Abstract
Acute renal failure due to ischemia/reperfusion involves disruption of integrin-mediated cellular adhesion and activation of the extracellular signal-regulated kinase (ERK) pathway. The dynamics of focal adhesion organization and phosphorylation during ischemia/reperfusion in relation to ERK activation are unknown. In control kidneys, protein tyrosine-rich focal adhesions, containing focal adhesion kinase, paxillin, and talin, were present at the basolateral membrane of tubular cells and colocalized with short F-actin stress fibers. Unilateral renal ischemia/reperfusion caused a reversible protein dephosphorylation and loss of focal adhesions. The focal adhesion protein phosphorylation rebounded in a biphasic manner, in association with increased focal adhesion kinase, Src, and paxillin tyrosine phosphorylation. Preceding phosphorylation of these focal adhesion proteins, reperfusion caused increased phosphorylation of ERK. The specific mitogen-activated protein kinase kinase 1/2 inhibitor U0126 prevented ERK activation and attenuated focal adhesion kinase, paxillin, and Src phosphorylation, focal adhesion restructuring, and ischemia/reperfusion-induced renal injury. We propose a model whereby ERK activation enhanced protein tyrosine phosphorylation during ischemia/reperfusion, thereby driving the dynamic dissolution and restructuring of focal adhesions and F-actin cytoskeleton during reperfusion and renal injury.
Ischemia/reperfusion (I/R) injury is an important life-threatening clinical problem that may occur in various vital organs such as heart, brain, and kidney. One of the primary events during I/R is mitochondrial dysfunction leading to ATP depletion. A general phenomenon during I/R is compromised cell adhesion.1,2,3,4 Cell adhesion is tightly controlled by proper integrity of the actin cytoskeletal network, which requires ATP.5,6 Likewise, I/R causes cytoskeletal disruption in various tissues.7 It is important to understand better the basic mechanisms that drive disruption of cytoskeletal organization and cell adhesion in the course of I/R. In contrast to most other organs, the kidney can completely and efficiently regenerate after I/R injury, making it a unique model to unravel the dynamics of the F-actin cytoskeleton and the cell-extracellular matrix (ECM) interactions and molecular events involved in this process.
I/R injury is one of the most important causes of acute renal failure, and the proximal tubular cells (PTCs) are the main target.8,9 Early after the ischemic period the basolateral-apical protein polarity is disturbed, and the microvilli brush border is lost in conjunction with loss of cytoskeletal integrity.7,10 Renal I/R injury also results in perturbations of cell-cell contacts at adherens and tight junctions11,12 and the integrin-mediated cell-ECM adhesions.4,13,14 These changes are requirements for detachment and exfoliation of epithelial cells into the lumen, leaving a denuded proximal tubule that requires regeneration. To better understand cell adhesion in vivo and the role that adhesion plays during I/R, it is necessary to unravel the molecular and cellular mechanisms that underlie the cellular injury and regeneration during I/R.
Cell-ECM adhesions are mediated by the integrin family of cell adhesion receptors at focal adhesions (FAs). FAs consist of a large number of both cytoskeletal and signal transduction (adapter) proteins and are rich in tyrosine phosphorylated proteins. β1-Integrin is the most prominent integrin in PTC-mediated cell-ECM interactions. β1-Integrins are lost from the PTC basolateral membrane region during the ischemic period and return to this side during reperfusion.4,15,16 Localization of β1 integrin at the cell-ECM contacts is regulated by the integrity of the F-actin cytoskeletal network as well as signal transduction pathways. Given the fact that I/R results in ATP depletion coupled to intracellular stress responses and modulation of β1-integrins, adhesion of PTCs will largely be regulated via inside-out signaling. Protein phosphorylation is one of the principal regulatory mechanisms that control cell adhesion dynamics. Therefore, determining differential kinase activities as well as protein (de)phosphorylation events is essential in understanding the mechanisms of cell detachment during I/R.
Focal adhesion kinase (FAK) is a ubiquitously expressed nonreceptor protein tyrosine kinase that is essential in cell-ECM signaling toward cell migration, survival, proliferation, and stress pathways.17,18 Recruitment to clustered integrins at FAs allows FAK autophosphorylation on tyrosine residue Y397. This residue forms a binding site for the SH2 domain of Src kinase, which is activated and subsequently induces phosphorylation of FAK on other tyrosine residues, including Y576/Y577 in the kinase domain and Y861 in the C-terminal domain. Together the FAK/Src kinase complex phosphorylates downstream targets such as the signaling adapter protein paxillin.19,20 FAK is dephosphorylated after chemical anoxia in isolated rabbit proximal tubules21 and during nephrotoxicant exposure in primary cultured rat PTCs.22 So far, the involvement of FAK in I/R in vivo remains unclear. Moreover, the differential phosphorylation of FAK at different tyrosine residues and molecular mechanisms involved in the context of restructuring of both adhesion complexes and the F-actin cytoskeleton in renal I/R injury and regeneration have not been investigated.
The family of mitogen-activated protein kinases, including p38, c-Jun NH2-terminal kinase, and extracellular signal-regulated kinase (ERK), are activated during I/R.23 Although the ERK pathway plays a role in cell growth and differentiation, giving cells a survival advantage, there is growing evidence suggesting that activation of ERK may contribute to injury and apoptotic cell death.24,25 ERK is reported to localize at the FAs where it binds to paxillin and is required for FA disassembly and turnover together with FAK, paxillin, and Src kinase.26,27,28 Although this suggests a possible link between ERK activation and FA restructuring during I/R, this has never been investigated in relation to either renal protection or pathophysiology.
In this study, we report the abundant presence of protein tyrosine phosphorylation-rich FAs at the basolateral membrane of PTCs in vivo, containing FAK, paxillin, and talin that colocalize with F-actin stress fibers. Tyrosine phosphorylation at FAs was lost directly after ischemia, which was associated with reorganization of the FAs and followed by a drastic increase in phosphorylation during reperfusion, together with an increase in FA size. The ischemia-induced FAK dephosphorylation was followed by a differential phosphorylation of the different FAK tyrosine residues during reperfusion. ERK was phosphorylated directly after ischemia. Inhibition of the mitogen-activated protein kinase kinase (MEK)/ERK pathway with U0126 attenuated the early changes of protein tyrosine phosphorylation (pTyr) proteins FAK and paxillin in association with the onset of FA restructuring and renal failure. These data indicate an ERK-dependent dynamic restructuring of FAs in association with differential tyrosine phosphorylation of FA-associated structural and signaling proteins.
Materials and Methods
Renal Ischemia/Reperfusion Injury
For this study, a unilateral rat model of renal ischemia/reperfusion was used.4,29,30 Male Wistar rats (170 to 220 g) were anesthetized with S-ketamine (25 mg/kg body weight s.c.) and metadomine hydrochloride (0.04 mg/animal i.m.). A small incision was made over the left flank, and the left renal artery was prepared and clamped with a hemostatic clamp (5 to 15 g/mm2; Fine Science Tools, Heidelberg, Germany) for 30 or 45 minutes, whereas right kidneys were unaffected and served as internal controls. After removal of the clamp, the kidney was reperfused for indicated time intervals. After reperfusion, both left and right kidneys were harvested and prepared for further analysis as described below. Control kidneys were obtained from animals that underwent sham surgery without ischemia/reperfusion. Each group consisted of five animals.
Intraperitoneal Injection of the ERK1/2 Inhibitor U0126
Thirty minutes before clamping the left renal artery, rats were injected intraperitoneally with either vehicle (2% dimethylsulfoxide in phosphate-buffered saline or the ERK1/2 inhibitor U0126 (Promega, Leiden, The Netherlands) (1 mg/kg in 2% dimethylsulfoxide in phosphate-buffered saline). After 30 minutes, the procedure was followed as described above.
Tissue Preparation
After harvesting, both kidneys were briefly washed in ice-cold phosphate-buffered saline to remove excess blood and sliced into two equal halves. One-half was frozen in liquid nitrogen and stored at −80°C for Western blot analysis or immunohistochemistry. One-half was fixated in ice-cold Carnoy’s solution [60% (v/v) absolute ethanol, 30% (v/v) chloroform, and 10% (v/v) glacial acetic acid] for 3 hours and thereafter transferred to 70% (v/v) ethanol and stored at 4°C for histopathological evaluation.
Histopathological Evaluation
Kidneys were embedded in paraffin and sectioned (3 μm) onto 3-aminopropyltriethoxysilane-coated slides. Paraffin sections were deparaffinized and rehydrated before staining for hematoxylin and eosin and examined for tubular injury resulting from I/R injury using light microscopy (Leica DM6000B, ×400 magnification; Wetzlar, Germany). To assess tubulointerstitial injuries, kidney sections were arbitrarily divided into three regions, ie, cortex, outer medulla, and inner medulla. Using semiquantitative indices, sections were analyzed for the evaluation of acute tubulointerstitial damage. In each region, extent of tubular cast formation, tubular dilatation, and tubular degeneration (vacuolar change, loss of brush border, detachment of tubular epithelial cells, and condensation of tubular nuclei) were scored according to following criteria by two blinded observers: 0, normal; 1, <30%; 2, 30 to 70%; and 3, >70% of the pertinent area. After scoring, the scores were summed to show the overall tubular damage in the kidney.
Immunohistochemistry
Frozen sections (10 μm) were cut with cryostat and thaw-settled on 3-aminopropyltriethoxysilane-coated slides and fixed in 4% formaldehyde for 10 minutes. After washing with Tris-buffered saline, sections were blocked in 5% (v/v) normal goat serum (NGS; Vector Laboratories, Burlingame, CA) for 1 hour and incubated overnight at 4°C in a humidified chamber with primary antibody: total protein tyrosine phosphorylation (PY99; Santa Cruz Biotechnology, Santa Cruz, CA); FAK (clone 77; Transduction Laboratories, Lexington, KY); PY397-FAK (BioSource International, Camarillo, CA); paxillin (Transduction Laboratories); PY118-paxillin (BioSource); collagens I and III (Sigma, St. Louis, MO); ERK1/2 and PSer17/221-ERK1/2 (Cell Signaling Technology Inc., Danvers, MA). Thereafter, slides were washed and incubated for 1 hour with secondary antibody: Alexa Fluor 488-labeled goat anti-mouse or anti-rabbit antibodies (Invitrogen, Carlsbad, CA); Cy3-labeled goat anti-mouse or anti-rabbit antibodies (Jackson Laboratories). Rhodamine/phalloidin (Invitrogen) was used for F-actin staining. After removing the secondary antibody, slides were washed and mounted on Poly Aquamount (Polysciences, Inc., Warrington, PA). Images were made using a Bio-Rad Radiance 2100 confocal system (Bio-Rad, Hercules, CA) with a 60× Plan Apo (NA 1.4; Nikon, Tokyo, Japan) objective lens. All images were processed with Image-Pro Plus (version 5.1; Media Cybernetics, Bethesda, MD).
Western Blot Analysis
Frozen sections were lysed in 250 μl of Triton lysis buffer (20 mmol/L Tris, pH 7.4, 137 mmol/L NaCl, 2 mmol/L ethylenediamine tetraacetic acid, 1% Triton X-100, 25 mmol/L β-glycerophosphate, and 10% glycerol) with inhibitors and incubated at 4°C for 2 hours. Lysates were syringed four times though a 26-gauge needle, centrifuged (20 minutes at 10,000 rpm, 4°C) and immediately boiled in sample preparation buffer (125 mmol/L Tris-HCl, pH 6.8, 20% glycerol, 4% sodium dodecyl sulfate, and bromphenol blue). Protein concentrations were determined using a Bradford assay with IgG as a standard. Equal amounts of protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane (Schleicher & Schuell, Keene, NH). Blots were blocked in 5% (w/v) bovine serum albumin in Tris-buffered saline/Tween 20 [0.5 mol/L NaCl, 20 mmol/L Tris-HCl, and 0.05% (v/v) Tween 20, pH 7.4] for 1 hour. Primary antibody incubation was performed overnight at 4°C in anti-PY99 (0.04 μg/ml, monoclonal; Santa Cruz Biotechnology); anti-PY397-FAK (1 μg/ml, polyclonal; BioSource); anti-FAK (monoclonal, 1 μg/ml; Transduction Laboratories); anti-PY118-paxillin (0.75 μg/ml, polyclonal; BioSource); anti-paxillin (monoclonal, 0.5 μg/ml; Transduction Laboratories). Thereafter blots were incubated with horseradish peroxidase-conjugated secondary antibody (GE Healthcare, Little Chalfont, Buckinghamshire, UK) in Tris-buffered saline/Tween 20 for 1 hour at room temperature. Protein signals were detected with the ECL Plus method (GE Healthcare) followed by scanning of the blots with the Typhoon 9400 (GE Healthcare). Ratios of the protein band intensity were obtained using ImageQuant analysis (GE Healthcare).
Results
Focal Adhesions in Vivo Are Tyrosine-Phosphorylated Structures Connected to F-Actin Stress Fibers
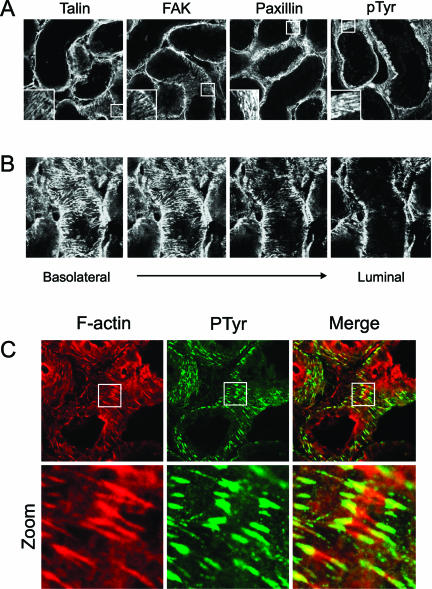
In vitro, FAs are located at the closest contact site between the cell and the ECM and are connected to the F-actin cytoskeleton. To demonstrate the presence of FAs in vivo, we stained frozen sections of control kidneys for the focal adhesion proteins talin, FAK, and paxillin. These three proteins were organized in a similar stripe-like manner at the basolateral cell surface of the proximal tubules, demonstrating the existence of FA-like structures in vivo (Figure 1A). To visualize the localization of FAK-containing FAs in more detail, a Z-scan was made starting at the basolateral side of a proximal tubule and ending with a luminal cross section (Figure 1B). Collagen I and III staining supported the localization of FAs at the basolateral membrane of the proximal tubulus (see Supplemental Figure S1, A and B, at http://ajp.amjpathol.org). FAs were solely present at this collagen-rich basolateral side where cells adhere to the ECM and not at the luminal side of the cells, thereby surrounding the proximal tubulus. Because the function of FA proteins is often regulated by tyrosine phosphorylation, frozen sections were stained for total pTyr to determine whether in vivo FAs are pTyr-containing structures. pTyr staining was organized in similar stripe-like structures as observed for the FA proteins talin, FAK, and paxillin (Figure 1A). Focal adhesion proteins such as FAK and paxillin are generally located at the end of F-actin stress fibers.20,31 In vivo, short stress fibers were found at the basolateral side of PTCs, and the tips colocalized with the phosphotyrosine-positive FAs structures (Figure 1C).
Figure 1.
In vivo colocalization of tyrosine-phosphorylated focal adhesion proteins with F-actin stress fibers. To determine localization of FAs in the proximal tubules, frozen sections (10 μm) of control kidneys were stained for the FA proteins talin, FAK, and paxillin and for total tyrosine phosphorylation (pTyr) (A). A Z-scan was created from the basolateral site toward the lumen of the tubule to show localization of FAK (B). To indicate colocalization between pTyr containing FAs and F-actin, sections were costained for pTyr (green) and F-actin (red); colocalization is yellow (C). All sections were imaged using a confocal laser scanning microscope. Sections are representative of proximal tubules in three different rats and observed in two different stainings.
Reversible Focal Adhesion Protein Tyrosine Dephosphorylation and Focal Adhesion Dissolution during Ischemia/Reperfusion
Next, we determined the dynamics of F-actin organization and focal adhesions during ischemia/reperfusion injury. In PTCs of normal rat kidneys, F-actin is concentrated mainly in the brush border microvilli at the apical membrane, at cell-cell junction sites, and at the basolateral membrane together with FAs (Figure 1C and Supplemental Figure S2 at http://ajp.amjpathol.org). Directly after mild ischemia (30 minutes), which by itself did not result in acute tubular necrosis at 24 hours, the proximal tubules were dilated in both the outer stripe of the outer medulla (OSOM) and cortex region (see Supplemental Figure S2A at http://ajp.amjpathol.org). In contrast, 45 minutes of ischemia caused severe acute tubular necrosis at 24 hours associated with atrophic and denuded tubules and fragmented nuclei pointing to apoptosis (see Supplemental Figure S2B at http://ajp.amjpathol.org). Under these latter conditions, 2 weeks after the ischemic insult all tubules were relined with proximal tubular cells mostly resembling control conditions. Renal sections from both mild and severe ischemia/reperfusion conditions were stained for F-actin. Directly after 30 minutes of ischemia, the F-actin network was reorganized with a loss of stress fibers (zoom Supplemental Figure S3A at http://ajp.amjpathol.org) and a reduction in F-actin at the microvilli. After 24 hours of reperfusion, more pronounced stress fibers reappeared at the basolateral membrane, which were not observed in sham-operated animals (zoom Supplemental Figure S3A at http://ajp.amjpathol.org). Similar F-actin reorganization, although more severe, took place in kidneys subjected to 45 minutes of ischemia (see Supplemental Figure S3B at http://ajp.amjpathol.org). Reperfusion times of up to 2 weeks were necessary to completely regenerate the F-actin network (data not shown).
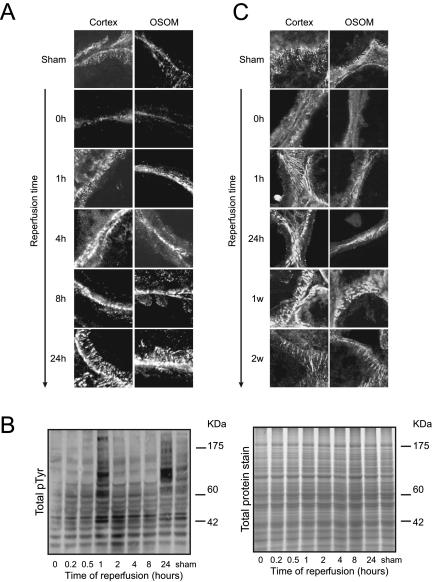
Both 30 as well as 45 minutes of ischemia resulted in dephosphorylation of proteins located at the FAs of the renal cells, leaving only little pTyr at the basolateral side of the cells (Figure 2, A–C). Talin was still present at the focal adhesions, indicating that the focal adhesions were still intact, although differently shaped (data not shown). For mild ischemia (30 minutes), an increase in pTyr was observed after 1 hour and 24 hours of reperfusion, whereas pTyr was lower at intermediate time points (Figure 2B). The phosphorylation pattern at 1 hour of reperfusion slightly differed from the pattern observed at 24 hours of reperfusion, suggesting that different phosphoproteins may be activated. After 1 hour of reperfusion, pTyr was not solely located on the FAs but also was more cytosolic (Figure 2A). During 4 and 8 hours of reperfusion, the overall intensity of the phosphotyrosine staining decreased at FAs, which were now organized in dot-like structures. In addition, we observed more cytosolic staining with some pTyr in the microvilli of the tubular cells (Figure 2A). The intensity of the phosphotyrosine staining increased drastically after 24 hours of reperfusion, with thick and striped organized focal adhesion structures (Figure 2A) that colocalized with F-actin stress fibers (data not shown). Similar findings were obtained for 45 minutes of ischemia, although the time frame of FA reorganization differed. Here, the OSOM region was severely injured, resulting in a slower recovery of FA structures. After 24 hours of reperfusion, thick, bulky, and intensely stained FAs were mainly found in the cortex. After 1 week of reperfusion, the FAs became very round, large, and hyperphosphorylated in both OSOM and cortex. FAs almost fully recovered after 2 weeks of reperfusion. At this time point, tyrosine-phosphorylated FAs were organized like FAs in the sham-operated animals but appeared slightly more elongated (Figure 2C).
Figure 2.
I/R caused a biphasic increase in protein tyrosine phosphorylation and restructuring of focal adhesions. Rats were subjected to 30 (A) or 45 minutes (C) of ischemia followed by reperfusion for the indicated time periods. Thereafter, frozen kidney sections (10 μm) were stained for total tyrosine phosphorylation using an anti-pTyr antibody. Both OSOM and cortex region were evaluated for differential tyrosine phosphorylation using confocal laser scanning microscopy (A and C) and Western blot analysis by staining blots with an anti-pTyr antibody (B). Total protein was measured by staining a sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel with Sypro Ruby (B). Data are representative of three different rats and observed in two different stainings.
Time-Dependent Differential Phosphorylation of FAK, Src, and Paxillin during Ischemia/Reperfusion Injury
FAK activity mediates focal adhesion turnover, and its activity is controlled by differential phosphorylation of several tyrosine residues.32,33 Because both pTyr protein expression and FA organization were affected, we determined the effect on FAK phosphorylation, ie, activation and function. Staining of sham control kidneys indicated that PY397-FAK colocalized with FAK in FA-like structures (Figure 3) in a similar fashion to talin and paxillin. Thirty minutes of ischemia resulted in a loss of PY397-FAK localization at these FAs. However, small and thin FAK-containing FAs were still present, indicating that FAs were not completely disrupted during ischemia. After 1 hour of reperfusion PY397-FAK increased (Figures 3 and 4) and was located on the basolateral membrane of the proximal tubule cells organized in small, thin, and elongated structures. Staining of PY397-FAK increased 24 hours after ischemia and was organized in thick and bulky FAs and was colocalizing with FAK (Figure 3A). Quantitative analysis of renal cortex PY397-FAK levels confirmed the immunohistochemical analysis (Figure 3B). Moreover, FAK phosphorylation followed a biphasic pattern like that observed for total pTyr staining (Figures 2 and 3B).
Figure 3.
I/R induces dynamic phosphorylation of FAK, paxillin, and Src. Rats were subjected to 30 minutes of ischemia followed by reperfusion for 0 to 24 hours. Frozen sections (10 μm) were stained for FAK (green) and PY397-FAK (red) to determine differential tyrosine phosphorylation and colocalization (yellow) at FAs (A). In addition, frozen sections were prepared for Western blot analysis and stained for PY397-FAK and FAK (B), PY118-paxillin and paxillin (D), and PY576-FAK, PY861-FAK PY416-Src kinase, and PSer17/221-ERK (C). All sections were imaged using a confocal laser scanning microscope. Data are representative of proximal tubules in three different rats.
Figure 4.
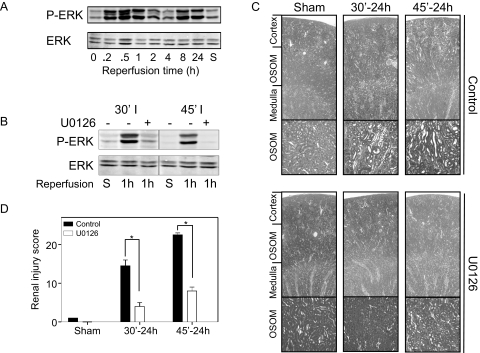
I/R-induced injury is inhibited by the ERK inhibitor U0126. Rats were pretreated with U0126 (1 mg/kg) 30 minutes before I/R for indicated time points. For untreated (A) and treated rats (B), frozen sections were prepared for Western blot analysis and stained for PSer17/221-ERK. Paraffin sections were stained for H&E to determine tubular damage (C). Sections are representative of proximal tubules in three different rats. Pictures were made using light microscopy (Leica DM6000B; magnifications, ×25 and ×100). Sections were scored double-blind and semiquantitatively to assess tubulointerstitial injury (D). Data are presented as means ± SE (n = 3 rats per group). *P < 0.05 compared with control.
FAK phosphorylation at Y397 promotes the Src homology domain 2 (SH2)-dependent binding of Src kinase, thereby activating the protein via phosphorylation of Y416.34 On its turn, Src mediates the phos-phorylation of other FAK residues as well as the phosphorylation of other FA proteins, including paxillin. Therefore, we next determined systematically whether the differential FAK phosphorylation was associated with Src activation, differential FAK tyrosine phosphorylation, and phosphorylation of downstream effectors. During ischemia, Src was dephosphorylated at Y416, but unlike FAK and paxillin, this dephosphorylation was not complete (Figure 3C). Src kinase was phosphorylated at 1 hour and 24 hours after reperfusion, the time points at which FAK was also phosphorylated at Y397. Activation of Src kinase results in phosphorylation of FAK at multiple residues, among which are Y576, thereby enhancing FAK catalytic activity and Y861, thereby creating additional interaction sites for SH2-containing proteins.35,36 Both Y576 and Y861 were dephosphorylated during ischemia and phosphorylated after 1 hour of reperfusion, with high phosphorylation content for Y576 and phosphorylation levels just above control for Y861. Like the other FA proteins, Y861 was phosphorylated in a biphasic expression pattern during reperfusion, since it was highly phosphorylated after 24 hours of reperfusion (Figure 3C). In contrast, Y576 phosphorylation levels resembled that of control kidneys at 24 hours after reperfusion, indicating that FAK catalytic activity is probably less compared with its activity after 1 hour of reperfusion. A key downstream effector of the FAK/Src-kinase complex is paxillin, which is phosphorylated on both tyrosine residues 31 and 118.37 In a similar fashion to FAK and Src, paxillin phosphorylation followed a biphasic phosphorylation pattern (Figure 3D), whereas paxillin phosphorylation at 24 hours was three times higher than in sham controls. Immunostaining against paxillin indicated that the localization of PY118-paxillin after ischemia/reperfusion was at FAs in a similar manner as for total pTyr and PY397-FAK staining (data not shown). These results indicate that the different components of the FA complex that regulate both turnover and downstream signaling from focal adhesions are all phosphorylated in a biphasic manner, which is directly related to their localization at and dynamic reorganization of focal adhesions.
Inhibition of ERK Activation Protects against Protein Tyrosine Kinase Activation, Focal Adhesion Restructuring, and Renal Injury
In addition to these focal adhesion proteins, the mitogen-activated protein kinase family member ERK is known to be activated in response to I/R injury.23 Moreover, ERK phosphorylation and activation is important for paxillin phosphorylation and its association with FAK.28 This prompted us to verify the activation of ERK in relation to phosphorylation of FAK, paxillin, and Src. ERK is phosphorylated within 10 minutes of reperfusion, before phosphorylation of FAK and paxillin (Figure 4A). This phosphorylation dropped to intermediate values between 2 and 4 hours and increased again after 8 and 24 hours of reperfusion.
To clarify a possible relationship of early activation of the ERK pathway, tyrosine phosphorylation of focal adhesion proteins, and renal injury, rats were injected with a specific MEK1/2 inhibitor, U0126, 30 minutes before clamping of the left renal artery. U0126 pretreatment reduced the phosphorylation of ERK in rats that were subjected to 30 or 45 minutes of ischemia and 1 hour of reperfusion (Figure 4B). In the absence of U0126 ischemia/reperfusion resulted in dilated tubules, loss of brush border, and cast formation. U0126 significantly attenuated injury to the kidney (Figure 4, C and D); almost no dilated tubules in the OSOM region compared with control kidneys were observed on 30 minutes of ischemia and 24 hours of reperfusion, whereas U0126 completely protected against injury in the cortex region. In addition, the severe injury observed in both OSOM and cortex region caused by 45 minutes of ischemia and 24 hours of reperfusion was significantly attenuated by U0126 pretreatment (Figure 4, C and D).
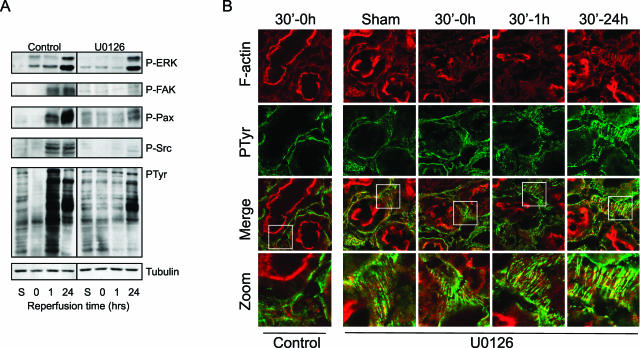
Given the role of ERK in renal injury and the regulation of the FA organization, our model allowed us to investigate the mechanism by which FA protein phosphorylation is controlled during ischemia/reperfusion and its relationship to renal injury. In the absence of U0126, overall protein tyrosine phosphorylation was lost on ischemia and dramatically increased after 1 hour of reperfusion (Figure 5A), consistent with the results shown in Figure 2. In contrast, U0126 almost completely prevented such a loss of phosphorylation as well as the increase in tyrosine phosphorylation (Figure 5A). This was also associated with an attenuation of FAK, Src, and paxillin phosphorylation at this early time point. At 24 hours of reperfusion, U0126 pretreatment no longer blocked ERK phosphorylation. FAK, Src, and paxillin phosphorylation were still clearly decreased at this time point compared with untreated control, albeit at a slightly higher level that the sham control (Figure 5A). In addition, the overall protein tyrosine phosphorylation was slightly increased at this time point. Finally, we determined whether this protection by U0126 against the dynamic changes in FA protein phosphorylation was linked to a protection against disturbances of FA organization. In the absence of U0126, 30 minutes of ischemia resulted in F-actin reorganization and dephosphorylation of proteins located at the FAs (Figures 2 and 5B, left). Inhibition of ERK activation using U0126 pretreatment protected against F-actin reorganization and disruption of phosphotyrosine-rich focal adhesions (Figure 5B). In agreement, kidneys of U0126 treated rats did not show any sign of protein dephosphorylation after 30 minutes (Figure 5B) as well as 45 minutes of reperfusion (data not shown).
Figure 5.
U0126 prevents I/R-mediated loss of FA tyrosine phosphorylation in conjunction with maintenance of FA structure. Rats were pretreated with U0126 (1 mg/kg), and both control and U0126-treated rats were subjected to 30 minutes of ischemia followed by reperfusion for the indicated time periods. Thereafter, frozen kidney sections (10 μm) were prepared for Western blotting and stained for pTyr, P-FAK, paxillin, and Src (A) and stained with rhodamine/phalloidin for F-actin and total tyrosine phosphorylation using anti-pTyr antibody (B). Sections were evaluated for F-actin reorganization and differential pTyr using confocal laser scanning microscopy. The bottom panel indicates a zoom of the areas containing stress fibers and tyrosine-phosphorylated focal adhesions. Sections are representative of proximal tubules in three different rats.
Discussion
In this study, we show for the first time an ERK-dependent temporal and spatial reorganization of FAs and F-actin stress fibers as well as phosphorylation of FAK and other focal adhesion proteins during I/R injury and regeneration. First, our data indicate the existence of FAs under in vivo conditions at the basolateral membrane of the renal proximal tubular epithelial cells. The FA structures are enriched in FAK and paxillin as well as their respective tyrosine phosphorylated forms and are connected to basolateral F-actin stress fibers. Second, protein tyrosine phosphorylation events at the FAs were lost directly after the ischemic event, which coincided with a disruption of the FA structures and the F-actin network. During the reperfusion period, levels of protein tyrosine phosphorylation increased in association with an increase in FA size and F-actin stress fiber formation. Preceding this phosphorylation, the MEK/ERK pathway is activated. Inhibition of this pathway by pretreatment of rats with a specific MEK inhibitor, U0126, attenuated dephosphorylation and enhanced rephosphorylation and dissolution of FAs in conjunction with decreased renal injury.
Our data clearly show that activation of the MEK/ERK pathway is linked to renal injury. This supports growing evidence suggesting that activation of ERK in renal cells is involved in injury and apoptosis rather than contributing to cell survival.24,38 Likewise, adenovirus-mediated antisense ERK2 gene therapy attenuated chronic allograft nephropathy, thereby protecting against acute renal failure39; pretreatment of mice with U0126 reduced tissue damage and improved renal function after cisplatin treatment.25
I/R-induced ERK activation was associated with a temporal restructuring of FAs coinciding with phosphorylation of FAK, paxillin, and Src. Generally, FA organization and signaling is studied in vitro. Although FA-like signaling complexes are likely to be present in a variety of cells in vivo, the classical FA contacts are not well studied. Our data not only indicate the existence of FAK and paxillin containing FAs in vivo, but they also show that these FAs are tyrosine phosphorylated and connected to F-actin stress fibers. In vitro, FA size and stability are linked to the amount of cytoskeletal contractility.40,41 This suggests that the presence FAs and their attachment to F-actin stress fibers in vivo may allow regulation of tension in the proximal tubulus,41 thereby providing it with a possibility to regulate tubular pressure and ultrafiltration rate.
Renal ischemia caused disruption of the F-actin cytoskeleton together with dephosphorylation and restructuring of FAs. The dephosphorylation of basolateral associated proteins on Tyr residues is consistent with other studies that have shown dephosphorylation in vitro21 and in other organs such as brain and heart.2,3,42 The tyrosine dephosphorylation of proteins was blocked by ERK inhibition. In addition, reperfusion of the kidney resulted in early ERK activation and subsequent tyrosine phosphorylation of FA proteins such as FAK and paxillin. In vitro, ERK activation is associated with increased phosphorylation of paxillin, resulting in increased association between paxillin and FAK, whereas inhibition of ERK resulted in disruption of the complex and dephosphorylation of FAK.28 Because ERK is activated early during reperfusion before FAK and paxillin phosphorylation in vivo, ERK may well be involved in FA organization by activating signaling pathways leading to phosphorylation of FA proteins on Tyr residues, including FA-associated proteins. In cell culture models it is well established that increased activation of FA-associated kinases such as FAK and Src increases turnover of FA structures.26,37 Therefore, the drastic increase in tyrosine phosphorylation early in the reperfusion period (1 hour) most likely affects the turnover of the FA complexes, resulting in their dissolution. U0126 pretreatment, which prevented ERK activation early after reperfusion, was associated with protection against FA protein phosphorylation and reorganization (Figure 5). Together, these data suggest that maintenance of focal adhesion complexes is important for renal function and dependent on ERK activation.
After ischemic injury, a biphasic protein tyrosine phosphorylation wave was observed: after 1 and 24 hours of reperfusion, the amount of total pTyr was increased above sham-operated control levels. The increase at an early time point is most likely related to the reversal of cellular ATP levels, which can be used by protein tyrosine kinases to phosphorylate cellular proteins. Activation of the EGF receptor is already observed after 5 to 30 minutes of reperfusion,43 suggesting that activation of receptor protein tyrosine kinases participates in the increased protein tyrosine phosphorylation observed at an early time point. Moreover, activation of the ERK pathway, which can occur in response to epidermal growth factor receptor activation or reactive oxygen species formation, has been known to contribute to the modulation of protein tyrosine phosphorylation,44 suggesting that the rapid activation of ERK in the reperfusion period further stimulates tyrosine phosphorylation. The increased levels of protein pTyr at later time points are most likely directly related to increased amounts of growth factors (ie, epidermal growth factor, hepatocyte growth factor, and insulin-like growth factor) that are generated in the renal cell response to injury.45 These factors will activate receptor tyrosine kinases that promote downstream activation of signal cascades, including activation of FAK and the c-Met receptor.18,46 In addition, after 2 to 8 hours of protein phosphorylation, levels were lower compared with 1 and 24 hours of reperfusion. Although it has been shown that protein phosphatase activity can decrease after an ischemic insult,47 we did not observe changes in overall phosphatase activity (data not shown). Although we cannot exclude that the activity of some phosphatases is affected during I/R injury, this suggests that the increase in tyrosine phosphorylation at 1 and 24 hours is mainly caused by activation of kinases.
The FAK phosphorylation observed during the reperfusion period was both temporal and tyrosine residue site-specific. In control kidney, primarily Y397 was phosphorylated. At later time points, an increase in Y861 phosphorylation was observed. The latter occurred in conjunction with increased activation of Src kinase (ie, Y416 phosphorylation). These data suggest a potential dualistic function of FAK during the reperfusion period. Autophosphorylation of FAK at Y397 occurs on binding of FAK to FAs through integrin and/or talin and paxillin adaptor protein binding.19,20 Phospho-Y397 serves as a SH2 docking site for Src kinase, resulting in activation of the latter. Subsequently, Src kinase will phosphorylate other tyrosine residues of FAK, including Y576/Y577 in the kinase domain and Y861 in the C-terminal domain. Y576/Y577 phosphorylation results in increased FAK kinase activity.34,35,36 Paxillin can be phosphorylated on Y31 and Y118 and is important for FA turnover and cell motility.20,31 Interestingly, PY861-FAK was low under control conditions but increased considerably after I/R. PY861-FAK seems important in the migratory processes and c-Jun N-terminal kinase-mediated expression of matrix metalloproteinase 9.48 The coordinated and differential phosphorylation of FAK and downstream substrates that takes place before renal injury may indicate a requirement for reorganization of FAs and the actin cytoskeletal network early after ischemia-reperfusion and drives a cellular stress response that can result in renal tissue injury. This needs further investigation.
In summary, our current data support a model whereby an ischemic insult causes loss of tyrosine phosphorylation of FA-associated signaling and adapter proteins followed by a MEK/ERK pathway-dependent burst in protein tyrosine kinase activation and loss of FA structures. Inhibition of the MEK/ERK pathway and/or specific protein tyrosine kinases during the ischemic period may be a potential therapeutic means to protect against renal failure caused by ischemic insults.
Supplementary Material
Acknowledgments
We thank Emile de Heer for suggestions and for kindly providing the collagen antibodies and the members of the Division of Toxicology of the Leiden/Amsterdam Center for Drug Research for valuable discussion and support.
Footnotes
Address reprint requests to Prof. Dr. Bob van de Water, Division of Toxicology, Leiden/Amsterdam Center for Drug Research, Leiden University, Einsteinweg 55, P.O. Box 9502, 2300 RA Leiden, The Netherlands. E-mail: b.water@lacdr.leidenuniv.nl.
Supported by grants 902-21-229, 908-02-107, and 911-02-022 from The Netherlands Organization for Scientific Research. B.W. was supported by a fellowship of the Royal Netherlands Academy for Arts and Sciences.
M.A. and M.d.G. contributed equally to this work.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Molina A, Ubeda M, Escribese MM, Garcia-Bermejo L, Sancho D, de Lema GP, Liano F, Cabanas C, Sanchez-Madrid F, Mampaso F. Renal ischemia/reperfusion injury: functional tissue preservation by anti-activated β1 integrin therapy. J Am Soc Nephrol. 2005;16:374–382. doi: 10.1681/ASN.2004070528. [DOI] [PubMed] [Google Scholar]
- Zalewska T, Makarewicz D, Janik B, Ziemka-Nalecz M. Neonatal cerebral hypoxia-ischemia: involvement of FAK-dependent pathway. Int J Dev Neurosci. 2005;23:657–662. doi: 10.1016/j.ijdevneu.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Zalewska T, Ziemka-Nalecz M, Domanska-Janik K. Transient forebrain ischemia effects interaction of Src FAK, and PYK2 with the NR2B subunit of N-methyl-D-aspartate receptor in gerbil hippocampus. Brain Res. 2005;1042:214–223. doi: 10.1016/j.brainres.2005.02.025. [DOI] [PubMed] [Google Scholar]
- Zuk A, Bonventre JV, Brown D, Matlin KS. Polarity, integrin, and extracellular matrix dynamics in the postischemic rat kidney. Am J Physiol. 1998;275:C711–C731. doi: 10.1152/ajpcell.1998.275.3.C711. [DOI] [PubMed] [Google Scholar]
- Raman N, Atkinson SJ. Rho controls actin cytoskeletal assembly in renal epithelial cells during ATP depletion and recovery. Am J Physiol. 1999;276:C1312–C1324. doi: 10.1152/ajpcell.1999.276.6.C1312. [DOI] [PubMed] [Google Scholar]
- Hallett MA, Dagher PC, Atkinson SJ. Rho GTPases show differential sensitivity to nucleotide triphosphate depletion in a model of ischemic cell injury. Am J Physiol. 2003;285:C129–C138. doi: 10.1152/ajpcell.00007.2003. [DOI] [PubMed] [Google Scholar]
- Sutton TA, Molitoris BA. Mechanisms of cellular injury in ischemic acute renal failure. Semin Nephrol. 1998;18:490–497. [PubMed] [Google Scholar]
- Lieberthal W, Levine JS. Mechanisms of apoptosis and its potential role in renal tubular epithelial cell injury. Am J Physiol. 1996;271:F477–F488. doi: 10.1152/ajprenal.1996.271.3.F477. [DOI] [PubMed] [Google Scholar]
- Paller MS. The cell biology of reperfusion injury in the kidney. J Invest Med. 1994;42:632–639. [PubMed] [Google Scholar]
- Fish EM, Molitoris BA. Extracellular acidosis minimizes actin cytoskeletal alterations during ATP depletion. Am J Physiol. 1994;267:F566–F572. doi: 10.1152/ajprenal.1994.267.4.F566. [DOI] [PubMed] [Google Scholar]
- Bush KT, Tsukamoto T, Nigam SK. Selective degradation of E-cadherin and dissolution of E-cadherin-catenin complexes in epithelial ischemia. Am J Physiol. 2000;278:F847–F852. doi: 10.1152/ajprenal.2000.278.5.F847. [DOI] [PubMed] [Google Scholar]
- Tsukamoto T, Nigam SK. Tight junction proteins form large complexes and associate with the cytoskeleton in an ATP depletion model for reversible junction assembly. J Biol Chem. 1997;272:16133–16139. doi: 10.1074/jbc.272.26.16133. [DOI] [PubMed] [Google Scholar]
- Gailit J, Colflesh D, Rabiner I, Simone J, Goligorsky MS. Redistribution and dysfunction of integrins in cultured renal epithelial cells exposed to oxidative stress. Am J Physiol. 1993;264:F149–F157. doi: 10.1152/ajprenal.1993.264.1.F149. [DOI] [PubMed] [Google Scholar]
- Lieberthal W, McKenney JB, Kiefer CR, Snyder LM, Kroshian VM, Sjaastad MD. Beta1 integrin-mediated adhesion between renal tubular cells after anoxic injury. J Am Soc Nephrol. 1997;8:175–183. doi: 10.1681/ASN.V82175. [DOI] [PubMed] [Google Scholar]
- Kelly KJ, Williams WW, Jr, Colvin RB, Bonventre JV. Antibody to intercellular adhesion molecule 1 protects the kidney against ischemic injury. Proc Natl Acad Sci USA. 1994;91:812–816. doi: 10.1073/pnas.91.2.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noiri E, Romanov V, Forest T, Gailit J, DiBona GF, Miller F, Som P, Oster ZH, Goligorsky MS. Pathophysiology of renal tubular obstruction: therapeutic role of synthetic RGD peptides in acute renal failure. Kidney Int. 1995;48:1375–1385. doi: 10.1038/ki.1995.426. [DOI] [PubMed] [Google Scholar]
- Ben Mahdi MH, Andrieu V, Pasquier C. Focal adhesion kinase regulation by oxidative stress in different cell types. IUBMB Life. 2000;50:291–299. doi: 10.1080/713803721. [DOI] [PubMed] [Google Scholar]
- Parsons JT, Martin KH, Slack JK, Taylor JM, Weed SA. Focal adhesion kinase: a regulator of focal adhesion dynamics and cell movement. Oncogene. 2000;19:5606–5613. doi: 10.1038/sj.onc.1203877. [DOI] [PubMed] [Google Scholar]
- Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- Wozniak MA, Modzelewska K, Kwong L, Keely PJ. Focal adhesion regulation of cell behavior. Biochim Biophys Acta. 2004;1692:103–119. doi: 10.1016/j.bbamcr.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Weinberg JM, Venkatachalam MA, Roeser NF, Senter RA, Nissim I. Energetic determinants of tyrosine phosphorylation of focal adhesion proteins during hypoxia/reoxygenation of kidney proximal tubules. Am J Pathol. 2001;158:2153–2164. doi: 10.1016/S0002-9440(10)64687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de WB, Nagelkerke JF, Stevens JL. Dephosphorylation of focal adhesion kinase (FAK) and loss of focal contacts precede caspase-mediated cleavage of FAK during apoptosis in renal epithelial cells. J Biol Chem. 1999;274:13328–13337. doi: 10.1074/jbc.274.19.13328. [DOI] [PubMed] [Google Scholar]
- Park KM, Kramers C, Vayssier-Taussat M, Chen A, Bonventre JV. Prevention of kidney ischemia/reperfusion-induced functional injury, MAPK and MAPK kinase activation, and inflammation by remote transient ureteral obstruction. J Biol Chem. 2002;277:2040–2049. doi: 10.1074/jbc.M107525200. [DOI] [PubMed] [Google Scholar]
- Zhuang S, Schnellmann RG. A death-promoting role for extracellular signal-regulated kinase. J Pharmacol Exp Ther. 2006;319:991–997. doi: 10.1124/jpet.106.107367. [DOI] [PubMed] [Google Scholar]
- Jo SK, Cho WY, Sung SA, Kim HK, Won NH. MEK inhibitor, U0126, attenuates cisplatin-induced renal injury by decreasing inflammation and apoptosis. Kidney Int. 2005;67:458–466. doi: 10.1111/j.1523-1755.2005.67102.x. [DOI] [PubMed] [Google Scholar]
- Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, Horwitz AF. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol. 2004;6:154–161. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- Ishibe S, Joly D, Liu ZX, Cantley LG. Paxillin serves as an ERK-regulated scaffold for coordinating FAK and Rac activation in epithelial morphogenesis. Mol Cell. 2004;16:257–267. doi: 10.1016/j.molcel.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Liu ZX, Yu CF, Nickel C, Thomas S, Cantley LG. Hepatocyte growth factor induces ERK-dependent paxillin phosphorylation and regulates paxillin-focal adhesion kinase association. J Biol Chem. 2002;277:10452–10458. doi: 10.1074/jbc.M107551200. [DOI] [PubMed] [Google Scholar]
- Witzgall R, Brown D, Schwarz C, Bonventre JV. Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J Clin Invest. 1994;93:2175–2188. doi: 10.1172/JCI117214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk A, Matlin KS. Induction of a laminin isoform and α(3)β(1)-integrin in renal ischemic injury and repair in vivo. Am J Physiol. 2002;283:F971–F984. doi: 10.1152/ajprenal.00176.2002. [DOI] [PubMed] [Google Scholar]
- Brown MC, Turner CE. Paxillin: adapting to change. Physiol Rev. 2004;84:1315–1339. doi: 10.1152/physrev.00002.2004. [DOI] [PubMed] [Google Scholar]
- Hamadi A, Bouali M, Dontenwill M, Stoeckel H, Takeda K, Ronde P. Regulation of focal adhesion dynamics and disassembly by phosphorylation of FAK at tyrosine 397. J Cell Sci. 2005;118:4415–4425. doi: 10.1242/jcs.02565. [DOI] [PubMed] [Google Scholar]
- Liu G, Guibao CD, Zheng J. Structural insight into the mechanisms of targeting and signaling of focal adhesion kinase. Mol Cell Biol. 2002;22:2751–2760. doi: 10.1128/MCB.22.8.2751-2760.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean GW, Fincham VJ, Frame MC. v-Src induces tyrosine phosphorylation of focal adhesion kinase independently of tyrosine 397 and formation of a complex with Src. J Biol Chem. 2000;275:23333–23339. doi: 10.1074/jbc.M909322199. [DOI] [PubMed] [Google Scholar]
- Calalb MB, Polte TR, Hanks SK. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol Cell Biol. 1995;15:954–963. doi: 10.1128/mcb.15.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calalb MB, Zhang X, Polte TR, Hanks SK. Focal adhesion kinase tyrosine-861 is a major site of phosphorylation by Src. Biochem Biophys Res Commun. 1996;228:662–668. doi: 10.1006/bbrc.1996.1714. [DOI] [PubMed] [Google Scholar]
- Brown MC, Cary LA, Jamieson JS, Cooper JA, Turner CE. Src and FAK kinases cooperate to phosphorylate paxillin kinase linker, stimulate its focal adhesion localization, and regulate cell spreading and protrusiveness. Mol Biol Cell. 2005;16:4316–4328. doi: 10.1091/mbc.E05-02-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang S, Yan Y, Daubert RA, Han J, Schnellmann RG. ERK promotes hydrogen peroxide-induced apoptosis through caspase-3 activation and inhibition of Akt in renal epithelial cells. Am J Physiol. 2007;292:F440–F447. doi: 10.1152/ajprenal.00170.2006. [DOI] [PubMed] [Google Scholar]
- Gong N, Dong C, Chen Z, Chen X, Guo H, Zeng Z, Ming C, Klaus CZ. Adenovirus-mediated antisense-ERK2 gene therapy attenuates chronic allograft nephropathy. Transplant Proc. 2006;38:3228–3230. doi: 10.1016/j.transproceed.2006.10.141. [DOI] [PubMed] [Google Scholar]
- de Rooij J, Kerstens A, Danuser G, Schwartz MA, Waterman-Storer CM. Integrin-dependent actomyosin contraction regulates epithelial cell scattering. J Cell Biol. 2005;171:153–164. doi: 10.1083/jcb.200506152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffin JM, Pittet P, Csucs G, Lussi JW, Meister JJ, Hinz B. Focal adhesion size controls tension-dependent recruitment of alpha-smooth muscle actin to stress fibers. J Cell Biol. 2006;172:259–268. doi: 10.1083/jcb.200506179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebas E, Lachowicz L, Mussur M, Szkudlarek J. The activity of protein tyrosine kinases of rat heart after ischemia and reperfusion. Med Sci Monit. 2001;7:884–888. [PubMed] [Google Scholar]
- Yano T, Yazima S, Hagiwara K, Ozasa H, Ishizuka S, Horikawa S. Activation of epidermal growth factor receptor in the early phase after renal ischemia-reperfusion in rat. Nephron. 1999;81:230–233. doi: 10.1159/000045281. [DOI] [PubMed] [Google Scholar]
- Katz M, Amit I, Yarden Y: Regulation of MAPKs by growth factors and receptor tyrosine kinases. Biochim Biophys Acta 2007, [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonventre JV. Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J Am Soc Nephrol. 2003;14(Suppl 1):S55–S61. doi: 10.1097/01.asn.0000067652.51441.21. [DOI] [PubMed] [Google Scholar]
- Zhang YW, Vande Woude GF. HGF/SF-met signaling in the control of branching morphogenesis and invasion. J Cell Biochem. 2003;88:408–417. doi: 10.1002/jcb.10358. [DOI] [PubMed] [Google Scholar]
- Keshavjee S, Zhang XM, Fischer S, Liu M. Ischemia reperfusion-induced dynamic changes of protein tyrosine phosphorylation during human lung transplantation. Transplantation. 2000;70:525–531. doi: 10.1097/00007890-200008150-00022. [DOI] [PubMed] [Google Scholar]
- Lim Y, Han I, Jeon J, Park H, Bahk YY, Oh ES. Phosphorylation of focal adhesion kinase at tyrosine 861 is crucial for Ras transformation of fibroblasts. J Biol Chem. 2004;279:29060–29065. doi: 10.1074/jbc.M401183200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.