Abstract
Envenomation by the sea anemone Phyllodiscus semoni causes fulminant dermatitis and, rarely, acute renal failure in humans. Here, we investigated whether the venom extracted from the nematocysts (PsTX-T) was nephrotoxic when administered intravenously in rats and whether PsTX-T induced activation of the complement system. Although small dose of PsTX-T induced acute tubular necrosis in rats resembling pathology seen in patients, kidneys displayed glomerular injury with glomerular endothelial damage, thrombus formation, mesangiolysis, and partial rupture of glomerular basement membrane, accompanied by severe tubular necrosis at 24 hours after administration of 0.03 mg of PsTX-T per animal, similar to the glomerular findings typical of severe hemolytic uremic syndrome. The early stage injury was accompanied by specific PsTX-T binding, massive complement C3b, and membrane attack complex deposition in glomeruli in the regions of injury and decreased glomerular expression of complement regulators. A pathogenic role for complement was confirmed by demonstrating that systemic complement inhibition reduced renal injury. The isolated nephrotoxic component, a 115-kd protein toxin (PsTX-115), was shown to cause identical renal pathology. The demonstration that PsTX-T and PsTX-115 were highly nephrotoxic acting via induction of complement activation suggests that inhibition of complement might be used to prevent acute renal damage following envenomation by P. semoni.
Sea anemones, members of the phylum Coelenterata, are armed with venom-secreting nematocytes to aid in the capture of prey and to protect from predators. Most of these venoms are harmless to humans or induce mild dermatitis. However, a few species of sea anemones possess highly toxic venoms hazardous for humans. Phyllodiscus semoni is one of the most dangerous.1 P. semoni is commonly called “night sea anemone” and is distributed in the Western Pacific ocean; it is also called in Japanese “unbachi-isoginchaku,” which means “wasp-sea anemone,” in Okinawa (South Japan). The sting induces severe dermatitis with local ulceration and swelling that often takes months to resolve.2 We recently reported a more serious sequela of envenomation by P. semoni; the victim developed unexplained acute renal failure without evidence of dysfunction of other organs.2 From hospital records covering the last 6 years in the Okinawa area, there were at least three other cases with acute renal failure in individuals stung by PsTX-T, including our case report.2 Unfortunately, we have no information for frequency in other countries. We believe that this may be an under-reported consequence of envenomation by this organism. Several hemolytic protein toxins have been isolated from P. semoni venom, including a 20-kd molecular weight protein, PsTX-20A, and 60-kd molecular weight proteins 60A and 60B,3 whereas other sea anemone venoms contain hemolytic, neurotoxic, and cardiotoxic protein toxins.4,5,6 However, there have been no previous reports describing nephrotoxic factors in sea anemone venom.
Renal injury has been reported following envenomation by snakes, spiders, and scorpions.7,8,9,10,11,12,13 In some instances these effects are associated with complement (C)-activating components in the venoms that indirectly contribute to tissue damage.14,15,16,17 A well-known example is the C3-like protein cobra venom factor, purified from the venom of the Egyptian or Thai cobra, which activates C to completion in experimental animals.18 No direct association between sea anemone venoms and C activation has been reported, although the sea anemone-derived toxin AvTX-60A was recently reported to have structural similarities to terminal pathway C proteins.19 We here tested the nephrotoxic activity of P. semoni venom in rodents to identify the mechanisms of nephrotoxicity and the toxic principle in the venom.
Materials and Methods
Animals
Female Wistar rat weighing ∼150 g (Chubu Kagaku Shizai, Nagoya, Japan) were used in the present study. All experiments described were performed according to The Animal Experimentation Guide of Nagoya University School of Medicine.
Extraction of P. semoni Venom (PsTX-T) from Isolated Nematocysts
Sea anemones, P. semoni, were collected along the Pacific coast at Itoman, Okinawa, Japan. Isolation of nematocysts and extraction of venom was performed as previously described.3,20 In brief, isolated nematocysts were incubated in 10 mmol/L phosphate buffer (pH 6.0) and centrifuged (12,000 × g, 15 minutes). The supernatant was sterile-filtered; this venom extract was termed PsTX-T. Protein concentration was measured by Coomassie assay (Pierce, Rockford, IL). Presence of endotoxin was tested using the Wako ES EndoToxinometer MT-358 (Wako Pure Chemical Industries, Tokyo, Japan). All samples were below the detection level for endotoxin (<0.5 EU/ml).
To investigate C-activating activity of PsTX-T in the fluid phase, 1 ml of serum from untreated rats was incubated with 4.0 × 10−2, 1.0 × 10−2, 1.0 × 10−3, 1.0 × 10−4, 1.0 × 10−5, 1.0 × 10−6, 1.0 × 10−7, and 1.0 × 10−8 mg/ml of PsTX-T. Residual serum hemolytic activity (CH50) was measured in a commercial assay according to the manufacturer’s instructions (Ishizu Pharmaceutical Co., Osaka, Japan).
Administration of PsTX-T to Rats
Rats (nine per group) were intravenously (i.v.) injected with 0.3, 0.03, or 0.003 mg of PsTX-T per animal in 0.3 ml of isotonic saline and observed at least daily for up to 7 days. Histological changes caused by PsTX-T administration were assessed in three randomly selected rats sacrificed at 24 hours by examining kidney, heart, liver, and lung tissue. To analyze the time course of renal injury, a separate group of 16 rats were injected i.v. with 0.03 mg of PsTX-T per animal and the kidneys harvested at intervals from 10 minutes, 6 hours, 24 hours, 3 days, and 7 days after injection. In another experiment, four rats were followed up to 7 days to measure serum creatinine before PsTX-T injection and at 24 hours and on 7 days after administration with 0.03 mg of PsTX-T per animal. In the above experiments, four rats were injected with vehicle instead of 0.03 mg of PsTX-T per animal and/or used untreated rats as the control. Tissue was processed for histological and immunohistochemical analysis and/or electron microscopic (EM) analysis. Blood samples were also collected from the tail vein in rats treated with 0.03 mg of animal PsTX-T per animal.
Nephrotoxicity was also assessed in rats injected with fractions obtained from the purification of nephrotoxic components from PsTX-T. For these analyses, nine rats were injected i.v. with 0.03, 0.015, or 0.003 mg of protein (three rats for each) and killed after 24 hours. Kidneys were harvested and renal injuries assessed under light microscopy (LM).
In another experiment, systemic C activity was inhibited by i.v. administration of sCR1 (AVANT Immunotherapeutics, Inc., Needham, MA) at 20 mg/kg in 0.5 ml of sterile isotonic saline, a dose known to cause C inhibition in rats,21,22,23 at 30 minutes before PsTX-T injection and every 12 hours after administration. As the control for sCR1 injection, 0.5 ml of sterile isotonic saline was administered instead of sCR1. The number of rats used is stated in the relevant figure legends. Suppression of serum C activity was confirmed by measurement of CH50.
Generation of Mouse Polyclonal Antibody against PsTX-T and Detection of PsTX-T Binding in Rat Organs
BALB/c mice were immunized with PsTX-T in Freund’s adjuvant. Immune mice were exsanguinated and serum prepared. Anti-PsTX-T serum was used to detect binding of PsTX-T following administration.
To confirm the reactivity of the anti-PsTX-T, Western blot analysis was performed. Briefly, PsTX-T was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under nonreduced conditions on 12.5% gels and transferred to nitrocellulose membrane (Pall Corporation, Pensacola, FL). The membrane was blocked with phosphate-buffered saline/milk (PBS-M) and then probed with anti-PsTX-T (×1/2000), followed by incubation with horseradish peroxidase-conjugated donkey anti-mouse Ig (The Jackson Laboratory, Bar Harbor, ME), diluted in PBS-M. After further washing in PBS/Tween, blots were developed using enhanced chemiluminescence (Perbio Science, Helsingborg, Sweden) and images captured on Hyperfilm enhanced chemiluminescence (Amersham Biosciences, Buckinghamshire, UK).
To investigate the distribution of administered PsTX-T, rats were sacrificed 10 minutes (n = 3), 6 hours (n = 3), and 24 hours (n = 3) after i.v. administration of 0.03 mg of PsTX-T or vehicle (n = 2) per animal. Kidneys, livers, hearts, and lungs were harvested and snap-frozen. Sections were prepared as described below and incubated with the mouse anti-PsTX-T serum (1:100 dilution) or nonimmune serum as a staining negative control followed by fluorescein isothiocyanate-labeled anti-mouse IgG (1:200) previously adsorbed using normal rat serum (1:1, v/v). To confirm the specific binding of anti-PsTX-T, frozen kidney sections from three vehicle-administered rats were used as controls. They were also incubated with the mouse anti-PsTX-T serum (1:100 dilution) and fluorescein isothiocyanate-labeled anti-mouse IgG as described above. Sections were viewed under a fluorescence microscope.
To confirm further the specific binding of PsTX-T (10 minutes after PsTX-T or vehicle injection) and decrease of CReg expression (24 hours after PsTX-T or vehicle injection), lysates of kidney, lung, heart, and liver were prepared in 2% Nonidet P-40/PBS with proteinase inhibitor cocktail [containing 4-(2-aminoethyl)benzenesulfonyl fluoride, pepstatin A, E-64, bestatin, leupeptin, and aprotinin; Sigma-Aldrich, St. Louis, MO]. Equal amounts of protein from each lysate were spotted on nitrocellulose membranes and dried for dot blot analysis to confirm PsTX-T distribution. The membrane was blocked with PBS-M, incubated with anti-PsTX-T or untreated mouse serum, followed by horseradish peroxidase-conjugated donkey anti-mouse Ig (The Jackson Laboratory), and developed as described above.
Histological and Immunohistochemical Methods
For LM analyses, tissues were fixed in methacarn overnight and embedded in paraffin. Two-micrometer sections were stained with periodic acid-Schiff for the kidney and with hematoxylin and eosin for heart, liver, and lung. Kidney pathology was assessed separately in glomeruli, cortex, outer medulla, and inner medulla. For immunohistological analysis, kidney was embedded in OCT compound (Sakura Finetechnical Co., Tokyo, Japan), snap-frozen in liquid nitrogen, cryostat-sectioned at 2 μm, and fixed with acetone for 10 minutes at room temperature. To assess the presence of C activation in the areas of renal injury induced by PsTX-T, tissue sections were incubated either with fluorescein isothiocyanate-labeled goat anti-rat C3 (Cappel Laboratories, Cochranville, PA), or with an in-house rabbit anti-rat C9 followed by fluorescein isothiocyanate-labeled anti-rabbit IgG (Zymed Laboratories, South San Francisco, CA), as described.24
To investigate the expression of CReg, sections were incubated with anti-rat Crry (5I2; gift of Dr. H. Okada and Dr. N. Okada, Nagoya City University, Nagoya, Japan),25 anti-rat DAF (RDIII-7),26 or anti-rat CD59 (6D1).27 To count infiltrating cells in glomeruli, anti-leukocyte common antigen (clone OX1; Dainippon Pharmaceutical Company, Osaka, Japan) was used. Bound antibodies were detected using fluorescein isothiocyanate-labeled rabbit anti-mouse IgG (Cappel Laboratories) absorbed with normal rat serum. The CReg CD46 is not expressed in rat kidney.28 Leukocyte common antigen-positive cells were counted in 20 glomeruli, and the average was calculated in rats treated with PsTX-T or with vehicle (four per group) at 24 hours after injection.
To investigate ex vivo effects of PsTX-T on expression of CReg, 10-μm unfixed sections of fresh-frozen kidney were incubated with 4 μg of PsTX-T in 50 μl of PBS or with 4 μg of PsTX-T mixed with 10 μl/2 ml of protease inhibitor cocktail (including 4-(2-aminoethyl)benzenesulfonyl fluoride, ethylenediamine tetraacetic acid, bestatin, E-64, leupeptin, and aprotinin; Sigma-Aldrich) in 50 μl of PBS for 60 minutes at 37°C. Control sections were incubated with PBS alone. After PsTX-T exposure, sections were washed in PBS and then stained for CReg expression.
Western Blot Analysis to Confirm Decrease of CReg Expression after PsTX-T Injection
Western blot analysis was performed to confirm decreased CReg expression in the kidney 24 hours after PsTX-T administration. Protein concentration was measured, and equal protein amounts from lysates were loaded on the gels, separated by SDS-PAGE under nonreducing conditions on 5 to 18% gradient gels, and transferred to nitrocellulose membrane. The membrane was blocked with PBS-M, incubated with monoclonal antibody RDIII-7 to detect rat CD55, monoclonal antibody CLT1C-11 to detect rat Crry, a polyclonal antibody (pcAb) rabbit anti-rat CD59, a mouse monoclonal antibody OX-7 to recognize rat Thy1.1 antigen as a GPI-anchored protein control,29 a rabbit anti-thrombomodulin30 as a transmembrane protein control, or nonimmune mouse serum, followed by incubation with horseradish peroxidase-conjugated donkey anti-mouse Ig or horseradish peroxidase-conjugated donkey anti-rabbit Ig (The Jackson Laboratory), and developed as described above.
Electron Microscopy
Specimens for EM were fixed with glutaraldehyde, postfixed with osmium tetraoxide, and embedded in Epon 812 (Nisshin EM Co., Tokyo, Japan). Ultrathin sections were viewed with a H7100 electron microscope (Hitachi Co. Ltd., Ibaraki, Japan).
Measurement of Serum Creatinine Level, Serum C Activity, and Urinary Protein
Blood samples were collected from tail veins and serum creatinine measured by “Kinos creatinine” kit (Kinos Co., Tokyo, Japan) following the manufacturer’s protocols. Serum C hemolytic activity (CH50) was measured in a commercial assay according to the manufacturer’s instructions (Ishizu Pharmaceutical Co., Osaka, Japan).
Spot urine samples from selected rats were directly harvested from the bladder using 26-gauge needles at sacrifice. The samples were semiquantitatively analyzed by dip and read stick method (Multisticks; AMES, Tokyo, Japan). The results were read as 0, +, 2+, 3+, and 4+ (30, 2+; 100, 3+; 300, 4+; 1000 mg/dl, respectively). Statistical analysis was performed using Student’s unpaired t-test.
Identification of the Nephrotoxic Protein Fractions from PsTX-T
PsTX-T was applied to an ion-exchange high-performance liquid chromatography, protein pak G-DEAE column (5 × 100 mm, 1.0 ml/min; Waters, Milford, MA), equilibrated with 10 mmol/L phosphate buffer, pH 7.0. The column was washed with 10 ml of the same buffer, and bound components were eluted with a linear NaCl gradient to 0.5 mol/L over 25 ml. Protein peaks were collected. PsTX-T was also subjected to size exclusion chromatography on a Superdex 200 fast-performance liquid chromatography column (10 × 300 mm, 0.5 ml/min; Amersham Pharmacia Biotech, Uppsala, Sweden), equilibrated with 10 mmol/L phosphate buffer, pH 7.0, containing 0.15 mol/L NaCl. Protein peaks were pooled, dialyzed into PBS, and concentrated.
To test hemolytic activity, rabbit erythrocytes at 20% in sterile isotonic saline were mixed with dilutions of the various pools (2:1, v/v) and incubated at room temperature for 1 hour. Tubes were then centrifuged, and absorbance in supernatant was measured at 540 nm to assess percentage of hemolysis. Hemolytic activity was scored as −, negative; +/−, hemolysis >50% at twofold dilution of sample; +, hemolysis >50% between four- and eightfold dilution; ++, hemolysis >50% beyond 16-fold dilution.
To estimate nephrotoxic activity, rats were injected i.v. with 0.003, 0.01, or 0.03 mg of each pool per animal, kidneys harvested at 24 hours, and processed as described above. The degree of renal injury was scored as −, minimal change, through +++ according to the number of affected glomeruli in 50 glomeruli examined: +, glomerular injury involving less than 25% of total glomeruli; ++, glomerular injury involving between 25 and 75% of glomeruli; +++, widespread injury with severe damage involving over 75% of glomeruli in the kidney. Tubular changes were broadly found from the cortex to outer medullar similar to the injuries caused by PsTX-T administration. Nephrotoxic pools scoring (+++) on this scale at the lowest administered dose were taken to the next stage of purification. Protein peaks from DEAE chromatography and from size exclusion chromatography were collected, dialyzed into PBS, and concentrated. Nephrotoxic activity in these pools was tested as described above by administration at 0.03 mg per animal in three rats for each fraction.
In the next step for purification of nephrotoxic components, the strongest nephrotoxic pool from DEAE chromatography was subjected to size exclusion chromatography on a Superdex 200 fast-performance liquid chromatography column equilibrated with 10 mmol/L phosphate buffer pH 7.0 containing 0.15 mol/L NaCl, as described above. Each fraction collected from size exclusion chromatography was tested for nephrotoxic activity in three rats as described above. In addition, the titer of the strongest nephrotoxic fraction (PsTX-115) was estimated by dilution and retesting for nephrotoxicity. The most active fraction was reapplied separately to the Superdex 200 column and separated on SDS-PAGE and Coomassie-stained to confirm purity.
Amino Acid Sequencing of Nephrotoxic Protein
From a Coomassie-stained SDS-PAGE gel, the major protein band at 115 kd was excised and digested with lysyl endopeptidase in 0.1 mol/L Tris buffer, pH 8.5, at 35°C overnight. The digested sample was separated on reversed-phase high-performance liquid chromatography (TSKgel ODS-80Ts QA, 2.0 × 250 mm; Tosho Co., Tokyo, Japan) in 0.1% trifluoroacetic acid at 200 μl/min. Abundant peptides were analyzed on a Procise 494 HT protein sequencing system (column: 2.1 mm I.D. × 22 cm; program: pulse liquid; APRO Science Co., Tokushima, Japan). Nonstained pieces from the same gel were used as a control.
Results
PsTX-T Causes a Dose-Dependent Nephrotoxicity in Rats
All rats injected intravenously with 0.3 mg of PsTX-T developed a shock-like syndrome and died within 20 minutes of injection. This rapidly lethal effect was not further examined. When rats were injected with 0.03 mg of the venom, all survived through day 5, and two of nine died by day 7. At 24 hours after injection of this dose, severe destructive glomerular changes with concomitant interstitial injury were found in kidney (Figure 1). Proteinuria (2+ to 4+) was detected in all of these rats. Histological analyses did not reveal any pathological change in other major organs, including heart, lung, and liver (data not shown), suggesting that the injury was specific to the kidney. The only other pathology detected was a mild and variable degree of intravascular hemolysis. All rats injected with 0.003 mg of venom survived at least 7 days and did not show pathology in kidney or any other organ. Three of nine rats receiving 0.015 mg of venom had renal tubular necrosis with minor glomerular abnormalities under LM (data not shown). A dose of 0.03 mg of venom was thus chosen for subsequent studies in vivo.
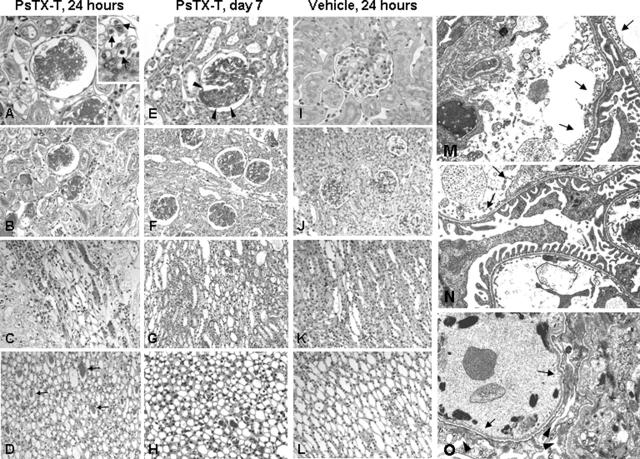
Figure 1.
Nephrotoxicity in rats after PsTX-T injection. Under light microscopy 24 hours after PsTX-T injection, glomerular damage with thrombus formation was found with severe tubular necrosis extending from cortex to outer medulla (A–D). A magnified glomerulus is shown on the upper right side of A; arrows indicate infiltrating cells in glomerular capillaries. On day 7 after PsTX-T, focal and lobular glomerular damage was found (E, arrowheads, and F). At this time point, tubular epithelial healing was found (G and H). In vehicle controls at 24 hours, no significant pathological changes were found (I–L). Under EM, 10 minutes after PsTX-T administration, epithelial cells were well preserved, but segmental glomerular endothelial cell detachment and infiltrating cells in the glomeruli were found (M, arrows), apparent to a similar degree in sCR1-treated rats exposed to PsTX-T (N, arrows). By EM at 24 hours after PsTX-T administration, severe endothelial detachment (O, arrows) and mesangiolysis were found with accompanying epithelial damage (O, arrowheads). A, E, and I: Glomerulus. B, F, and J: Cortex. C, G, and K: Outer medulla. D, H, and L: Inner medulla. Original magnifications:×400 (A, E, and I); ×200 (B, C, D, F, G, H, J, K, and L); ×1500 (M, N, and O).
Under LM at 6 hours after injection, the pathological changes were minimal (data not shown). At 24 hours after injection, partial disruption of the glomerular basement membrane, massive thrombus formation in glomerular capillaries, severe mesangiolysis, and infiltrating cells were found in the majority of glomeruli (Figure 1, A and B). At 24 hours, infiltrating cell number, detected by anti-leukocyte common antigen, was 1.58 ± 0.35 (n = 4, mean ± SE) per glomerulus in PsTX-T-administered rats; in contrast, there were 0.38 ± 0.08 infiltrating cells per glomerulus in vehicle-injected rats (n = 4, P < 0.05). Extensive and severe tubular damage, including epithelial cell vacuolization and degeneration and epithelial detachment, was found from cortex to outer medulla (Figure 1, A–C). Tubular damage in the inner medulla was less marked, although there were numerous casts inside the tubular lumen (Figure 1D, arrows).
Glomerular changes were detected at EM level even 10 minutes after PsTX-T injection. Swelling and partial detachment of glomerular endothelium with accumulation of platelets and infiltrating leukocytes and early mesangial degeneration were seen in a minority of glomeruli at this time (Figure 1M). Glomerular epithelial cells appeared intact, and there were no obvious changes in tubular epithelium and peritubular capillaries, except for sparse infiltrating inflammatory cells. Of note, endothelium of peritubular capillaries showed minimal changes at 10 minutes. At 24 hours, the majority of glomeruli showed severe endothelial detachment, fibular extraction, and thrombus formation, supporting the LM appearance (Figure 1O). Fusion of glomerular epithelial cell foot process, mesangiolysis, and abundant degenerated fragments inside glomerular capillaries were present at this time.
In the glomeruli on day 3, the pathological changes of tubular epithelial damage were still apparent (see Figure 6, D and H). On day 7 after PsTX-T administration, segmental increase of cell number in glomeruli (Figure 1E, arrowheads) was observed, indicating segmental glomerular injury (Figure 1, E and F), although damage to tubular epithelium was much reduced (Figure 1, G and H).
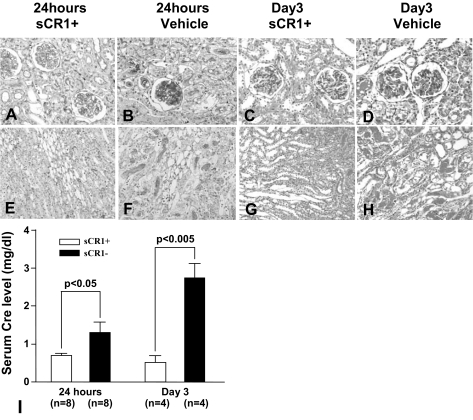
Figure 6.
Treatment with sCR1 inhibits PsTX-T-induced nephrotoxicity. Renal injury both in glomeruli and interstitium of kidney in sCR1-treated rats after 24 hours of PsTX-T administration (A and E) and after 3 days of PsTX-T administration (C and G) was much reduced compared with rats administered vehicle alone with PsTX-T administration at these time points (B, D, F, and H). Original magnifications: ×400 (A–D); ×200 (E–H). I: Serum creatinine level of toxin-exposed rats treated with sCR1 or with vehicle control. Number of rats in each group is shown in I; error bars represent SE, and significant differences between groups are indicated (P < 0.05 or P < 0.005).
Serum creatinine as a measure of renal function at 24 hours after PsTX-T administration was increased to 1.77 ± 0.52 mg/dl compared with 0.40 ± 0.03 mg/dl in control rats (mean ± SE; P < 0.05, n = 4 for each). On day 7 after PsTX-T, serum creatinine was still high (0.95 ± 0.03 mg/dl; n = 3, one of four rats died on day 5; P < 0.001 by comparison with control rats), supporting the pathological changes described above.
The Nephrotoxicity of PsTX-T Is Associated with C Activation and Diminished Membrane C Regulator (CReg) Expression
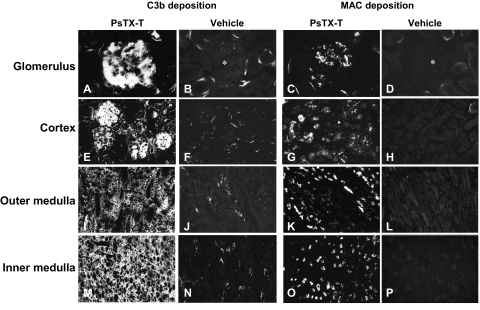
At 6 hours after injection, when LM changes were minimal, small but consistent deposits of C3b and membrane attack complex (MAC) were present in glomerular capillaries and tubular epithelium; after 24 hours, C3b and MAC deposition was abundant and widespread, coincident with injured glomeruli and tubuli (Figure 2, A, C, E, G, I, K, M, and O). Deposition of C3b and MAC peaked at 24 hours after injection; by day 3, C3b deposition was decreased and MAC deposition was absent (data not shown).
Figure 2.
C3b and MAC deposition in the kidney at 24 hours after PsTX-T administration. Twenty-four hours after PsTX-T injection, massive C3b deposition was found in the glomeruli and tubular epithelium (A, E, I, and M) and strong MAC deposition was present in glomeruli and tubular epithelium (C, G, K, and O). Vehicle controls are represented in B, D, F, H, J, L, N, and P. The tissue locations are shown on the left side. Legends at the top show what was detected and what was injected. Original magnifications: ×400 (A–D); ×200 (E–P).
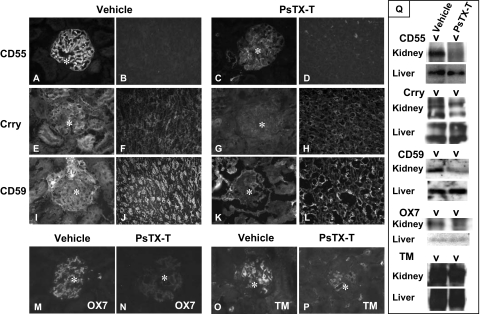
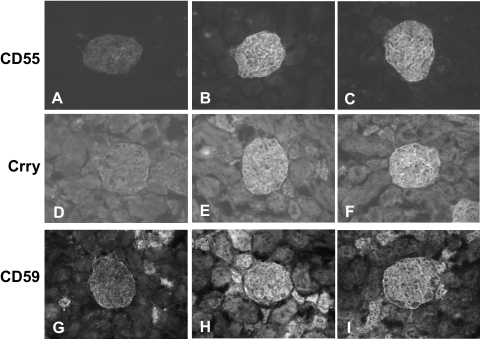
The expression of each of the CReg, CD55, Crry, and CD59 was markedly decreased in glomeruli 24 hours after injection when compared with controls (Figure 3). CD55 is only expressed in glomeruli in rat26; however, expression of Crry and CD59 was also diminished in the interstitium, including tubuli of inner medulla (Figure 3). The expression of Thy1.1 antigen (recognized by OX-7) and thrombomodulin was also clearly decreased in glomeruli 24 hours after PsTX-T injection compared with the vehicle injection (Figure 3, M–P).
Figure 3.
Expression of CReg 24 hours after PsTX-T administration under Immunofluorescence and Western blot analyses. Sections were stained for CD55 (A–D), Crry (E–H), CD59 (I–L), Thy 1.1 antigen (M and N), or thrombomodulin (O and P). A, B, E, F, I, J, M, and N show sections from rats injected with vehicle, whereas C, D, G, H, K, L, O, and P are from rats injected with PsTX-T. Exposure times and other parameters were kept constant to permit comparison. The expression of CD55, Crry, and CD59 was clearly decreased in glomeruli after PsTX-T exposure. In contrast, the expression of Crry and CD59 in tubular epithelium was preserved except where the epithelium was damaged (H and L). Original magnifications: ×400 (A, C, E, G, I, K, and M–P); ×200 (B, D, F, H, J, N, and O). Asterisk, glomerulus. TM, thrombomodulin. Q: Results of Western blot analysis. The left of each blotting set was the lysate from rats injected with PsTX-T, and the right was from rats injected with vehicle. The samples and the probed antigens are displayed at the left.
Western blot analysis of expression of CRegs using tissue lysates showed CD55, Crry, and CD59 expression in the kidney at 24 hours after PsTX-T exposure was reduced compared with vehicle controls (Figure 3Q). Thy1.1 expression as a control GPI-anchored protein and thrombomodulin expression as a control transmembrane protein in kidney were also decreased after PsTX-T injection compared with the vehicle injection (Figure 3Q). In contrast, expression of CD55, Crry, CD59, Thy1.1, and thrombomodulin was not altered in other major tissues such as liver, lung, and heart after PsTX-T injection compared with the vehicle injection.
To examine whether venom components directly caused loss of CReg, unfixed tissue sections were incubated with PsTX-T. The expression of each of the CReg, CD55, Crry, and CD59 in glomeruli was clearly decreased after incubation with PsTX-T (Figure 4, A, D, and G). However, Crry and CD59 in tubular epithelium were comparatively preserved. When protease inhibitors were included with PsTX-T, the expression of the CReg was preserved (Figure 4, B, E, and H) and was similar to that in buffer controls (Figure 4, C, F, and I).
Figure 4.
CReg expression after ex vivo exposure of kidney sections to PsTX-T. Sections were stained for CD55 (A–C), Crry (D–F), or CD59 (G–I). Expression of each of the CRegs was decreased in glomeruli following PsTX-T treatment (A, D, and G). Tubular expression of Crry and CD59 were preserved. In contrast, all CRegs were preserved when sections were incubated with PsTX-T mixed with protease inhibitor cocktail (B, E, and H) compared with buffer only controls (C, F, and I). Original magnification, ×400.
To test whether PsTX-T directly activated C, doses in the range 4.0 × 10−2 and 1.0 × 10−8mg/ml were incubated with aliquots of rat serum. No loss of C activity occurred during these incubations as assessed by measuring residual CH50 levels, demonstrating that PsTX-T did not directly activate C (data not shown).
PsTX-T Specifically Binds in the Renal Glomerulus
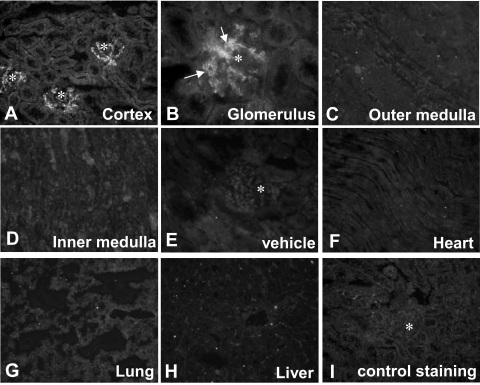
PsTX-T administered intravenously was detected in renal glomeruli but not in other parts of the kidney within 10 minutes of administration (Figure 5A). PsTX-T was located on the luminal side of glomerular capillaries (Figure 5B, arrows) and the mesangial area. At 6 hours after exposure, PsTX-T was clearly found in glomeruli and also in the apical region of parts of the tubuli. The strength of PsTX-T staining in glomeruli was decreased by 24 hours. No significant binding of PsTX-T was detected in heart, liver, or lung at any time after administration (Figure 5, F–H). In contrast, no staining for PsTX-T was found in kidney specimens from control rats (Figure 5E) or, when nonimmune mouse serum was substituted for anti-PsTX-T, in kidneys of rats treated with PsTX-T (Figure 5I).
Figure 5.
The tissue distribution of PsTX-T after intravenous administration. Intravenously administered PsTX-T was detected using specific antiserum in glomerular endothelium (A and B) 10 minutes after injection. At this time, there was no specific staining in the interstitium of cortex (A), in outer medulla (C), or in inner medulla (D). There was also no detectable staining in heart (F), lung (G), and liver (H) after administration of toxin. Asterisks in A, E, and I show positions of glomeruli. E: Anti-PsTX-T staining in kidney with vehicle administration instead of PsTX-T. I: Staining of PsTX-T-treated kidney with nonimmune mouse serum. Original magnifications: ×200 (A and C–H); ×400 (B).
For the further confirmation of the tissue-specific distribution of PsTX-T, we analyzed tissue lysates of heart, lung, liver, and kidney from rats treated with PsTX-T or vehicle using dot blot analysis. The anti-PsTX-T was spe-cifically bound in the kidney lysate but not heart, lung, or liver (data not shown).
Systemic C Inhibition Decreases the Renal Damage Caused by PsTX-T
At 10 minutes after PsTX-T injection, C3b and MAC deposition was negative or minimal in glomeruli (data not shown). At this time, glomerular endothelial injuries were detectable by EM (Figure 1M) and were not inhibited when systemic complement was suppressed by sCR1 (Figure 1N). In contrast, at 24 hours after injection in rats pretreated with sCR1 to inhibit C activation, the glomerular injury induced by PsTX-T injection was markedly reduced compared with untreated rats (Figure 6, A, B, E, and F). On day 3 after injection, glomerular changes were still severe and the damage to renal tubular epithelium remained (Figure 6, D and H). In sCR1-treated rats on day 3 after injection, glomerular damage and renal tubular epithelial damage were both clearly suppressed (Figure 6, C and G). The serum creatinine level was significantly lower in sCR1-treated rats compared with controls at 24 hours and at 3 days, supporting the histological data (Figure 6I).
Nephrotoxicity in PsTX-T Is Mediated by a Single 115-kd Protein Component
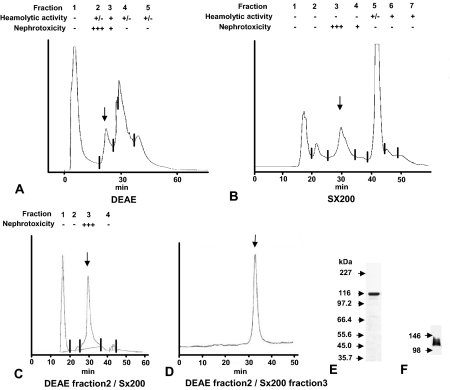
To identify the specific nephrotoxic components of PsTX-T, samples were fractionated by ion exchange and size exclusion chromatography and pools tested for nephrotoxicity in vivo (Figure 7, A and B). The first fractionation step separated venom hemolytic activity from nephrotoxicity by ion exchange (Figure 7) or size exclusion chromatography (Figure 7B). In the next step, sample separation was performed by ion exchange chromatography. The nephrotoxic fraction from this step (fraction 2, Figure 7A, arrow; extracted by 0.24 mol/L NaCl) was reseparated on size exclusion column, and fraction 3 showed strong nephrotoxicity (Figure 7C, arrow). This fraction gave a homogeneous profile when rerun on size exclusion columns (Figure 7D). SDS-PAGE under reducing conditions followed by Coomassie staining identified a single major band in the final nephrotoxic fraction with apparent molecular mass (Mr) of 115 kd, termed PsTX-115 (Figure 7E). The anti-PsTX-T antiserum that detected PsTX-T binding (Figure 5) also detected PsTX-115 in Western blot analysis (Figure 7F). The data for hemolytic and nephrotoxic activities in the fractions are summarized in Figure 7. Titration of nephrotoxicity of the purified PsTX-115 showed that 0.0015 mg of PsTX-115 per animal gave nephrotoxicity equivalent to 0.03 mg of the original PsTX-T per animal, meaning that nephrotoxicity of PsTX-115 was ∼20-fold that of PsTX-T. At 24 hours after PsTX-115 treatment, renal pathology was indistinguishable from that induced by PsTX-T treatment (data not shown). At this time point, C3 and MAC deposition were clearly detected in the kidneys treated with PsTX-115, as with PsTX-T (data not shown).
Figure 7.
Purification of the nephrotoxic fraction from PsTX-T. The crude PsTX-T was fractionated either by DEAE chromatography (A) or gel filtration on Superdex 200 (Sx200) (B). Both fractionations separated hemolytic from nephrotoxic activities. For further purification, the nephrotoxic fraction from DEAE chromatography (fraction 2, arrow in A, eluting at 0.24 mol/L NaCl) was applied to the Sx200 column (C). Nephrotoxic activity was found in a single fraction (fraction 3, arrow in C). To confirm homogeneity, this fraction was reapplied to the Sx200 column and again eluted as a single, tight peak (arrow in D). This final product was separated on SDS-PAGE and stained with Coomassie Blue (E). A single, strong protein band was detected at 115 kd. This same band was detected when the purified protein was Western blotted and probed with polyclonal anti-PsTX-T (F).
Partial Protein Sequences of PsTX-115
The isolated PsTX-115 protein band was cut from a SDS-PAGE gel, protease digested, and the resultant peptides sequenced. Sequence was obtained from two fragments, yielding 12 and 11 amino acids (RDFTHTIIDNSD and LFSESRNTRLG, respectively). No significant homology was obtained with other known proteins using BLAST search, suggesting that PsTX-115 is a previously unidentified protein.
Discussion
Venoms cause diverse effects in victims and have provided important lessons in understanding mammalian physiology and pathology. Several venoms and venom-derived toxins from snakes, spiders, and other venomous animals have been described that target the kidney in man and rodents.7,8,9,10,11,12,13 The reasons why venoms target the kidney are uncertain, although glomerular filtration might concentrate the venom at this site, whereas tissue-specific binding sites would retain toxin. In some cases, venom toxins have been directly localized in the damaged kidney.13 We recently described the first case of nephrotoxicity following envenomation by a marine animal.2 The patient developed acute renal failure without evidence of damage to other organs following envenomation by the sea anemone P. semoni, a native of the warm seas around Cebu Island.2 The organ specificity of the observed pathology led us to explore nephrotoxicity in a rat model. Here, we show that the venom of P. semoni, termed PsTX-T, localized in the renal glomerulus and induced severe renal injuries when injected intravenously in rats. The severity of the renal injuries was dependent on the amount of toxin delivered; when rats received 0.015 mg of the toxin, the renal pathology comprised minor glomerular abnormalities with renal tubular necrosis, similar to pathogenic changes described in our patient.2 When rats received twice the amount of PsTX-T, severe glomerular endothelial damages were induced, and the pathological changes included mesangiolysis and glomerular epithelial damage with extensive tubular epithelial necrosis from cortex to outer medulla. The glomerular endothelial injuries induced by PsTX-T in the kidney resembled the typical pathological glomerular changes of human hemolytic uremic syndrome (HUS). Rats receiving 0.03 mg of PsTX-T also had anemia when assessed at 24 hours (data not shown). No pathology was found in the other major organs. Renal injury rapidly followed administration of venom with EM changes detectable within 10 minutes in glomerular endothelium and obvious glomerular and tubular pathology at LM level by 24 hours. PsTX-T was dominantly bound in glomerular endothelium and in mesangial area in which PsTX-T might be infiltrated following initial glomerular endothelial damage and was not retained in other major organs. The tissue-specific binding of PsTX-T in kidney was confirmed by dot blot analysis. Despite abundant early complement deposition, we could not detect antibodies against PsTX-T in treated rats earlier than 7 days after treatment, and no antibody deposition was detected in the kidney under IF and enzyme-linked immunosorbent assay analysis, eliminating the possibility of immune complex deposits (data not shown). The rapid time course and characteristic PsTX-T binding pattern suggested a direct nephrotoxic effect of the venom.
Previous studies have described sea anemone-derived toxins that had hemolytic activity,31,32,33,34 cardiac toxicity,5,35 and neurotoxicity,4,36,37,38 but there have been no previous descriptions of nephrotoxins from sea anemone. Hemolytic toxins purified from P. semoni venom were proteins of apparent Mr of 20 kd (termed PsTX-20A) and 60 kd (PsTx-60A and 60B).3,20 To characterize the nephrotoxin further, we used a two-step purification and isolated a single protein toxin, termed PsTx-115 because of its apparent molecular mass, in keeping with earlier terminology.3,20 Renal pathology caused by purified toxin was indistinguishable from that caused by whole venom, but purified toxin was 20-fold more active on a protein weight-for-weight basis (100% incidence of renal injury at 1.5 μg/animal compared with 30 μg/animal for unfractionated venom). The glomerular injuries induced by PsTx-115 were identical to those of PsTX-T and also displayed similar decreased CReg expression and complement deposition. The one difference was that PsTX-115 treatment was not associated with hemolysis. We therefore conclude that PsTX-115 is the major nephrotoxic component in the venom PsTX-T of P. semoni.
Deposition of C3 fragments and MAC in the glomeruli and interstitium was seen early in the injured kidney, absent or minimal at 10 minutes, easily detectable by 6 hours, and peaking at 24 hours. To ascertain whether C activation was driving pathology, rats were treated with sCR1 before administration of venom and at 12-hour intervals thereafter.21,22,23 This treatment markedly reduced renal damage at 24 hours and at day 3, demonstrating that C activation was contributing to the observed renal injuries. Of note, the ultrastructural damage to the glomerular endothelium seen at 10 minutes was not suppressed by sCR1, suggesting that this hyperacute phase was C-independent. It is possible that C is involved in the renal injuries induced by PsTX-T but might not be the initiator of the injuries and that other effectors contribute early and perhaps at later times. Indeed, in our index patient, no significant C deposition in glomeruli was found in the renal biopsy taken on day 4 after envenomation and the serum C activity was preserved,2 whereas in our model, C deposition was decreased at day 3. It is therefore not surprising that C activation was no longer detectable in renal biopsy performed in our human case on day 4 after envenomation. We have no information regarding renal pathology in human kidney on days 1 and 2 after envenomation. Given that there are currently no specific therapies for P. semoni envenomation, consideration should be given to the use of complement inhibition.
Renal expression of CReg has been shown to be important in restricting C injury in several rodent models of nephritis, and neutralization of individual CReg markedly exacerbated disease in these models.39,40,41,42 We were provoked to examine whether PsTx-115 modulated renal expression of CReg by a recent report describing a spider venom toxin that caused profound loss of membrane CReg from cells and tissues via a metalloproteinase-like activity.43 We found that expression of each membrane CReg in rat kidney was much reduced at 24 hours following administration of venom. Time-course studies showed that CD55 loss was detected even at 6 hours, when injury was minimal (data not shown). These data imply that venom directly causes a loss of CReg, rendering the kidney susceptible to C damage. In support of this possibility, incubation of kidney sections with venom ex vivo caused loss of CReg that was inhibited by inclusion of protease inhibitors, suggesting that the venom toxin has protease activity. CD55 and CD59 are GPI-anchored molecules and Crry a transmembrane protein; expression of a control GPI-anchored protein (Thy1.1) and a transmembrane control (thrombomodulin) were also decreased in glomeruli during the progression of renal injuries, demonstrating that the toxin activity was not specifically targeting CReg. Nevertheless, loss of CReg would enhance the C-driven renal injury. Of note, we have not observed marked loss of CReg accompanying renal injury in other rodent models used in our laboratory, whereas in human glomerular diseases increase of CReg were found.41,44 C deposition was much decreased on day 3, although glomerular injuries still remained and serum creatinine was also still high compared with the controls. Only focal and segmental glomerular changes were found on day 7 in surviving animals, suggesting that the damage caused by the toxin is largely reversible.
Research on marine animals has yielded interesting and clinically relevant data of therapeutic value. For example, dideoxpetrasynol A, a protein toxin from the sponge Petrosia sp., caused apoptosis in human melanoma cells45; sticholysin I, a sea anemone-derived cytolysin, has been coupled to tumor-specific antibody to target cancer cells46; and several agents have been shown to have antitumor activities.47,48,49,50,51 Although it is difficult to envisage therapeutic roles for the nephrotoxin PsTX-115, knowledge of the mechanisms of injury might inform understanding of other renal diseases with thrombotic microangiopathy, including HUS. The earliest detectable effect of PsTX-T (and PsTX-115) is glomerular endothelial damage progressing to acute renal failure. Similarly, HUS is an acute renal injury caused by glomerular endothelial damage. The common diarrhea-associated HUS is mediated by an Escherichia coli-derived verotoxin that targets kidney and damages glomerular endothelial cells both directly and as a consequence of local activation of C. Atypical nondiarrheal forms of HUS have been ascribed to defects in CReg, including factor H and CD46, resulting in dysregulation of C activation in the glomerulus.52,53 Modulation of CReg expression in the glomerulus caused by PsTX-T (and PsTX-115) may therefore mimic dysregulation of C seen in HUS with resultant acute renal failure, particularly when the dose of venom received by the victim is high. Inhibiting C activation might thus be an effective way of preventing renal injury following envenomation with PsTX-T. Anti-C therapies may perhaps also be effective in nondiarrheal and diarrheal forms of HUS.
Acknowledgments
We greatly appreciate N. Asano, T. Katahara, and Y. Fujitani for technical help and Dr. D.V. Tambourgi (Brazil) for helpful discussions.
Footnotes
Address reprint requests to Masashi Mizuno, M.D., Ph.D., Department of Nephrology, Nagoya University Graduate School of Medicine, School of Medicine, 65 Tsuruma-cho, Showa-ku, Nagoya 466-8550, Japan. E-mail: mmizu@med.nagoya-u.ac.jp or masashim1jp@yahoo.co.jp.
Supported in part by Wellcome Programme number 068590N. N.S. was financially supported by Tawada Clinic (Nagoya).
References
- Kwietniewski CR. Actiniaria von ambon und thursday island. Jena: Gustav Fischer,; Zoologische Forschungsreisen in Australien und dem Malayischen Archipelago von Richard Semon. 1897:pp 385–430. [Google Scholar]
- Mizuno M, Nishikawa K, Yuzawa Y, Kanie T, Mori H, Araki Y, Hotta N, Matsuo S. A case report of acute renal failure following a sting presumably by a sea anemone. Am J Kidney Dis. 2000;36:E10. doi: 10.1053/ajkd.2000.9006. [DOI] [PubMed] [Google Scholar]
- Nagai H, Oshiro N, Takuwa-Kuroda K, Iwanaga S, Nozaki M, Nakajima T. Novel proteinaceous toxins from the nematocyst venom of the Okinawan sea anemone Phyllodiscus semoni Kwietniewski. Biochem Biophys Res Commun. 2002;294:760–763. doi: 10.1016/S0006-291X(02)00547-8. [DOI] [PubMed] [Google Scholar]
- Wang L, Ou J, Peng L, Zhong X, Du J, Liu Y, Huang Y, Liu W, Zhang Y, Dong M, Xu AL. Functional expression and characterization of four novel neurotoxins from sea anemone Anthopleura sp. Biochem Biophys Res Commun. 2004;313:163–170. doi: 10.1016/j.bbrc.2003.11.102. [DOI] [PubMed] [Google Scholar]
- Bunc M, Drevensek G, Budihna M, Suput D. Effects of equinatoxin II from Actinia equina (L.) on isolated rat heart: the role of direct cardiotoxic effects in equinatoxin II lethality. Toxicon. 1999;37:109–123. doi: 10.1016/s0041-0101(98)00168-8. [DOI] [PubMed] [Google Scholar]
- Huerta V, Morera V, Guanche Y, Chinea G, Gonzalez LJ, Betancourt L, Martinez D, Alvarez C, Lanio ME, Besada V. Primary structure of two cytolysin isoforms from Stoichodactyla helianthus differing in their hemolytic activity. Toxicon. 2001;39:1253–1256. doi: 10.1016/s0041-0101(00)00247-6. [DOI] [PubMed] [Google Scholar]
- Amaral CF, Da Silva OA, Goody P, Miranda D. Renal cortical necrosis following Bothrops jararaca and B. jararacussu snake bite. Toxicon. 1985;23:877–885. doi: 10.1016/0041-0101(85)90379-4. [DOI] [PubMed] [Google Scholar]
- Tu AT. Biotoxicology of sea snake venoms. Ann Emerg Med. 1987;16:1023–1028. doi: 10.1016/s0196-0644(87)80752-7. [DOI] [PubMed] [Google Scholar]
- Ratcliffe PJ, Pukrittayakamee S, Ledingham JG, Warrell DA. Direct nephrotoxicity of Russell’s viper venom demonstrated in the isolated perfused rat kidney. Am J Trop Med Hyg. 1989;40:312–319. doi: 10.4269/ajtmh.1989.40.312. [DOI] [PubMed] [Google Scholar]
- Zimmerman SE, Yong LC. Nephrotoxicity of notexin in experimental mice. Exp Toxicol Pathol. 1995;47:149–155. doi: 10.1016/S0940-2993(11)80305-2. [DOI] [PubMed] [Google Scholar]
- Burdmann EA, Antunes I, Saldanha LB, Abdulkader RC. Severe acute renal failure induced by the venom of Lonomia caterpillars. Clin Nephrol. 1996;46:337–339. [PubMed] [Google Scholar]
- Martins AM, Toyama MH, Havt A, Novello JC, Marangoni S, Fonteles MC, Monteiro HS. Determination of Crotalus durissus cascavella venom components that induce renal toxicity in isolated rat kidneys. Toxicon. 2002;40:1165–1171. doi: 10.1016/s0041-0101(02)00119-8. [DOI] [PubMed] [Google Scholar]
- Luciano MN, da Silva PH, Chaim OM, dos Santos VL, Franco CR, Soares MF, Zanata SM, Mangili OC, Gremski W, Veiga SS. Experimental evidence for a direct cytotoxicity of Loxosceles intermedia (brown spider) venom in renal tissue. J Histochem Cytochem. 2004;52:455–467. doi: 10.1177/002215540405200404. [DOI] [PubMed] [Google Scholar]
- Tambourgi DV, de Femandes Pedrosa FM, van den Berg CW, Goncalves-de-Andrade RM, Ferracini M, Paixao-Cavalcante D, Morgan BP, Rushmere NK. Molecular cloning, expression, function and immunoreactivities of members of a gene family of sphingomyelinases from Loxosceles venom glands. Mol Immunol. 2004;41:831–840. doi: 10.1016/j.molimm.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Rodrigues FG, Petretski JH, Kanashiro MM, Lemos L, de Silva WD, Kipnis TL. The complement system is involved in acute inflammation but not in the hemorrhage produced by a Bothrops atrox snake venom low molecular mass proteinase. Mol Immunol. 2004;40:1149–1156. doi: 10.1016/j.molimm.2003.11.032. [DOI] [PubMed] [Google Scholar]
- Bertazzi DT, de Assis-Pandochi AI, Azzolini AE, Talhaferro VL, Lazzarini M, Arantes EC. Effect of Tityus serrulatus scorpion venom and its major toxin, TsTX-I, on the complement system in vivo. Toxicon. 2003;41:501–508. doi: 10.1016/s0041-0101(02)00391-4. [DOI] [PubMed] [Google Scholar]
- Yamamoto C, Tsuru D, Oda-Ueda N, Ohno M, Hattori S, Kim ST. Flavoxobin, a serine protease from Trimeresurus flavoviridis (habu snake) venom, independently cleaves Arg726-Ser727 of human C3 and acts as a novel, heterologous C3 convertase. Immunology. 2002;107:111–117. doi: 10.1046/j.1365-2567.2002.01490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering RJ, Wolfson MR, Good RA, Gewurz H. Passive hemolysis by serum and cobra venom factor: a new mechanism inducing membrane damage by complement. Proc Natl Acad Sci USA. 1969;62:521–527. doi: 10.1073/pnas.62.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshiro N, Kobayashi C, Iwanaga S, Nozaki M, Namikoshi M, Spring J, Nagai H. A new membrane-attack complex/perforin (MACPF) domain lethal toxin from the nematocyst venom of the Okinawan sea anemone Actineria villosa. Toxicon. 2004;43:225–228. doi: 10.1016/j.toxicon.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Nagai H, Oshiro N, Takuwa-Kuroda K, Iwanaga S, Nozaki M, Nakajima T. A new polypeptide toxin from the nematocyst venom of an Okinawa sea anemone Phyllodiscus semoni (Japanese name “unbachi-isoginchaku”). Biosci Biotechnol Biochem. 2002;66:2621–2625. doi: 10.1271/bbb.66.2621. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Nishikawa K, Morgan BP, Mastuo S. Comparison of the suppressive effects of soluble CR1 and C5a receptor antagonist in acute arthritis induced by anti-rat CD59 antibody. Clin Exp Immunol. 2000;119:368–375. doi: 10.1046/j.1365-2249.2000.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno M, Nishikawa K, Okada N, Matsuo S, Okada H. Soluble CR1 protects rats from lethal shock induced by anti-Crry antibody following LPS priming. Int Arch Allergy Immunol. 2002;127:55–62. doi: 10.1159/000048169. [DOI] [PubMed] [Google Scholar]
- Nomura A, Morita Y, Maruyama S, Hotta N, Nadai M, Wang L, Hasegawa T, Matsuo S. Role of complement in acute tubulointerstitial injury of rats with aminonucleoside nephrosis. Am J Pathol. 1997;151:539–547. [PMC free article] [PubMed] [Google Scholar]
- Kondo C, Mizuno M, Nishikawa K, Yuzawa Y, Hotta N, Matsuo S. The role of C5a in the development of thrombotic glomerulonephritis in rats. Clin Exp Immunol. 2001;124:323–329. doi: 10.1046/j.1365-2249.2001.01513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa H, Okada N, Okada H. Complement inhibitor of rat cell membrane resembling mouse Crry/p65. J Immunol. 1994;152:3032–3038. [PubMed] [Google Scholar]
- Spiller OB, Hanna SM, Morgan BP. Tissue distribution of the rat analogue of decay-accelerating factor. Immunology. 1999;97:374–384. doi: 10.1046/j.1365-2567.1999.00776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes TR, Piddlesden SJ, Williams JD, Harrison RA, Morgan BP. Isolation and characterization of a membrane protein from rat erythrocytes which inhibits lysis by the membrane attack complex of rat complement. Biochem J. 1992;284:169–176. doi: 10.1042/bj2840169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno M, Harris CL, Johnson PM, Morgan BP. Rat membrane cofactor protein (MCP; CD46) is expressed only in the acrosome of developing and mature spermatozoa and mediates binding to immobilised activated C3. Biol Reprod. 2004;71:1374–1383. doi: 10.1095/biolreprod.104.030114. [DOI] [PubMed] [Google Scholar]
- Mizutani M, Yuzawa Y, Maruyama I, Sakamoto N, Matsuo S. Glomerular localization of thrombomodulin in human glomerulonephritis. Lab Invest. 1993;69:193–202. [PubMed] [Google Scholar]
- Miyata T, Isobe K, Inagi R, Taguchi R, Ikezawa H, Takai I, Fujita Y, Iwamoto T, Hasegawa T, Oda O, Yamanaka N, Sugiyama S, Maeda K, Yamada K, Nakashima I. Rat mesangial cells actively produce phosphatidylinositol anchored Thy-1. Immunology. 1989;67:531–533. [PMC free article] [PubMed] [Google Scholar]
- Bernheimer AW, Avigad LS. Properties of a toxin from the sea anemone Stoichacis helianthus, including specific binding to sphingomyelin. Proc Natl Acad Sci USA. 1976;73:467–471. doi: 10.1073/pnas.73.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebs D, Gebauer E. Isolation of proteinase inhibitory, toxic and hemolytic polypeptides from a sea anemone, Stoichacis sp. Toxicon. 1980;18:97–106. doi: 10.1016/0041-0101(80)90035-5. [DOI] [PubMed] [Google Scholar]
- Macek P, Lebez D. Kinetics of hemolysis induced by equinatoxin, a cytolytic toxin from the sea anemone Actinia equina: effect of some ions and pH. Toxicon. 1981;19:233–240. doi: 10.1016/0041-0101(81)90026-x. [DOI] [PubMed] [Google Scholar]
- Mebs D, Liebrich M, Reul A, Samejima Y. Hemolysins and proteinase inhibitors from sea anemones of the Gulf of Aqaba. Toxicon. 1983;21:257–264. doi: 10.1016/0041-0101(83)90010-7. [DOI] [PubMed] [Google Scholar]
- Galettis P, Norton RS. Biochemical and pharmacological studies of the mechanism of action of tenebrosin-C, a cardiac stimulatory and haemolytic protein from the sea anemone, Actinia tenebrosa. Toxicon. 1990;28:695–706. doi: 10.1016/0041-0101(90)90258-9. [DOI] [PubMed] [Google Scholar]
- Lagos P, Duran R, Cervenansky C, Freitas JC, Silveira R. Identification of hemolytic and neuroactive fractions in the venom of the sea anemone Bunodosoma cangicum. Braz J Med Biol Res. 2001;34:895–902. doi: 10.1590/s0100-879x2001000700009. [DOI] [PubMed] [Google Scholar]
- Kawai N, Konno K. Molecular determinants of two neurotoxins that regulate sodium current inactivation in rat hippocampal neurons. Neurosci Lett. 2004;361:44–46. doi: 10.1016/j.neulet.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Fogh RH, Kem WR, Norton RS. Solution structure of neurotoxin I from the sea anemone Stichodactyla helianthus: a nuclear magnetic resonance, distance geometry, and restrained molecular dynamics study. J Biol Chem. 1990;265:13016–13028. doi: 10.2210/pdb2sh1/pdb. [DOI] [PubMed] [Google Scholar]
- Matsuo S, Nishikage H, Yoshida F, Nomura A, Piddlesden SJ, Morgan BP. Role of CD59 in experimental glomerulonephritis in rats. Kidney Int. 1994;46:191–200. doi: 10.1038/ki.1994.259. [DOI] [PubMed] [Google Scholar]
- Nishikage H, Baranyi L, Okada H, Okada N, Isobe K, Nomura A, Yoshida F, Matsuo S. The role of a complement regulatory protein in rat mesangial glomerulonephritis. J Am Soc Nephrol. 1995;6:234–241. doi: 10.1681/ASN.V62234. [DOI] [PubMed] [Google Scholar]
- Hatanaka Y, Yuzawa Y, Nishikawa K, Fukatsu A, Okada N, Okada H, Mizuno M, Matsuo S. Role of a rat membrane inhibitor of complement in anti-basement membrane antibody-induced renal injury. Kidney Int. 1995;48:1728–1738. doi: 10.1038/ki.1995.471. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Morita Y, Mizuno M, Nishikawa K, Yuzawa Y, Hotta N, Morgan BP, Okada N, Okada H, Matsuo S. CD59 protects kidneys from complement mediated injury in collaboration with Crry in rats. Kidney Int. 2000;58:1569–1579. doi: 10.1046/j.1523-1755.2000.00318.x. [DOI] [PubMed] [Google Scholar]
- Van Den Berg CW, de Andrade RM, Magnoli FC, Marchbank KJ, Tambourgi DV. Loxosceles spider venom induces metalloproteinase mediated cleavage of MCP/CD46 and MHCI and induces protection against C-mediated lysis. Immunology. 2002;107:102–110. doi: 10.1046/j.1365-2567.2002.01468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dixhoorn MG, Salazar-Exaire D, Sato T, Daha MR, Qigg RJ, Bruijn JA, Couser WG, DeHeer E. Anti-vitronectin antibodies enhance anti-Thy-1-induced proteinuria in PVG/c, but not in Wistar rats. J Am Soc Nephrol. 1998;9:994–1007. doi: 10.1681/ASN.V96994. [DOI] [PubMed] [Google Scholar]
- Choi HJ, Bae SJ, Kim ND, Jung JH, Choi YH. Induction of apoptosis by dideoxypetrosynol A, a polyacetylene from the sponge Petrosia sp., in human skin melanoma cells. Int J Mol Med. 2004;14:1091–1096. [PubMed] [Google Scholar]
- Tejuca M, Diaz I, Figueredo R, Roque L, Pazos F, Martinez D, Iznaga-Escobar N, Perez R, Alvarez C, Lanio ME. Construction of an immunotoxin with the pore forming protein StI and/or C5, a monoclonal antibody against a colon cancer cell line. Int Immunopharmacol. 2004;4:731–744. doi: 10.1016/j.intimp.2004.02.010. [DOI] [PubMed] [Google Scholar]
- De Souza MV. (+)-Discodermolide: a marine natural product against cancer. ScientificWorldJournal. 2004;4:415–436. doi: 10.1100/tsw.2004.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami N, Tamura S, Koyama K, Sugimoto M, Maekawa R, Kobayashi M. New analogue of arenastatin A, a potent cytotoxic spongean depsipeptide, with anti-tumor activity. Bioorg Med Chem Lett. 2004;14:2597–2601. doi: 10.1016/j.bmcl.2004.02.080. [DOI] [PubMed] [Google Scholar]
- Shimizu W, Antzelevitch C. Cellular and ionic basis for T-wave alternans under long-QT conditions. Circulation. 1999;99:1499–1507. doi: 10.1161/01.cir.99.11.1499. [DOI] [PubMed] [Google Scholar]
- Wang HW, Zheng YQ, Yang ZF, Li CZ, Liu YM. Effect of mexiletine on long QT syndrome model. Acta Pharmacol Sin. 2003;24:316–320. [PubMed] [Google Scholar]
- Platou ES, Refsum H, Hotvedt R. Class III antiarrhythmic action linked with positive inotropy: antiarrhythmic, electrophysiological, and hemodynamic effects of the sea-anemone polypeptide ATX II in the dog heart in situ. J Cardiovasc Pharmacol. 1986;8:459–465. [PubMed] [Google Scholar]
- Caprioli J, Castelletti F, Bucchioni S, Bettinaglio P, Bresin E, Pianetti G, Gamba S, Brioschi S, Daina E, Remuzzi G, Noris M, International Registry of Recurrent and Familial HUS/TTP Complement factor H mutations and gene polymorphisms in haemolytic uraemic syndrome: the C-257T, the A2089G and the G2881T polymorphisms are strongly associated with the disease. Hum Mol Genet. 2003;12:3385–3395. doi: 10.1093/hmg/ddg363. [DOI] [PubMed] [Google Scholar]
- Goodship T. Inherited dysregulation of the complement system. Bull Mem Acad R Med Belg. 2004;159:195–198. [PubMed] [Google Scholar]