Abstract
Understanding the genetic origin of cancer at the molecular level has facilitated the development of novel targeted therapies. Aberrant activation of the ErbB family of receptors is implicated in many human cancers and is already the target of several anticancer therapeutics. The use of mAbs specific for the extracellular domain of ErbB receptors was the first implementation of rational targeted therapy. The cytoplasmic tyrosine kinase domain is also a preferred target for small compounds that inhibit the kinase activity of these receptors. However, current therapy has not yet been optimized, allowing for opportunities for optimization of the next generation of targeted therapy, particularly with regards to inhibiting heteromeric ErbB family receptor complexes.
Traditional cancer therapy is predominantly defined by cytotoxic chemotherapeutic agents. Cytotoxic events occur as a consequence of the disruption of various aspects of DNA synthesis and repair or as a consequence of the disturbance of mitosis. As these cellular processes are common to all dividing cells, most chemotherapeutic agents are frequently accompanied by substantial adverse side effects.
The discovery of molecular changes at the gene level represented a critical milestone toward the development of novel targeted therapy specific to cancer cells. The identification of the Philadelphia chromosome in 1960 provided the first evidence of genetic defects associated with chronic myelogenous leukemia (CML), a cancer of the blood that is characterized by an excessive production of immature white blood cells (1). The reciprocal chromosomal translocation [t(9;22)(q34;q11)], which causes the Philadelphia chromosome phenomenon, creates a constitutively active breakpoint cluster region–v-Abl Abelson murine leukemia viral oncogene homolog (BCR-ABL) fusion kinase (2). Recent efforts, which have focused on disabling the abnormal BCR-ABL kinase with the kinase inhibitor imatinib mesylate (also known as Gleevec), have proven effective for treating individuals with CML (3).
Aberrant signal transduction through activated growth factor receptors, for example the ErbB family of receptor tyrosine kinases (RTKs), is a common feature of many types of solid tumors (4). Therapeutic reagents tailored to target these receptors selectively attack malignant cells and, for the most part, spare normal cells. In addition, ErbB-targeted therapeutic strategies have been shown to sensitize tumor cells to traditional chemotherapy and radiotherapy (5, 6).
The continuous process of refining targeted therapeutics will permit even more effective reversal of oncogenic transformation. This Review provides both historical and current insights into the understanding of the fundamental features of transformation at the molecular level caused by the ErbB family of RTKs.
The ErbB family of receptors
Discovery of the ErbB receptors.
In search of the oncogene that led to neuroblastoma, Shih and colleagues observed that the transfer of high molecular weight DNA from ethylnitrosourea-induced neuroblastomas conferred a transformed phenotype to immortalized NIH3T3 cells (7). The oncogene that caused the transformation was later identified and termed neu (8). Studies by Drebin and colleagues identified a cell surface tumor antigen encoded by neu that had a molecular weight of 185 kDa (9), establishing that tumor antigens could be linked to the expression of transforming genes. The oncogenic protein was different from the cellular proto-oncogene–encoded protein p185her2/neu by a single point mutation in the transmembrane region (valine to glutamic acid at residue 664) (10). The protein product is also alternatively called HER2 (11), HER2/neu, and ErbB2 but will only be referred to in this Review as p185her2/neu and the gene as her2/neu. How the introduction of a negative charge into the transmembrane domain caused the protein to gain transforming activity was solved biochemically by Weiner and colleagues shortly thereafter, when they showed that the mutation promoted homodimer formation (12).
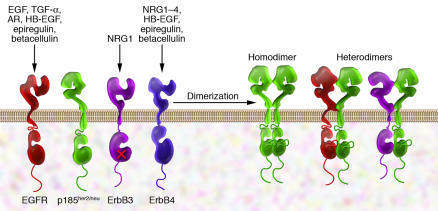
The her2/neu oncogene bears homology to another viral oncogene v-erbB (8), which was isolated from avian erythroblastosis virus but encodes a fragment of the chicken EGFR. EGFR and p185her2/neu are members of the ErbB family of RTKs (known as the HER family of RTKs in humans) that are fundamental to cell proliferation and survival (Figure 1). The two other members of this family, ErbB3 (13) and ErbB4 (14), were identified later.
Figure 1. ErbB receptors and their ligands.
All four members of the ErbB receptor family share high homology in the extracellular domain and the kinase domain. However, ErbB3 is not kinase active. So far no ligand has been found for p185her2/neu, which has been found to be the preferred dimerization partner for other receptors. Only a few examples of receptor dimers are shown here. Ectodomain-truncated receptors also exist in some cancer cells. NRG1, neuregulin 1.
Several ligands are relevant to activation of ErbB receptors and can promote receptor dimer formation. Under certain conditions, such as in malignant transformed cells, dimer complexes can also form irrespective of ligands. EGFR is activated by EGF, TGF-α, and several other ligands. ErbB3 and ErbB4 are the principal receptors for the neuregulins. Although none of the neuregulins directly binds p185her2/neu, activation of EGFR, ErbB3, and ErbB4 can facilitate transactivation of p185her2/neu through ligand-induced heterodimerization (15, 16).
In humans, overexpression of human p185her2/neu in tumors seems to be the predominant transformation-activating mechanism and achieves the same effect as the oncogenic mutations observed in the rat her2/neu gene. An important set of studies described almost simultaneously by 3 laboratories revealed that the her2/neu gene was amplified in primary human breast tumors, and amplification correlated with poor prognosis and disease progression (17–20). Specifically, an association between the extent of her2/neu amplification and the presence of tumor in lymph nodes was observed (18). Furthermore, in individuals with tumors in their lymph nodes, her2 gene amplification was found to be a valuable predictive factor for overall survival and time to relapse.
Homo- and heterodimerization of ErbB receptors.
ErbB receptors achieve activation by forming homo- or heterodimeric receptor complexes, a process that can be encouraged by ligands. The dimerization of ErbB receptors represents the fundamental mechanism that drives transformation. In fact, the oncogenic mutation causes p185her2/neu to form aggregates at the plasma membrane in a manner similar to that of ligand-induced receptor aggregation of the other ErbB family members (12), and it becomes kinase active as a consequence. Dimeric receptors become catalytically active and are able to phosphorylate the C terminus and initiate downstream signaling pathways (4, 21). Mutations in the extracellular juxtamembrane region of p185her2/neu that lead to interreceptor disulfide bonds and receptor aggregation have also been observed in her2/neu transgenic mice (22, 23).
In 1990, Wada and colleagues reported that p185her2/neu formed heteromeric associations with EGFR (15). Even in the absence of exogenously supplied ligand, slight overexpression of p185her2/neu and EGFR led to heterodimeric receptor complexes with activated kinases (15). This set of studies changed the understanding of monomorphic receptor systems because it demonstrated that diversification of receptor signaling can occur through combinatorial associations.
Heteromers have been found with p185her2/neu and all other members of the ErbB family, and p185her2/neu is the preferred heterodimerization partner for each of the family members (24). This notion is further supported by crystallographic studies that have revealed two types of conformations for the ectodomains of ErbB receptors: tethered and extended. Only p185her2/neu adopts the active and dimerization-prone extended conformation similar to ligand-bound EGFR (25–28). EGFR, ErbB3, and ErbB4 all stay in the inactive tethered conformation in the absence of ligand (29–31). Because of its central role in the ErbB family, p185her2/neu became a primary target for developing therapeutics that target ErbB receptors. The mechanism of receptor heterodimerization also suggests that an efficient way to treat ErbB-mediated transformation is to find an inhibitor, or a cocktail of inhibitors, that can disable the interactions of all members of the ErbB family simultaneously.
ErbB signaling and malignant transformation.
Dimerization of ErbB receptors leads to induction of the kinase activity. As a result, a number of tyrosine residues at the C terminal end of the ErbB molecules become phosphorylated. The phosphorylated tyrosine residues serve as docking sites for an array of signaling molecules that contain Src homology 2 (SH2) domains or phosphotyrosine-binding domains. The 2 major signaling pathways activated by ErbB receptors are the MAPK and PI3K-AKT pathways (4) (Figure 2). In addition, ErbB receptors also trigger signaling through other effectors, such as mammalian target of rapamycin (mTOR) (32). The general paradigm is that the specific combination of ErbB receptors in the dimer defines the downstream signaling network as well as the intensity and the duration of the stimulation. For example, heterodimers that contain ErbB3 favor activation of the PI3K pathway (33–35). The signaling events triggered by the ErbB receptors promote cell proliferation and survival, which are the driving forces of malignant transformation.
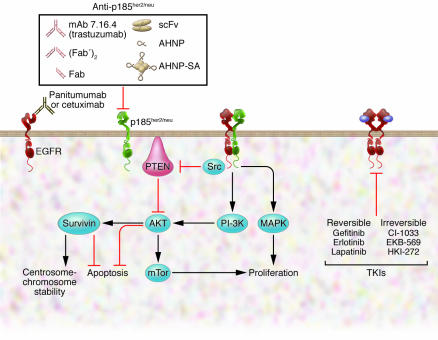
Figure 2. Molecules in the ErbB signaling pathways as targets for cancer therapies.
FDA-approved drugs include three mAbs targeting the extracellular domain of ErbB and three TKIs targeting the kinase domains. Trastuzumab targets p185her2/neu. Cetuximab and panitumumab target EGFR. Some TKIs (gefitinib, erlotinib) are only specific for EGFR, while lapatinib and HKI-272 also broadly inhibit other receptors in the family. Inhibitors to molecules downstream of ErbB signaling pathways, such as Src, AKT, survivin, and mTor, are also potential therapeutics for ErbB-mediated transformation. AHNP-SA, AHNP-streptavidin; scFv, single-chain variable fragment.
In addition, activation of ErbB receptors might contribute to gross chromosomal changes, leading to aneuploidy. Chromosome instability and aneuploidy are frequently seen in human cancers overexpressing ErbB receptors (36). Similarly, in her2/neu-transgenic mice, chromosome instability is associated with her2/neu-positive tumors (37). Amplification of the centrosome, the organelle that plays a critical role in spindle assembly and proper chromosomal segregation during mitosis, is also commonly detected in her2/neu-transgenic mice (38). Aberrant ErbB receptor activities can facilitate accumulation of genomic abnormalities by suppressing the cell death that is normally associated with mitotic failure. Moreover, dysregulation of ErbB receptor signaling might disrupt mitotic checkpoints that are essential for maintaining chromosome integrity.
A series of recent studies indicated that survivin might be a target for ErbB receptor signaling during mitosis (36, 39–41). Survivin, initially identified as a member of the inhibitor of apoptosis protein (IAP) family, is a component of the chromosome passenger proteins involved in the mitotic checkpoint (40). Survivin has been implicated as having a role in both suppressing apoptosis and maintaining chromosome stability. Activation of p185her2/neu or EGFR can elevate the levels of survivin (36, 39), whereas inactivation of EGFR signaling using a dominant-negative form of oncogenic p185her2/neu or the kinase inhibitor lapatinib (Tykerb) can reduce survivin expression in cancer cells (41, 42). Interestingly, upregulation of survivin by the ErbB receptors is dependent on the PI3K signaling pathway but not the MAPK signaling pathway (36, 41). Collectively, these observations raise the possibility that the ErbB family of receptors might contribute to transformation by affecting survivin and its associated proteins (Figure 2).
The development of cancer therapeutics targeting the ErbB receptor extracellular domains
p185her2/neu-specific mAbs.
p185her2/neu mAbs were first created by immunizing mice with the oncogenic her2/neu- transformed cell line B104-1-1 (9). In 1985, Drebin et al. found that several such mAbs, including the mAb 7.16.4, were capable of inducing a rapid and reversible downmodulation of cell surface expression of the receptor, and this led to a reversal of the transformed phenotype of tumor cells growing in soft agar (43). This defining study indicated that downregulating the expression of the oncogenic protein responsible for maintaining the malignant phenotype would allow cells to manifest more normal properties and was followed by the demonstration that these p185her2/neu-specific mAbs were capable of inhibiting the growth of tumors overexpressing p185her2/neu implanted in athymic mice and syngeneic rats of the BDIX strain (44). We believe that this was the first time that an experimental targeted therapy was successfully used to downmodulate the function of an oncoprotein in a syngeneic system. A consequence of these studies was that they laid the foundation for the use of mAbs as a molecular approach to inhibiting tumor growth for the treatment of human malignancies. These findings also validated oncogenes as viable targets for therapeutic agents.
Although antibodies derived for the oncogenic rat receptor established the principle of rational, targeted disabling of oncoproteins and bound to an epitope shared by human p185her2/neu (45), they were not optimal agents for the treatment of human cancer due to their reduced affinity for the human homolog. Shortly after this discovery several laboratories developed mAbs specific for the extracellular domain of human p185her2/neu. One of these antibodies, 4D5 (46), demonstrated the same desirable features as the mAb developed by Drebin et al. (43). The mAb 4D5 specifically inhibited the in vitro growth of tumor cells only when they overexpressed p185her2/neu. In athymic mice with human tumor xenografts, 4D5 localized to the tumor and inhibited tumor growth (46). Furthermore, 4D5, which was of the IgG1 subtype, was able to inhibit the growth of breast cancer cell lines by antibody-dependent cellular cytotoxicity (ADCC) in a manner dependent on the level of p185her2/neu overexpression (47).
To overcome the immunological consequences of administering mouse antibodies to humans, Carter et al. engineered a recombinant humanized version of 4D5, rhumAb4D5, consisting of the complementarity determining regions (CDRs) of the parental mouse mAb built on human framework regions (48). The resulting molecule possessed a three-fold greater affinity for p185her2/neu and could much more effectively mediate ADCC of p185her2/neu-expressing tumor cell lines than could the mouse 4D5 (48). The humanization of 4D5 (later named trastuzumab; also known as Herceptin) greatly expanded its potential for use in the clinic. Trastuzumab was approved for use in combination with first line chemotherapeutic agents in patients with metastatic breast cancer expressing high levels of p185her2/neu (5).
Most importantly, the activity of trastuzumab is not limited to late-stage tumors. Using a transgenic mouse model in which the oncogenic her2/neu was expressed specifically in the mammary tissues, Katsumata and colleagues demonstrated that early administration of mAb 7.16.4 prior to tumor appearance reduced the development of oncogenic her2/neu–mediated breast cancers by 50% (49). mAbs to human p185her2/neu receptors demonstrated similar effect. In recent clinical studies of her2/neu-positive breast cancer, trastuzumab in combination with standard adjuvant therapy substantially reduced the risk of breast cancer recurrence by approximately 50%, as compared with standard adjuvant therapy alone (50, 51). These studies indicated that in tissues that overexpress her2/neu but that are still not fully malignant, mAb could prevent subsequent processes of transformation. Trastuzumab is now used as an adjuvant treatment for individuals with her2/neu-expressing breast cancer that is also detectable in lymph nodes to prevent tumor emergence.
The use of a combination of two mAbs targeting p185her2/neu has been studied in rodent models (52). Antibodies that bound to different epitopes of the p185her2/neu ectodomain were able to synergistically disable the kinase complex in vitro. In vivo studies were even more dramatic, leading to cures in about 50% of animals treated with 2 mAbs. This approach has now been extended in early phase trials to human breast cancer that is resistant to the effects of trastuzumab alone.
EGFR-specific mAbs.
EGFR overexpression has been documented in many tumors, although in rodent cells overexpression is unable to induce a completely transformed cell to grow in vivo (53). However, even slight EGFR overexpression on the cell surface is able to cause cellular transformation in the presence of proto-oncogenic p185her2/neu, as originally shown by Kokai and colleagues (53).
Sato et al. produced mAbs that bound A431, a human epithelial carcinoma cell line with high EGFR levels, and obtained several antagonistic EGFR-specific mAbs that were capable of blocking the binding of EGF to its receptor and, consequently, inhibited EGF-induced activation of EGFR tyrosine kinase activity and cell proliferation (54). The EGFR-specific mAbs were also capable of substantially inhibiting the growth of EGFR-overexpressing human tumor xenografts in athymic mice (54). The possible mechanisms by which mAb 225 reverses the malignant phenotype have been reviewed in detail by Mendelsohn and Baselga (55).
As in the case of trastuzumab, the immunogenicity of these EGFR-specific mAbs needed to be reduced prior to further development for clinical use. The strategy employed was to make a chimeric antibody consisting of the variable region of one of these mAbs, clone 225, and the human IgG1 constant region (56). The chimeric version, cetuximab (formerly called C225; also known as Erbitux), had a greater affinity for EGFR and was more effective at inhibiting tumor xenografts than the parent antibody. In some cases cetuximab caused regression of EGFR-overexpressing human tumors. Cetuximab has been approved to treat patients with advanced metastatic colorectal cancer.
Panitumumab (also known as Vectibix) is another EGFR-specific mAb that is currently used to treat individuals with metastatic colorectal cancer. Panitumumab, known as ABX-EGF during development (57), was generated using transgenic mice (XenoMouse) expressing human Ig genes and is fully humanized (reviewed in ref. 58). Panitumumab has a very high affinity for EGFR (5 × 10–11 M) and was able to eradicate established tumors in mice as a monotherapy (57). By contrast, combination therapy with chemotherapeutic agents is required for cetuximab to mediate activity on well established tumors (59).
Antibody-like molecules as therapeutics in preclinical development.
mAb-based therapies, although successful as demonstrated by trastuzumab, cetuximab, and panitumumab, face certain limitations. For example, they penetrate poorly into solid tumors (reviewed in ref. 60) and the cost of manufacturing the recombinant proteins is high. Several short forms of trastuzumab have been reported, including F(ab′)2, Fab, and single-chain variable fragment (scFv) (Figure 2), but they appear to be more useful as imaging or drug delivery tools (61–63).
Our laboratory designed anti-HER2/Neu peptidomimetic (AHNP) from the 3D structure of the heavy chain CDR3 loop of trastuzumab (64, 65) (Figure 2). As with the parental mAb, AHNP is capable of disabling p185her2/neu receptor function in vitro and in vivo, although it has lower affinity for p185her2/neu. The activity of AHNP has been verified in several other labs (66, 67). Unlike the parental mAb, AHNP was unable to downregulate p185her2/neu expression at the cell surface, indicating that the small molecule functioned differently to inhibit p185her2/neu.
Peptide mimetics are not limited to the CDR loops from antibodies and can be extended to functional loops on receptors. Peptides designed from potential dimerization surfaces in the extracellular domain of ErbB receptors have been shown to selectively bind to all four members of the ErbB receptor and to inhibit heregulin-induced interactions of ErbB3 with other ErbB receptors (68). The target loop is within the membrane-proximal part of subdomain IV. This study demonstrated the possibility of preventing the dimerization of ErbB receptors to disable receptor activity by structure-based targeting of the dimerization interfaces (68).
The major disadvantage for peptide mimetics is the reduced affinity to the receptor, much shorter half-life in vivo, and lack of the elements required for ADCC. By recombinantly displaying AHNP peptide units on the surface of a tetrameric streptavidin scaffold, Masuda et al. improved the affinity and in vivo antitumor activity of AHNP (69). In fact, we hypothesize that if CDR mimetics are integrated into elements involved in ADCC, the new species can be as effective as an intact antibody.
The development of cancer therapeutics targeting the ErbB receptor tyrosine kinase domain
A critical feature of ErbB receptors (except ErbB3) is the inducible kinase activity that trans-phosphorylates tyrosine residues in the C terminal domain and leads to activation of downstream signaling pathways (70, 71). Receptor overexpression in tumor cells also greatly enhances kinase activity, even in the absence of ligand. Conversely, it has been shown that EGF-mediated cellular activities, such as receptor downregulation, activation of gene transcription, and cell proliferation, are lost as a result of a single amino acid mutation rendering the EGFR kinase deficient (72).
There are two classes of tyrosine kinase inhibitors (TKIs) for ErbB family receptors, reversible and irreversible inhibitors. Reversible inhibitors only compete with ATP binding to the kinase, whereas the irreversible inhibitors can also alkylate a single cysteine residue (cysteine 773 of EGFR) within the ATP-binding pocket and inactivate the kinase permanently.
Another feature of the latest TKIs is their broad activity against multiple receptors in the ErbB family. Kinase-deficient receptors have been shown to cause cellular transformation by recruiting a truncated receptor that has intact kinase domains (73). As tumor cells usually contain several ErbB family members and heterodimerization occurs routinely, the inhibition effectiveness of a TKI on cellular transformation mediated by ErbB family members is limited if it is only specific to one receptor, even if it completely blocks the kinase activity of that receptor. For this reason, it is not surprising that pan–ErbB TKIs work more effectively (74).
Reversible inhibitors.
Current reversible TKIs are derivatives of quinazolines. Gefitinib (also known as Iressa) and erlotinib (also known as Tarceva) are selectively active against the EGFR tyrosine kinase (75, 76), whereas lapatinib shows comparable activity against both EGFR and p185her2/neu (77). In crystallographic studies, only lapatinib locked the EGFR kinase domain into an inactive conformation (78) that resembles that of inactive SRC family kinases (79, 80). The erlotinib-bound EGFR kinase still retained an active conformation (81), although it no longer autophosphorylated EGFRs in treated tumor cells (82). There is virtually no structural information on the interaction of the p185her2/neu kinase domain with any of these inhibitors.
In clinical use on non–small cell lung cancer patients, gefitinib and erlotinib were discovered to be effective in individuals carrying multiple somatic mutations, namely a deletion mutation (del 747–750) or a point mutation L858R (83–85). These mutations slightly increase the activity of EGFR. Both types of mutation most probably reposition critical kinase domain residues around the ATP-binding cleft and thus enhance the affinity of the kinase domain for the inhibitors. However, a secondary point mutation, T790M, was found in patients who became resistant to the effects of gefitinib and had a relapse after two years of complete remission (86). In vitro experiments clearly showed that gefitinib, erlotinib, and lapatinib were not able to inhibit EGFR kinase activity of T790M EGFR or the more frequently found EGFR VIII mutant (87, 88).
By potentially targeting both EGFR and p185her2/neu, lapatinib has, in principle, advantages over gefitinib and erlotinib. Lapatinib caused potent inhibition of the activation of EGFR and p185her2/neu and, subsequently, the critical downstream effectors MAPK and AKT, resulting in marked death of human head, neck, and breast cancer cells in vitro and in vivo (74). Importantly, since lapatinib can inhibit AKT phosphorylation by blocking the activating signaling from both EGFR and p185her2/neu kinases, its activity no longer depends on the expression of phosphatase and tensin homolog (PTEN), the phosphatase that negatively regulates AKT and is required for trastuzumab antitumor activity (89) (Figure 2). However, it is not clear whether lapatinib simultaneously inhibits both kinases found in a dimeric complex or whether it affects p185her2/neu-ErbB3 heterodimers. If that does not occur, lapatinib will also be found to induce resistance to its own effects, as the uninhibited kinase in the dimer can still trans-phosphorylate the other receptor and activate the PI3K/AKT pathway that let the cells escape the erlotinib therapy (90).
Irreversible inhibitors.
This class of inhibitors that irreversibly bind to and permanently modify the tyrosine kinase domain were originally developed to achieve durable suppression of ligand-dependent EGFR autophosphorylation (91). The advantage of irreversible inhibitors became apparent after they were found to inhibit EGFR mutants (such as T790M) that are resistant to reversible inhibitors (87) and show clinical activity in gefitinib-resistant Japanese non–small cell lung cancer patients (92). Another advantage of irreversible inhibitors is that they inhibit all of the ErbB family receptor kinases.
However, the effective inhibitory concentration of the early compounds (CI-1033 and EKB-569) for p185her2/neu was not impressive. In a colon cancer model, CI-1033 blocked EGFR signaling but did not completely inhibit the p185her2/neu-AKT signaling pathway (93). CI-1033 failed a trial for unscreened advanced ovarian cancer (94). Recently, EKB-569 was modified to become HKI-272 in order to improve its inhibitory activity against p185her2/neu. As expected, HKI-272 demonstrated better antitumor activity on p185her2/neu-expressing tumors and works on tumors expressing the EGFR VIII mutant (88). HKI-272 and a similar irreversible dual inhibitor, BIBW, are in phase II clinical trials for the treatment of advanced breast cancer and other solid tumors (95).
TKI resistance.
Most patients with non–small cell lung cancer who are initially sensitive to gefitinib or erlotinib treatment eventually have a relapse (86, 96). Lapatinib is able to inhibit both EGFR and p185her2/neu, but there is insufficient clinical data on relapse. In vitro studies have already indicated that the gefitinib-resistant mutation T790M is also resistant to lapatinib (87).
However, the mechanism of acquired resistance to the reversible TKIs is not limited to the secondary EGFR mutation T790M, which is only observed in a fraction of recurrent tumors (97). Available evidence points to receptor internalization, heterodimerization with p185her2/neu, and recently a pathway that also involves ErbB3 and Akt. Sergina et al. showed that gefitinib and erlotinib treatment had a sustained inhibition on the phosphorylation of EGFR, p185her2/neu, JNK, and MAPK, but phosphorylated ErbB3 and AKT levels recovered 12–24 hours after treatment (90). Activation of MET was also reported to induce consistent ErbB3 phosphorylation in gefitinib-resistant cells, while a specific MET inhibitor restored the sensitivity to gefitinib (98). Knocking down p185her2/neu expression with siRNA also suppressed the drug refractory ErbB3 signaling and gefitinib resistance (97).
Irreversible inhibitors, working by a slightly different mechanism, have shown some promise in initial studies on the gefitinib- or erlotinib-resistant mutations and might lessen the rapidly acquired resistance.
Targeted therapy: what target?
When the concept of targeted therapy was developed 20 years ago with mAbs specific for p185her2/neu, the expectation was simple and naive: the cancer, as long as it expressed the receptor (target), would be destroyed (or at least inhibited from further growth) by the therapy. Initially, experiments with animal models optimistically supported this (44). The focus at that stage was to refine the reagents for use in humans, including optimizing the affinity to the target, humanization of the antibody, and modification of glycosylation of the antibody to extend its half-life. It was not until the therapy was in clinical practice that a different kind of question emerged: how good is the target?
As a single agent, trastuzumab has moderate activity for the treatment of breast cancer (99). This is not simply because it cannot reach the target, as the response rate increases when the antibody is administered together with a chemotherapeutic agent. The problem is variations among signaling proteins present downstream of the p185her2/neu.
Drebin et al. first noted in 1985 that cells transformed by both oncogenic her2/neu and ras were not susceptible to p185her2/neu mAb therapy and predicted that cells with mutations in multiple pathways contributing to cancer would be problematic to treat (43). Nagata et al. (100) discovered that patients with p185her2/neu-expressing breast cancer who also had PTEN deficiency, which happens in 48% of breast cancers (101), had a substantially lower response rate to trastuzumab than did patients with p185her2/neu-expressing breast cancer who had no PTEN deficiency. Overexpression of the IGF1 receptor and the ectodomain-missing p95her2/neu receptor, both of which also lead to AKT activation, has also been implicated in trastuzumab resistance (102, 103).
The clinical use of gefitinib and erlotinib represents another example of how the genetic background (e.g., del 747–750 and L858R) can affect the response rate to a particular therapeutic (83–85). In addition, genetic changes affecting the protein level are also critically informative. Recently, after retrospectively studying lung cancer patients enrolled in the phase III trial of gefitinib, Hirsch et al. reported that EGFR FISH-positive or IHC-positive patients had better rates of response and survival than did EGFR FISH-negative patients (104). The lesson learned is that the target needs to be defined more specifically.
Since RTKs are susceptible to somatic mutations or drug-induced resistance, a successful strategy of ErbB-targeted therapy might require inhibiting other targets related to ErbB signaling pathways, such as survivin. Overexpression of survivin is implicated in increased paclitaxel (Taxol) resistance in cancers (105), but downregulation of survivin sensitizes cancer cells to γ-irradiation (106). These observations justify targeting survivin as a therapeutic approach to treat human cancers. Based on the three-dimensional structure of survivin (107–109), we have designed several small-molecule compounds that modulate survivin subcellular localization and function. It would be interesting to test whether combination of survivin inhibitors with anti-ErbB antibodies or TKIs can produce a synergistic effect on inhibition of tumor growth.
Early detection technologies
Novel detection methods are needed to delineate the genetic and proteomic profiles of human cancers. Currently, the most widely used clinical p185her2/neu tests are immunohistochemistry and FISH (110). However, both are invasive, low-throughput methodologies. Furthermore, it has been reported that serum p185her2/neu levels are a better indicator for stage IV breast cancer than immunohistochemistry scores (111). Accordingly, a sensitive serum p185her2/neu test has been developed for screening patients for trastuzumab-based therapy and monitoring post-treatment p185her2/neu levels (112). This platform, which is suitable for high-throughput analyses of clinical samples, could be further improved to test a panel of biomarkers. For example, a multiplex test for the expression of PTEN, IGF1 receptor, FGF receptor 2, and p95her2/neu together with EGFR and p185her2/neu, from serum or tissue extracts, will help physicians and patients on their treatment decisions regarding trastuzumab and TKIs.
The future of ErbB receptor–targeted therapy
As we have discussed here, current clinical therapeutic reagents targeting ErbB family receptors fall into two categories: mAbs and small molecules, with antibodies targeting the extracellular domain and small molecules targeting the kinase domain in the cytoplasmic region (Figure 2). More mAb-like small forms of preclinical proteins or peptides have been developed to target the extracellular domains (63, 64, 69). With more available therapeutics, we envision that cocktail therapies will be the best way to treat human tumors mediated by ErbB family receptors. In mice, utilization of combinations of p185her2/neu-specific mAbs reactive to distinct epitopes revealed a synergistic inhibitory effect on the growth of tumors in vivo (52). Combinations of EGFR and p185her2/neu mAbs also led to remarkable antitumor activities as a consequence of simultaneously inhibiting the heteromeric assemblies that cause transformation (113, 114). A combination therapy with trastuzumab and kinase inhibitors might also increase the response rate in patients who also have the expression of a truncated receptor p95her2/neu, which no longer has the epitope for trastuzumab but is kinase active and can be inhibited by TKIs such as lapatinib (103). This combination treatment paradigm has emerged as the next wave of cancer therapeutics.
Successful treatment of CML using imatinib provided impetus for RTK-targeted therapy. However, the effectiveness of current reversible TKIs is limited on the majority of tumors that express WT EGFR. Erlotinib and gefitinib have been shown to have higher binding affinity to the sensitive mutant (L858R) than to WT EGFR or the T790M EGFR mutant (87, 115). Rapid dissociation of erlotinib from WT EGFR has been observed (78). One way to address this problem is to redesign compounds specific for the WT or resistant mutants. Alternatively, currently available drugs can be made more active with agents that enhance their binding kinetics.
To date, targeting ErbB receptors has led to several FDA-approved therapeutics for cancers. Although issues like resistance tarnished the initial excitement for these agents, the cancer research community has gained insights into mechanisms of action and ideas for developing better drugs. The next generation of ErbB therapeutics should be not only more potent but also more cost effective to relieve the burden on the patients and the health care system.
Acknowledgments
Our thanks to Francisco X. Real for helpful comments on this manuscript. This work was supported by the Abramson Family Cancer Research Institute and NIH grants CA089480, CA089481, and CA055306 to M.I. Greene.
Footnotes
Nonstandard abbreviations used: ADCC, antibody-dependent cellular cytotoxicity; AHNP, anti-HER2/Neu peptidomimetic; CDR, complementarity determining region; CML, chronic myelogenous leukemia; HB-EGF, heparin-binding EGF-like growth factor; PTEN, phosphatase and tensin homolog; RTK, receptor tyrosine kinase; SH2, Src homology 2; TKI, tyrosine kinase inhibitor.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 117:2051–2058 (2007). doi:10.1172/JCI32278.
References
- 1.Nowell P.C., Hungerford D.A. A minute chromosome in human chronic granulocytic leukemia. Science. 1960;132:1497. doi: 10.1126/science.144.3623.1229. [DOI] [PubMed] [Google Scholar]
- 2.Rowley J.D. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973;243:290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- 3.Druker B.J., et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat. Med. 1996;2:561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 4.Yarden Y., Sliwkowski M.X. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 5.Slamon D.J., et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 6.O’Rourke D.M., et al. Conversion of a radioresistant phenotype to a more sensitive one by disabling erbB receptor signaling in human cancer cells. Proc. Natl. Acad. Sci. U. S. A. 1998;95:10842–10847. doi: 10.1073/pnas.95.18.10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shih C., Shilo B.Z., Goldfarb M.P., Dannenberg A., Weinberg R.A. Passage of phenotypes of chemically transformed cells via transfection of DNA and chromatin. Proc. Natl. Acad. Sci. U. S. A. 1979;76:5714–5718. doi: 10.1073/pnas.76.11.5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schechter A.L., et al. The neu oncogene: an erb-B-related gene encoding a 185,000-Mr tumour antigen. Nature. 1984;312:513–516. doi: 10.1038/312513a0. [DOI] [PubMed] [Google Scholar]
- 9.Drebin J.A., Stern D.F., Link V.C., Weinberg R.A., Greene M.I. Monoclonal antibodies identify a cell-surface antigen associated with an activated cellular oncogene. Nature. 1984;312:545–548. doi: 10.1038/312545a0. [DOI] [PubMed] [Google Scholar]
- 10.Bargmann C.I., Weinberg R.A. Oncogenic activation of the neu-encoded receptor protein by point mutation and deletion. EMBO J. 1988;7:2043–2052. doi: 10.1002/j.1460-2075.1988.tb03044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.King C.R., Kraus M.H., Aaronson S.A. Amplification of a novel v-erbB-related gene in a human mammary carcinoma. Science. 1985;229:974–976. doi: 10.1126/science.2992089. [DOI] [PubMed] [Google Scholar]
- 12.Weiner D.B., Liu J., Cohen J.A., Williams W.V., Greene M.I. A point mutation in the neu oncogene mimics ligand induction of receptor aggregation. Nature. 1989;339:230–231. doi: 10.1038/339230a0. [DOI] [PubMed] [Google Scholar]
- 13.Kraus M.H., Issing W., Miki T., Popescu N.C., Aaronson S.A. Isolation and characterization of ERBB3, a third member of the ERBB/epidermal growth factor receptor family: evidence for overexpression in a subset of human mammary tumors. Proc. Natl. Acad. Sci. U. S. A. 1989;86:9193–9197. doi: 10.1073/pnas.86.23.9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plowman G.D., et al. Ligand-specific activation of HER4/p180erbB4, a fourth member of the epidermal growth factor receptor family. Proc. Natl. Acad. Sci. U. S. A. 1993;90:1746–1750. doi: 10.1073/pnas.90.5.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wada T., Qian X.L., Greene M.I. Intermolecular association of the p185neu protein and EGF receptor modulates EGF receptor function. Cell. 1990;61:1339–1347. doi: 10.1016/0092-8674(90)90697-d. [DOI] [PubMed] [Google Scholar]
- 16.Carraway K.L., 3rd, Cantley L.C. A neu acquaintance for erbB3 and erbB4: a role for receptor heterodimerization in growth signaling. Cell. 1994;78:5–8. doi: 10.1016/0092-8674(94)90564-9. [DOI] [PubMed] [Google Scholar]
- 17.McGuire W.L. Prognostic factors for recurrence and survival in human breast cancer. Breast Cancer Res. Treat. 1987;10:5–9. doi: 10.1007/BF01806129. [DOI] [PubMed] [Google Scholar]
- 18.Slamon D.J., et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 19.van de Vijver M., et al. Amplification of the neu (c-erbB-2) oncogene in human mammmary tumors is relatively frequent and is often accompanied by amplification of the linked c-erbA oncogene. Mol. Cell. Biol. 1987;7:2019–2023. doi: 10.1128/mcb.7.5.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varley J.M., Swallow J.E., Brammar W.J., Whittaker J.L., Walker R.A. Alterations to either c-erbB-2(neu) or c-myc proto-oncogenes in breast carcinomas correlate with poor short-term prognosis. Oncogene. 1987;1:423–430. [PubMed] [Google Scholar]
- 21.Qian X., LeVea C.M., Freeman J.K., Dougall W.C., Greene M.I. Heterodimerization of epidermal growth factor receptor and wild-type or kinase-deficient Neu: a mechanism of interreceptor kinase activation and transphosphorylation. Proc. Natl. Acad. Sci. U. S. A. 1994;91:1500–1504. doi: 10.1073/pnas.91.4.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siegel P.M., Dankort D.L., Hardy W.R., Muller W.J. Novel activating mutations in the neu proto-oncogene involved in induction of mammary tumors. Mol. Cell. Biol. 1994;14:7068–7077. doi: 10.1128/mcb.14.11.7068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siegel P.M., Muller W.J. Mutations affecting conserved cysteine residues within the extracellular domain of Neu promote receptor dimerization and activation. Proc. Natl. Acad. Sci. U. S. A. 1996;93:8878–8883. doi: 10.1073/pnas.93.17.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graus-Porta D., Beerli R.R., Daly J.M., Hynes N.E. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 1997;16:1647–1655. doi: 10.1093/emboj/16.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho H.S., et al. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature. 2003;421:756–760. doi: 10.1038/nature01392. [DOI] [PubMed] [Google Scholar]
- 26.Ogiso H., et al. Crystal structure of the complex of human epidermal growth factor and receptor extracellular domains. Cell. 2002;110:775–787. doi: 10.1016/s0092-8674(02)00963-7. [DOI] [PubMed] [Google Scholar]
- 27.Garrett T.P., et al. The crystal structure of a truncated ErbB2 ectodomain reveals an active conformation, poised to interact with other ErbB receptors. Mol. Cell. 2003;11:495–505. doi: 10.1016/s1097-2765(03)00048-0. [DOI] [PubMed] [Google Scholar]
- 28.Garrett T.P., et al. Crystal structure of a truncated epidermal growth factor receptor extracellular domain bound to transforming growth factor alpha. Cell. 2002;110:763–773. doi: 10.1016/s0092-8674(02)00940-6. [DOI] [PubMed] [Google Scholar]
- 29.Cho H.S., Leahy D.J. Structure of the extracellular region of HER3 reveals an interdomain tether. Science. 2002;297:1330–1333. doi: 10.1126/science.1074611. [DOI] [PubMed] [Google Scholar]
- 30.Bouyain S., Longo P.A., Li S., Ferguson K.M., Leahy D.J. The extracellular region of ErbB4 adopts a tethered conformation in the absence of ligand. Proc. Natl. Acad. Sci. U. S. A. 2005;102:15024–15029. doi: 10.1073/pnas.0507591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li S., et al. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell. 2005;7:301–311. doi: 10.1016/j.ccr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Klos K.S., et al. ErbB2 increases vascular endothelial growth factor protein synthesis via activation of mammalian target of rapamycin/p70S6K leading to increased angiogenesis and spontaneous metastasis of human breast cancer cells. Cancer Res. 2006;66:2028–2037. doi: 10.1158/0008-5472.CAN-04-4559. [DOI] [PubMed] [Google Scholar]
- 33.Alimandi M., et al. Cooperative signaling of ErbB3 and ErbB2 in neoplastic transformation and human mammary carcinomas. Oncogene. 1995;10:1813–1821. [PubMed] [Google Scholar]
- 34.Wallasch C., et al. Heregulin-dependent regulation of HER2/neu oncogenic signaling by heterodimerization with HER3. EMBO J. 1995;14:4267–4275. doi: 10.1002/j.1460-2075.1995.tb00101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones R.B., Gordus A., Krall J.A., MacBeath G. A quantitative protein interaction network for the ErbB receptors using protein microarrays. Nature. 2006;439:168–174. doi: 10.1038/nature04177. [DOI] [PubMed] [Google Scholar]
- 36.Asanuma H., et al. Survivin expression is regulated by coexpression of human epidermal growth factor receptor 2 and epidermal growth factor receptor via phosphatidylinositol 3-kinase/AKT signaling pathway in breast cancer cells. Cancer Res. 2005;65:11018–11025. doi: 10.1158/0008-5472.CAN-05-0491. [DOI] [PubMed] [Google Scholar]
- 37.Jeruss J.S., et al. Characterization and chromosomal instability of novel derived cell lines from a wt-erbB-2 transgenic mouse model. Carcinogenesis. 2003;24:659–664. doi: 10.1093/carcin/bgg001. [DOI] [PubMed] [Google Scholar]
- 38.Montagna C., Andrechek E.R., Padilla-Nash H., Muller W.J., Ried T. Centrosome abnormalities, recurring deletions of chromosome 4, and genomic amplification of HER2/neu define mouse mammary gland adenocarcinomas induced by mutant HER2/neu. Oncogene. 2002;21:890–898. doi: 10.1038/sj.onc.1205146. [DOI] [PubMed] [Google Scholar]
- 39.Xia W., et al. Regulation of survivin by ErbB2 signaling: therapeutic implications for ErbB2-overexpressing breast cancers. Cancer Res. 2006;66:1640–1647. doi: 10.1158/0008-5472.CAN-05-2000. [DOI] [PubMed] [Google Scholar]
- 40.Vagnarelli P., Earnshaw W.C. Chromosomal passengers: the four-dimensional regulation of mitotic events. Chromosoma. 2004;113:211–222. doi: 10.1007/s00412-004-0307-3. [DOI] [PubMed] [Google Scholar]
- 41.Wang Q., Greene M.I. EGFR enhances Survivin expression through the phosphoinositide 3 (PI-3) kinase signaling pathway. Exp. Mol. Pathol. 2005;79:100–107. doi: 10.1016/j.yexmp.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 42.Xia W., et al. Combining lapatinib ( GW572016), a small molecule inhibitor of ErbB1 and ErbB2 tyrosine kinases, with therapeutic anti-ErbB2 antibodies enhances apoptosis of ErbB2-overexpressing breast cancer cells. Oncogene. 2005;24:6213–6221. doi: 10.1038/sj.onc.1208774. [DOI] [PubMed] [Google Scholar]
- 43.Drebin J.A., et al. Down-modulation of an oncogene protein product and reversion of the transformed phenotype by monoclonal antibodies. . Cell. 1985;41:697–706. doi: 10.1016/s0092-8674(85)80050-7. [DOI] [PubMed] [Google Scholar]
- 44.Drebin J.A., Link V.C., Weinberg R.A., Greene M.I. Inhibition of tumor growth by a monoclonal antibody reactive with an oncogene-encoded tumor antigen. Proc. Natl. Acad. Sci. U. S. A. 1986;83:9129–9133. doi: 10.1073/pnas.83.23.9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang H., et al. Shared antigenic epitopes and pathobiological functions of anti-p185(her2/neu) monoclonal antibodies. Exp. Mol. Pathol. 1999;67:15–25. doi: 10.1006/exmp.1999.2266. [DOI] [PubMed] [Google Scholar]
- 46.Hudziak R.M., et al. p185HER2 monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor. Mol. Cell. Biol. 1989;9:1165–1172. doi: 10.1128/mcb.9.3.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lewis G.D., et al. Differential responses of human tumor cell lines to anti-p185HER2 monoclonal antibodies. Cancer Immunol. Immunother. 1993;37:255–263. doi: 10.1007/BF01518520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carter P., et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc. Natl. Acad. Sci. U. S. A. 1992;89:4285–4289. doi: 10.1073/pnas.89.10.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Katsumata M., et al. Prevention of breast tumour development in vivo by downregulation of the p185neu receptor. Nat. Med. 1995;1:644–648. doi: 10.1038/nm0795-644. [DOI] [PubMed] [Google Scholar]
- 50.Slamon D.J., Romond E.H., Perez E.A. Advances in adjuvant therapy for breast cancer. Clin. Adv. Hematol. Oncol. 2006;4(Suppl. 1):4–9; discussion Suppl. 10. [PubMed] [Google Scholar]
- 51.Romond E.H., et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N. Engl. J. Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 52.Drebin J.A., Link V.C., Greene M.I. Monoclonal antibodies reactive with distinct domains of the neu oncogene-encoded p185 molecule exert synergistic anti-tumor effects in vivo. Oncogene. 1988;2:273–277. [PubMed] [Google Scholar]
- 53.Kokai Y., et al. Synergistic interaction of p185c-neu and the EGF receptor leads to transformation of rodent fibroblasts. Cell. 1989;58:287–292. doi: 10.1016/0092-8674(89)90843-x. [DOI] [PubMed] [Google Scholar]
- 54.Sato J.D., et al. Biological effects in vitro of monoclonal antibodies to human epidermal growth factor receptors. Mol. Biol. Med. 1983;1:511–529. [PubMed] [Google Scholar]
- 55.Mendelsohn J., Baselga J. The EGF receptor family as targets for cancer therapy. Oncogene. 2000;19:6550–6565. doi: 10.1038/sj.onc.1204082. [DOI] [PubMed] [Google Scholar]
- 56.Goldstein N.I., Prewett M., Zuklys K., Rockwell P., Mendelsohn J. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin. Cancer Res. 1995;1:1311–1318. [PubMed] [Google Scholar]
- 57.Yang X.D., Jia X.C., Corvalan J.R., Wang P., Davis C.G. Development of ABX-EGF, a fully human anti-EGF receptor monoclonal antibody, for cancer therapy. Crit. Rev. Oncol. Hematol. 2001;38:17–23. doi: 10.1016/s1040-8428(00)00134-7. [DOI] [PubMed] [Google Scholar]
- 58.Cohenuram M., Saif M.W. Panitumumab the first fully human monoclonal antibody: from the bench to the clinic. Anticancer Drugs. 2007;18:7–15. doi: 10.1097/CAD.0b013e32800feecb. [DOI] [PubMed] [Google Scholar]
- 59.Fan Z., Baselga J., Masui H., Mendelsohn J. Antitumor effect of anti-epidermal growth factor receptor monoclonal antibodies plus cis-diamminedichloroplatinum on well established A431 cell xenografts. Cancer Res. 1993;53:4637–4642. [PubMed] [Google Scholar]
- 60.Beckman R.A., Weiner L.M., Davis H.M. Antibody constructs in cancer therapy: protein engineering strategies to improve exposure in solid tumors. Cancer. 2007;109:170–179. doi: 10.1002/cncr.22402. [DOI] [PubMed] [Google Scholar]
- 61.Spiridon C.I., Guinn S., Vitetta E.S. A comparison of the in vitro and in vivo activities of IgG and F(ab’)2 fragments of a mixture of three monoclonal anti-Her-2 antibodies. Clin. Cancer Res. 2004;10:3542–3551. doi: 10.1158/1078-0432.CCR-03-0549. [DOI] [PubMed] [Google Scholar]
- 62.Dennis M.S., et al. Imaging tumors with an albumin-binding Fab, a novel tumor-targeting agent. Cancer Res. 2007;67:254–261. doi: 10.1158/0008-5472.CAN-06-2531. [DOI] [PubMed] [Google Scholar]
- 63.Neve R.M., et al. Biological effects of anti-ErbB2 single chain antibodies selected for internalizing function. Biochem. Biophys. Res. Commun. 2001;280:274–279. doi: 10.1006/bbrc.2000.4104. [DOI] [PubMed] [Google Scholar]
- 64.Park B.W., et al. Rationally designed anti-HER2/neu peptide mimetic disables P185HER2/neu tyrosine kinases in vitro and in vivo. Nat. Biotechnol. 2000;18:194–198. doi: 10.1038/72651. [DOI] [PubMed] [Google Scholar]
- 65.Berezov A., Zhang H.T., Greene M.I., Murali R. Disabling erbB receptors with rationally designed exocyclic mimetics of antibodies: structure-function analysis. J. Med. Chem. 2001;44:2565–2574. doi: 10.1021/jm000527m. [DOI] [PubMed] [Google Scholar]
- 66.Fantin V.R., et al. A bifunctional targeted peptide that blocks HER-2 tyrosine kinase and disables mitochondrial function in HER-2-positive carcinoma cells. Cancer Res. 2005;65:6891–6900. doi: 10.1158/0008-5472.CAN-05-0395. [DOI] [PubMed] [Google Scholar]
- 67.Tan M., et al. Selective inhibition of ErbB2-overexpressing breast cancer in vivo by a novel TAT-based ErbB2-targeting signal transducers and activators of transcription 3-blocking peptide. Cancer Res. 2006;66:3764–3772. doi: 10.1158/0008-5472.CAN-05-2747. [DOI] [PubMed] [Google Scholar]
- 68.Berezov A., et al. Disabling receptor ensembles with rationally designed interface peptidomimetics. J. Biol. Chem. 2002;277:28330–28339. doi: 10.1074/jbc.M202880200. [DOI] [PubMed] [Google Scholar]
- 69.Masuda K., et al. AHNP-streptavidin: a tetrameric bacterially produced antibody surrogate fusion protein against p185her2/neu. Oncogene. 2006;25:7740–7746. doi: 10.1038/sj.onc.1209745. [DOI] [PubMed] [Google Scholar]
- 70.Baserga R. Oncogenes and the strategy of growth factors. Cell. 1994;79:927–930. doi: 10.1016/0092-8674(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 71.Heldin C.H. Dimerization of cell surface receptors in signal transduction. Cell. 1995;80:213–223. doi: 10.1016/0092-8674(95)90404-2. [DOI] [PubMed] [Google Scholar]
- 72.Chen W.S., et al. Requirement for intrinsic protein tyrosine kinase in the immediate and late actions of the EGF receptor. Nature. 1987;328:820–823. doi: 10.1038/328820a0. [DOI] [PubMed] [Google Scholar]
- 73.Qian X., Dougall W.C., Fei Z., Greene M.I. Intermolecular association and trans-phosphorylation of different neu-kinase forms permit SH2-dependent signaling and oncogenic transformation. Oncogene. 1995;10:211–219. [PubMed] [Google Scholar]
- 74.Xia W., et al. Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene. 2002;21:6255–6263. doi: 10.1038/sj.onc.1205794. [DOI] [PubMed] [Google Scholar]
- 75.Fry D.W., et al. A specific inhibitor of the epidermal growth factor receptor tyrosine kinase. Science. 1994;265:1093–1095. doi: 10.1126/science.8066447. [DOI] [PubMed] [Google Scholar]
- 76.Ward W.H., et al. Epidermal growth factor receptor tyrosine kinase. Investigation of catalytic mechanism, structure-based searching and discovery of a potent inhibitor. Biochem. Pharmacol. 1994;48:659–666. doi: 10.1016/0006-2952(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 77.Rusnak D.W., et al. The characterization of novel, dual ErbB-2/EGFR, tyrosine kinase inhibitors: potential therapy for cancer. Cancer Res. 2001;61:7196–7203. [PubMed] [Google Scholar]
- 78.Wood E.R., et al. A unique structure for epidermal growth factor receptor bound to GW572016 (lapatinib): relationships among protein conformation, inhibitor off-rate, and receptor activity in tumor cells. Cancer Res. 2004;64:6652–6659. doi: 10.1158/0008-5472.CAN-04-1168. [DOI] [PubMed] [Google Scholar]
- 79.Schindler T., et al. Crystal structure of Hck in complex with a Src family-selective tyrosine kinase inhibitor. Mol. Cell. 1999;3:639–648. doi: 10.1016/s1097-2765(00)80357-3. [DOI] [PubMed] [Google Scholar]
- 80.Xu W., Doshi A., Lei M., Eck M.J., Harrison S.C. Crystal structures of c-Src reveal features of its autoinhibitory mechanism. Mol. Cell. 1999;3:629–638. doi: 10.1016/s1097-2765(00)80356-1. [DOI] [PubMed] [Google Scholar]
- 81.Stamos J., Sliwkowski M.X., Eigenbrot C. Structure of the epidermal growth factor receptor kinase domain alone and in complex with a 4-anilinoquinazoline inhibitor. J. Biol. Chem. 2002;277:46265–46272. doi: 10.1074/jbc.M207135200. [DOI] [PubMed] [Google Scholar]
- 82.Moyer J.D., et al. Induction of apoptosis and cell cycle arrest by CP-358,774, an inhibitor of epidermal growth factor receptor tyrosine kinase. Cancer Res. 1997;57:4838–4848. [PubMed] [Google Scholar]
- 83.Lynch T.J., et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 84.Paez J.G., et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 85.Pao W., et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc. Natl. Acad. Sci. U. S. A. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kobayashi S., et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 87.Carter T.A., et al. Inhibition of drug-resistant mutants of ABL, KIT, and EGF receptor kinases. Proc. Natl. Acad. Sci. U. S. A. 2005;102:11011–11016. doi: 10.1073/pnas.0504952102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ji H., et al. Epidermal growth factor receptor variant III mutations in lung tumorigenesis and sensitivity to tyrosine kinase inhibitors. Proc. Natl. Acad. Sci. U. S. A. 2006;103:7817–7822. doi: 10.1073/pnas.0510284103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xia W., et al. Lapatinib antitumor activity is not dependent upon phosphatase and tensin homologue deleted on chromosome 10 in ErbB2-overexpressing breast cancers. Cancer Res. 2007;67:1170–1175. doi: 10.1158/0008-5472.CAN-06-2101. [DOI] [PubMed] [Google Scholar]
- 90.Sergina N.V., et al. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature. 2007;445:437–441. doi: 10.1038/nature05474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fry D.W., et al. Specific, irreversible inactivation of the epidermal growth factor receptor and erbB2, by a new class of tyrosine kinase inhibitor. Proc. Natl. Acad. Sci. U. S. A. 1998;95:12022–12027. doi: 10.1073/pnas.95.20.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yoshimura N., et al. EKB-569, a new irreversible epidermal growth factor receptor tyrosine kinase inhibitor, with clinical activity in patients with non-small cell lung cancer with acquired resistance to gefitinib. Lung Cancer. 2006;51:363–368. doi: 10.1016/j.lungcan.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 93.Rajput A., et al. A novel mechanism of resistance to epidermal growth factor receptor antagonism in vivo. Cancer Res. 2007;67:665–673. doi: 10.1158/0008-5472.CAN-06-2773. [DOI] [PubMed] [Google Scholar]
- 94.Campos S., et al. Multicenter, randomized phase II trial of oral CI-1033 for previously treated advanced ovarian cancer. J. Clin. Oncol. 2005;23:5597–5604. doi: 10.1200/JCO.2005.08.091. [DOI] [PubMed] [Google Scholar]
- 95.Shaw H., et al. A phase I dose escalation study of BIBW 2992, an irreversible dual EGFR/HER2 receptor tyrosine kinase inhibitor, in patients with advanced solid tumours [abstract]. J. Clin. Oncol. 2006;24:3025. [Google Scholar]
- 96.Uramoto H., et al. Resistance to gefitinib. Int. J. Clin. Oncol. 2006;11:487–491. doi: 10.1007/s10147-006-0609-y. [DOI] [PubMed] [Google Scholar]
- 97.Kwak E.L., et al. Irreversible inhibitors of the EGF receptor may circumvent acquired resistance to gefitinib. Proc. Natl. Acad. Sci. U. S. A. 2005;102:7665–7670. doi: 10.1073/pnas.0502860102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Engelman J.A., et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 99.Vogel C.L., et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. . J. Clin. Oncol. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 100.Nagata Y., et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117–127. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 101.Depowski P.L., Rosenthal S.I., Ross J.S. Loss of expression of the PTEN gene protein product is associated with poor outcome in breast cancer. Mod. Pathol. 2001;14:672–676. doi: 10.1038/modpathol.3880371. [DOI] [PubMed] [Google Scholar]
- 102.Nahta R., Yuan L.X., Zhang B., Kobayashi R., Esteva F.J. Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res. 2005;65:11118–11128. doi: 10.1158/0008-5472.CAN-04-3841. [DOI] [PubMed] [Google Scholar]
- 103.Scaltriti M., et al. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J. Natl. Cancer Inst. 2007;99:628–638. doi: 10.1093/jnci/djk134. [DOI] [PubMed] [Google Scholar]
- 104.Hirsch F., et al. Combination of EGFR gene copy number and protein expression predicts outcome for advanced non-small-cell lung cancer patients treated with gefitinib. Ann. Oncol. 2007;18:752–760. doi: 10.1093/annonc/mdm003. [DOI] [PubMed] [Google Scholar]
- 105.Zaffaroni N., et al. Expression of the anti-apoptotic gene survivin correlates with taxol resistance in human ovarian cancer. Cell. Mol. Life Sci. 2002;59:1406–1412. doi: 10.1007/s00018-002-8518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pennati M., et al. Radiosensitization of human melanoma cells by ribozyme-mediated inhibition of survivin expression. J. Invest. Dermatol. 2003;120:648–654. doi: 10.1046/j.1523-1747.2003.12082.x. [DOI] [PubMed] [Google Scholar]
- 107.Sun C., Nettesheim D., Liu Z., Olejniczak E.T. Solution structure of human survivin and its binding interface with Smac/Diablo. Biochemistry. 2005;44:11–17. doi: 10.1021/bi0485171. [DOI] [PubMed] [Google Scholar]
- 108.Chantalat L., et al. Crystal structure of human survivin reveals a bow tie-shaped dimer with two unusual alpha-helical extensions. Mol. Cell. 2000;6:183–189. [PubMed] [Google Scholar]
- 109.Muchmore S.W., et al. Crystal structure and mutagenic analysis of the inhibitor-of-apoptosis protein survivin. Mol. Cell. 2000;6:173–182. [PubMed] [Google Scholar]
- 110.Di Leo A., Dowsett M., Horten B., Penault-Llorca F. Current status of HER2 testing. Oncology. 2002;63:25–32. doi: 10.1159/000066204. [DOI] [PubMed] [Google Scholar]
- 111.Luftner D., et al. Discordant results obtained for different methods of HER-2/neu testing in breast cancer — a question of standardization, automation and timing. Int. J. Biol. Markers. 2004;19:1–13. doi: 10.1177/172460080401900101. [DOI] [PubMed] [Google Scholar]
- 112.Zhang H., Cheng X., Richter M., Greene M.I. A sensitive and high-throughput assay to detect low-abundance proteins in serum. Nat. Med. 2006;12:473–477. doi: 10.1038/nm1378. [DOI] [PubMed] [Google Scholar]
- 113.Wada T., et al. Anti-receptor antibodies reverse the phenotype of cells transformed by two interacting proto-oncogene encoded receptor proteins. . Oncogene. 1990;5:489–495. [PubMed] [Google Scholar]
- 114.Ye D., Mendelsohn J., Fan Z. Augmentation of a humanized anti-HER2 mAb 4D5 induced growth inhibition by a human-mouse chimeric anti-EGF receptor mAb C225. Oncogene. 1999;18:731–738. doi: 10.1038/sj.onc.1202319. [DOI] [PubMed] [Google Scholar]
- 115.Ji H., et al. The impact of human EGFR kinase domain mutations on lung tumorigenesis and in vivo sensitivity to EGFR-targeted therapies. Cancer Cell. 2006;9:485–495. doi: 10.1016/j.ccr.2006.04.022. [DOI] [PubMed] [Google Scholar]