Macrophages have long been known as key contributors of inflammation following infection, and they play a central role as effector cells during the engulfment of pathogens and cellular debris.1 Moreover, inappropriate macrophage or microglia activation may be responsible for harmful inflammatory processes that occur in a number of diverse autoimmune diseases, including rheumatoid arthritis,2 multiple sclerosis,3 and type I diabetes.4
In this issue of the American Journal of Pathology, Copland et al5 used their model of experimental autoimmune uveoretinitis (EAU) to demonstrate that the administration of a CD200 receptor (CD200R) agonist antibody can suppress macrophage activation and greatly diminish disease. EAU is considered to be a murine model for human endogenous uveitis, a common sight-threatening intraocular disease that involves the cell-mediated destruction of retinal tissues.6,7
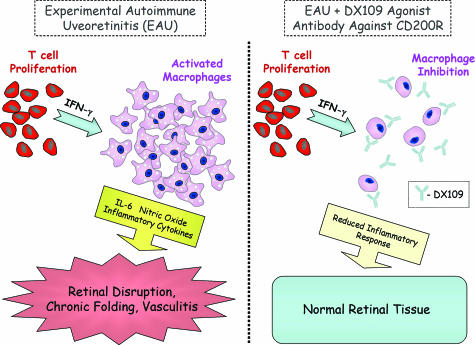
Autoreactive lymphocytes are routinely induced in this autoimmune model by immunization with retinal proteins emulsified in complete Freund’s adjuvant plus injection of pertussis toxin.8 The agonist antibody used in the current study, a monoclonal rat anti-mouse CD200R antibody called DX109, exerts its effects on EAU by delivering a negative signal to macrophages normally provided by CD200, which in turn may lead to the suppression of interferon-γ-mediated interleukin-6 and nitric oxide production during the inflammatory response (Figure 1).
Figure 1.
Systemic administration of DX109 inhibited macrophage activation and suppressed EAU. B10.RIII mice were used by Copland et al5 to test the efficacy of DX109 in their EAU model of autoimmune disease due to their increased susceptibility to autoimmune disease. Immunization of B10.RIII mice with hRBP-3 peptide led to the proliferation of CD4+ autoimmune T cells, macrophage activation, and EAU induction (left). In contrast, B10.RIII mice treated with DX109 displayed reduced signs of disease and fewer infiltrating macrophages (right). Furthermore, interferon (IFN)-γ-treated macrophages incubated with DX109 showed reduced levels of the proinflammatory cytokine interleukin (IL)-6 as well as nitric oxide, as compared with controls.
CD200/CD200R Interactions and Macrophage Inhibition
CD200, a membrane glycoprotein formerly known as OX2, has a broad distribution and expression in activated T cells, B cells, dendritic cells, and endothelium. The interaction between CD200 and CD200R has been previously shown to deliver an inhibitory signal to cells of the myeloid lineage through CD200/CD200R interaction.9,10 Consequently, mice deficient for CD200 (CD200−/− mice) display dysregulated macrophage function and increased susceptibility to autoimmune diseases. Moreover, recent studies suggest that a spontaneously occurring strain of mice (called Wlds), having a unique phenotype of protection against axonal injury, may be protected due to the elevated levels of CD200 expression by neurons.11 The CD200/CD200R interactions may also play a role in the “danger model” of immune recognition by the expression of CD200 on keratinocytes and Langerhans cells.12 Hence, providing the necessary ligand for activation of the CD200R in macrophages and microglia may be essential in managing the inflammatory response in a wide spectrum of diseases.13
Copland et al5 first demonstrate that CD200−/− mice displayed increased numbers of infiltrating macrophages and earlier EAU onset compared with control strain mice, thereby showing a role for CD200 in the exacerbation of disease. EAU was induced in these mice following immunization with peptides derived from the retinoid-binding protein (hRBP-3), which has previously been shown to induce CD4+ T-cell-mediated destruction of the neuroretina and photoreceptors of the eye.14 Remarkably, the disease outcome was strikingly reduced in highly susceptible B10.RIII mice following the systemic administration of DX109, and the majority of treated animals seemed normal and healthy. Furthermore, local administration of DX109 was able to lessen severity of disease with far less amounts of antibody. These results demonstrate the profound effect of the agonist antibody on sequestering macrophages and the inflammatory process. Additional experiments by Copland et al5 suggested that DX109 may act on interferon-γ-dependent signaling to inhibit the production of nitric oxide and the proinflammatory cytokine interleukin-6, both major contributors to inflammation and disease.15,16 These were carefully performed studies, and the therapeutic uses of DX109 may be far reaching; namely, the use of DX109 may be expanded to other diseases whereby macrophage activation is linked to immunopathology and autoimmune disease.17
DX109 effectively curbed the disease progression despite the presence of retinal antigen-specific T cells during EAU. These intriguing results suggest that the suppression of macrophage activation by DX109 may go a long way in inhibiting autoaggressive T-cell responses in other T-cell-mediated autoimmune diseases such as experimental autoimmune encephalomyelitis. Given that T-cell proliferation and cytokine production appeared normal following the administration of DX109, the inhibition of macrophage activation may be sufficient to modulate T-cell effector function, similar to T-cell modulation by mast cells.18 However, this point may not be entirely elucidated and may require additional studies for clarification. Of note, mast cells also express CD200R, and their activation might also be down-regulated following administration of DX109 during EAU.19 Regardless, the therapeutic potential of DX109, and perhaps a humanized form of the antibody, is a remedial path well worth visiting.
Limitations and Potential Difficulties
The utilization of DX109 or similar agonist antibodies directed against human CD200R is not without problems. In fact, antibody-based drugs continue to pose technical difficulties in terms of administration, systemic distribution, and stability. This issue becomes even more problematic if the therapeutic uses of DX109 or other large molecules are expanded to down-regulate chronically activated microglia associated with neurodegenerative diseases, such as multiple sclerosis. In essence, the difficulty of breaching the blood-brain barrier remains complicated, although not entirely unfeasible.20
In addition, it is not clear what unintended immunological consequences may occur following systemic administration of DX109. As with any immunomodulatory reagent, potential side effects may include the inadvertent suppression of the immune response and emergence of opportunistic infections, which may limit the use of DX109 or similar drugs used in a clinical setting. For example, natalizumab (Tysabri; Biogen Idec, Cambridge, MA), an antibody engineered against integrin α4 to block immune cells that cause nerve damage from entering nervous tissue, inadvertently led to the reactivation of latent JC virus in the central nervous system. The reactivation of JC virus was responsible for development of progressive multifocal leukoencephalopathy in some patients receiving natalizumab.21 By quantifying the proliferative and cytokine response of splenocytes to an immunizing peptide, Copland et al5 show that the administration of DX109 did not lead to any adverse affects on the peripheral immune system. However, it may be more informative in future studies to test the ability of the immune system to respond to a viral or bacterial pathogen in the presence of systemic DX109 antibody administration.
Future Directions
In summary, it will be exciting to determine whether the DX109 agonist antibody can lessen pathology for other diseases in which macrophages are thought to play a primary role. For example, can DX109 shut down macrophage activation and inflammation in animal models of rheumatoid arthritis? Can the progressive plaque lesions of atherosclerosis be prevented or diminished by reducing macrophage recruitment? In addition, follow-up studies may help determine whether macrophage suppression by DX109 may limit autoimmune diseases whereby CD4 and CD8 T-cell responses play a primary role. What about the ability of DX109 to suppress autoimmune diseases induced following viral or bacterial infections? The possibilities for the treatment of disease seem to be endless.
Footnotes
Address reprint requests to Ralph Feuer, Department of Biology, Cell & Molecular Biology Doctoral Program, San Diego State University, 5500 Campanile Dr., San Diego, CA 92182-4614. E-mail: rfeuer@sciences.sdsu.edu.
See related article on page 580
This commentary relates to Copland et al, Am J Pathol 2007, 171:580–588, published in this issue.
References
- Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- Szekanecz Z, Koch AE. Macrophages and their products in rheumatoid arthritis. Curr Opin Rheumatol. 2007;19:289–295. doi: 10.1097/BOR.0b013e32805e87ae. [DOI] [PubMed] [Google Scholar]
- Jack C, Ruffini F, Bar-Or A, Antel JP. Microglia and multiple sclerosis. J Neurosci Res. 2005;81:363–373. doi: 10.1002/jnr.20482. [DOI] [PubMed] [Google Scholar]
- Calderon B, Suri A, Unanue ER. In CD4+ T-cell-induced diabetes, macrophages are the final effector cells that mediate islet β-cell killing: studies from an acute model. Am J Pathol. 2006;169:2137–2147. doi: 10.2353/ajpath.2006.060539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copland DA, Calder CJ, Raveney BJE, Nicholson LB, Phillips J, Cherwinski H, Jenmalm M, Sedgwick JD, Dick AD. Monoclonal antibody-mediated CD200 receptor signalling suppresses macrophage activation and tisue damage in experimental autoimmune uveoretinitis (EAU). Am J Pathol. 2007;171:580–588. doi: 10.2353/ajpath.2007.070272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick AD, Carter D, Robertson M, Broderick C, Hughes E, Forrester JV, Liversidge J. Control of myeloid activity during retinal inflammation. J Leukoc Biol. 2003;74:161–166. doi: 10.1189/jlb.1102535. [DOI] [PubMed] [Google Scholar]
- Caspi RR. Th1 and Th2 responses in pathogenesis and regulation of experimental autoimmune uveoretinitis. Int Rev Immunol. 2002;21:197–208. doi: 10.1080/08830180212063. [DOI] [PubMed] [Google Scholar]
- Forrester JV, Liversidge J, Dua HS, Dick A, Harper F, McMenamin PG. Experimental autoimmune uveoretinitis: a model system for immunointervention: a review. Curr Eye Res. 1992;11(Suppl):33–40. doi: 10.3109/02713689208999509. [DOI] [PubMed] [Google Scholar]
- Minas K, Liversidge J. Is the CD200/CD200 receptor interaction more than just a myeloid cell inhibitory signal? Crit Rev Immunol. 2006;26:213–230. doi: 10.1615/critrevimmunol.v26.i3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek RM, Ruuls SR, Murphy CA, Wright GJ, Goddard R, Zurawski SM, Blom B, Homola ME, Streit WJ, Brown MH, Barclay AN, Sedgwick JD. Down-regulation of the macrophage lineage through interaction with OX2 (CD200). Science. 2000;290:1768–1771. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- Chitnis T, Imitola J, Wang Y, Elyaman W, Chawla P, Sharuk M, Raddassi K, Bronson RT, Khoury SJ. Elevated neuronal expression of CD200 protects Wlds mice from inflammation-mediated neurodegeneration. Am J Pathol. 2007;170:1695–1712. doi: 10.2353/ajpath.2007.060677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum MD, Yancey KB, Olasz EB, Truitt RL. CD200, a “no danger” signal for hair follicles. J Dermatol Sci. 2006;41:165–174. doi: 10.1016/j.jdermsci.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Gorczynski RM, Chen Z, Yu K, Hu J. CD200 immunoadhesion suppresses collagen-induced arthritis in mice. Clin Immunol. 2001;101:328–334. doi: 10.1006/clim.2001.5117. [DOI] [PubMed] [Google Scholar]
- Broderick C, Hoek RM, Forrester JV, Liversidge J, Sedgwick JD, Dick AD. Constitutive retinal CD200 expression regulates resident microglia and activation state of inflammatory cells during experimental autoimmune uveoretinitis. Am J Pathol. 2002;161:1669–1677. doi: 10.1016/S0002-9440(10)64444-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther. 2006;8(Suppl 2):S3. doi: 10.1186/ar1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenmalm MC, Cherwinski H, Bowman EP, Phillips JH, Sedgwick JD. Regulation of myeloid cell function through the CD200 receptor. J Immunol. 2006;176:191–199. doi: 10.4049/jimmunol.176.1.191. [DOI] [PubMed] [Google Scholar]
- Sayed BA, Brown MA. Mast cells as modulators of T-cell responses. Immunol Rev. 2007;217:53–64:53–64. doi: 10.1111/j.1600-065X.2007.00524.x. [DOI] [PubMed] [Google Scholar]
- Cherwinski HM, Murphy CA, Joyce BL, Bigler ME, Song YS, Zurawski SM, Moshrefi MM, Gorman DM, Miller KL, Zhang S, Sedgwick JD, Phillips JH. The CD200 receptor is a novel and potent regulator of murine and human mast cell function. J Immunol. 2005;174:1348–1356. doi: 10.4049/jimmunol.174.3.1348. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Molecular Trojan horses for blood-brain barrier drug delivery. Curr Opin Pharmacol. 2006;6:494–500. doi: 10.1016/j.coph.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Khalili K, White MK, Lublin F, Ferrante P, Berger JR. Reactivation of JC virus and development of PML in patients with multiple sclerosis. Neurology. 2007;68:985–990. doi: 10.1212/01.wnl.0000257832.38943.2b. [DOI] [PubMed] [Google Scholar]