Abstract
MHC class I–restricted CD8+ T cells are necessary to mount an immune response against Mycobacterium tuberculosis. M. tuberculosis antigens can enter MHC class I cross-processing pathways through a number of different mechanisms, including via the uptake of antigen-containing apoptotic vesicles released by infected cells. A study in this issue of the JCI by Hinchey and colleagues shows that M. tuberculosis inhibits host cell apoptosis and thus may interfere with optimal cross-priming and action of CD8+ T cells (see the related article beginning on page 2279). M. tuberculosis genetically modified to induce apoptosis is shown to be more effective in priming CD8+ T cells in vivo and therefore may be a more effective vaccine against tuberculosis than the currently utilized M. bovis BCG vaccine.
Mycobacterium tuberculosis continues to cause widespread morbidity and mortality in children and adults worldwide, despite the availability of relatively simple diagnostic tools, inexpensive and effective drugs, and public health infrastructures in most countries for control and treatment of tuberculosis (TB) (1). In adolescents and adults, TB is primarily caused by reactivation of latent/persistent M. tuberculosis bacilli and progression to active pulmonary disease. M. bovis bacille Calmette-Guérin (BCG), widely used as TB vaccine for newborns and effective in preventing disseminated M. tuberculosis disease in young children, is unable to prevent pulmonary (reactivation) TB in adolescents and adults (2, 3). The latter finding was reconfirmed in a recent study of BCG revaccination of more than 15,000 7- to 14-year-old school children in Brazil (4). Thus, an effective vaccine for the prevention of pulmonary TB in adolescents and adults, many of whom are latently infected with M. tuberculosis in countries in which TB is endemic, is urgently needed to control the TB pandemic.
Macrophage apoptosis and M. tuberculosis
During the last 20 years, great progress has been made in areas essential for new TB vaccine development, including mycobacterial genetics, TB immunology, and animal models of M. tuberculosis infection. Completion of the M. tuberculosis genome sequence combined with genetic tools to delete, add back, or complement mycobacterial genes allows one to determine the M. tuberculosis genes essential for survival in macrophages and animal models and those genes involved in resisting host immune responses (5, 6). M. tuberculosis readily infects macrophages, and macrophage apoptosis has developed as one host defense mechanism against infection. However, virulent M. tuberculosis has evolved to be capable of inhibiting macrophage apoptosis. The study by Hinchey et al. in this issue of the JCI (7) represents an elegant example of a combination of approaches from the 3 areas of research described above to determine the role of mycobacterial genes secA2 and sodA in resisting macrophage apoptosis and to determine whether enhanced apoptosis of secA2 gene–deleted M. tuberculosis (ΔsecA2) is associated with increased cross-presentation of antigens to CD8+ T cells and improved immunity against an aerosol challenge with M. tuberculosis in vivo (7). Earlier studies established that SecA2 was required for secretion of superoxide dismutase A (SodA) by M. tuberculosis and that knocking out secA resulted in a less virulent organism (8). Superoxide anions can kill mycobacteria directly and induce macrophage apoptosis. Apoptosis kills intracellular mycobacteria by a superoxide-independent mechanism. Hinchey et al. (7) now show that, in vitro, a ΔsecA2 mutant causes increased caspase expression and macrophage apoptosis compared with WT M. tuberculosis. When extracellular SodA expression was restored in the ΔsecA2 mutant by adding an N-terminal signal sequence to sodA, the level of macrophage apoptosis were reduced to that observed in response to WT M. tuberculosis. Thus a link between SecA2-dependent SodA secretion and inhibition of macrophage apoptosis was established.
Cross-processing of M. tuberculosis for CD8+ T cells
Adaptive immunity mediated by T cells and the cytokines they secrete is essential for controlling initial M. tuberculosis infection (usually in the lungs) and preventing reactivation of latent/persistent M. tuberculosis bacilli residing in granulomas. T cell failure induced by malnutrition, aging, HIV-1 infection, or immune-suppressive drugs allows latent infection to progress to active TB. Multiple T cell subsets are activated by M. tuberculosis antigens, including MHC class II–restricted CD4+ and MHC class I–restricted CD8+ T cells, as well as γδ TCR+ T cells, CD1-restricted T cells, CD25+CD4+ Tregs, and others (9). CD4+ and CD8+ T cells are essential for protective immunity to M. tuberculosis and thus a major focus for vaccine development (10). It is speculated, but not proven, that an inability to adequately prime CD8+ T cells is responsible for the failure of M. bovis BCG to adequately protect against TB and that optimal activation of both CD8+ and CD4+ T cells is necessary for developing an improved TB vaccine.
The antigen repertoire for CD8+ T cells and the processing mechanisms of M. tuberculosis antigens for MHC class I presentation by dendritic cells and macrophages remain poorly defined. Conventional MHC class I antigen processing requires de novo synthesized antigens (e.g., viral proteins) in the cytosol for proteolysis by proteasomes and transport of peptides into the ER by a transporter associated with antigen processing (TAP) molecule for loading onto MHC class I. An alternative mechanism allows processing of exogenous (i.e., those taken up by phagocytosis) or vacuolar antigens (i.e., those from M. tuberculosis bacilli in phagosomes) for presentation by MHC class I molecules to CD8+ T cells (11, 12). This alternative form of MHC class I antigen processing is called cross-processing and is responsible for in vivo cross-priming. Cross-processing of M. tuberculosis antigens can occur through a number of distinct mechanisms: Antigens may translocate directly from phagosomes to the cytosol for processing or they may remain entirely within the vacuolar compartment. In a recently described pathway, the ER was shown to deliver protein translocation channels and peptide loading components to phagosomes. M. tuberculosis antigens then could transfer to the cytosol for proteasomal processing and peptides could be imported into phagosomes via TAP for binding to MHC class I (12).
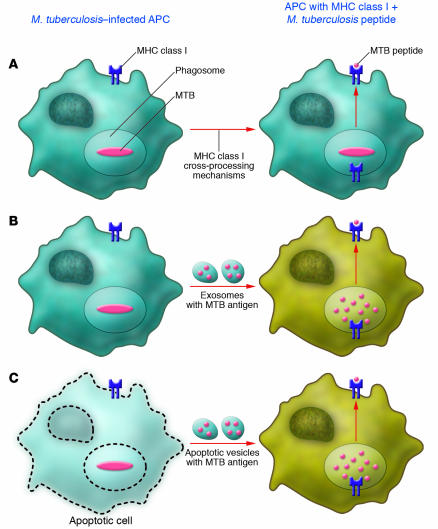
These are at least three mechanisms through which M. tuberculosis antigens can enter these cellular cross-processing mechanisms. Via the first mechanism, antigens can directly be cross-processed by cells that have taken up M. tuberculosis bacilli (Figure 1A), as shown for human macrophages (13). Via the second mechanism, M. tuberculosis–infected cells can produce exosomes containing mycobacterial antigens, which can be taken up by bystander dendritic cells or macrophages for MHC class I cross-processing (Figure 1B) (14). Via the third mechanism, M. tuberculosis–infected cells can apoptose and release apoptotic vesicles with mycobacterial antigens for uptake by bystander APCs (Figure 1C) (15). Just which of these three mechanism(s) is operative or dominant during M. tuberculosis infection in vivo likely depends on the type and in vivo location of the APC. For vaccines, the adjuvant and/or vector used to deliver antigen will determine which mechanism will be used for CD8+ T cell priming.
Figure 1. M. tuberculosis antigen entry into MHC class I cross-processing pathways for CD8+ T cell activation.
(A) Direct MHC class I cross-processing of M. tuberculosis antigens in an infected APC. (B) Cross-processing by a bystander APC of M. tuberculosis antigen–containing exosomes extruded by infected cells. (C) Cross-processing by a bystander APC of M. tuberculosis antigen–containing apoptotic vesicles. MTB, M. tuberculosis.
In their current study, Hinchey et al. (7) sought to determine whether increased macrophage apoptosis in vitro translated into increased MHC class I–restricted CD8+ T cell responses in vivo. By adoptively transferring OT-I TCR-transgenic T cells, which recognize the SIINFEKL peptide of OVA presented by H-2Kb MHC class I molecules, into mice infected with mutant and WT M. tuberculosis expressing the SIINFEKL peptide (16), the authors performed a series of elegant in vivo experiments. After i.v. infection with these different M. tuberculosis strains, increased levels of SIINFEKL-specific CD8+ T cells were detected in spleens of ΔsecA2-OVA–infected mice compared with WT M. tuberculosis–infected mice. These CD8+ T cells proliferated and were cytotoxic in vivo. Subcutaneous immunization with ΔsecA2-OVA increased the number of SIINFEKL-specific CD8+ memory T cells as measured by H-2Kb tetramer, CD44, and CD62 ligand staining during the first 1–2 months, with a suggestion of increased long-term persistence of CD8+ T cell memory in ΔsecA2-OVA– compared with M. tuberculosis H37Rv–OVA–immunized mice. Apoptosis is difficult to detect in vivo, and thus it isn’t clear whether apoptosis was responsible for the increased cross-priming of CD8+ T cells observed in vivo in ΔsecA-OVA–infected mice.
Animal models of M. tuberculosis infection
Mouse, guinea pig, and primates are the species most commonly used for experimental M. tuberculosis infection for pathogenesis and vaccine studies (17). These animals generally do not develop latent infection with reactivation TB as seen in humans, but they are useful as models of acute infection and for determining a vaccine’s immunogenicity and efficacy in reducing mycobacterial growth after an aerosol challenge. For animal studies, vaccination with M. bovis BCG remains the gold standard against which all other vaccines need to be compared. Establishing the superiority of a new TB vaccine over M. bovis BCG in these animal models is difficult, as demonstrated by the study by Hinchey et al. (7). Modest differences in mycobacterial CFU in the lungs after 1 month between ΔsecA- and M. bovis BCG–vaccinated mice translated into significant differences in survival. In guinea pigs, vaccination with M. bovis BCG or ΔsecA yielded similar levels of protection (decrease in CFU) in lungs and spleen for the two vaccines. Vaccination with ΔsecA reduced CFU and pathology in mediastinal lymph nodes, suggesting that ΔsecA might be better at limiting pulmonary pathology and bacterial dissemination.
New TB vaccines
Progress in standardizing animal models of M. tuberculosis infection has allowed ready comparison of genetically manipulated mycobacteria and new TB vaccines across studies and research centers around the world. This has resulted in rapid development of a new generation of TB vaccines using four general approaches (reviewed in ref. 18): (a) developing subunit vaccines of fused M. tuberculosis proteins (72F and ESAT6-85B, both fusions of 2 proteins) with novel adjuvants (19, 20); (b) developing heterologous vectors such as modified vaccinia Ankara (MVA) or adenovirus expressing M. tuberculosis proteins (21); (c) improving the efficacy of M. bovis BCG by overexpressing M. tuberculosis proteins or heterologous proteins such as listeriolysin (22, 23); and (d) attenuating M. tuberculosis by removing virulence genes such as secA (18). A number of these new TB vaccines are beyond preclinical testing and in phase I and II clinical trials in uninfected and latently M. tuberculosis–infected tuberculin skin test–positive (TST+) healthy volunteers. The challenge will be to select vaccine(s) for phase III trials that will require 10,000 or more participants in TB-endemic settings. There are no surrogate markers for protection against progression of M. tuberculosis infection and development of TB to use in phase II studies to triage vaccine candidates. Whether enhanced cross-priming of MHC class I–restricted CD8+ T cells is a requirement for new TB vaccines or can be considered a surrogate for vaccine efficacy remains to be determined. The study by Hinchey et al. (7) increases our understanding of the role of MHC class I–restricted CD8+ T cells, the antigens they recognize, and their antigen-processing requirements in immunity against M. tuberculosis and indicates that activation of these cells is important and should be considered as new TB vaccines are designed and developed.
Acknowledgments
Special thanks to David Canaday and Cliff Harding for reviewing the manuscript. W. Henry Boom is supported by NIH grants AI27243 and HL55967 and NIAID-DMID contract HHSN266200700022C/AI70022.
Footnotes
Nonstandard abbreviations used: BCG, bacille Calmette-Guérin; ΔsecA2, secA2 gene–deleted Mycobacterium tuberculosis; SodA, superoxide dismutase A; TB, tuberculosis.
Conflict of interest: The author has declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 117:2092–2094 (2007). doi:10.1172/JCI32933.
See the related article beginning on page 2279.
References
- s. [Anonymous]. 2007. Global tuberculosis control — surveillance, planning, financing. Report of a WHO expert committee. WHO. Geneva, Switzerland. http://www.who.int/tb/publications/global_report/2007/en/index.html. [Google Scholar]
- 2.Colditz G.A., et al. The efficacy of bacillus Calmette-Guerin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics. 1995;96:29–35. [PubMed] [Google Scholar]
- 3.Colditz G.A., et al. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994;271:698–702. [PubMed] [Google Scholar]
- 4.Rodrigues L.C., et al. Effect of BCG revaccination on incidence of tuberculosis in school-aged children in Brazil: the BCG-REVAC cluster-randomised trial. Lancet. 2005;366:1290–1295. doi: 10.1016/S0140-6736(05)67145-0. [DOI] [PubMed] [Google Scholar]
- 5.Cole S.T. Comparative and functional genomics of the Mycobacterium tuberculosis complex. Microbiology. 2002;148:2919–2928. doi: 10.1099/00221287-148-10-2919. [DOI] [PubMed] [Google Scholar]
- 6.Hingley-Wilson S.M., Sambandamurthy V.K., Jacobs W.R., Jr. Survival perspectives from the world’s most successful pathogen, Mycobacterium tuberculosis. Nat. Immunol. 2003;4:949–955. doi: 10.1038/ni981. [DOI] [PubMed] [Google Scholar]
- 7.Hinchey J., et al. Enhanced priming of adaptive immunity by a proapoptotic mutant of Mycobacterium tuberculosis. . J. Clin. Invest. . 2007;117:2279–2288. doi: 10.1172/JCI31947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braunstein M., et al. SecA2 functions in the secretion of superoxide dismutase A and in the virulence of Mycobacterium tuberculosis. Mol. Microbiol. 2003;48:453–464. doi: 10.1046/j.1365-2958.2003.03438.x. [DOI] [PubMed] [Google Scholar]
- 9.Boom W.H., et al. Human immunity to M. tuberculosis: T cell subsets and antigen processing. Tuberculosis. (Edinb.). 2003;83:98–106. doi: 10.1016/s1472-9792(02)00054-9. [DOI] [PubMed] [Google Scholar]
- 10.Sud D., et al. Contribution of CD8+ T cells to control of Mycobacterium tuberculosis infection. J. Immunol. 2006;176:4296–4314. doi: 10.4049/jimmunol.176.7.4296. [DOI] [PubMed] [Google Scholar]
- 11.Ramachandra L., et al. Phagocytic antigen processing and effects of microbial products on antigen processing and T-cell responses. Immunol. Rev. 1999;168:217–239. doi: 10.1111/j.1600-065x.1999.tb01295.x. [DOI] [PubMed] [Google Scholar]
- 12.Rock K.L. Exiting the outside world for cross-presentation. Immunity. 2006;25:523–525. doi: 10.1016/j.immuni.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Canaday D.H., et al. Activation of human CD8+ alpha beta TCR+ cells by Mycobacterium tuberculosis via an alternate class I MHC antigen-processing pathway. J. Immunol. 1999;162:372–379. [PubMed] [Google Scholar]
- 14.Russell D.G. Who puts the tubercle in tuberculosis? Nat. Rev. Microbiol. 2007;5:39–47. doi: 10.1038/nrmicro1538. [DOI] [PubMed] [Google Scholar]
- 15.Winau F., et al. Apoptotic vesicles crossprime CD8 T cells and protect against tuberculosis. Immunity. 2006;24:105–117. doi: 10.1016/j.immuni.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Hogquist K.A., et al. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 17.Flynn J.L. Lessons from experimental Mycobacterium tuberculosis infections. Microbes Infect. 2006;8:1179–1188. doi: 10.1016/j.micinf.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 18.Kaufmann S.H., Baumann S., Nasser Eddine A. Exploiting immunology and molecular genetics for rational vaccine design against tuberculosis. Int. J. Tuberc. Lung Dis. 2006;10:1068–1079. [PubMed] [Google Scholar]
- 19.Olsen A.W., et al. Protective effect of a tuberculosis subunit vaccine based on a fusion of antigen 85B and ESAT-6 in the aerosol guinea pig model. Infect. Immun. 2004;72:6148–6150. doi: 10.1128/IAI.72.10.6148-6150.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skeiky Y.A., et al. Differential immune responses and protective efficacy induced by components of a tuberculosis polyprotein vaccine, Mtb72F, delivered as naked DNA or recombinant protein. J. Immunol. 2004;172:7618–7628. doi: 10.4049/jimmunol.172.12.7618. [DOI] [PubMed] [Google Scholar]
- 21.McShane H., et al. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat. Med. 2004;10:1240–1244. doi: 10.1038/nm1128. [DOI] [PubMed] [Google Scholar]
- 22.Grode L., et al. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guérin mutants that secrete listeriolysin. . J. Clin. Invest. 2005;115:2472–2479. doi: 10.1172/JCI24617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horwitz M.A., Harth G. A new vaccine against tuberculosis affords greater survival after challenge than the current vaccine in the guinea pig model of pulmonary tuberculosis. Infect. Immun. 2003;71:1672–1679. doi: 10.1128/IAI.71.4.1672-1679.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]