080/147342202320883588. [DOI] [PubMed] [Google Scholar]
- Wüllner U, Weller M, Schulz JB, Krajewski S, Reed JC, Klockgether T. Bcl-2, Bax and Bcl-x expression in neuronal apoptosis: a study of mutant weaver and lurcher mice. Acta Neuropathol (Berl) 1998;96:233–238. doi: 10.1007/s004010050889. [DOI] [PubMed] [Google Scholar]
- Yuan J, Lipinski M, Degterev A. Diversity in the mechanisms of neuronal cell death. Neuron. 2003;40:401–413. doi: 10.1016/s0896-6273(03)00601-9. [DOI] [PubMed] [Google Scholar]
- Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, Peter ME. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selimi F, Vogel MW, Mariani J. Bax inactivation in Lurcher mutants rescues cerebellar granule cells but not Purkinje cells or inferior olivary neurons. J Neurosci. 2000;20:5339–5345. doi: 10.1523/JNEUROSCI.20-14-05339.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doughty ML, De Jager PL, Korsmeyer SJ, Heintz N. Neurodegeneration in Lurcher mice occurs via multiple cell death pathways. J Neurosci. 2000;20:3687–3694. doi: 10.1523/JNEUROSCI.20-10-03687.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selimi F, Lohof AM, Heitz S, Lalouette A, Jarvis CI, Bailly Y, Mariani J. Lurcher GRID2-induced death and depolarization can be dissociated in cerebellar Purkinje cells. Neuron. 2003;37:813–819. doi: 10.1016/s0896-6273(03)00093-x. [DOI] [PubMed] [Google Scholar]
- Yue Z, Horton A, Bravin M, DeJager PL, Selimi F, Heintz N. A novel protein complex linking the δ2 glutamate receptor and autophagy: implications for neurodegeneration in Lurcher mice. Neuron. 2002;35:921–933. doi: 10.1016/s0896-6273(02)00861-9. [DOI] [PubMed] [Google Scholar]
- Norman DJ, Feng L, Cheng SS, Gubbay J, Chan E, Heintz N. The lurcher gene induces apoptotic death in cerebellar Purkinje cells. Development. 1995;121:1183–1193. doi: 10.1242/dev.121.4.1183. [DOI] [PubMed] [Google Scholar]
- Selimi F, Campana A, Weitzman J, Vogel MW, Mariani J. Bax and p53 are differentially involved in the regulation of caspase-3 expression and activation during neurodegeneration in Lurcher mice. C R Acad Sci III. 2000;323:967–973. doi: 10.1016/s0764-4469(00)01243-9. [DOI] [PubMed] [Google Scholar]
- Dusart I, Guenet JL, Sotelo C. Purkinje cell death: differences between developmental cell death and neurodegenerative death in mutant mice. Cerebellum. 2006;5:163–173. doi: 10.1080/14734220600699373. [DOI] [PubMed] [Google Scholar]
- Lee JW, Beebe K, Nangle LA, Jang J, Longo-Guess CM, Cook SA, Davisson MT, Sundberg JP, Schimmel P, Ackerman SL. Editing-defective tRNA synthetase causes protein misfolding and neurodegeneration. Nature. 2006;443:50–55. doi: 10.1038/nature05096. [DOI] [PubMed] [Google Scholar]
- Martinou JC, Dubois-Dauphin M, Staple JK, Rodriguez I, Frankowski H, Missotten M, Albertini P, Talabot D, Catsicas S, Pietra C, Huarte J. Overexpression of BCL-2 in transgenic mice protects neurons from naturally occurring cell death and experimental ischemia. Neuron. 1994;13:1017–1030. doi: 10.1016/0896-6273(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Marangos PJ, Schmechel DE. Neuron specific enolase, a clinically useful marker for neurons and neuroendocrine cells. Annu Rev Neurosci. 1987;10:269–295. doi: 10.1146/annurev.ne.10.030187.001413. [DOI] [PubMed] [Google Scholar]
- Flechsig E, Hegyi I, Leimeroth R, Zuniga A, Rossi D, Cozzio A, Schwarz P, Rulicke T, Gotz J, Aguzzi A, Weissmann C. Expression of truncated PrP targeted to Purkinje cells of PrP knockout mice causes Purkinje cell death and ataxia. EMBO J. 2003;22:3095–3101. doi: 10.1093/emboj/cdg285. [DOI] [PMC free article] [PubMed] [Google Scholar]