Abstract
Increased tissue or serum levels of oxidized phospholipids have been detected in a variety of chronic and acute pathological conditions such as hyperlipidemia, atherosclerosis, heart attack, cell apoptosis, acute inflammation and injury. We have recently described signaling cascades activated by oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (OxPAPC) in the human pulmonary artery endothelial cells (EC) and reported potent barrier-protective effects of OxPAPC, which were mediated by small GTPases Rac and Cdc42. In this study we have further characterized signal transduction pathways involved in the OxPAPC-mediated endothelial barrier protection. Inhibitors of small GTPases, protein kinase A (PKA), protein kinase C (PKC), Src family kinases and general inhibitors of tyrosine kinases attenuated OxPAPC-induced barrier-protective response and EC cytoskeletal remodeling. In contrast, small GTPase Rho, Rho kinase, Erk-1,2 MAP kinase and p38 MAP kinase and PI3-kinase were not involved in the barrier-protective effects of OxPAPC. Inhibitors of PKA, PKC, tyrosine kinases and small GTPase inhibitor toxin B suppressed OxPAPC-induced Rac activation and decreased phosphorylation of focal adhesion kinase (FAK) and paxillin. Barrier-protective effects of OxPAPC were not reproduced by platelet activating factor (PAF), which at high concentrations induced barrier dysfunction, but were partially attenuated by PAF receptor antagonist A85783. These results demonstrate for the first time upstream signaling cascades involved in the OxPAPC-induced Rac activation, cytoskeletal remodeling and barrier regulation and suggest PAF receptor-independent mechanisms of OxPAPC-mediated endothelial barrier protection.
Keywords: PKC, PKA, Rac, FAK, paxillin, phosphorylation, endothelium, permeability, actin, cytoskeleton
INTRODUCTION
The vascular endothelial monolayer forms a semi-selective permeable barrier between the blood and the interstitial space, which regulates macromolecule transport and leukocyte trafficking through the vessel wall. A number of agonists including phospholipid products (Zhang, Magnusson et al. 1997; Huang, Subbaiah et al. 2005) and bioactive peptides (Garcia, Siflinger-Birnboim et al. 1986; van Nieuw Amerongen, Draijer et al. 1998; Petrache, Verin et al. 2001; Moy, Blackwell et al. 2002; Birukova, Adyshev et al. 2005) increase vascular endothelial permeability, which my lead to alveolar flooding and pulmonary edema. However, much smaller number of bioactive molecules capable of enhancing endothelial barrier properties have been reported so far (Garcia, Liu et al. 2001; Liu, Schaphorst et al. 2002; Vouret-Craviari, Bourcier et al. 2002; Birukov, Bochkov et al. 2004; Kolosova, Mirzapoiazova et al. 2005), and signaling mechanisms underlying these barrier protective effects have been a major focus of ongoing studies (Lee, Lee et al. 2000; Garcia, Liu et al. 2001; Vouret-Craviari, Bourcier et al. 2002; Birukov, Leitinger et al. 2004; Dudek, Jacobson et al. 2004; Kolosova, Mirzapoiazova et al. 2005; Mehta, Konstantoulaki et al. 2005; Singleton, Dudek et al. 2005).
Phosphocholines represent a major group of cell membrane structural phospholipids. Enhanced lipid peroxidation leading to formation of oxidized phospholipids was observed in acute lung injury syndromes such as ARDS, ventilator-induced lung injury, and asthma (Chabot, Mitchell et al. 1998; Wood, Gibson et al. 2003). An increased release of membrane vesicles containing oxidized phospholipids has been detected as a result of tissue injury and apoptosis (Holme, Solum et al. 1994; Huber, Vales et al. 2002), and such membrane vesicles were also observed in patients with acute lung injury, cardiac ischemia, acute coronary syndrome and in pathological settings with platelet activation (Holme, Solum et al. 1994; Mallat, Benamer et al. 2000).
We have recently described that oxidized phospholipids derived from oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine (OxPAPC) exhibited potent barrier-protective effects towards human pulmonary endothelial monolayers (Birukov, Bochkov et al. 2004). Formation of rigid peripheral cytoskeletal structures is a morphological basis of endothelial barrier enhancement and is regulated by several signaling pathways with small GTPases Rac and Cdc42 playing a major role in the triggering of EC barrier-protective response (Lum and Malik 1996; Dudek and Garcia 2001; Garcia, Liu et al. 2001; Birukov, Bochkov et al. 2004; Kouklis, Konstantoulaki et al. 2004). Using molecular approaches including ectopic expression of mutant small GTPases and siRNA-based small GTPase protein depletion, we linked barrier-protective effects of OxPAPC to Rac/Cdc42-mediated enhancement of peripheral actin cytoskeleton (Birukov, Bochkov et al. 2004).
In various types of endothelial cells, OxPAPC also activates small GTPase R-Ras (Cole, Subbanagounder et al. 2003), stimulates protein kinase C, protein kinase A, protein tyrosine kinases, and induces the Raf / MEK1,2 / Erk-1,2 and JNK MAP kinase signaling cascades (Cole, Subbanagounder et al. 2003; Birukov, Bochkov et al. 2004; Birukov, Leitinger et al. 2004), as well as transcriptional STAT3 pathway (Yeh, Gharavi et al. 2004). Importantly, these effects are not reproduced by non-oxidized PAPC (Birukov, Leitinger et al. 2004). Activation of PKC and the MEK/Erk-1,2 MAP kinase cascade by OxPAPC triggers expression of early growth response factor 1 (Egr-1) and tissue factor in HUVEC monolayers (Bochkov, Mechtcheriakova et al. 2002). OxPAPC-induced PKA activation is involved in the OxPAPC-induced heme oxygenase-1 induction (Kronke, Bochkov et al. 2003) and deposition of CS-1 fibronectin on apical αvβ1 integrin critical for monocyte binding to arterial endothelium from systemic circulation (Cole, Subbanagounder et al. 2003). In addition, OxPAPC induces activation of tyrosine kinases including Src kinase and focal adhesion kinase (FAK) (Birukov, Leitinger et al. 2004; Yeh, Gharavi et al. 2004), resulting in activation of focal adhesion complexes and cytoskeletal remodeling. Thus, a number of signaling pathways induced by OxPAPC have been reported so far. However, specific protein kinase pathways involved in OxPAPC-mediated EC barrier-protective response have not been yet characterized.
A small proportion of phospholipids contain sn-1 residues attached to glycerol by an ether bond. Upon oxidative fragmentation of sn-2 unsaturated fatty acid residues, these alkyl-oxidized phospholipids become agonists for the platelet-activating factor (PAF) receptor (Marathe, Davies et al. 1999). These “PAF-like lipids” are formed in vivo and induce many effects typical of PAF (Marathe, Prescott et al. 2001). However, potential involvement of PAF receptor in OxPAPC-mediated EC permeability responses has not been yet studied.
In this study, we characterized protein kinase signaling cascades involved in barrier-protective response to OxPAPC, studied relations between protein kinase A, protein kinase C, Src kinase and Rac GTPase in OxPAPC-induced endothelial barrier regulation and examined involvement of PAF receptor in the OxPAPC-mediated EC barrier regulation.
MATERIALS AND METHODS
Reagents and cell culture
All biochemical reagents including PAF, A85783, and PAPC were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise indicated. PAPC was oxidized by exposure of dry lipid to air for 72 hours. The extent of oxidation was monitored by positive ion electrospray mass spectrometry as described previously (Watson, Leitinger et al. 1997). Each batch of oxidized phospholipids was standardized by thin layer chromatography. In addition, quality control of the OxPAPC composition in each batch was also performed by positive ion electrospray mass spectrometry and judged by a standard pattern of characteristic peaks with m/z 616.4; 632.4; 810.5; 828.5; corresponding to major oxygenated and fragmented products of PAPC oxidation. Specific effects of oxygenated (PECPC, PEIPC) and fragmented (POVPC, PGPC, lyso-PC) components present in standard OxPAPC preparations have been characterized previously (see (Bochkov and Leitinger 2003; Birukov 2006; Bochkov, Leitinger et al. 2006) for review). PAPC and OxPAPC preparations were shown negative for endotoxin by the limulus amebocyte assay performed after PAPC oxidation prior to in vivo and in vitro experiments (BioWhittaker, Frederick, MD). All reagents used for immunofluorescent staining were purchased from Molecular Probes (Eugene, OR). Toxin B, genistein, PP2, Y27632, SB 203580, LY294002 were purchased from Calbiochem (La Jolla, CA), U0126 and cell permeant myristoylated PKA and PKC inhibitory peptides were purchased from Promega (Madison, WI), phospho-FAK (Tyr576/577), FAK, phospho-Src (Tyr416), Src, and p-paxillin (Tyr118) antibodies were obtained from Cell Signaling (Beverly, MA), phospho-tyrosine antibody was purchased from Upstate Biotechnology (Lake Placid, NY), paxillin antibody was purchased from BD Transduction Laboratories (San Diego, CA). Human pulmonary artery endothelial cells were obtained from Clonetics, BioWhittaker Inc. (Frederick, MD), maintained according to vendor’s protocol, and used at passages 6–10.
Measurement of transendothelial electrical resistance
The cellular barrier properties were measured according to the method described elsewhere (Tiruppathi, Malik et al. 1992). The total transendothelial electrical resistance (TER) was measured dynamically across the monolayer using an electrical cell-substrate impedance sensing system (Applied Biophysics, Troy, NY) and was determined by the combined resistance between the basal surface of the cell and the electrode, reflective of focal adhesion, and the resistance between the cells, as described previously (Birukov, Bochkov et al. 2004; Birukova, Smurova et al. 2004).
Immunofluorescent staining
EC monolayers were fixed after agonist treatment in 3.7% formaldehyde solution in PBS for 10 min at 4° C, washed three times with PBS, permeabilized with 0.2% triton X-100 in PBS-Tween (PBST) for 30 min at room temperature, and blocked with 2% BSA in PBST for 30 min. Incubation with antibody of interest was performed in 2% BSA–PBST for 1 hr at room temperature followed by staining with Texas Red-conjugated phalloidin (Molecular Probes, Eugene, OR) to visualize actin filaments. After immunostaining, the glass slides were analyzed using Nikon video-imaging system (Nikon Instech Co., Tokyo, Japan). The images were recorded and processed using Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA) software.
Rac activation assay
Activation of Rac GTPase in pulmonary EC culture were analyzed using Rac activation assay kit available from Upstate Biotechnology (Lake Placid, NY) according to manufacturer’s protocol, as previously described (Birukova, Smurova et al. 2004; Shikata, Rios et al. 2005).
Western immunoblotting
Protein extracts were separated by SDS-PAGE on 10% gels, transferred to nitrocellulose membrane (100 V for 1 h), and probed with specific antibodies. Immunoreactive proteins were detected using enhanced a chemiluminescent detection system (ECL) according to the manufacturer’s protocol (Amersham, Little Chalfont, UK). The relative intensities of the protein in the bands were quantified by scanning densitometry.
Statistical Analysis
Results are expressed as means ± SD of three to five independent experiments. Stimulated samples were compared with controls by unpaired Student’s t-test. For multiple-group comparisons, one-way analysis of variance (ANOVA) followed by the post hoc Fisher’s test were used. P<0.05 was considered statistically significant.
RESULTS
Involvement of cellular signaling pathways in barrier enhancement induced by oxidized phospholipids
We have previously shown involvement of Rac and Cdc42 in the OxPAPC-induced EC barrier protection (Birukov, Bochkov et al. 2004). Furthermore, we have described OxPAPC-induced activation of protein kinase A, protein kinase C, protein tyrosine phosphorylation, FAK and paxillin phosphorylation, as well as activation of Erk-1,2, p38 and JNK MAP kinase cascades in the human pulmonary EC (Birukov, Leitinger et al. 2004). In these experiments we studied OxPAPC-induced signaling pathways, which mediate OxPAPC-induced EC barrier protection. We performed inhibitory analysis and examined effects of cell-permeable peptide inhibitors of PKA (20 μM), PKC (20 μM) (Promega, Madison, WI), broad range tyrosine kinase inhibitor genistein (100 μM), inhibitor of Src family tyrosine kinases PP2 (2 μM), MEK inhibitor U0126 (5 μM), p38 MAPK inhibitor SB203580 (20μM), and PI3 kinase inhibitor LY294002 (25 μM) on OxPAPC-induced increases in transendothelial resistance across EC monolayers. Because Rac and Cdc42, but not Rho mediate OxPAPC- induced barrier enhancement (Birukov, Bochkov et al. 2004), in this study we used a general Rac, Rho and Cdc42 inhibitor from C. difficile toxin B (20 ng/ml) and Rho kinase inhibitor Y27632 (5 μM) as controls. EC monolayers pre-treated with specific inhibitors were stimulated with OxPAPC (20 μg/ml), and TER changes monitored during 2 hours were expressed as percent of maximal barrier-protective response induced by OxPAPC. EC pretreatment with toxin B or inhibitors of PKA, PKC, or tyrosine phosphorylation significantly attenuated OxPAPC-induced increases in TER (Figure 1). In contrast, inhibition of Rho- and Erk-1,2-dependent pathways, p38 MAPK, and PI3 kinase were without effect. To further evaluate an involvement of specific signaling pathways in OxPAPC-mediated cytoskeletal changes associated with EC barrier protective response, pulmonary EC were pretreated with pharmacological inhibitors followed by OxPAPC stimulation (20 μg/ml, 30 min), and F-actin remodeling was analyzed by immunofluorescent staining with Texas Red conjugated phalloidin (Figure 2). Consistent with previous studies (Birukov, Bochkov et al. 2004), OxPAPC stimulation caused disappearance of central actin stress fibers and pronounced accumulation of F-actin at the cell periphery (shown by arrows), as compared to quiescent EC monolayers. Remarkably, EC pre-incubation with toxin B or inhibitors of PKA, PKC and tyrosine kinase not only abolished disappearance of central actin stress fibers, but significantly attenuated F-actin accumulation at the areas of intercellular junctions. In contrast, inhibition of Erk-1,2 and p38 MAPK kinases, Rho-kinase and PI3-kinase did not affect the OxPAPC-induced cytoskeletal remodeling consistent with the lack of effects on OxPAPC-induced TER increases. These results strongly suggest an essential role of tyrosine phosphorylation, PKA and PKC-dependent signaling in OxPAPC-mediated barrier enhancement.
Figure 1. Inhibitory analysis of signaling pathways involved in OxPAPC-mediated increase in EC resistance.
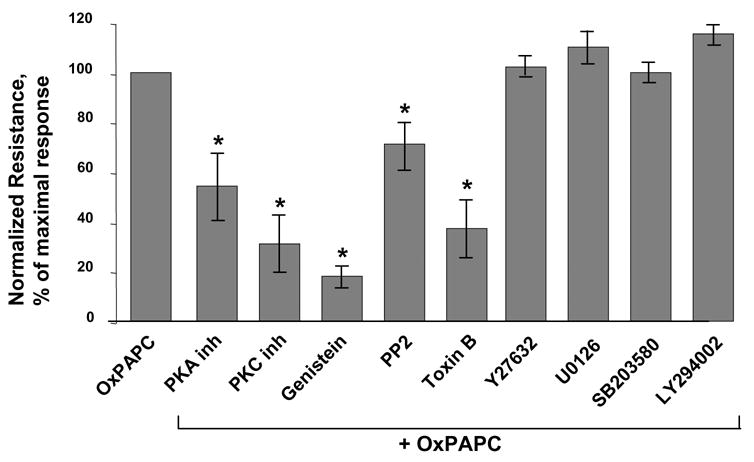
Transendothelial resistance was monitored across confluent endothelial monolayers pretreated with vehicle, PKA inhibitor (20 μM), PKC inhibitor (20 μM), genistein (100 μM), PP2 (2 μM), toxin B (20 ng/ml), Y27632 (5 μM), U0125 (5 μM), SB 203580 (20 μM), or LY294002 (25 μM) for 30 min followed by addition of OxPAPC (20 μg/ml). Bar graphs represent measurements of TER after 30 min of OxPAPC stimulation. Shown are results of three to seven independent experiments represented as mean ± SE. *p<0.01 vs OxPAPC alone.
Figure 2. Analysis of signaling pathways involved in OxPAPC-induced cytoskeletal remodeling.
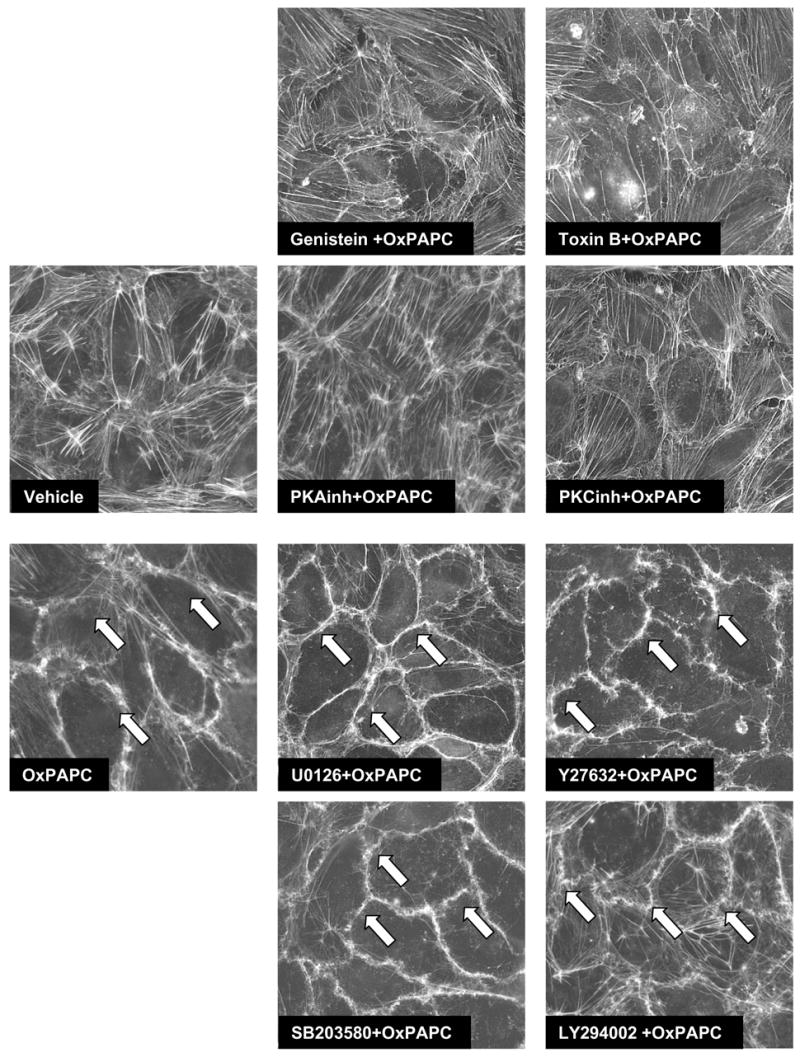
EC grown on glass coverslips were preincubated with genistein (100 μM), small GTPase inhibitor toxin B (20 ng/ml), PKA inhibitor (20 μM), PKC inhibitor (20 μM), Rho kinase inhibitor Y27632 (5 μM), MEK/Erk1,2 inhibitor U0125 (5 μM), p38 MAP kinase inhibitor SB203580 (20 μM), or PI3-kinase inhibitor LY294002 (25 μM) for 30 min followed by addition of OxPAPC (20 μg/ml) for 30 min. Cytoskeletal remodeling was assessed by immunofluorescent staining for F-actin with Texas Red phalloidin. Results are representative of three independent experiments.
Involvement of PKA and PKC-dependent pathways in OxPAPC-induced Rac activation
Activation of small GTPase Rac has been previously described as a leading mechanism of barrier protection in response to various stimuli including OxPAPC, sphingosine 1-phosphate, hepatocyte growth factor (HGF), and shear stress (Garcia, Liu et al. 2001; Birukov, Birukova et al. 2002; Liu, Schaphorst et al. 2002; Birukov, Bochkov et al. 2004; Shikata, Rios et al. 2005). In the next studies we investigated the roles of protein kinases A and C and tyrosine kinases in the regulation of OxPAPC-induced Rac activation. HPAEC preincubated with cell permeable peptide inhibitors of PKA and PKC, or pretreated with general tyrosine kinase inhibitor genistein for 30 min were next stimulated with OxPAPC. Toxin B pretreatment served as a positive control for Rac inhibition. Inhibition of PKA, PKC and protein tyrosine phosphorylation significantly attenuated OxPAPC-induced Rac activation (Figure 3).
Figure 3. Effect of PKA and PKC inhibition on OxPAPC-induced Rac activation.
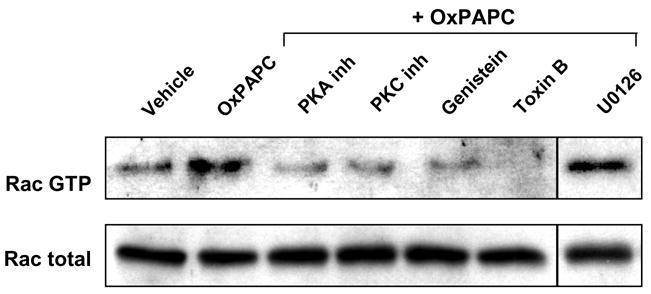
HPAEC were preincubated with PKA inhibitor (20 μM), PKC inhibitor (20 μM), toxin B (20 ng/ml), genistein (100 μM) or U0125 (5 μM) for 30 min followed by addition of OxPAPC (20 μg/ml) for 5 min. Rac activation was analyzed as described in Methods section. Results are representative of three independent experiments.
Effect of PKA and PKC on OxPAPC-induced protein tyrosine phosphorylation
Tyrosine phosphorylation of focal adhesion and cytoskeletal proteins is essential for regulation of endothelial barrier (Mehta, Tiruppathi et al. 2002; Shikata, Birukov et al. 2003; van Nieuw Amerongen, Natarajan et al. 2004). Previous study showed increased FAK and paxillin phosphorylation in response to OxPAPC (Birukov, Leitinger et al. 2004). We next examined involvement of PKA and PKC signaling in the OxPAPC-induced protein tyrosine phosphorylation. EC preincubated for 30 min with toxin B (20 ng/ml) or cell permeable peptide inhibitors of PKA (20 μM) and PKC (20 μM) were treated with OxPAPC (20 μg/ml, 30 min). Genistein (100 μM, 1 hr) was used as a broad inhibitor of tyrosine kinases. Obtained cell lysates were probed in immunoblot with site-specific phosphotyrosine antibodies to Src-Tyr416, FAK-Tyr576/577 (Figure 4A,B), and paxillin-Tyr118 (Figure 4C). Inhibition of PKA and PKC, as well as inhibition of small GTPases by toxin B significantly reduced site-specific tyrosine phosphorylation of Src, FAK and paxillin in response to OxPAPC challenge.
Figure 4. Effect of PKA and PKC on OxPAPC-induced protein tyrosine phosphorylation.
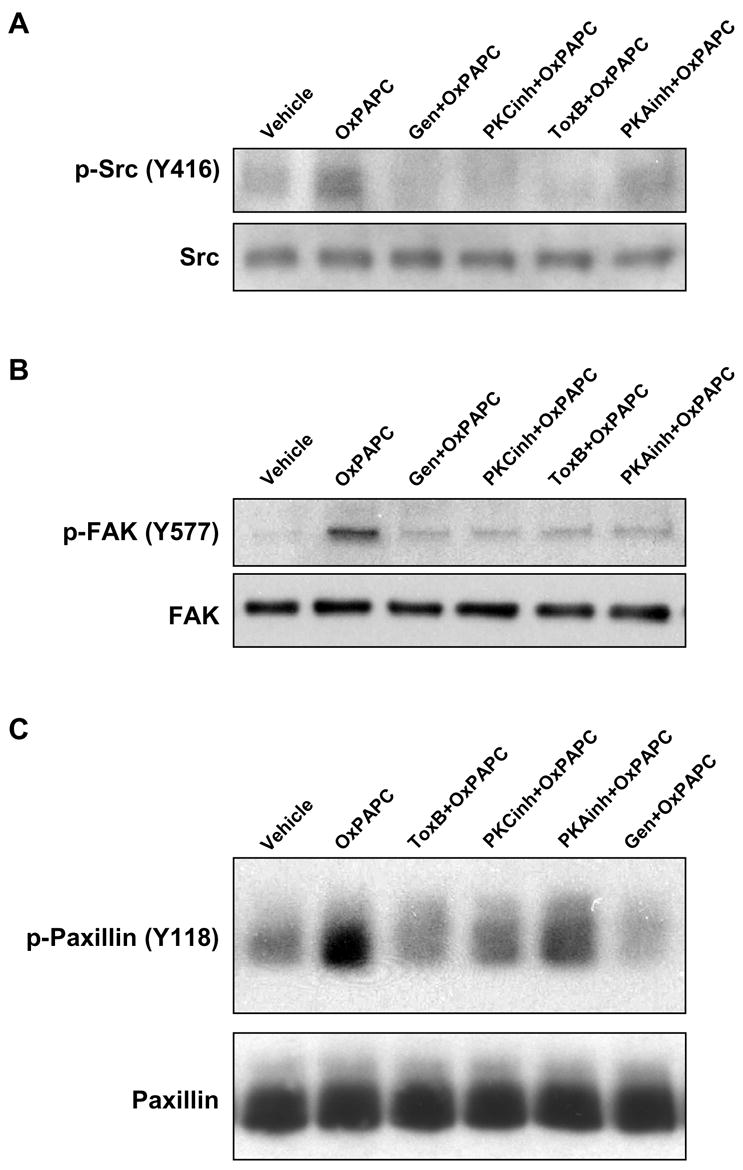
EC were pretreated with genistein (100 μM), PKA inhibitor (20 μM), PKC inhibitor (20 μM), or toxin B (20 ng/ml) for 30 min followed by stimulation with OxPAPC (20 μg/ml) for 30 min. Phosphorylation of specific proteins was detected by western blot with specific antibodies to phospho-Src (Tyr416) (panel A), phospho-FAK (Tyr576/577) (panel B) and phospho-paxillin (Tyr118) (panel C). Equal protein loadings were confirmed by reprobing the membranes with Src, FAK or paxillin antibodies, respectively. Shown are representative results of three independent experiments.
Role of PAF receptor-dependent signaling in the regulation of OxPAPC-mediated barrier protection
Because selected biological effects of OxPAPC can be partially inhibited by platelet activating factor (PAF) receptor antagonists (Leitinger, Watson et al. 1997; Subbanagounder, Leitinger et al. 1999; Kadl, Huber et al. 2002), we next tested effects of PAF in the model of pulmonary EC permeability and examined potential involvement of PAF receptor in OxPAPC-mediated barrier-protective responses, cytoskeletal remodeling, and protein phosphorylation. In contrast to OxPAPC, PAF stimulation did not induce TER increases in HPAEC monolayers (Figure 5A) at hormonal concentrations (10 – 50 nM), whereas at high non-physiological concentrations (5 – 100 μg/ml), PAF caused dose-dependent TER decline reflective of pronounced EC barrier dysfunction (Figure 5A), which was not affected by PAF receptor antagonist A85783 (5 μM, 1 hr) (Figure 5B,D). In contrast to effects on PAF responses, A85783 partially attenuated OxPAPC-induced increase in transendothelial resistance (Figure 5C,D). Next, we investigated effects of PAF receptor antagonist A85783 on OxPAPC-induced cytoskeletal remodeling. A85783 alone did not change EC morphology, but partially attenuated OxPAPC-induced peripheral F-actin accumulation (Figure 5E). Interestingly, PAF receptor antagonist also partially decreased OxPAPC-induced paxillin phosphorylation and general protein tyrosine phosphorylation.
Figure 5. Analysis of potential involvement of PAF receptor in OxPAPC-induced signaling and endothelial barrier protection.
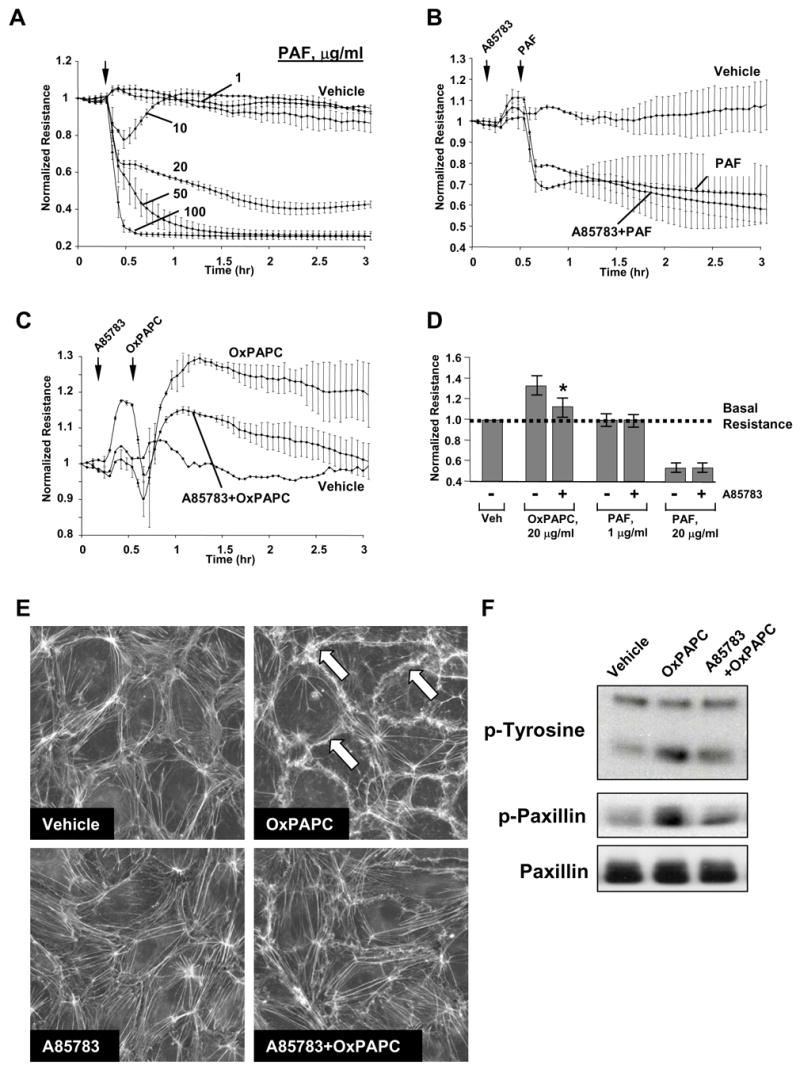
Panels A – D: TER measurements in EC monolayers. Cells grown on gold microelectrodes were stimulated with PAF (1 μg/ml, 5 μg/ml, 20 μg/ml, 50 μg/ml, 100 μg/ml) (panel A); or were preincubated with PAF receptor antagonist A85783 (5 μM, 30 min) followed by stimulation with 20 μg/ml of PAF (panel B) or OxPAPC (panel C). Effects of PAF receptor antagonist on PAF- and OxPAPC-mediated permeability changes are summarized in panel D. Shown are pooled data of three independent experiments represented as mean + SD. *p<0.01 vs OxPAPC alone. Panel E: EC were preincubated with A85783 (5 μM, 30 min) followed by stimulation with OxPAPC (20 μg/ml, 30 min) and immunofluorescent analysis of F-actin remodeling by staining with Texas Red phalloidin. Panel F: EC preincubated with vehicle or A85783 (5 μM, 30 min) were treated with OxPAPC, and total protein tyrosine phosphorylation and site-specific paxillin phosphorylation was detected by immunoblotting with phospho-tyrosine or phospho-paxillin (Tyr118) antibodies. Equal protein loading was confirmed by reprobing the membranes with paxillin antibody.
Discussion
The results of this study demonstrate for the first time the role for PKA and PKC as upstream regulators of Rac activity in response to OxPAPC stimulation and indicate that Rac activity was required for OxPAPC-induced tyrosine phosphorylation of Src, FAK, paxillin, which was essential for EC cytoskeletal remodeling and barrier protective response.
Previous studies have shown that Src kinase inhibition abolished OxPAPC-induced phosphorylation of FAK and its substrate paxillin, a focal adhesion protein, which functions as structural and adaptor protein (Birukov, Leitinger et al. 2004). This study shows that inhibitors of PKA and PKC abolished OxPAPC-induced Rac activation, whereas MEK/Erk-1,2 and p38 MAP kinase inhibitors were without effect. In turn, downregulation of small GTPases by cell pretreatment with toxin B attenuates phosphorylation of Src, FAK and paxillin. Since OxPAPC does not activate Rho, these results suggest involvement of Rac-dependent mechanism in OxPAPC-induced tyrosine phosphorylation of Src, FAK, and paxillin, which may be an important part of coordinated focal adhesion and cytoskeletal remodeling associated with barrier-protective effects of OxPAPC. Rac-dependent mechanisms of tyrosine kinase activation are not well understood, but may involve induction of NADPH oxidase, production of reactive oxygen species (ROS) and ROS-induced stimulation of tyrosine phosphorylation (Rhee, Chang et al. 2003).
Regulation of Rac activation by PKC and PKA may involve modulation of specific guanosine nucleotide exchange factors (GEFs). Tiam1 is a Rac-specific GEF regulated by diverse mechanisms including phosphorylation by Ca2+/calmodulin–dependent kinase II (CaMKII) or related kinases, interaction with phosphatidylinositol-3-kinase (PI3K) product PtdIns(3,4,5)P3, binding to cell surface molecule CD44 or cytoskeletal protein ankyrin, as well as by direct binding to activated Ras (Zheng 2001). Tiam1 activity may be also regulated by tyrosine phosphorylation (Servitja, Marinissen et al. 2003). Src-dependent phosphorylation of Tiam1 has been recently implicated in the Rac activation induced by another barrier-protective agonist, sphingosine 1-phosphate (Gonzalez, Kou et al. 2006). Thus, control of Tiam1 activity by PKC-, tyrosine kinase-, and PKA-dependent mechanisms may be a potential mechanism of OxPAPC-induced Rac regulation. Ongoing studies from our lab are addressing these important questions.
Upon oxidative fragmentation of sn-2 unsaturated fatty acid residues, OxPL may become agonists for the platelet-activating factor (PAF) receptor (Marathe, Davies et al. 1999). These “PAF-like lipids” are formed in vivo and induce many effects typical of PAF (Marathe, Prescott et al. 2001). In the case of fragmented acyl-oxidized phospholipids, however, many biological actions can not be explained solely by the ability to stimulate PAF receptors, because many effects of these acyl-oxidized phospholipids are not reproduced by PAF (Marathe, Davies et al. 1999, Leitinger, Watson et al. 1997; Subbanagounder, Leitinger et al. 1999; Subbanagounder, Watson et al. 2000). In this study we examined potential involvement of PAF receptor signaling in the barrier-protective effects of OxPAPC. Our results indicate that OxPAPC-induced barrier-protective responses were not mimicked by PAF, which did not decrease endothelial permeability at hormonal concentrations (0.1–1.0 μg/ml), but instead, induced dramatic barrier dysfunction and cytotoxic effects at non-physiologically high concentrations (20– 50 μg/ml), possibly by disrupting endothelial barrier in a nonspecific fashion or by acting as detergent. These effects of high PAF concentrations were not affected by PAF receptor antagonist A85783 (data not shown). However, PAF receptor antagonist partially attenuated barrier-protective effects of OxPAPC. These results suggest an inhibitory effect of A85783 on a putative OxPAPC receptor involved in EC barrier protective response, which is distinct from PAF receptor. Our results also do not exclude a possibility that several receptors may bind different components of OxPAPC and trigger different pathways of OxPAPC-mediated signal transduction (Leitinger, Tyner et al. 1999). For example, recent report (Cole, Subbanagounder et al. 2003) proposes a hypothetical G protein-coupled receptor involved in activation of CS-1 fibronectin production by OxPAPC. Other study indicates that epoxyisoprostane-containing products of PAPC oxidation may engage prostaglandin E2 (EP2) receptor and elevate intracellular cAMP levels (Li, Mouillesseaux et al. 2006), which may promote endothelial barrier function (Moy A. B. 1998; Birukova, Liu et al. 2004). However, EP2-mediated effects can not be blocked by PAF receptor antagonists suggesting additional A85783-sensitive receptors mediating barrier protective effects of OxPAPC. Importantly, putative OxPAPC receptor mediating barrier protective response appears to be specifically activated by epoxycyclopenthenone-containing products of PAPC oxidation such as PEIPC and PECPC, but not by fragmented OxPAPC derivates, such as lyso-phosphocholine, POVPC or PGPC, which exhibit barrier-disruptive properties (Birukov, Bochkov et al. 2004).
In summary, this study defined signaling pathways involved in OxPAPC-induced EC barrier protective response and described PKA, PKC and tyrosine kinases as upstream activators of Rac/Cdc42 pathway of OxPAPC-induced signaling in focal adhesions, cytoskeletal remodeling and barrier protection. Our results indicate that OxPAPC-induced barrier-protective responses are not mediated by PAF receptor. Based on the results of this study and previous works, we propose a hypothetical scheme of EC barrier regulation by OxPAPC (Figure 6). Interaction of oxygenated PAPC products such as PEIPC and PECPC with putative receptor stimulates protein tyrosine kinases, PKA and PKC, which activate Rac specific GEF(s) and promote GDP/GTP exchange in the nucleotide binding center of the Rac leading to its activation. Other effects of tyrosine kinase activation include phosphorylation of focal adhesion proteins and focal adhesion redistribution essential for anchoring of peripheral actin cytoskeleton and enhancement of EC barrier (Shikata, Birukov et al. 2003). Additional effects of PKC activation include stimulation of Erk-1,2 MAP kinase cascade (Birukov, Leitinger et al. 2004), which is however not involved in OxPAPC-mediated EC cytoskeletal remodeling and barrier protective responses (Figures 1–3). Activated Rac interacts with downstream cytoskeletal and cell adhesion effectors and promotes cytoskeletal remodeling and EC barrier enhancement. In addition, activation of Rac stimulates Src kinase and leads to phosphorylation of FAK and paxillin. These two signaling focal adhesion proteins are essential for initiation of focal adhesion remodeling observed in response to OxPAPC stimulation.
Figure 6. Proposed mechanism of OxPAPC-mediated activation of Rac and regulation of cytoskeletal rearrangement and barrier function.
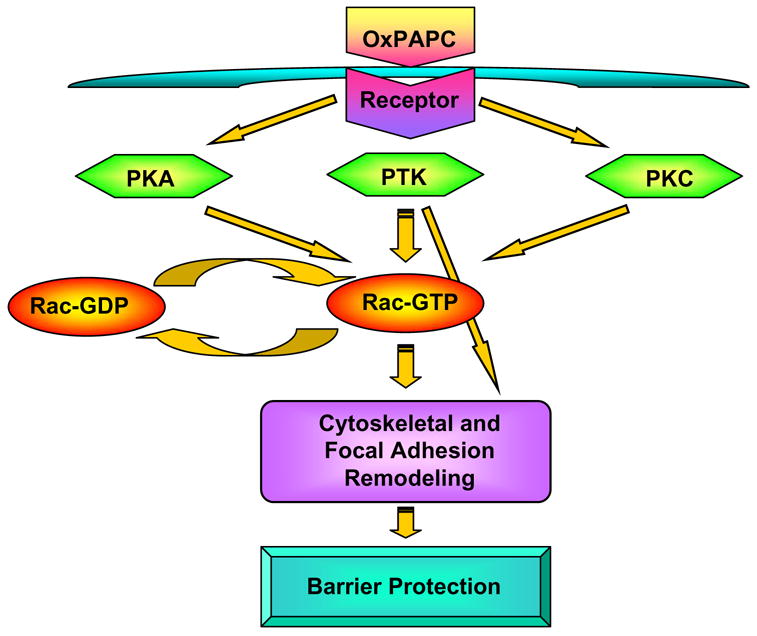
OxPAPC treatment stimulates PKA, PKC and protein tyrosine kinases. PKA, PKC and tyrosine kinases promote Rac activation via stimulation of Rac specific GEF(s). Activated Rac interacts with downstream cytoskeletal and cell adhesion effectors and promotes cytoskeletal remodeling and EC barrier enhancement. In addition, activation of Rac stimulates Src kinase and leads to phosphorylation of FAK and paxillin, which may further promote assembly of new focal adhesion protein complexes and attachment of newly formed actin filaments to the peripherally redistributed focal adhesions. Mechanisms of tyrosine kinase activation upstream of Rac may be also involved in OxPAPC-induced tyrosine phosphorylation of focal adhesion proteins These events result in formation of peripheral actin rim, focal adhesion remodeling and increased EC monolayer barrier properties.
Acknowledgments
This work was supported by grants from National Heart, Lung, and Blood Institutes (HL076259 and HL075349). AAB is a recipient of AHA Scientist Development Grant. The authors wish to thank Nurgul Moldobaeva for superb laboratory assistance.
Footnotes
Grants
This work was supported by NIH NHLBI grants HL076259 and HL075349 and National Scientist Developing Grant from American Heart Association for AAB
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Birukov KG. Oxidized lipids: the two faces of vascular inflammation. Curr Atheroscler Rep. 2006;8(3):223–31. doi: 10.1007/s11883-006-0077-x. [DOI] [PubMed] [Google Scholar]
- Birukov KG, Birukova AA, et al. Shear stress-mediated cytoskeletal remodeling and cortactin translocation in pulmonary endothelial cells. Am J Respir Cell Mol Biol. 2002;26(4):453–64. doi: 10.1165/ajrcmb.26.4.4725. [DOI] [PubMed] [Google Scholar]
- Birukov KG, V, Bochkov N, et al. Epoxycyclopentenone-containing oxidized phospholipids restore endothelial barrier function via Cdc42 and Rac. Circ Res. 2004;95(9):892–901. doi: 10.1161/01.RES.0000147310.18962.06. [DOI] [PubMed] [Google Scholar]
- Birukov KG, Leitinger N, et al. Signal transduction pathways activated in human pulmonary endothelial cells by OxPAPC, a bioactive component of oxidized lipoproteins. Microvasc Res. 2004;67(1):18–28. doi: 10.1016/j.mvr.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Adyshev D, et al. ALK5 and Smad4 are involved in TGF-beta1-induced pulmonary endothelial permeability. FEBS Lett. 2005;579(18):4031–7. doi: 10.1016/j.febslet.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Liu F, et al. Protein kinase A attenuates endothelial cell barrier dysfunction induced by microtubule disassembly. Am J Physiol Lung Cell Mol Physiol. 2004;287(1):L86–93. doi: 10.1152/ajplung.00441.2003. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Smurova K, et al. Role of Rho GTPases in thrombin-induced lung vascular endothelial cells barrier dysfunction. Microvasc Res. 2004;67(1):64–77. doi: 10.1016/j.mvr.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Bochkov VN, Leitinger N. Anti-inflammatory properties of lipid oxidation products. J Mol Med. 2003;81(10):613–26. doi: 10.1007/s00109-003-0467-2. [DOI] [PubMed] [Google Scholar]
- Bochkov VN, Leitinger N, et al. Role of oxidized phospholipids in acute lung injury. Curr Resp Med Rev. 2006;2(1):27–37. [Google Scholar]
- Bochkov VN, Mechtcheriakova D, et al. Oxidized phospholipids stimulate tissue factor expression in human endothelial cells via activation of ERK/EGR-1 and Ca(++)/NFAT. Blood. 2002;99(1):199–206. doi: 10.1182/blood.v99.1.199. [DOI] [PubMed] [Google Scholar]
- Chabot F, Mitchell JA, et al. Reactive oxygen species in acute lung injury. Eur Respir J. 1998;11(3):745–57. [PubMed] [Google Scholar]
- Cole AL, Subbanagounder G, et al. Oxidized phospholipid-induced endothelial cell/monocyte interaction is mediated by a cAMP-dependent R-Ras/PI3-kinase pathway. Arterioscler Thromb Vasc Biol. 2003;23(8):1384–90. doi: 10.1161/01.ATV.0000081215.45714.71. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol. 2001;91(4):1487–500. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Jacobson JR, et al. Pulmonary endothelial cell barrier enhancement by sphingosine 1-phosphate: roles for cortactin and myosin light chain kinase. J Biol Chem. 2004;279(23):24692–700. doi: 10.1074/jbc.M313969200. [DOI] [PubMed] [Google Scholar]
- Garcia JG, Liu F, et al. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest. 2001;108(5):689–701. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JG, Siflinger-Birnboim A, et al. Thrombin-induced increase in albumin permeability across the endothelium. J Cell Physiol. 1986;128(1):96–104. doi: 10.1002/jcp.1041280115. [DOI] [PubMed] [Google Scholar]
- Gonzalez E, Kou R, et al. Rac1 modulates sphingosine 1-phosphate-mediated activation of phosphoinositide 3-kinase/Akt signaling pathways in vascular endothelial cells. J Biol Chem. 2006;281(6):3210–6. doi: 10.1074/jbc.M510434200. [DOI] [PubMed] [Google Scholar]
- Holme PA, Solum NO, et al. Demonstration of platelet-derived microvesicles in blood from patients with activated coagulation and fibrinolysis using a filtration technique and western blotting. Thromb Haemost. 1994;72(5):666–71. [PubMed] [Google Scholar]
- Huang F, Subbaiah PV, et al. Lysophosphatidylcholine increases endothelial permeability: role of PKCalpha and RhoA cross talk. Am J Physiol Lung Cell Mol Physiol. 2005;289(2):L176–85. doi: 10.1152/ajplung.00003.2005. [DOI] [PubMed] [Google Scholar]
- Huber J, Vales A, et al. Oxidized membrane vesicles and blebs from apoptotic cells contain biologically active oxidized phospholipids that induce monocyte-endothelial interactions. Arterioscler Thromb Vasc Biol. 2002;22(1):101–7. doi: 10.1161/hq0102.101525. [DOI] [PubMed] [Google Scholar]
- Kadl A, Huber J, et al. Analysis of inflammatory gene induction by oxidized phospholipids in vivo by quantitative real-time RT-PCR in comparison with effects of LPS. Vascul Pharmacol. 2002;38(4):219–27. doi: 10.1016/s1537-1891(02)00172-6. [DOI] [PubMed] [Google Scholar]
- Kolosova IA, Mirzapoiazova T, et al. Signaling pathways involved in adenosine triphosphate-induced endothelial cell barrier enhancement. Circ Res. 2005;97(2):115–24. doi: 10.1161/01.RES.0000175561.55761.69. [DOI] [PubMed] [Google Scholar]
- Kouklis P, Konstantoulaki M, et al. Cdc42 regulates the restoration of endothelial barrier function. Circ Res. 2004;94(2):159–66. doi: 10.1161/01.RES.0000110418.38500.31. [DOI] [PubMed] [Google Scholar]
- Kronke G, V, Bochkov N, et al. Oxidized phospholipids induce expression of human heme oxygenase-1 involving activation of cAMP-responsive element-binding protein. J Biol Chem. 2003;278(51):51006–14. doi: 10.1074/jbc.M304103200. [DOI] [PubMed] [Google Scholar]
- Lee OH, Lee DJ, et al. Sphingosine 1-phosphate stimulates tyrosine phosphorylation of focal adhesion kinase and chemotactic motility of endothelial cells via the G(i) protein-linked phospholipase C pathway. Biochem Biophys Res Commun. 2000;268(1):47–53. doi: 10.1006/bbrc.2000.2087. [DOI] [PubMed] [Google Scholar]
- Leitinger N, Tyner TR, et al. Structurally similar oxidized phospholipids differentially regulate endothelial binding of monocytes and neutrophils. Proc Natl Acad Sci U S A. 1999;96(21):12010–5. doi: 10.1073/pnas.96.21.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitinger N, Watson AD, et al. Monocyte binding to endothelial cells induced by oxidized phospholipids present in minimally oxidized low density lipoprotein is inhibited by a platelet activating factor receptor antagonist. Adv Exp Med Biol. 1997;433:379–82. doi: 10.1007/978-1-4899-1810-9_82. [DOI] [PubMed] [Google Scholar]
- Li R, Mouillesseaux KP, et al. Identification of prostaglandin E2 receptor subtype 2 as a receptor activated by OxPAPC. Circ Res. 2006;98(5):642–50. doi: 10.1161/01.RES.0000207394.39249.fc. [DOI] [PubMed] [Google Scholar]
- Liu F, Schaphorst KL, et al. Hepatocyte growth factor enhances endothelial cell barrier function and cortical cytoskeletal rearrangement: potential role of glycogen synthase kinase-3beta. Faseb J. 2002;16(9):950–62. doi: 10.1096/fj.01-0870com. [DOI] [PubMed] [Google Scholar]
- Lum H, Malik AB. Mechanisms of increased endothelial permeability. Can J Physiol Pharmacol. 1996;74(7):787–800. doi: 10.1139/y96-081. [DOI] [PubMed] [Google Scholar]
- Mallat Z, Benamer H, et al. Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation. 2000;101(8):841–3. doi: 10.1161/01.cir.101.8.841. [DOI] [PubMed] [Google Scholar]
- Marathe GK, Davies SS, et al. Inflammatory platelet-activating factor-like phospholipids in oxidized low density lipoproteins are fragmented alkyl phosphatidylcholines. J Biol Chem. 1999;274(40):28395–404. doi: 10.1074/jbc.274.40.28395. [DOI] [PubMed] [Google Scholar]
- Marathe GK, Prescott SM, et al. Oxidized LDL contains inflammatory PAF-like phospholipids. Trends Cardiovasc Med. 2001;11(3–4):139–42. doi: 10.1016/s1050-1738(01)00100-1. [DOI] [PubMed] [Google Scholar]
- Mehta D, Konstantoulaki M, et al. Sphingosine 1-phosphate-induced mobilization of intracellular Ca2+ mediates rac activation and adherens junction assembly in endothelial cells. J Biol Chem. 2005;280(17):17320–8. doi: 10.1074/jbc.M411674200. [DOI] [PubMed] [Google Scholar]
- Mehta D, Tiruppathi C, et al. Modulatory role of focal adhesion kinase in regulating human pulmonary arterial endothelial barrier function. J Physiol. 2002;539(Pt 3):779–89. doi: 10.1113/jphysiol.2001.013289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy ABBJE, Blackwell K, Shasby SS, Shasby DM. cAMP protects endothelial barrier function independent of inhibiting MLC20-dependent tension development. american journal of physiology. 1998;274(18):L1024–L1029. doi: 10.1152/ajplung.1998.274.6.L1024. [DOI] [PubMed] [Google Scholar]
- Moy AB, Blackwell K, et al. Differential effects of histamine and thrombin on endothelial barrier function through actin-myosin tension. Am J Physiol Heart Circ Physiol. 2002;282(1):H21–9. doi: 10.1152/ajpheart.2002.282.1.H21. [DOI] [PubMed] [Google Scholar]
- Petrache I, Verin AD, et al. Differential effect of MLC kinase in TNF-alpha-induced endothelial cell apoptosis and barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2001;280(6):L1168–78. doi: 10.1152/ajplung.2001.280.6.L1168. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Chang TS, et al. Cellular regulation by hydrogen peroxide. J Am Soc Nephrol. 2003;14(8 Suppl 3):S211–5. doi: 10.1097/01.asn.0000077404.45564.7e. [DOI] [PubMed] [Google Scholar]
- Servitja JM, Marinissen MJ, et al. Rac1 function is required for Src-induced transformation. Evidence of a role for Tiam1 and Vav2 in Rac activation by Src. J Biol Chem. 2003;278(36):34339–46. doi: 10.1074/jbc.M302960200. [DOI] [PubMed] [Google Scholar]
- Shikata Y, Birukov KG, et al. Involvement of site-specific FAK phosphorylation in sphingosine-1 phosphate- and thrombin-induced focal adhesion remodeling: role of Src and GIT. FASEB J. 2003;17(15):2240–9. doi: 10.1096/fj.03-0198com. [DOI] [PubMed] [Google Scholar]
- Shikata Y, Rios A, et al. Differential effects of shear stress and cyclic stretch on focal adhesion remodeling, site-specific FAK phosphorylation, and small GTPases in human lung endothelial cells. Exp Cell Res. 2005;304(1):40–9. doi: 10.1016/j.yexcr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Singleton PA, Dudek SM, et al. Regulation of sphingosine 1-phosphate-induced endothelial cytoskeletal rearrangement and barrier enhancement by S1P1 receptor, PI3 kinase, Tiam1/Rac1, and alpha-actinin. Faseb J. 2005;19(12):1646–56. doi: 10.1096/fj.05-3928com. [DOI] [PubMed] [Google Scholar]
- Subbanagounder G, Leitinger N, et al. Evidence that phospholipid oxidation products and/or platelet-activating factor play an important role in early atherogenesis : in vitro and In vivo inhibition by WEB 2086. Circ Res. 1999;85(4):311–8. doi: 10.1161/01.res.85.4.311. [DOI] [PubMed] [Google Scholar]
- Subbanagounder G, Watson AD, et al. Bioactive products of phospholipid oxidation: isolation, identification, measurement and activities. Free Radic Biol Med. 2000;28(12):1751–61. doi: 10.1016/s0891-5849(00)00233-1. [DOI] [PubMed] [Google Scholar]
- Tiruppathi C, Malik AB, et al. Electrical method for detection of endothelial cell shape change in real time: assessment of endothelial barrier function. Proc Natl Acad Sci U S A. 1992;89(17):7919–23. doi: 10.1073/pnas.89.17.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nieuw Amerongen GP, Draijer R. Transient and prolonged increase in endothelial permeability induced by histamine and thrombin: role of protein kinases, calcium, and RhoA. Circ Res. 1998;83(11):1115–23. doi: 10.1161/01.res.83.11.1115. [DOI] [PubMed] [Google Scholar]
- van Nieuw Amerongen GP, Natarajan K, et al. GIT1 mediates thrombin signaling in endothelial cells: role in turnover of RhoA-type focal adhesions. Circ Res. 2004;94(8):1041–9. doi: 10.1161/01.RES.0000125627.77235.0C. [DOI] [PubMed] [Google Scholar]
- Vouret-Craviari V, Bourcier C, et al. Distinct signals via Rho GTPases and Src drive shape changes by thrombin and sphingosine-1-phosphate in endothelial cells. J Cell Sci. 2002;115(Pt 12):2475–84. doi: 10.1242/jcs.115.12.2475. [DOI] [PubMed] [Google Scholar]
- Watson AD, Leitinger N, et al. Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. J Biol Chem. 1997;272(21):13597–607. doi: 10.1074/jbc.272.21.13597. [DOI] [PubMed] [Google Scholar]
- Wood LG, Gibson PG, et al. Biomarkers of lipid peroxidation, airway inflammation and asthma. Eur Respir J. 2003;21(1):177–86. doi: 10.1183/09031936.03.00017003a. [DOI] [PubMed] [Google Scholar]
- Yeh M, Gharavi NM, et al. Oxidized phospholipids increase interleukin 8 (IL-8) synthesis by activation of the c-src/signal transducers and activators of transcription (STAT)3 pathway. J Biol Chem. 2004;279(29):30175–81. doi: 10.1074/jbc.M312198200. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Magnusson MK, et al. Lysophosphatidic acid and microtubule-destabilizing agents stimulate fibronectin matrix assembly through Rho-dependent actin stress fiber formation and cell contraction. Mol Biol Cell. 1997;8(8):1415–25. doi: 10.1091/mbc.8.8.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y. Dbl family guanine nucleotide exchange factors. Trends Biochem Sci. 2001;26(12):724–32. doi: 10.1016/s0968-0004(01)01973-9. [DOI] [PubMed] [Google Scholar]


