Abstract
Recombination contributes significantly to diversity within virus populations and ultimately to viral evolution. Here we use a recently developed statistical test to perform exploratory analysis of recombination in fourteen feline immunodeficiency virus (FIVpco) genomes derived from a wild population of cougars. We use both the global and local Phi statistical test as an overall guide to predict where recombination may have occurred. Further analyses, including similarity plots and phylogenetic incongruence tests, confirmed that three FIVpco lineages were derived from recombinant events. Interestingly, the regions of mosaic origin were clustered in the area encoding lentiviral accessory genes and largely spared the viral structural genes. Because some of the mosaic strains are currently geographically disparate, our data indicate that the dispersal of cougars infected with these strains was preceded by recombination events. These results suggest that recombination has played an important role in the evolution of FIVpco for this wild population of cougars.
Introduction
Recombination creates new genotypes by combining genetic material from distinct lineages and is a principal mechanism to enhance population genetic diversity (Negroni and Buc, 2001; Worobey and Holmes, 1999). A major force in viral evolution, recombination can also affect viral pathogenicity and confound therapeutic strategies (Awadalla, 2003; Liu et al., 2002; Peeters et al., 1999; Posada, 2002; Zetterberg et al., 2004). Indeed, the astounding diversity in viral populations is in part a result of extensive recombination in the viral world (Apetrei, Robertson, and Marx, 2004; Bachmann et al., 1997; Hamilton, 2006).
It is important to identify recombination in viral sequences because it complicates phylogenetic analysis (Posada, Crandall, and Holmes, 2002). Recombination creates signals consistent with exponential growth and may cause the time to the most recent common ancestor to be biased (Schierup and Hein, 2000). Because of the confounding effects of substitution rate heterogeneity, rigorous statistical analysis must be used to separate recombination from other processes (Anderson et al., 2000). Thus, an important problem in viral evolutionary analysis is to detect whether recombination has occurred (Awadalla, 2003; Posada, 2002) and a number of approaches have been developed to detect and analyze recombinant sequences (Posada, 2002; Posada and Crandall, 2001). If strains originate from a single population (in an infected host, for instance) population genetic approaches for inferring the rate and presence of recombination are appropriate (Bruen, Philippe, and Bryant, 2006). A number of approaches based on phylogenetic principles and summary statistics are applicable to analyze divergent strains. Phylogenetic approaches such as bootscanning (Salminen et al., 1995) are popular for recombinant analysis, but compared to other methods they have low sensitivity and can produce an excess of false positives (Posada, 2002; Posada and Crandall, 2001). Moreover recently it has been shown that summary statistics such as Max Chi Squared (Smith, 1992), although hitherto considered the most powerful methods (Posada, 2002), may produce an excess of false positives in certain cases and fail to detect recombination in other circumstances (Bruen, Philippe, and Bryant, 2006).
A statistical approach called the Phi statistic has been recently developed to analyze recombinant sequences (Bruen, Philippe, and Bryant, 2006). The Phi test accurately determines whether recombination has occurred or not a in a given sequence alignment. This method is very sensitive, does not produce false positives in excess, and can be applied to divergent or closely related strains (Bruen, Philippe, and Bryant, 2006). These characteristics make it ideal to distinguish between recurrent mutation and recombination in viral genomes. However, the Phi test as proposed in previous work simply determines whether or not recombination has occurred within a set of sequences (Bruen, Philippe, and Bryant, 2006). The original Phi test can thus be thought of as a global test for recombination and should be used as a first step in analyzing sequences.
In this report, we extend the use of the Phi statistic to test smaller regions for recombination resulting in local tests for recombination. Testing local regions for recombination accomplishes two goals. Firstly, regions that contain a recombinant signal (potential breakpoints) can be easily identified. Secondly, the region between suspected breakpoints can be subjected to phylogenetic analysis to determine whether the genome is derived from different ancestral sequences. Additional data analysis can then be done efficiently using techniques such as similarity plots. There is a tradeoff however for the gain in knowledge of where recombination may occur because testing smaller regions for recombination decreases sensitivity (Wiuf, Christensen, and Hein, 2001). Further, multiple test correction must be applied making the procedure much more conservative. This could potentially result in a situation where there is global evidence for recombination but it is difficult to pinpoint the exact location of the recombinant signal.
Here we apply the Phi test and other approaches to study recombination in feline immunodeficiency virus (FIVpco) derived from naturally infected cougars (Puma concolor). FIV was first described in domestic cats (Pedersen et al., 1987) and has subsequently been found in a wide variety of wild felines (Brown et al., 1994; Olmsted et al., 1992). Whereas FIV infection of domestic cats can lead to immune dysfunction as well as neurological disease (Burkhard and Dean, 2003), there is no evidence of disease in wild felines (Biek et al., 2006a; Biek et al., 2006b) possibly due to a lengthy coadaptation of virus and host (Biek et al., 2003; Carpenter and O′Brien, 1995). There is evidence that recombinant subtypes circulate globally in FIV-infected domestic cats (Bachmann et al., 1997; Reggeti and Bienzle, 2004) although these data are based on topological incongruity of gene fragments derived from an infected animal and not genomic comparisons. In FIVpco however, phylogenies based on gene fragments from different portions of the viral genome gave concordant trees, and thus there was no evidence for recombination among the genetically diverse sequences (Biek, Drummond, and Poss, 2006; Biek et al., 2003). Cougar populations in North America were extirpated principally due to bounty hunting throughout the late 1800’s and early 1900’s. Populations are currently expanding in the Rocky Mountain States subsequent to changes in hunting regulations (Biek, Drummond, and Poss, 2006). In this study, we report on the analysis of the entire viral genomes of fourteen FIVpco isolates to determine the role of recombination in the viral population during expansion of the host population.
Results
Identification of putative recombinant regions using the Phi statistic
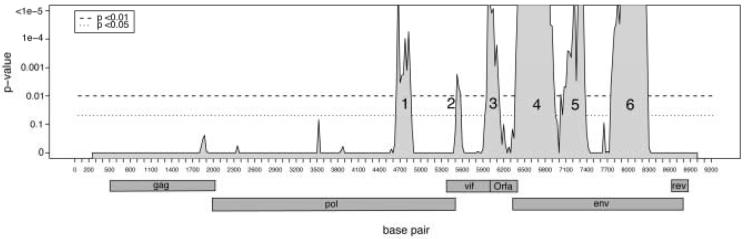
Initially all positions of the fourteen viral genomes were tested for recombination using the Phi statistic (Bruen, Philippe, and Bryant, 2006). Overwhelming evidence of recombination within the entire alignment was found (p < 10-20). Individual local regions were then tested for recombination using the Phi statistic with a window size of 500 base pairs. This analysis clearly demarcated six distinct smaller regions exhibiting statistically significant evidence of recombination within the fourteen FIVpco genomes (Fig 1). The strongest local evidence of recombination occurs in the second half of the genomes after base 4500.
Figure 1.
Statistically significant regions (peaks that contain at least one breakpoint) containing a recombinant signal of fourteen FIVpco genomes determined by Phi statistic. The window size was 500 bp and step size was 25 bp. p values are multiple test corrected using Hommel’s modified Bonferroni procedure (Hommel, 1988). Numbers on the x-axis indicate nucleotide position in the genome alignment. The position of open reading frames is indicated beneath the figure. The first exon of rev encompasses approximately the first 200 bp of the env ORF.
Because there was no evidence for recombination in the 5’ portion of the viral genome, a maximum likelihood tree was constructed based on sites 1-4500 (Fig 2). This tree represents an estimate of the actual phylogeny of the FIVpco sequences and can be used as a ‘reference’ tree. Sequences in putative recombinant regions that are misplaced with respect to this topology can be identified as tentatively recombinant. There are two sister groups in the tree. Sequences JM01/YM137, JF6 and YF125 form a monophyletic clade with the lineage SR631/SR631B placed as a strong out-group to this clade. Similarly, CoLV, Mc100, Mc121, Mc350 and Gc34 are monophyletic and sequences YM29 and YF16 are distantly related to this group. The divergence within and between the two sister groups ranges from 5-13% and 16-17%, respectively. PLV is 18-20% divergent from all other sequences.
Figure 2.
Maximum likelihood tree inferred for the first 4500 bp (before the first recombinant region or ‘peak’ in Figure 1) for fourteen FIVpco sequences. A substitution model of GTR+Γ was selected using ModelTest (Posada and Crandall, 1998) (with Γ equal to 0.26) and was used to infer the tree. The location of each infected cougar is listed next to the isolate name and is referenced to a schematic map of the area.
Exploratory analysis of recombinant regions identified by Phi statistic
To identify which sequences are recombinant, seven different neighbor-joining trees (Saitou and Nei, 1987) were built based on regions in-between the six statistically significant recombinant regions (Fig 3a-g). Only major lineages are shown for simplicity. CoLV represents the cluster of sequences Mc100, Mc121, Mc350 and Gc34, which always grouped together although the relationship within the cluster changed (results not shown). Closely related sequences SR631/SR631b are represented by SR631 and YM29 represents the YM29/YF16 group. Note that YM29 and CoLV serve as ‘anchor’ sequences on one branch, and YF125 and PLV serve as anchor’ sequences on the other branch. The putative recombinants change position relative to these sequences.
Figure 3.
Exploratory neighbor-joining trees corresponding to the regions in between ‘peaks’ of Figure 1. Only major lineages are shown for readability. Because the width of the peaks differs in length, the midpoint of each peak was chosen to delineate the region. Lineages that have boxes around them (JM01, SR631 and JF6) appear to be recombinant. The trees suggest an initial estimate of which lineages are recombinant.
Both the first and last trees display a history consistent with the reference tree (compare Fig 3a and 3g to Fig 2). The tree representing peaks 1 and 2 (Fig 3b) groups JM01 with SR631, in contrast to the reference tree that groups JM01 with JF6 and YF125, which indicates that either JM01 or SR631 derives from a recombinant event. Because the position of JM01 relative to the ‘anchor’ sequences YF125 and PLV also changes, the second tree suggests that at least JM01 is derived from a recombinant event.
Comparison of the second tree to the third tree (Fig 3b and 3c) provides preliminary evidence that peak 2-3 in both JM01 and SR631 is derived from a recombinant event with the ancestor to YM29. This suggests that SR631 is also a candidate recombinant sequence. In the fourth tree (Figure 3d), the affiliation of JF6 changes from YF125 to YM29 suggesting that JF6 is also a recombinant sequence in the region of peaks 3-4. The close relationship of JF6 with YM29 is also seen in the region between peaks 5-6 (Fig 3g). However, this exploratory analysis does not precisely identify where the recombination has occurred, partly because the regions containing a breakpoint are quite large and there is the possibility of overlapping recombinant events. Nonetheless, the exploratory analysis suggests that sequences JM01, SR631 and JF6 demand further study.
Fine-scale recombinant analysis and exact breakpoint identification
Similarity plots can be used to refine the hypothesis of recombinant origin for a particular sequence by suggesting where recombination may have occurred (Anderson et al., 2000). Because the exploratory analysis suggested that JF6, SR631, and JM01 were derived from recombinant events, similarity plots (Lole et al., 1999) were constructed using these sequences (Fig 4). JF6 is a putative recombinant sequence with the ancestral sequence to YM29 as one parent (Fig 3d and 3f). However, the other parent, the ancestral sequence to JM01 (Fig 3a, 3e, and 3g) appears to be itself a recombinant sequence (Fig 3b). Instead of JM01, an ‘anchor’ lineage, YF125 was chosen as a non-recombinant lineage similar to JF6 (Fig 3a -3c). The similarity plot of JF6, YM29 and YF125 (Fig 4a) suggests that JF6 has a mosaic origin, with the majority of the sequence similar to YF125 but with two distinct regions of the sequence appearing to originate from an ancestral sequence closely related to YM29. A maximum likelihood procedure in program LARD (Holmes, Worobey, and Rambaut, 1999) was used to identify the exact breakpoints (Fig 4a, dashed lines). Note that the breakpoints in Figure 4a fall into peaks of recombinant activity 3, 5 and 6 identified by the Phi statistic (Fig 1).
Figure 4.
Similarity plots of suspected recombinant and parental sequences. All sequences are compared to putative parental sequence YF125 and YM29 except in b. a) JF6, b) JM01 with SR631 and YF125, c) SR631, and d) JM01. Window size was 200 bp and step size was 20 bp. The dashed lines correspond to maximum likelihood estimate of recombinant points. Note their correspondence to the areas of significant recombination (shaded regions) in Figure 1.
JM01 was also identified as a recombinant sequence with one parent as a sequence closely related to SR631 (Fig3b-d). Because SR631 is also a putative recombinant (Fig 3c and 3e), the ‘anchor’ sequence YF125 was chosen for similarity plot comparison. The similarity plot (Fig 4b) indicates that one large region of JM01 is much more similar to SR631 than to YF125, which contradicts the reference tree in Figure 2. The maximum likelihood breakpoints found using LARD (Holmes, Worobey, and Rambaut, 1999) correspond to regions of recombinant activity peaks 1 through 4 identified by the Phi statistic (Fig 1).
The trees in Figures 3c and 3d indicate that the history of JM01 with respect to YM29 is complex. The similarity plot of JM01, YM29, and YF125 (Fig 4d) suggests a region that closely groups JM01 and YM29, again contradicting the reference tree in Figure 2. Interestingly, this region is fully contained within the JM01 and SR631 putative recombinant region (Fig 4b and 4d). The breakpoints as identified with LARD (Holmes, Worobey, and Rambaut, 1999) are shown with dashed lines and correspond to peaks 2 through 4 identified with the Phi statistic (Fig 1).
Finally, exploratory analysis indicated that SR631 was a putative recombinant sequence related to YM29 (Fig 3c and 3d). However, in the reference tree, SR631 appears to be closely related to YF125 (Fig 2, 3a, and 3g). The similarity plot of these three sequences shows that there is a region in SR631 that has higher sequence similarity to YM29 than to YF125 (Fig 4c). Interestingly, this region corresponds to the region in JM01 that is similar to YM29 suggesting that SR631 and JM01 have a shared history for this recombination event.
Data partitions
The previous analyses support the recombinant histories of JF6, JM01, and SR631 and identified the locations of recombination events. However, phylogenetic histories cannot be determined from similarity plots (Anderson et al., 2000). In order to confirm that JF6, JM01, and SR631 are recombinant sequences, topological incongruence tests must be performed showing that different regions of the sequence have different ancestral origins.
Five different partitions of the alignment were identified based upon the breakpoint identification discussed above. Closely related sequences, which were excluded from earlier analyses, were included in the partition analysis. To allow thorough evaluation of JF6, which has a unique recombinant history, two of the partitions (Partition_A and Partition_B) only involved JF6 (Table 1). Partition_A consists of all the regions where JF6 group closely with YF125 as shown in the reference tree (Fig 2) and similarity plot (Fig 4a). Likewise, Partition_B consists of both regions where JF6 groups with YM29 (Fig 3d, 3f, 4a). Partition_C consists of the regions where JM01, SR631, and YF125 have a relationship consistent with the reference tree (Fig 2 and Fig 4b, c). Partition_D consists of the regions where JM01, SR631, and YF125 appear to be closely related, whereas Partition_E consists of the regions where JM01, SR631, and YM29 appear to be closely related (Fig 4b-d).
Table 1.
Parameters for sequence partitions.
| Name of partition | Recombinant sequence(s) | Locationa b | Recombinationc | Substitution modeld | αd | Pinvd |
|---|---|---|---|---|---|---|
| Partition_A | JF6 | 1-5996, | 1.6 × 10-4* | GTR+G+I | 2.7 | 0.50 |
| 6273-7237, | ||||||
| 8120-9270 | ||||||
| Partition_B JF6 | 5997-6273 | 2.0 × 10-5* | GTR+I | 0.52 | ||
| 7238-8119 | ||||||
| Partition_C | JM01/YM137 | 1-4700 | 3.0 × 10-7* | GTR+G+I | 2.1 | 0.48 |
| SR631/SR631B | 6858-9270 | |||||
| Partition_D | JM01/YM137 | 4701-5452 | 0.17 | GTR+G | 0.3 | |
| SR631/SR631B | 6462-6857 | |||||
| Partition_E | JM01/YM137 | 5453-6461 | 1.9 × 10-5* | GTR+G+I | 2.9 | 0.46 |
| SR631/SR631B |
Breakpoints estimated by maximum likelihood using LARD (Holmes, Worobey, and Rambaut, 1999).
Distinct regions are concatenated together within each partition.
p-values for recombination within each partition found with the Phi statistic (Bruen, Philippe, and Bryant, 2006). Significant p values are indicted with *.
Estimated using ModelTest based on AIC. GTR; general time reversible. G; gamma distribution. I; invariable sites.
p < .05
Each partition was tested for recombination using the Phi statistic. The Phi statistic indicated that each of the partitions except Partition_D still contained a signal for recombination (Table 2). This suggests that there is evidence for recombination within each of the partitions.
Table 2.
Comparison of phylogenetic support for different partitions.
| Name of partition | Recombinant sequence(s) | Possible treesa | -Log likelihoodb | p-valuesc |
|---|---|---|---|---|
| Partition_A | JF6 | Partition_A | 27504 | |
| Partition_B | 28385 | 0.000 | ||
| Partition_B | JF6 | Partition_B | 3942 | |
| Partition_A | 3970 | 0.012 | ||
| Partition_C | JM01/YM137, | Partition_C | 26915 | |
| SR631/SR631B | Partition_D | 27062 | 0.002 | |
| Partition_E | 27935 | 0.000 | ||
| Partition_D | JM01/YM137, | Partition_D | 4567 | |
| SR631/SR631B | Partition_C | 4618 | 0.007 | |
| Partition_E | 4633 | 0.001 | ||
| Partition_E | JM01/YM137, | Partition_E | 4041 | |
| SR631/SR631B | Partition_C | 4079 | 0.000 | |
| Partition_D | 4076 | 0.000 |
Phylogenetic incongruence - confirmation of mosaic sequences
Five different maximum likelihood phylogenetic trees were built using Partition_A to Partition_E, with the substitution model of best fit (Table 1). The maximum likelihood trees of Partition_A and Partition_B differ in their placement of JF6 (Fig 5a and 5b). A Shimodaira-Hasegawa (SH) test (Shimodaira and Hasegawa, 1999) confirmed that JF6 is closely related to YF125 in Partition_A and to YM29 in Partition_B. Thus, JF6 has two statistically significant histories based on different regions of the sequence (p-value 0.01, Table 2).
Figure 5.
Maximum likelihood trees inferred for a) Partition_A and b) Partition_B. The trees differ in their placement of recombinant sequence JF6. Partitions (including nucleotide substitution models) are described in Table 1. Recombinant viruses are boxed.
Partition_C supports the reference topology, which places JM01/YM137 with YF125 as a monophyletic clade to the exclusion of SR631/SR631B (Fig 2 and Fig 6a). Conversely, Partition_D and Partition_E support JM01/YM137 forming a monophyletic clade with SR631/SR631B (Fig 6b and 6c). Furthermore, Partition_E also supports the monophyletic grouping of YM29/YF16 with the JM01/YM137/SR631/SR631B monophyletic clade (Fig 6c). The SH shows that the tree found for Partition_C provides a significantly better fit to the data in Partition_C than both the alternative topologies found for Partition_D and Partition_E (p-value < 0.01, Table 2). Likewise, the SH test shows that this is also true for the trees in Partition_D and Partition_E (p-value < 0.01, Table 2). This demonstrates that both the JM01 /YM137 and SR631/SR631B lineages have multiple ancestry.
Figure 6.
Maximum likelihood trees (drawn to scale) for partition a) Partition_C, b) Partition_D and c) Partition_E with recombinant sequences JM01/YM137 and SR631/SR631B. Partitions (including nucleotide substitution models) described in Table 1. Recombinant viruses are boxed.
Discussion
In this paper we used the Phi statistic to determine if recombination was present in full-length FIVpco genomes derived from cougars in the northern Rocky Mountain ecosystem. We then extended the use of the Phi test to localize the regions containing a recombinant signal. From a methodological standpoint, testing local regions for recombination provides a statistically coherent step to ascertain recombinant regions. Traditional visual methods, similarity plots, which are applicable to only three sequences at a time, were then used to estimate the locations of recombination breakpoints (Holmes, Worobey, and Rambaut, 1999). The regions between breakpoints were evaluated for phylogenetic discordance to confirm that they had unique phylogenetic histories.
Three lineages in the FIVpco data set (JF6, JM01/YM137, and SR631/SR631B) were derived from recombinant events. JF6 is a mosaic sequence with most of the 5’ portion of the sequence originating from a sequence ancestral to YF125. However, two small portions of the JF6 sequence originated from a sequence ancestral to YM29/YF16. One of these regions encompasses the majority of the open reading frame for the putative transactivator protein, OrfA, (position 6009-6353) and the other generates a mosaic env gene.
The JM01/YM137 lineage also has a recombinant history. In the reference tree, JM01/YM137 is monophyletic with YF125 but this cluster does not contain SR631/SR631B. However, portions of the JM01/YM137 genomes are monophyletic with SR631/SR631B and do not include YF125. This suggests that the JM01/YM137 lineage derives from a sequence ancestral to YF125 and from a sequence ancestral to SR631/SR631B. However, the history of the JM01/YM137 lineage is more complex than that of JF6 because in Partition_E, JM01/YM137 and SR631/SR631B sequences form a monophyletic clade with YM29/YF16 whereas in Partition_C and _D, they cluster with YF125. Similarity plots show the same phenomenon; that both strains JM01 and SR631 appear at one point to share much more genetic similarity with YM29 than YF125. The regions of conversion of both JM01 and SR631 are identical suggesting that the recombination events are not independent. Thus the JM01 lineage appears to be derived from two historical recombinant events. The first event created a recombinant ancestor of SR631/SR631B that derived the accessory gene portion of the genome (position 5304-6350) from a sequence ancestral to the YM29/YF16 lineage. The strain ancestral to JM01/YM137 then acquired a portion of this recombinant ancestor spanning approximately from the integrase-encoding portion of pol through the end of rev through an additional recombinant event. Thus the region between the reverse transcriptase encoding portion of pol and the beginning of env, which encodes accessory genes unique to the lentiviruses, has undergone multiple recombinant events in the past, suggesting that susceptibility to recombination in this region may be high.
Expression of lentivirus accessory genes requires splicing or internal ribosome entry sites, both of which are defined by specific secondary structure in the RNA. These stable RNA structures may facilitate recombination by decreasing processivity of the viral reverse transcriptase (DeStefano et al., 1992). The outcome of most strand switches is not detected in natural infection because many recombinant progeny will be replication incompetent. However, the number of recombinant HIV-1 strains currently circulating globally indicates that recombination can lead to highly fit viruses. Among the circulating HIV-1 recombinants there is evidence for sequential recombination events occurring among primary recombinant strains, which is similar to our findings for strains JM01 and SR631. Additionally, four of the circulating recombinant HIV-1 strains share complete gag and pol with one parental strain but carry accessory genes from a different strain.
It is interesting to note that the ancestral sequences to YM29/YF16 were involved in all recombination events that led to the mosaic strains found in JF6, JM01/YM137, and SR631/SR631B, making these important forbearers to the viruses circulating today. This lineage was prevalent during the early sampling period of Greater Yellowstone Ecosystem (GYE) cougars (1990-1994) but only one representative of this strain was identified in the area in subsequent sampling (1999-2004) (Biek, Drummond, and Poss, 2006). In each case, the recombinant progeny have maintained the accessory gene OrfA or both vif and OrfA of the YM29/YF16 lineage. In addition, the SR631/SR631B lineage was obtained from cougars from the Snowy Mountains of Wyoming, (Biek et al., 2003) a region that is separated from the GYE by over 600 kM of inhospitable cougar habitat (Fig 2). The fact that this lineage derived ultimately from a recombinant event with FIVpco lineages from GYE suggests that cougars infected with the ancestral viruses must have been in geographical proximity in the recent past.
FIVpco from free-ranging cougars has provided a rich source material for which to investigate questions related to host population biology (Biek, Drummond, and Poss, 2006) and lentiviruses molecular genetics in natural populations (Biek et al., 2003). In this study, we apply both traditional and novel analytical methods to detect and localize recombination in full-length viral genomes. This expands our understanding of viral molecular evolution and demonstrates that recombination has contributed to the evolution of feline lentiviruses in a naturally expanding population of cougars.
Materials and Methods
Data Set
The primers used to sequence each isolate and source of the tissue from which the isolate was derived is described in supplementary material Tables 1 and 2. The alignment of the fourteen FIVpco genomes sequences (EF455603-EF455615) derived from northern Rocky Mountain cougars (GC34, MC100, MC121, MC350, YM29, YF16, JM01, YM137, JF6, YF125, SR631/SR631B, and CoLV) was done using Clustal W with manual adjustment. The PLV sequence (DQ192583) used as an out-group was collected from Vancouver Island in 1995 and has been previously described (Poss et al., 2006).
Exploratory Recombination Analysis with Phi statistic
Analysis of the recombinant events that shaped all fourteen FIVpco genomes proceeded as follows. Firstly, the entire alignment was tested for recombination using the Phi statistic (Bruen, Philippe, and Bryant, 2006). Regions containing a recombinant signal (at least one breakpoint) were identified as follows. Local regions of 500 base pairs (with 25 base pairs between each region) were tested for recombination with the Phi statistic, using the Profile program (Bruen, Philippe, and Bryant, 2006). To ensure significance in the presence of multiple tests, Hommel’s (Hommel, 1988) modified Bonferroni procedure was applied to the p-values. A maximum likelihood tree based on the first 4500 bp of all sequences was constructed in PAUP* (Felsenstein, 1981; Swofford, 2001) using the best fitting substitution model found with ModelTest (Posada and Crandall, 1998). Exploratory neighbor-joining trees were built for each of the regions that did not contain a recombinant signal using PAUP* to identify recombinant lineages (Saitou and Nei, 1987; Swofford, 2001). Trees were generated using a heuristic search with tree-bisections and reconnections and were drawn using the program TREEVIEW (Page, 1996).
Fine-scale recombination analysis
Diversity plots were created between the putative recombinant and its parent sequences using the program Simplot (Lole et al., 1999) in order to explore the recombination history. Simplot was run using a window-size of 200 base pairs and a step size of 20 base pairs. Breakpoints were identified using the putative recombinant and both parents under a maximum likelihood framework in the program LARD (Holmes, Worobey, and Rambaut, 1999), and the location of breakpoint regions was compared to those identified by the Phi statistic.
Finally, phylogenetic trees for the individual partitions were built using representatives of the major lineages. The partitions were constructed to compare a putative placement of a recombinant lineage and thus only contained a subset of the taxa. Each partition was evaluated for recombination using the Phi test (Bruen, Philippe, and Bryant, 2006). A best fitting nucleotide substitution model for each partition was found using the Akaike Information Criterion (AIC), implemented in ModelTest (Posada and Crandall, 1998) (Table 1). In each case, the General Time Reversible (GTR) model was chosen as the best fitting nucleotide substitution model and except in one case a Gamma model (Uzzell and Corbin, 1971; Yang, 1993) for substitution rate heterogeneity was used. Based on the inferred best model of substitution, maximum likelihood trees were found with PAUP*using the a heuristic search option and Tree-Bisections and Reconnections (Felsenstein, 1981; Swofford, 2001). Finally, for each region, the different possible trees were compared to each other using a one-sided Shimodaira-Hasegawa test (Shimodaira and Hasegawa, 1999) based on resampling-estimated log likelihoods (RELL) bootstrapping 10000 replicates available in PAUP* (Shimodaira and Hasegawa, 1999). Trees were drawn using the program TREEVIEW (Page, 1996).
Supplementary Material
Acknowledgements
This research was supported by NIH AI052055 and by a McGill Major scholarship to T. Bruen. We thank Kari Samuel, Sally Painter, and Evan Burkala for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson JP, Rodrigo AG, Learn GH, Madan A, Delahunty C, Coon M, Girard M, Osmanov S, Hood L, Mullins JI. Testing the Hypothesis of a Recombinant Origin of Human Immunodeficiency Virus Type 1 Subtype E. J. Virol. 2000;74(22):10752–10765. doi: 10.1128/jvi.74.22.10752-10765.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apetrei C, Robertson DL, Marx PA. The history of SIVS and AIDS: epidemiology, phylogeny and biology of isolates from naturally SIV infected non-human primates (NHP) in Africa. Front Biosci. 2004;9:225–54. doi: 10.2741/1154. [DOI] [PubMed] [Google Scholar]
- Awadalla P. The Evolutionary Genomics Of Pathogen Recombination. Nature Reviews Genetics. 2003;4(1):50–60. doi: 10.1038/nrg964. [DOI] [PubMed] [Google Scholar]
- Bachmann MH, Mathiason-Dubard C, Learn GH, Rodrigo AG, Sodora DL, Mazzetti P, Hoover EA, Mullins JI. Genetic diversity of feline immunodeficiency virus: dual infection, recombination, and distinct evolutionary rates among envelope sequence clades. J. Virol. 1997;71(6):4241–4253. doi: 10.1128/jvi.71.6.4241-4253.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biek R, Drummond AJ, Poss M. A Virus Reveals Population Structure and Recent Demographic History of Its Carnivore Host. Science. 2006;311(5760):538–541. doi: 10.1126/science.1121360. [DOI] [PubMed] [Google Scholar]
- Biek R, Rodrigo AG, Holley D, Drummond A, Anderson CR, Jr., Ross HA, Poss M. Epidemiology, Genetic Diversity, and Evolution of Endemic Feline Immunodeficiency Virus in a Population of Wild Cougars. J. Virol. 2003;77(17):9578–9589. doi: 10.1128/JVI.77.17.9578-9589.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biek R, Ruth TK, Murphy KM, Anderson CR, Jr., Johnson M, DeSimone R, Gray R, Hornocker MG, Gillin CM, Poss M. Factors associated with microparasite seroprevalence and exposure risk in Rocky Mountain cougars. J Wildlife Disease. 2006a;42:606–615. doi: 10.7589/0090-3558-42.3.606. [DOI] [PubMed] [Google Scholar]
- Biek R, Ruth TK, Murphy KM, Anderson CR, Jr., Poss M. Examining effects of persistent retrovirus infection on fitness and pathogen susceptibility in a natural feline host. Canadian Journal of Zoology. 2006b;84:365–373. [Google Scholar]
- Brown EW, Yuhki N, Packer C, O′Brien SJ. A lion lentivirus related to feline immunodeficiency virus: epidemiologic and phylogenetic aspects. J. Virol. 1994;68(9):5953–5968. doi: 10.1128/jvi.68.9.5953-5968.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruen T, Philippe H, Bryant D. A simple and robust statistical test for detecting recombination. Genetics. 2006;172:2665–2681. doi: 10.1534/genetics.105.048975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhard MJ, Dean GA. Transmission and immunopathogenesis of FIV in cats as a model for HIV. Curr HIV Res. 2003;1(1):15–29. doi: 10.2174/1570162033352101. [DOI] [PubMed] [Google Scholar]
- Carpenter MA, O′Brien SJ. Coadaptation and immunodeficiency virus: lessons from the Felidae. Current Opinion in Genetics & Development. 1995;5(6):739–745. doi: 10.1016/0959-437x(95)80006-q. [DOI] [PubMed] [Google Scholar]
- DeStefano JJ, Buiser RG, Mallaber LM, Fay PJ, Bambara RA. Parameters that influence processive synthesis and site-specific termination by human immunodeficiency virus reverse transcriptase on RNA and DNA templates. Biochim Biophys Acta. 1992;1131(3):270–80. doi: 10.1016/0167-4781(92)90025-u. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Evolutionary trees from DNA sequences: a maximum likelihood approach. Journal of Molecular Evolution. 1981;17(6):368–376. doi: 10.1007/BF01734359. [DOI] [PubMed] [Google Scholar]
- Hamilton G. Virology: The gene weavers. Nature. 2006;441(7094):683–685. doi: 10.1038/441683a. [DOI] [PubMed] [Google Scholar]
- Holmes EC, Worobey M, Rambaut A. Phylogenetic evidence for recombination in dengue virus. Mol Biol Evol. 1999;16(3):405–9. doi: 10.1093/oxfordjournals.molbev.a026121. [DOI] [PubMed] [Google Scholar]
- Hommel G. A stagewise rejective multiple test procedure based on a modified Bonferroni test. Biometrika. 1988;75(2):383–386. [Google Scholar]
- Liu SL, Mittler JE, Nickle DC, Mulvania TM, Shriner D, Rodrigo AG, Kosloff B, He X, Corey L, Mullins JI. Selection for human immunodeficiency virus type 1 recombinants in a patient with rapid progression to AIDS. J Virol. 2002;76(21):10674–84. doi: 10.1128/JVI.76.21.10674-10684.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lole KS, Bollinger RC, Paranjape RS, Gadkari D, Kulkarni SS, Novak NG, Ingersoll R, Sheppard HW, Ray SC. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J Virol. 1999;73(1):152–60. doi: 10.1128/jvi.73.1.152-160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negroni M, Buc H. Mechanisms of retroviral recombination. Annu Rev Genet. 2001;35:275–302. doi: 10.1146/annurev.genet.35.102401.090551. [DOI] [PubMed] [Google Scholar]
- Olmsted RA, Langley R, Roelke ME, Goeken RM, Adger-Johnson D, Goff JP, Albert JP, Packer C, Laurenson MK, Caro TM. Worldwide prevalence of lentivirus infection in wild feline species: epidemiologic and phylogenetic aspects. J. Virol. 1992;66(10):6008–6018. doi: 10.1128/jvi.66.10.6008-6018.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page RDM. TREEVIEW: An application to display phylogenetic trees on personal computers. Computer Applications in the Biosciences. 1996;12:357–58. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- Pedersen NC, Ho EW, Brown ML, Yamamoto JK. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science. 1987;235(4790):790–3. doi: 10.1126/science.3643650. [DOI] [PubMed] [Google Scholar]
- Peeters M, Liegeois F, Torimiro N, Bourgeois A, Mpoudi E, Vergne L, Saman E, Delaporte E, Saragosti S. Characterization of a highly replicative intergroup M/O human immunodeficiency virus type 1 recombinant isolated from a Cameroonian patient. J Virol. 1999;73(9):7368–75. doi: 10.1128/jvi.73.9.7368-7375.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posada D. Evaluation of Methods for Detecting Recombination from DNA Sequences: Empirical Data. Mol Biol Evol. 2002;19(5):708–717. doi: 10.1093/oxfordjournals.molbev.a004129. [DOI] [PubMed] [Google Scholar]
- Posada D, Crandall KA. MODELTEST: testing the model of DNA substitution. Bioinformatics. 1998;14(9):817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- Posada D, Crandall KA. Evaluation of methods for detecting recombination from DNA sequences: Computer simulations. PNAS. 2001;98(24):13757–13762. doi: 10.1073/pnas.241370698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posada D, Crandall KA, Holmes EC. Recombination In Evolutionary Genomics. Annual Review of Genetics. 2002;36(1):75–97. doi: 10.1146/annurev.genet.36.040202.111115. [DOI] [PubMed] [Google Scholar]
- Poss M, Ross HA, Painter SL, Holley DC, Terwee JA, Vandewoude S, Rodrigo A. Feline lentivirus evolution in cross-species infection reveals extensive G-to-A mutation and selection on key residues in the viral polymerase. J Virol. 2006;80(6):2728–37. doi: 10.1128/JVI.80.6.2728-2737.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggeti F, Bienzle D. Feline immunodeficiency virus subtypes A, B and C and intersubtype recombinants in Ontario, Canada. J Gen Virol. 2004;85(7):1843–1852. doi: 10.1099/vir.0.19743-0. [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Salminen MO, Carr JK, Burke DS, McCutchan FE. Identification of Breakpoints in Intergenotypic Recombinants of Hiv Type-1 by Bootscanning. Aids Research and Human Retroviruses. 1995;11(11):1423–1425. doi: 10.1089/aid.1995.11.1423. [DOI] [PubMed] [Google Scholar]
- Schierup MH, Hein J. Consequences of Recombination on Traditional Phylogenetic Analysis. Genetics. 2000;156(2):879–891. doi: 10.1093/genetics/156.2.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimodaira H, Hasegawa M. Multiple comparisons of log-likelihoods with applications to phylogenetic inference. Molecular Biology and Evolution. 1999;16:1114–1116. [Google Scholar]
- Smith J. Analyzing the mosaic structure of genes. Journal of Molecular Evolution. 1992;34:126–9. doi: 10.1007/BF00182389. [DOI] [PubMed] [Google Scholar]
- Swofford DL. ″PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods).″ Version 4. Sinauer Associates; Sunderland, Massachusetts: 2001. [Google Scholar]
- Uzzell T, Corbin KW. Fitting discrete probability distributions to evolutionary events. Science. 1971;172:1089–1096. doi: 10.1126/science.172.3988.1089. [DOI] [PubMed] [Google Scholar]
- Wiuf C, Christensen T, Hein J. A Simulation Study of the Reliability of Recombination Detection Methods. Mol Biol Evol. 2001;18(10):1929–1939. doi: 10.1093/oxfordjournals.molbev.a003733. [DOI] [PubMed] [Google Scholar]
- Worobey M, Holmes EC. Evolutionary aspects of recombination in RNA viruses. J Gen Virol. 1999;80(Pt 10):2535–43. doi: 10.1099/0022-1317-80-10-2535. [DOI] [PubMed] [Google Scholar]
- Yang Z. Maximum-likelihood estimation of phylogeny from DNA sequences when substitution rates differ over sites. Molecular Biology and Evolution. 1993;10(6):1396–1401. doi: 10.1093/oxfordjournals.molbev.a040082. [DOI] [PubMed] [Google Scholar]
- Zetterberg V, Ustina V, Liitsola K, Zilmer K, Kalikova N, Sevastianova K, Brummer-Korvenkontio H, Leinikki P, Salminen MO. Two viral strains and a possible novel recombinant are responsible for the explosive injecting drug use-associated HIV type 1 epidemic in Estonia. AIDS Res Hum Retroviruses. 2004;20(11):1148–56. doi: 10.1089/aid.2004.20.1148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.