Abstract
Defective proteolysis has been implicated in hearing loss through the discovery of mutations causing autosomal recessive nonsyndromic deafness in a type II transmembrane serine protease gene, TMPRSS3. To investigate their physiological function and the contribution of this family of proteases to the auditory function, we analyzed the hearing status of mice deficient for hepsin, also known as TMPRSS1. These mice exhibited profound hearing loss with elevated hearing thresholds compared with their heterozygous and wild-type littermates. Their cochleae showed abnormal tectorial membrane development, reduction in fiber compaction in the peripheral portion of the auditory nerve, and decreased expression of the myelin proteins myelin basic protein and myelin protein zero. In addition, reduced level of the large conductance voltage- and Ca2+-activated K+ channel was detected in the sensory hair cells of Tmprss1-null mice. We examined thyroid hormone levels in Tmprss1-deficient mice, as similar cochlear defects have been reported in animal models of hypothyroidism, and found significantly reduced free thyroxine levels. These data show that TMPRSS1 is required for normal auditory function. Hearing impairment present in Tmprss1-null mice is characterized by a combination of various structural, cellular, and molecular abnormalities that are likely to affect different cochlear processes.
Hearing loss occurs in approximately 1 in 1000 children, and more than half of these cases can be considered to have a genetic origin.1 The majority of the cases of he-reditary deafness (70%) are nonsyndromic where im-
paired auditory function is the only clinical manifestation. In addition, hearing impairment affects 50% of individuals older than 80 years.2 Most of the cases of early-onset inherited hearing loss are due to single gene defects, and it has been proposed that genes responsible for monogenically inherited hearing loss may also be implicated in age-related hearing impairment (presbyacusis).3 Therefore, identifying the genes underlying hearing loss represents a major objective of current biomedical research. Most studies on the human ear can only be performed postmortem, making the investigation of early stages of pathogenicity impossible. Thus, understanding the molecular mechanisms of normal hearing and the pathogenic processes that lead to hearing loss relies extensively on animal models. The identification and study of the function of proteins encoded by deafness genes can be performed on mouse models.
Recently, defective proteolysis has been implicated in the etiology of nonsyndromic hearing loss. Indeed, we and others have reported that deleterious mutations in the TMPRSS3 gene were responsible for nonsyndromic recessive hearing loss,4,5,6,7,8,9 suggesting that critical signaling pathways in the inner ear are controlled by proteolytic cleavage. TMPRSS3 is a member of the type II transmembrane serine proteases (TMPRSSs) family. TMPRSSs are membrane-bound proteolytic enzymes that are important in a variety of biological or pathological processes.10
Genes underlying nonsyndromic deafness can be categorized by function, and several protein members of the same family are often associated in the same functional group. The most striking example is the presence of seven myosin and myosin-related genes in the motor molecule functional category (Table 1). TMPRSS3 did not belong to any of the existing categories and could represent the first member of a new functional group tentatively named proteolysis component. To investigate this hypothesis, we have started to evaluate the auditory function of a knockout mouse for a Tmprss gene available for analysis. Here, we report on the electrophysiological, cellular, and molecular analysis of the auditory function of Tmprss1-deficient mice.
Table 1.
Nonsyndromic Autosomal Deafness Genes
| Locus | Gene | Reference no. |
|---|---|---|
| DFNB2, DFNA11 | MYO7A | 11,12 |
| DFNB3 | MYO15 | 13 |
| DFNB30 | MYO3A | 14 |
| DFNB36, DFNA22 | MYO6 | 15,16 |
| DFNA4 | MYH14 | 17 |
| DFNA17 | MYH9 | 18 |
| DFNA48 | MYO1A | 19 |
Materials and Methods
Tmprss1 Knockout Mice
Mice with targeted disruption of the Tmprss1 gene were obtained from Q.W. In these mice, exons 5 to 8, which encode the conserved activation site and most of the catalytic domain of Tmprss1, were deleted. Northern blot analysis showed absence of Tmprss1 mRNA in Tmprss1−/− mice.20
Hearing Evaluation by Click-Evoked Auditory Brainstem Responses (ABRs)
Procedures for ABR measurements have been previously described.21 A total of 20 mice of 7 to 8 weeks of age (five Tmprss1+/+, five Tmprss1+/−, and 10 Tmprss1−/−) were anesthetized with ketamine (75 mg/kg body weight; Parnell Laboratories, Australia) and xylazil (7.5 mg/kg body weight; Troy Laboratories, Smithfield, NSW, Australia). Computer-generated rarefaction clicks were channeled to a loudspeaker positioned 10 cm from the pinna of the measured ear. At this distance, the maximum sound level at the pinna was 98 decibels (dB) peak equivalent sound pressure level. Stimuli were presented at a rate of 33/second, and responses were amplified 105 fold and bandpass-filtered (150 Hz to 3 kHz). Threshold was determined as the smallest stimulus level required to give peak-trough response amplitude of >0.25 μV for wave III of the ABR. At each stimulus level, recording was repeated to ensure consistency.
Histology
The cochleae of six postnatal day-8 pups (three Tmprss1+/+ and three Tmprss1−/−) and eight mice of 7 to 8 weeks of age (two Tmprss1+/+, two Tmprss1+/−, and four Tmprss1−/−) were fixed with 10% formalin (BDH Laboratory, Darmstadt, Germany) in absolute ethanol neutralized with 0.5 g of calcium acetate (BDH Laboratory) per liter of fixative. Cochleae were decalcified in 10% ethylenediamine tetraacetic acid (BDH Laboratory) in phosphate-buffered saline (PBS) and dehydrated in alcohol before final embedding in resin. Mid-modiolar serial sections of 5 μm were obtained and stained with hematoxylin and eosin. The cross-sectional area of the Rosenthal’s canal, osseous spiral lamina, and tectorial membrane from the middle turn were determined using a morphometric program (AxioVision LE, Hallbergmoos, Germany). The same program was also used to measure the longest vertical distance between the dorsal and ventral surface of the tectorial membrane. Spiral ganglion neuron and Schwann cell densities, tectorial membrane area, and vertical length were normalized to the wild-type cohort.
Immunohistochemistry
The cochleae of eight adult wild-type and eight age-matched Tmprss1−/− mice were removed for fixation with 3% paraformaldehyde in PBS for 2 hours, decalcified in 10% ethylenediamine tetraacetic acid (BDH Laboratory) in PBS, and incubated overnight in 25% sucrose in PBS. For BK channel α-subunit immunohistochemistry, we decalcified cochleae from three wild-type and three Tmprss1−/− mice in Decal Stat (Decal Chemical, Tallman, NY) before overnight incubation in 25% sucrose in PBS. Cochleae were then embedded in O.C.T. compound (Sakura, Tokyo, Japan), cryosectioned in 12-μm thickness, and mounted on SuperFrost Plus microscopic slides.
Cochlear sections were thawed, permeabilized for 10 minutes at room temperature with 0.1% Triton X-100, blocked with 10% normal goat serum (Vector Laboratories, Burlingame, CA) in 0.1% Triton X-100 for 2 hours, and incubated overnight at 4°C with primary antibodies diluted in blocking solution. For BK channel α-subunit immunohistochemistry, 1% bovine serum albumin (Sigma, St. Louis, MO) in 0.1% Triton X-100 was used to block unspecific sites for 30 minutes before the application of primary antibody diluted in 0.5% bovine serum albumin. On the following day, sections were washed three times with PBS for 10 minutes before incubating for 2 hours at room temperature with the appropriate secondary antibodies. Sections were then rinsed three times in PBS for 20 minutes before mounting in Vectorshield (Vector Laboratories) containing the nuclear stain 4′,-6-diamidino-2-phenylindole dihydrochloride. All sections were viewed with a Zeiss Axioplan 2 microscope.
The following primary antibodies were used: mouse monoclonal antibodies against myelin basic protein (MBP) (MAB382, 1:100; Chemicon, Temecula, CA), synaptophysin (MAB368, 1:500; Chemicon); rabbit polyclonal antibody against myelin protein zero (P0; a gift from Maria T. Filbin, City University of New York, New York, NY; 1:3000), large conductance voltage- and BK channel α-subunit (APC021, 1:100; Alomone, Jerusalem, Israel) and tyrosine kinase B neurotrophin receptor (1:100; Santa Cruz Biotechnology, Santa Cruz, CA). The following fluorescein-conjugated secondary antibodies from Molecular Probes were used at a 1:500 dilution: highly cross-adsorbed Alexa Fluor 488 goat anti-mouse IgG (A-11029) and highly cross-adsorbed Alexa Fluor 594 goat anti-rabbit IgG (A-11037).
Western Blotting
The cochleae and hippocampal tissues of six wild-type and six age-matched Tmprss1−/− mice were homogenized before extracting the hydrophobic (membrane protein) and hydrophilic (cytosolic protein) fractions using Mem-PER eukaryotic membrane protein extraction kit (89826; Pierce Technologies). Hydrophobic and hydrophilic proteins were enriched using PAGEprep (catalog no. 26800; Pierce Technologies, Rockford, IL) and concentrations determined with a Bradford reagent (B6916; Sigma). Proteins were run at 200 V for 1 hour on a 12% bis-Tris gel in the reducing XT-3-(N-morpholino)propanesulfonic acid buffer system (3450117, 1610793; Bio-Rad, Hercules, CA). Separated proteins were then transferred to polyvinylidene difluoride membranes (1620238; Bio-Rad) at 30 V for 1 hour and 20 minutes using a 1× Tris/glycine buffer (1610771; Bio-Rad) with 20% methanol. Membranes were subsequently blocked for 60 minutes in 5% milk powder (1706404; Bio-Rad) before incubating overnight at 4°C with primary antibodies. Blots containing hydrophobic proteins were incubated with rabbit polyclonal antibody against P0 (a gift from Maria T. Filbin, 1:6000), whereas blots containing hydrophilic proteins were incubated with glyceraldehyde-3-phosphate dehydrogenase from Abcam (ab9485; Cambridge, MA) at a 1:20,000 dilution. On the following day, blots were washed three times for 20 minutes in 0.1% Tween 20/PBS before incubating for 1 hour with horseradish peroxidase-conjugated secondary antibodies, goat anti-rabbit IgG at a 1:10,000 dilution (1706515; Bio-Rad). The blots were next washed four times for 30 minutes in 0.1% Tween 20/PBS, and substrate development was performed using ECL Plus Western blotting detection reagents (RPN2132; GE Healthcare, Little Chalfont, Buckinghamshire, UK). Chemiluminescence was visualized using ImageQuant 400 (GE Healthcare). Densitometry measurements were performed using the α Imager (Alpha Innotech, San Leandro, CA). Statistical analyses between wild-type and Tmprss1−/− mice were determined using the two-tailed Student’s t-test (SigmaStat; Systat Software, Inc., Point Richmond, CA).
Thyroxine Determination
Blood was obtained by cardiac puncture from 15 7-week-old wild-type and 12 age-matched Tmprss1−/− mice. Blood samples were allowed to clot before centrifugation at 2500 rpm for 5 minutes at room temperature. Sera were then collected and stored at −20°C. Quantification of free thyroxine (FT4) levels in mouse serum was performed using an ADVIA Centaur apparatus (Bayer Corporation, Tarrytown, NY), which uses direct chemiluminescence immunoassay technology.
Results
Auditory Function Deficits in Tmprss1−/− Mice
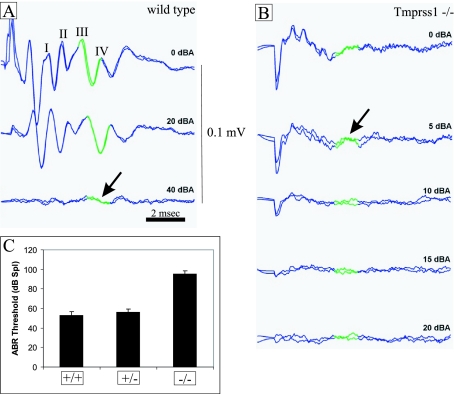
The click-evoked ABR gives an indication of overall auditory function. Both wild-type and Tmprss1+/− mice produced normal click-evoked waveforms when attenuation was set at 0 or 20 dBA (decibel attenuation), suggesting a functional auditory system (Figure 1A and data not shown). In contrast, Tmprss1−/− mice showed poorly defined ABR waveforms when attenuation was set at 0 or 5 dBA and no response at 10, 15, and 20 dBA (Figure 1B). The average ABR threshold for wild-type and Tmprss1+/− mice was 53 ± 3.92 and 56 ± 3.66 dB sound pressure level, respectively, whereas the ABR threshold for Tmprss1-null mice was 95.5 ± 2.68 dB sound pressure level (Figure 1C). Tmprss1−/− mice showed a 42 dB sound pressure level threshold increase compared with wild-type and Tmprss1+/− mice, indicating severe hearing impairment.
Figure 1.
Hearing function deficit in Tmprss1−/− mice. Adult wild-type, Tmprss1 heterozygous, and Tmprss1 knockout mice were subjected to click-evoked ABR measurements. The amplitude of the response (four peaks labeled from I to IV) is measured in millivolts. The latency is shown in milliseconds. Click stimulus intensity is indicated in decibel attenuation. Maximum stimulus intensity corresponds to 0 dBA. The green portion of the graph highlights the waveform chosen to determine threshold. A: Representative ABR response of wild-type mice at 0, 20, and 40 dBA. Arrow indicates ABR threshold. B: Representative ABR response of Tmprss1−/− mice at 0, 5, 10, 15, and 20 dBA. Arrow indicates ABR threshold. C: Quantification of the average ABR thresholds of wild-type, Tmprss1 heterozygote, and Tmprss1-null mice. Graph indicates mean ± SEM.
Abnormal Cochlear Morphology in Tmprss1−/− Mice
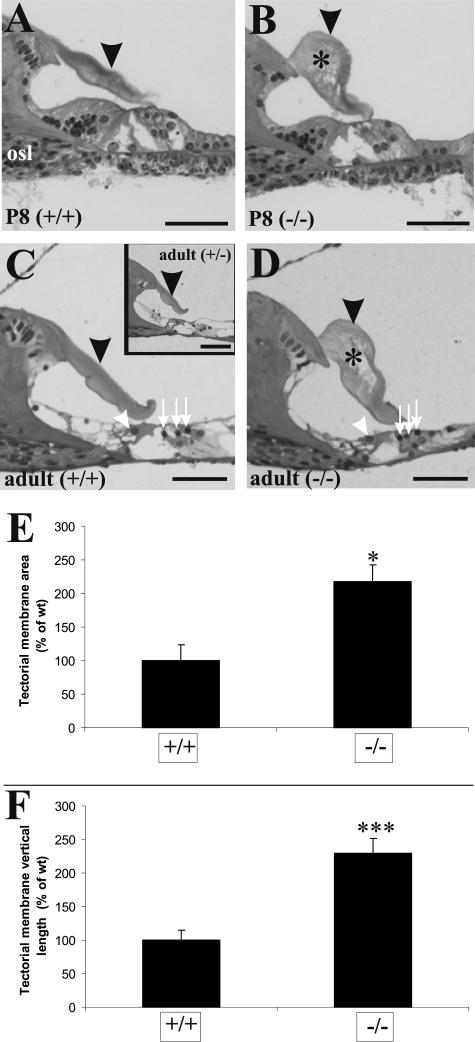
To understand the impaired auditory function of Tmprss1-null mice, a cochlear histological analysis was performed. Wild-type and Tmprss1+/− heterozygous mice showed normal tectorial membrane morphology at both postnatal day 8 and adulthood (Figure 2, A, C, and inset in C). In contrast, Tmprss1−/− mice have a deformed and enlarged tectorial membrane characterized by a reduced limbal attachment zone and large holes within its matrix (Figure 2, B and D). The organ of Corti appeared normal, as we could not observe any loss of inner and outer hair cells (Figure 2, C and D). The cross-sectional area of the tectorial membrane in Tmprss1−/− mice (218 ± 24%) significantly doubled that of the wild-type mice (Figure 2E, P = 0.011). The longest vertical distance measured between the dorsal and ventral surface of the tectorial membrane in Tmprss1−/− mice (229 ± 22%) also doubled that of the wild-type mice (Figure 2F, P = 0.002). Although the tectorial membranes of Tmprss1-null mice were deformed, they seemed to extend fully across the cavity of the inner sulcus and contact the sensory hair cells.
Figure 2.
Tectorial membrane defects in Tmprss1−/− mice. Histological analysis of cochleae from wild-type and Tmprss1−/− mice at postnatal day 8 (P8) (A and B, respectively) and adult (C and D, respectively) mice. Black arrowheads point to the tectorial membrane, and asterisks show holes found within the matrix of the membrane. Osseous spiral lamina is indicated by osl. White arrowheads show inner hair cells, and white arrows point to the outer hair cells in the organ of Corti. Scale bar = 50 μm. C, inset: Analysis of cochleae from adult Tmprss1+/− mice. E: Comparison of the cross-sectional area of the tectorial membrane in wild-type and Tmprss1−/− mice. Graph indicates mean ± SE, *P = 0.011. F: Comparison of the longest vertical distance measured between the dorsal and ventral surface of the tectorial membrane. Graph indicates mean ± SE, ***P = 0.002.
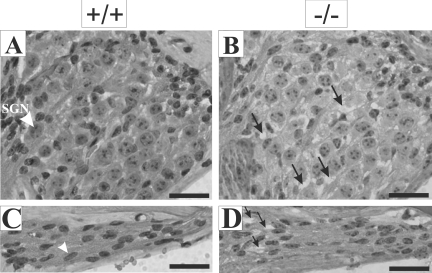
Interestingly, we observed that while spiral ganglion neurons and Schwann cells in wild-type mice tend to cluster closely with each other (Figure 3, A and C), empty spaces between these cells could be observed in Tmprss1−/− mice (Figure 3, B and D). This structural defect was not accompanied by cell loss, as spiral ganglion and Schwann cell density count did not differ between wild-type and Tmprss1−/− mice (data not shown). These observations suggest that Tmprss1−/− mice present a deficit in the compaction of the soma and fibers of spiral ganglion neurons.
Figure 3.
Morphological defects in the Rosenthal’s canal and osseous spiral lamina of Tmprss1−/− mice. Representative images of the Rosenthal’s canal and the osseous spiral lamina of adult wild-type (A and C, respectively) and adult Tmprss1−/− (B and D, respectively) mice. A: White arrowhead indicates spiral ganglion neuron (SGN). B: Black arrows indicate spaces between spiral ganglion neurons. C: White arrowhead indicates spindle-shaped nuclei of Schwann cells. D: Black arrows point to spaces between Schwann cells. Scale bar = 20 μm.
Reduced Expression of Myelin Genes in Tmprss1−/− Cochleae
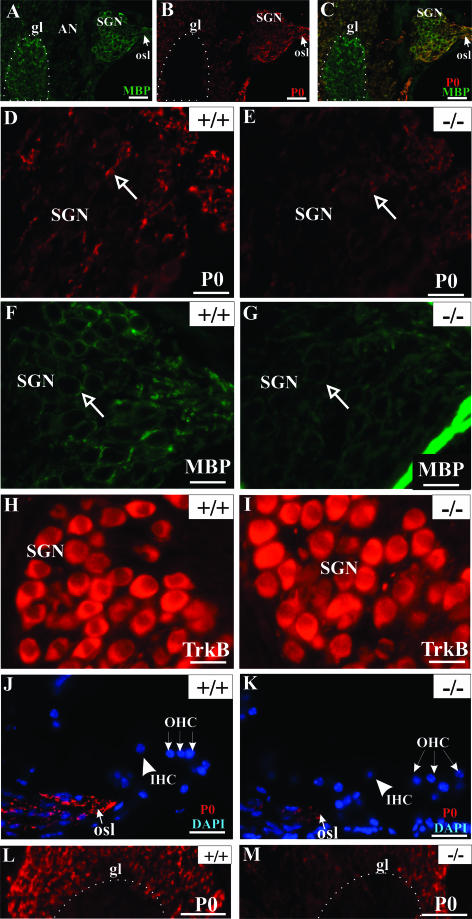
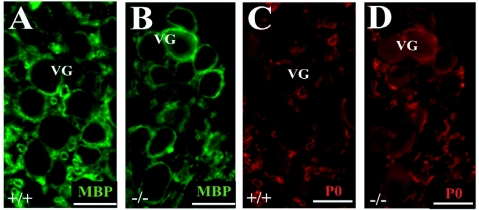
As myelin plays an important role in the fasciculation of neuronal processes, we examined the expression of MBP and P0 in the cochleae of Tmprss1−/− mice and wild-type littermates. In adult wild-type mice, strong MBP immunoreactivity was observed in the central portion of the auditory nerve, which is delimited by the glial transition zone, and in the auditory nerve inside the cochlea (Figure 4A), whereas P0 was absent in the central part of the auditory nerve (Figure 4B). Dual immunofluorescence showed that MBP and P0 immunoreactivities overlapped mainly in spiral ganglion neurons. MBP staining was predominant in the myelin sheath wrapping the cell body of spiral ganglion neurons in the Rosenthal’s canal. In contrast, P0 staining was rather weak around the soma of spiral ganglion neurons (Figure 4, A–C).
Figure 4.
MBP and P0 expression are reduced in the cochleae of Tmprss1−/− mice. MBP (A) and P0 (B) expression in the auditory nerve of wild-type mice. C: Superimposition of A and B. P0, MBP and tyrosine kinase receptor B expression in the Rosenthal’s canal of adult wild-type (D, F, and H, respectively) and adult Tmprss1−/− mice (E, G, and I, respectively). D and E: Arrows indicate a spiral ganglion neuron fiber (D and E) and a spiral ganglion neuron body (F and G). The bony boundary of the Rosenthal’s canal shows strong nonspecific staining (G). P0 expression in the osseous spiral lamina and fibers projecting to auditory nerve in the brainstem of adult wild-type and adult Tmprss1−/− mice (J and K, respectively, and L and M, respectively). Scale bar = 20 μm, except in A, B, C, L, and M, where scale bar = 50 μm. Glial transition zone (gl) is approximately outlined by the white dotted line. osl, osseous spiral lamina; SGN, spiral ganglion neuron; AN, auditory nerve; IHC, inner hair cell; OHC, outer hair cell.
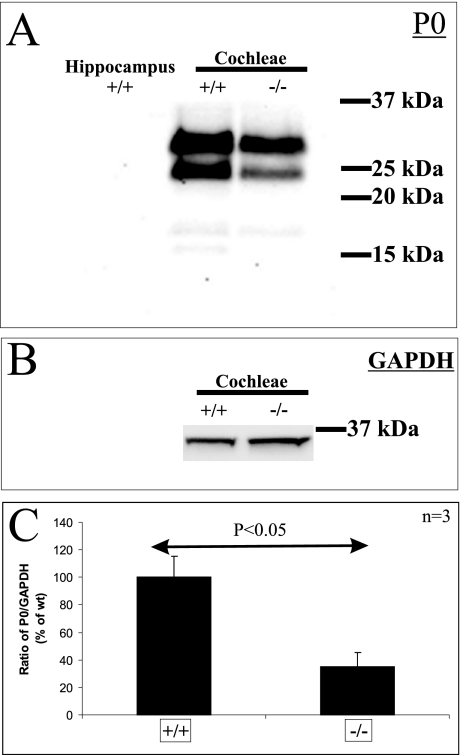
P0 and MBP expression were then analyzed in three different cochlear structures: the Rosenthal’s canal, the osseous spiral lamina, and the glial transition zone of the auditory nerve. Compared with wild-type mice, P0 expression in Tmprss1−/− mice was down-regulated in fibers of spiral ganglion neurons (Figure 4, D and E), in peripheral processes within the osseous spiral lamina (Figure 4, J and K), and in fibers projecting to the auditory nerve bundle in the brainstem (Figure 4, L and M). Analysis of MBP immunoreactivity in the Rosenthal’s canal yielded inconsistent results, with only half of the mutant mice showing down-regulation of MBP around spiral ganglion neuron bodies (Figure 4, F and G). In addition, analysis of MBP and P0 expression in the vestibular ganglion neurons did not reveal marked differences between wild-type and Tmprss1−/− mice (Figure 5), suggesting that the decline in expression of MBP and P0 is specific to the auditory system. Finally, the neurotrophin receptors tyrosine kinase B and p75, which are expressed in soma of spiral ganglion neurons, did not show clear differences in expression between wild-type and Tmprss1−/− mice (Figure 4, H and I; see Supplemental Figure 1, A and B, at http://ajp.amjpathol.org). We then confirmed and semiquantified the decline of myelin gene expression in adult Tmprss1−/− mice using Western blot analysis. Proteins extracted from cochleae of adult mice showed two major P0 protein species of ∼30 and 25 kd. Comparison of P0 levels between wild-type and Tmprss1−/− mice revealed a marked reduction in P0 expression levels in the mutant mice, whereas glyceraldehyde-3-phosphate dehydrogenase or p75 protein levels, which were used as cytosolic and membrane housekeeping proteins, respectively, did not vary considerably between wild-type and Tmprss1−/− mice (Figure 6, A–C; see Supplemental Figure 1C at http://ajp.amjpathol.org).
Figure 5.
MBP and P0 expression in the vestibular ganglion of adult wild-type (A and C, respectively) and Tmprss1−/− mice (B and D, respectively).
Figure 6.
Western blot quantification of the reduction of P0 expression in cochleae of Tmprss1−/− mice. A: Western blot analysis of P0 was performed on equal amount of membrane proteins isolated from hippocampus, wild-type (+/+), and Tmprss1−/− (−/−) mice cochleae. B: Western blot analysis of glyceraldehyde-3-phosphate dehydrogenase was performed on equal amounts of cytosolic proteins isolated from wild-type and Tmprss1−/− mice cochleae. C:The intensity of the 25-kd fragment was normalized to the GAPDH band in three independent experiments, and the mean ratio expressed as a relative percentage of the wild-type cohort. There was a significant reduction in this ratio in Tmprss1−/−mice, relative to wild-type mice (P= 0.024).
Collectively, these observations suggest that Tmprss1−/− mice present a deficit of compaction in both soma and processes of spiral ganglion neurons. This defect did not seem to be associated with a loss of spiral ganglion neurons or Schwann cells but rather with a specific down-regulation of myelin gene expression.
Tmprss1−/− Mice Are Hypothyroidic
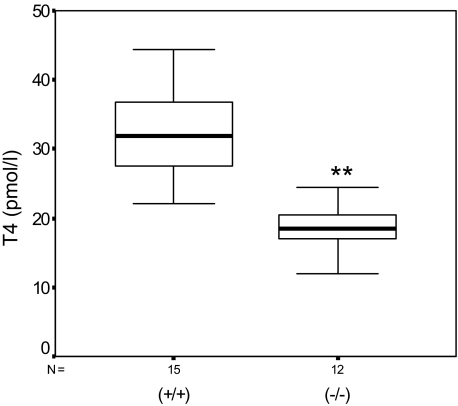
It has been shown that proper thyroid hormone supply is required for the development of the auditory system. In particular, the tectorial membrane and the peripheral auditory system are sensitive to thyroid hormone deficiency before the onset of hearing.22,23 Determination of fT4 levels in the serum of adult wild-type and Tmprss1−/− mice showed a significant 43% decline of fT4 in Tmprss1−/− (18.03 ± 4.05 pmol/L), compared with wild-type mice (31.53 ± 6.94 pmol/L; P = 2 × 10−5), suggesting that Tmprss1−/− mice showed a deficit in thyroid hormone metabolism (Figure 7).
Figure 7.
Tmprss1−/− mice are hypothyroidic. Box plot diagram of fT4 levels in the serum of 15 adult wild-type (+/+) and 12 Tmprss1−/− (−/−) mice. The distribution of fT4 levels between the two groups is statistically significant (**P = 2 × 10−5).
Reduced BK α-Subunit Expression in Tmprss1−/− Mice
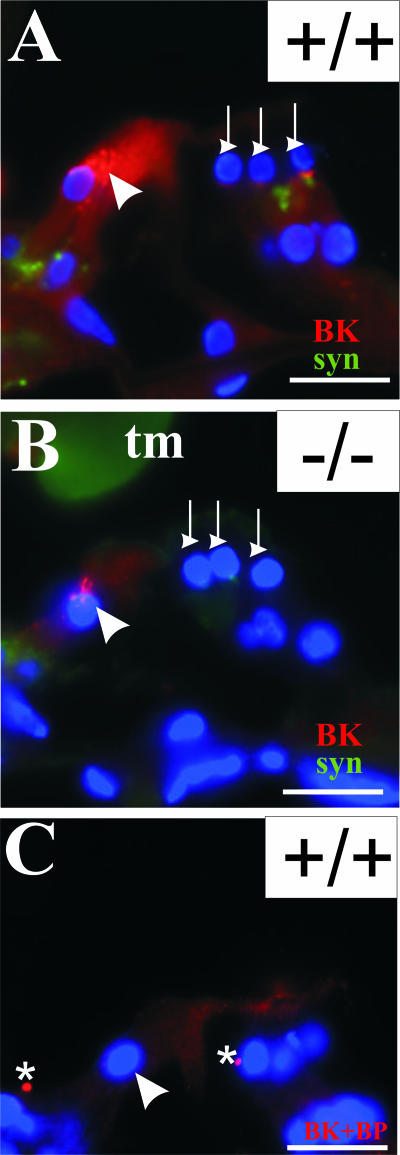
Expression of a fast-activating potassium conductance by the inner hair cells of mice lacking thyroid hormone β receptors is significantly retarded24; therefore, we examined whether Tmprss1−/− mice exhibit any defects in potassium channel expression. We focused on the large conductance voltage- and Ca2+-activated potassium channel because its expression has been characterized in inner hair cells,25,26 and mouse mutants lacking BK α-subunit have been shown to develop progressive hearing loss.27 BK α-subunit expression in wild-type mice was detected in the upper part of the inner hair cells, whereas weaker expression was detected at the base of outer hair cells, in contrast to synaptophysin-stained efferent projection fibers (Figure 8A). On preadsorption with the antigenic peptide, this staining pattern in the inner hair cells and base of outer hair cells was completely abolished, except for spotted background fluorescence that did not reside within inner hair cells or at the base of outer hair cells (Figure 8C). In Tmprss1−/− mice, BK α-subunit expression in the inner and outer hair cells was notably reduced (Figure 8B), suggesting possible functional deficit in potassium current within the organ of Corti, despite the absence of hair cell loss (Figure 2). Furthermore, this observation is consistent with reported findings of reduced BK α-subunit expression and conductance in inner hair cells of hypothyroid rats.28
Figure 8.
Reduced BK α-subunit expression in the organ of Corti of Tmprss1−/− mice. BK α-subunit (red) and synaptophysin (green) expression in the organ of Corti of adult wild-type (A) and age-matched Tmprss1−/− (B) mice. C: The BK α-subunit antibody was preincubated with the corresponding anti-peptide before being applied to sections. White arrowhead indicates the upper part of inner hair cells. White downward arrows point to the base of outer hair cells. Asterisks indicate background fluorescence, which does not localize within any cellular compartments. Cell nuclei are labeled with 4′,6-diamidino-2-phenylindole dihydrochloride (blue). Scale bar = 20 μm.
Discussion
In this article, we demonstrated the functional importance of Tmprss1 in the development of normal hearing by investigating the auditory function of Tmprss1-null mice. Mice deficient for Tmprss1 develop early-onset profound deafness and provide a valuable model to study the role of type II transmembrane serine proteases in the development of normal hearing. Our data demonstrate that various cochlear processes are affected by the absence of Tmprss1 function and suggest that Tmprss1 might exert both a direct and indirect role in the auditory system.
Hearing loss in Tmprss1−/− mice is accompanied by modest structural defect; apart from the abnormal tectorial membrane, the other structures of the cochlea appeared to develop normally. The tectorial membrane is an acellular membrane that is secreted and extends from the spiral limbus to contact the sensory hair cells of the organ of Corti.29 Tmprss1 expression was not detected in the spiral limbus or in the tectorial membrane (M. Guipponi, unpublished data), indicating that it may not play a direct role in the maintenance or the function of this cochlear membrane. The inner ear of Tmprss1-null mice was also characterized at the cellular level by a defect in the compaction of spiral ganglion neurons. This defect was associated with reduction of P0 and MBP levels along the peripheral portion of the auditory nerve. Tmprss1 is expressed in the soma and fibers of the spiral ganglion neurons (M. Guipponi, unpublished data) and could be directly involved in this myelination defect. Indeed, other transmembrane proteases have been shown to play important roles in myelination. For example, BACE1, a transmembrane neuronal protease, has recently been shown to be essential for peripheral nerve myelination by Schwann cells.30 Finally, mice deficient for Tmprss1 were also found to have reduced levels of BK α-subunit, suggesting potential functional deficits in ion channel conductance within the organ of Corti, despite the absence of morphological defects. Here again, a direct role of Tmprss1 is unlikely, as Tmprss1 expression was not detected in the sensory hair cells of the organ of Corti (M. Guipponi, unpublished data).
Based on our structural, cellular, and molecular evaluation of the hearing status of Tmprss1−/− mice, we hypothesize that the hearing loss in these mice could be partly due to abnormal thyroid hormone metabolism. In our hypothesis, the altered thyroid hormone metabolism in Tmprss1−/− mice would create a delayed or insufficient developmental rise of thyroid hormone plasma levels starting at birth to saturating level achieved at the onset of hearing at postnatal day 12 and lead to permanent hearing defects.31,32 Indeed, Tmprss1−/− mice showed a significant reduction of serum T4 levels, and their cochlear pathology recapitulated most of the cochlear phenotypes associated with experimental hypothyroidism in rodents. Different animal models have been generated to study the role of thyroid hormones in the development of the auditory function.22,31,32,33,34,35,36,37 In most of these animal models, hearing loss is associated with various degree of disruption in the morphogenesis of different cochlear structures, including the tectorial membrane and the organ of Corti. In addition to its morphogenetic effect, thyroid hormones play also an important role in the myelination of the auditory nerve. Indeed, developmental increase of thyroid hormone levels is necessary for a concomitant peak of myelin gene expression, which may guarantee nerve conduction and synchronized impulse transmission at the onset of hearing.23,38
Thyroid hormone metabolism is known to depend on processing of the prohormone thyroglobulin by sequential proteolytic events. It has been shown that mice deficient for proteinases cathepsins K and L have reduced levels of free T4.39 However, T4 was still present in the blood of these mice, suggesting that other proteases are involved in T4 metabolism. Therefore, Tmprss1 is an enzyme to consider in this respect, and further efforts to identify its role on thyroid function are clearly warranted.
In conclusion, we report on the cellular and molecular analysis of the pathogenic mechanisms of hearing loss present in TMPRSS1-deficient mice. This is a first detailed description of a phenotype in Tmprss1-deficient mice, as well as the first cellular and molecular description of hearing loss in mice deficient in a type II transmembrane serine protease. Important findings on TMPRSS1-related hearing loss are presented with evidence that Tmprss1 is essential for the development of normal auditory and thyroid function in mice. Mice deficient for Tmprss1 are hypothyroidic and present profound hearing loss that is characterized by defects in cochlear structures of which development and maturation are thyroid hormone-dependent. These findings are likely to better our understanding of the complex molecular and cellular mechanisms of normal auditory function but also help us identify the pathological processes of TMPRSS-related hearing loss. Proteases, including type II transmembrane serine proteases, are important therapeutic targets in cancer, particularly in metastases. Thus, hearing loss is a possible side effect of these therapies. In addition, TMPRSS1 may be involved in syndromic or nonsyndromic human hearing loss and common age-related hearing loss in humans.
Supplementary Material
Acknowledgments
We thank M. Fornito and R.J. Czajko for excellent technical assistance.
Footnotes
Address reprint requests to Dr. Hamish S. Scott, Division of Molecular Medicine, The Walter and Eliza Hall Institute of Medical Research, 1G Royal Parade, Parkville, 3050, VIC, Australia. E-mail: hscott@wehi.edu.au.
Supported by Swiss National Science Foundation grant 3100A0-114077-1 (to M.G.), a Garnett Passe and Rodney Williams Memorial Foundation Research Training Fellowship (to M.G.), and a project grant (to M.G. and H.S.S.); by National Health and Medical Research Council (NHMRC) fellowships 171601 and 461204, NHMRC grants (project no. 215305 and program no. 257501), and the Nossal Leadership Award from the Walter and Eliza Hall Institute (to H.S.S.); and by the National Institute on Deafness and Other Communication Disorders (grant NO1-DC-3-1005), a Medical Research and Technology grant (Victoria, Australia), and the Marion and E.H. Flack Trust (to J.T.).
M.G. and J.T. contributed equally to this work.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
Current address of M.G.: Division of Medical Genetics, University Hospital of Geneva, Geneva, Switzerland.
Current address of Q.W.: Molecular Cardiology/Nephrology & Hypertension Learner Research, Institute/ND50, The Cleveland Clinic Foundation, Cleveland, Ohio.
References
- Morton NE. Genetic epidemiology of hearing impairment. Ann NY Acad Sci. 1991;630:16–31. doi: 10.1111/j.1749-6632.1991.tb19572.x. [DOI] [PubMed] [Google Scholar]
- Seidman MD, Ahmad N, Bai U. Molecular mechanisms of age-related hearing loss. Ageing Res Rev. 2002;1:331–343. doi: 10.1016/s1568-1637(02)00004-1. [DOI] [PubMed] [Google Scholar]
- Gratton MA, Vazquez AE. Age-related hearing loss: current research. Curr Opin Otolaryngol Head Neck Surg. 2003;11:367–371. doi: 10.1097/00020840-200310000-00010. [DOI] [PubMed] [Google Scholar]
- Scott HS, Kudoh J, Wattenhofer M, Shibuya K, Berry A, Chrast R, Guipponi M, Wang J, Kawasaki K, Asakawa S, Minoshima S, Younus F, Mehdi SQ, Radhakrishna U, Papasavvas MP, Gehrig C, Rossier C, Korostishevsky M, Gal A, Shimizu N, Bonne-Tamir B, Antonarakis SE. Insertion of beta-satellite repeats identifies a transmembrane protease causing both congenital and childhood onset autosomal recessive deafness. Nat Genet. 2001;27:59–63. doi: 10.1038/83768. [DOI] [PubMed] [Google Scholar]
- Masmoudi S, Antonarakis SE, Schwede T, Ghorbel AM, Gratri M, Pappasavas MP, Drira M, Elgaied-Boulila A, Wattenhofer M, Rossier C, Scott HS, Ayadi H, Guipponi M. Novel missense mutations of TMPRSS3 in two consanguineous Tunisian families with non-syndromic autosomal recessive deafness. Hum Mutat. 2001;18:101–108. doi: 10.1002/humu.1159. [DOI] [PubMed] [Google Scholar]
- Ben-Yosef T, Wattenhofer M, Riazuddin S, Ahmed ZM, Scott HS, Kudoh J, Shibuya K, Antonarakis SE, Bonne-Tamir B, Radhakrishna U, Naz S, Ahmed Z, Riazuddin S, Pandya A, Nance WE, Wilcox ER, Friedman TB, Morell RJ. Novel mutations of TMPRSS3 in four DFNB8/B10 families segregating congenital autosomal recessive deafness. J Med Genet. 2001;38:396–400. doi: 10.1136/jmg.38.6.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattenhofer M, Di Iorio MV, Rabionet R, Dougherty L, Pampanos A, Schwede T, Montserrat-Sentis B, Arbones ML, Iliades T, Pasquadibisceglie A, D’Amelio M, Alwan S, Rossier C, Dahl HH, Petersen MB, Estivill X, Gasparini P, Scott HS, Antonarakis SE. Mutations in the TMPRSS3 gene are a rare cause of childhood nonsyndromic deafness in Caucasian patients. J Mol Med. 2002;80:124–131. doi: 10.1007/s00109-001-0310-6. [DOI] [PubMed] [Google Scholar]
- Wattenhofer M, Sahin-Calapoglu N, Andreasen D, Kalay E, Caylan R, Braillard B, Fowler-Jaeger N, Reymond A, Rossier BC, Karaguzel A, Antonarakis SE. A novel TMPRSS3 missense mutation in a DFNB8/10 family prevents proteolytic activation of the protein. Hum Genet. 2005;117:528–535. doi: 10.1007/s00439-005-1332-x. [DOI] [PubMed] [Google Scholar]
- Hutchin T, Coy NN, Conlon H, Telford E, Bromelow K, Blaydon D, Taylor G, Coghill E, Brown S, Trembath R, Liu XZ, Bitner-Glindzicz M, Mueller R. Assessment of the genetic causes of recessive childhood non-syndromic deafness in the UK: implications for genetic testing. Clin Genet. 2005;68:506–512. doi: 10.1111/j.1399-0004.2005.00539.x. [DOI] [PubMed] [Google Scholar]
- Wu Q. Type II transmembrane serine proteases. Curr Top Dev Biol. 2003;54:167–206. doi: 10.1016/s0070-2153(03)54009-1. [DOI] [PubMed] [Google Scholar]
- Liu XZ, Walsh J, Mburu P, Kendrick-Jones J, Cope MJ, Steel KP, Brown SD. Mutations in the myosin VIIA gene cause non-syndromic recessive deafness. Nat Genet. 1997;16:188–190. doi: 10.1038/ng0697-188. [DOI] [PubMed] [Google Scholar]
- Weil D, Kussel P, Blanchard S, Levy G, Levi-Acobas F, Drira M, Ayadi H, Petit C. The autosomal recessive isolated deafness, DFNB2, and the Usher 1B syndrome are allelic defects of the myosin-VIIA gene. Nat Genet. 1997;16:191–193. doi: 10.1038/ng0697-191. [DOI] [PubMed] [Google Scholar]
- Wang A, Liang Y, Fridell RA, Probst FJ, Wilcox ER, Touchman JW, Morton CC, Morell RJ, Noben-Trauth K, Camper SA, Friedman TB. Association of unconventional myosin MYO15 mutations with human nonsyndromic deafness DFNB3. Science. 1998;280:1447–1451. doi: 10.1126/science.280.5368.1447. [DOI] [PubMed] [Google Scholar]
- Walsh T, Walsh V, Vreugde S, Hertzano R, Shahin H, Haika S, Lee MK, Kanaan M, King MC, Avraham KB. From flies’ eyes to our ears: mutations in a human class III myosin cause progressive nonsyndromic hearing loss DFNB30. Proc Natl Acad Sci USA. 2002;99:7518–7523. doi: 10.1073/pnas.102091699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed ZM, Morell RJ, Riazuddin S, Gropman A, Shaukat S, Ahmad MM, Mohiddin SA, Fananapazir L, Caruso RC, Husnain T, Khan SN, Riazuddin S, Griffith AJ, Friedman TB, Wilcox ER. Mutations of MYO6 are associated with recessive deafness, DFNB37. Am J Hum Genet. 2003;72:1315–1322. doi: 10.1086/375122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchionda S, Ahituv N, Bisceglia L, Sobe T, Glaser F, Rabionet R, Arbones ML, Notarangelo A, Di Iorio E, Carella M, Zelante L, Estivill X, Avraham KB, Gasparini P. MYO6, the human homologue of the gene responsible for deafness in Snell’s waltzer mice, is mutated in autosomal dominant nonsyndromic hearing loss. Am J Hum Genet. 2001;69:635–640. doi: 10.1086/323156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaudy F, Snoeckx R, Pfister M, Zenner HP, Blin N, Di Stazio M, Ferrara A, Lanzara C, Ficarella R, Declau F, Pusch CM, Nurnberg P, Melchionda S, Zelante L, Ballana E, Estivill X, Van Camp G, Gasparini P, Savoia A. Nonmuscle myosin heavy-chain gene MYH14 is expressed in cochlea and mutated in patients affected by autosomal dominant hearing impairment (DFNA4). Am J Hum Genet. 2004;74:770–776. doi: 10.1086/383285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalwani AK, Goldstein JA, Kelley MJ, Luxford W, Castelein CM, Mhatre AN. Human nonsyndromic hereditary deafness DFNA17 is due to a mutation in nonmuscle myosin MYH9. Am J Hum Genet. 2000;67:1121–1128. doi: 10.1016/s0002-9297(07)62942-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaudy F, Ferrara A, Esposito L, Hertzano R, Ben-David O, Bell RE, Melchionda S, Zelante L, Avraham KB, Gasparini P. Multiple mutations of MYO1A, a cochlear-expressed gene, in sensorineural hearing loss. Am J Hum Genet. 2003;72:1571–1577. doi: 10.1086/375654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Yu D, Post J, Halks-Miller M, Sadler JE, Morser J. Generation and characterization of mice deficient in hepsin, a hepatic transmembrane serine protease. J Clin Invest. 1998;101:321–326. doi: 10.1172/JCI1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd RK, Coco A, Epp SB, Crook JM. Chronic depolarization enhances the trophic effects of brain-derived neurotrophic factor in rescuing auditory neurons following a sensorineural hearing loss. J Comp Neurol. 2005;486:145–158. doi: 10.1002/cne.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng L, Goodyear RJ, Woods CA, Schneider MJ, Diamond E, Richardson GP, Kelley MW, Germain DL, Galton VA, Forrest D. Hearing loss and retarded cochlear development in mice lacking type 2 iodothyronine deiodinase. Proc Natl Acad Sci USA. 2004;101:3474–3479. doi: 10.1073/pnas.0307402101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipper M, Zinn C, Maier H, Praetorius M, Rohbock K, Kopschall I, Zimmermann U. Thyroid hormone deficiency before the onset of hearing causes irreversible damage to peripheral and central auditory systems. J Neurophysiol. 2000;83:3101–3112. doi: 10.1152/jn.2000.83.5.3101. [DOI] [PubMed] [Google Scholar]
- Rusch A, Erway LC, Oliver D, Vennstrom B, Forrest D. Thyroid hormone receptor β-dependent expression of a potassium conductance in inner hair cells at the onset of hearing. Proc Natl Acad Sci USA. 1998;95:15758–15762. doi: 10.1073/pnas.95.26.15758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer P, Grunder S, Rusch A. Expression of Ca2+-activated BK channel mRNA and its splice variants in the rat cochlea. J Comp Neurol. 2003;455:198–209. doi: 10.1002/cne.10471. [DOI] [PubMed] [Google Scholar]
- Skinner LJ, Enee V, Beurg M, Jung HH, Ryan AF, Hafidi A, Aran JM, Dulon D. Contribution of BK Ca2+-activated K+ channels to auditory neurotransmission in the guinea pig cochlea. J Neurophysiol. 2003;90:320–332. doi: 10.1152/jn.01155.2002. [DOI] [PubMed] [Google Scholar]
- Rüttiger L, Sausbier M, Zimmermann U, Winter H, Braig C, Engel J, Knirsch M, Arntz C, Langer P, Hirt B, Müller M, Köpschall I, Pfister M, Münkner S, Rohbock K, Pfaff I, Rusch A, Ruth P, Knipper M. Deletion of the Ca2+-activated potassium (BK) α-subunit but not the BKβ1-subunit leads to progressive hearing loss. Proc Natl Acad Sci USA. 2004;101:12922–12927. doi: 10.1073/pnas.0402660101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt N, Kuhn S, Münkner S, Braig C, Winter H, Blin N, Vonthein R, Knipper M, Engel J. Thyroid hormone deficiency affects postnatal spiking activity and expression of Ca2+ and K+ channels in rodent inner hair cells. J Neurosci. 2007;21:3174–3186. doi: 10.1523/JNEUROSCI.3965-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GP, Russell IJ, Duance VC, Bailey AJ. Polypeptide composition of the mammalian tectorial membrane. Hear Res. 1987;25:45–60. doi: 10.1016/0378-5955(87)90078-5. [DOI] [PubMed] [Google Scholar]
- Willem M, Garratt AN, Novak B, Citron M, Kaufmann S, Rittger A, DeStrooper B, Saftig P, Birchmeier C, Haass C. Control of peripheral nerve myelination by the β-secretase BACE1. Science. 2006;314:664–666. doi: 10.1126/science.1132341. [DOI] [PubMed] [Google Scholar]
- Uziel A, Gabrion J, Ohresser M, Legrand C. Effects of hypothyroidism on the structural development of the organ of Corti in the rat. Acta Otolaryngol. 1981;92:469–480. doi: 10.3109/00016488109133286. [DOI] [PubMed] [Google Scholar]
- Uziel A. Periods of sensitivity to thyroid hormone during the development of the organ of Corti. Acta Otolaryngol. 1986;(Suppl) 429:23–27. doi: 10.3109/00016488609122726. [DOI] [PubMed] [Google Scholar]
- O’Malley BW, Jr, Li D, Turner DS. Hearing loss and cochlear abnormalities in the congenital hypothyroid (hyt/hyt) mouse. Hear Res. 1995;88:181–189. doi: 10.1016/0378-5955(95)00111-g. [DOI] [PubMed] [Google Scholar]
- Li D, Henley CM, O’Malley BW., Jr Distortion product otoacoustic emissions and outer hair cell defects in the hyt/hyt mutant mouse. Hear Res. 1999;138:65–72. doi: 10.1016/s0378-5955(99)00150-1. [DOI] [PubMed] [Google Scholar]
- Christ S, Biebel UW, Hoidis S, Friedrichsen S, Bauer K, Smolders JW. Hearing loss in athyroid pax8 knockout mice and effects of thyroxine substitution. Audiol Neurootol. 2004;9:88–106. doi: 10.1159/000076000. [DOI] [PubMed] [Google Scholar]
- Forrest D, Erway LC, Ng L, Altschuler R, Curran T. Thyroid hormone receptor beta is essential for development of auditory function. Nat Genet. 1996;13:354–357. doi: 10.1038/ng0796-354. [DOI] [PubMed] [Google Scholar]
- Rusch A, Ng L, Goodyear R, Oliver D, Lisoukov I, Vennstrom B, Richardson G, Kelley MW, Forrest D. Retardation of cochlear maturation and impaired hair cell function caused by deletion of all known thyroid hormone receptors. J Neurosci. 2001;21:9792–9800. doi: 10.1523/JNEUROSCI.21-24-09792.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipper M, Bandtlow C, Gestwa L, Kopschall I, Rohbock K, Wiechers B, Zenner HP, Zimmermann U. Thyroid hormone affects Schwann cell and oligodendrocyte gene expression at the glial transition zone of the VIIIth nerve prior to cochlea function. Development. 1998;125:3709–3718. doi: 10.1242/dev.125.18.3709. [DOI] [PubMed] [Google Scholar]
- Friedrichs B, Tepel C, Reinheckel T, Deussing J, von Figura K, Herzog V, Peters C, Saftig P, Brix K. Thyroid functions of mouse cathepsins B, K, and L. J Clin Invest. 2003;111:1733–1745. doi: 10.1172/JCI15990. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.