Abstract
An automatic fluorous solid-phase extraction (F-SPE) technique is developed by using FluoroFlash SPE cartridges on the RapidTrace workstation. A 10-module workstation has the capability to complete a maximum of one hundred SPEs each round in 1–2 h. Another important feature of the RapidTrace system is that it has the capability to load slurry samples onto the F-SPE cartridges. The F-SPE cartridge charged with 2 g of fluorous silica gel is used to purify up to 200 mg of crude sample. Sample loading, elution solvent, cartridge reuse, and SPE reproducibility are evaluated. The automatic SPE system is used for purification of a small urea library generated from amine scavenging reactions using fluorous dichlorotriazine (F-DCT), a 96-membered amide library generated using 2-chloro-4,6-bis[(perfluorohexyl)propyloxy]-1,3,5-triazine (F-CDMT) as the coupling agent, and another 96-membered library generated from fluorous Mitsunobu reactions. Approximately 90% of products have greater than 90% purity after F-SPE.
Introduction
Highly fluorinated (fluorous) organic molecules are lipophobic and hydrophobic. This unique property has been exploited for separation of fluorous molecules from mixtures containing non-fluorous molecules.1 Among several fluorous separation methods, fluorous solid-phase extraction (F-SPE) with FluoroFlash silica gel containing a -Si(Me)2CH2CH2C8F17 stationary phase has the capability to separate “light fluorous” molecules bearing perfluorinated chains such as C6F13 or C8F17.2 Since it was first introduced by the Curran group in 1997,3 F-SPE has been used for purification of reaction mixtures containing fluorous catalysts,4 scavengers,5 reagents,6 protecting groups,7 and biomolecules.8
Even though F-SPE has great potential for rapid separation, at the present time most F-SPEs are still performed manually on a 2 x 12 SPE manifold. In the chemical library synthesis setting, conducting F-SPEs in parallel or automatically can significantly improve efficiency. We have recently reported the development of a 24-well plate-to-plate F-SPE.9 Described in this paper is a complementary effort to use commercially available RapidTrace workstations for automatic purification of library compounds.
Results and Discussion
RapidTrace Workstation
The RapidTrace SPE workstation is a product of Caliper Life Sciences (Figure 1).10 It has been widely used for analytical and biological sample preparations. The RapidTrace system has the following features:
Figure 1.

RapidTrace SPE workstation. (left, single modular unit for 10 cartridges; right, ten units controlled by a PC)
The workstation has up to ten modular units; each unit conducts ten SPEs sequentially; a maximum of 10 x 10 = 100 SPE separations can be finished in 1–2 h unattended
Each SPE cartridge has up to 3 mL volume for up to 2 g of silica gel
Cartridge conditioning, sample loading, elution and rinsing are automated
The autosampler handles solution samples and slurry samples as well
The solvent pump operates at back pressure up to 100 psi and maximum flow rate at 30 mL/min
Each module controls eight elution solvents and may be programmed to use mixtures of them.
Ion exchange resins, normal and reverse-phase silica gels, and functionalized resins are the common packing materials for RapidTrace SPE. We envisioned that the RapidTrace system could be used for automatic F-SPE if fluorous silica gel was used as the separation medium.
Method development
Each cartridge placed on the RapidTrace workstation was charged with 2 g of fluorous silica gel (40–60 μm particle size). On the basis of previous experience of F-SPE, the mass loading (weight of crude sample compared to the weight of fluorous silica gel) for this kind of cartridge should be less than 10%. MeOH–H2O (80:20) was used as the fluorophobic solvent which elutes the nonfluorous components and leaves the fluorous component on the SPE cartridge. The fluorous component can be eluted with 100% MeOH from the cartridge. Another good fluorophobic solvent system is 90:10 DMF-H2O which has lower water content than the 80:20 MeOH-H2O system.
We first conducted a loading solvent test using 80:20 MeOH–H2O (6 mL) as the fluorophobic elution solvent and 100% MeOH (6 mL) as the fluorophilic solvent. The purpose of this study was to detect the fluorous compound breakthrough related to a loading solvent and its amount. The elution flow rate was set at 12 mL/min for the fluorophobic pass and 30 mL/min for the fluorophilic pass. A mixture of fluorous and non-fluorous triphenylphosphine oxide was used as a test sample. Each sample contained 0.1 mmole each of Ph3PO and p-C8F17CH2CH2PhP(O)Ph2 (F-PhP(O)Ph2). Six different solvents (THF, CH2Cl2, MeOH, MeCN, acetone, DMF) were tested in a volume range from 0.2 to 0.8 mL. The fluorophobic and fluorophilic fractions were submitted to LC-MS for purity analysis and also concentrated to determine the recovery. The results listed in Table 1 show that for a 2 g fluorous silica gel cartridge at 5% mass loading (28 mg of Ph3PO and 72 mg F-PhP(O)Ph2), F-PhP(O)Ph2 breakthrough was detected when 0.3 and 0.5 mL of THF, 0.5 mL of CH2Cl2 or acetone were used as the loading solvents. No F-PhP(O)Ph2 breakthrough was detected when MeOH or MeCN up to 0.5 mL, and DMF up to 0.8 mL were used as the loading solvents. These results are consistent with the fluorophilicity of these solvents: THF > acetone, CH2Cl2, MeCN, MeOH >DMF. Among the six loading solvents we have tested, the best one is DMF, which has low fluorophilicity and good solubility for many organic compounds. We found that when 2 mL DMF was used as the loading solvent and 90:10 DMF-H2O as the fluorophobic elution solvent, no fluorous breakthrough was detected. To allow a reasonable margin of error, we still recommend the use of <1 mL of DMF as loading solvent in common practice.
Table 1.
Loading test with a triphenylphosphine oxide mixture
| Loading solvent | CH2Cl2 | DMF | THF | ||||||
|---|---|---|---|---|---|---|---|---|---|
| volume (mL) | 0.2 | 0.3 | 0.5 | 0.4 | 0.5 | 0.8 | 0.2 | 0.3 | 0.5 |
| Ph3PO recovery (%)a | 100 | 96 | 100c | 100 | 100 | 100 | 93 | 93c | 125c |
| F-PhP(O)Ph2 recovery (%)b | 88 | 90 | 90 | 86 | 92 | 90 | 88 | 88 | 88 |
| Loading solvent | MeOH | MeCN | acetone | ||||||
| volume (mL) | 0.2 | 0.3 | 0.5 | 0.2 | 0.3 | 0.5 | 0.2 | 0.3 | 0.5 |
| Ph3PO recovery (%)a | 86 | 93 | 100 | 86 | 94 | 100 | 89 | 89 | 103c |
| F-PhP(O)Ph2 recovery (%)b | 77 | 85 | 92 | 74 | 83 | 92 | 82 | 86 | 91 |
From 80:20 MeOH–H2O fraction.
From 100% MeOH fraction.
F-PhP(O)Ph2 was detected
To test the reproducibility of the RapidTrace F-SPE process, samples of the same mixture of the two triphenylphosphine oxides in ten different sample vials were subjected to sequential F-SPE using ten different cartridges on two SPE units. The 90:10 DMF-H2O was used for fluorophobic elution and 100% MeOH was used for fluorophilic elution. Results in Table 2 show good recovery for both Ph3PO and F-PhP(O)Ph2. The reproducibility of ten samples on ten different cartridges is excellent.
Table 2.
Reproducibility test on ten SPE cartridgesc
| Cartridge # | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Ph3PO recovery (%)a | 93 | 93 | 93 | 96 | 93 | 96 | 96 | 93 | 93 | 96 |
| F-PhP(O)Ph2 recovery (%)b | 88 | 89 | 90 | 93 | 92 | 92 | 92 | 92 | 92 | 92 |
From 90:10 DMF–H2O fraction.
From 100% MeOH fraction.
Loading sample, Ph3PO (28 mg) and F-PhP(O)Ph2 (72 mg) in 0.5 mL of DMF.
The capability to reuse F-SPE cartridges is an attractive feature of fluorous technology. After collecting both non-fluorous and fluorous fractions, the cartridge can be washed with THF, reconditioned with 80:20 MeOH-H2O or 90:10 DMF-H2O, and is ready for the next round of F-SPE. All these operations are easily programmed and controlled by a PC. A ten round reuse test with one cartridge was conducted and results are listed in Table 3. Again, good recovery for both Ph3PO and F-PhP(O)Ph2 fractions were obtained. The reproducibility was excellent.
Table 3.
Single cartridge reuse testc
| F-SPE run # | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Ph3PO recovery (%)a | 93 | 89 | 93 | 93 | 93 | 93 | 93 | 93 | 93 | 93 |
| F-PhP(O)Ph2 recovery (%)b | 94 | 94 | 92 | 94 | 94 | 94 | 94 | 94 | 94 | 94 |
From 90:10 DMF–H2O fraction.
From 100% MeOH fraction.
Loading sample, Ph3PO (28 mg) and F-PhP(O)Ph2 (72 mg) in 0.5 mL of DMF.
Purification of Libraries
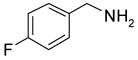
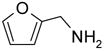
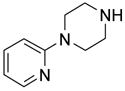
After method development, we used the automatic F-SPE system for purification of the products of a small library produced by 3 x 6 parallel reactions of three isocyanates and six amines. The amines were used in slight excess (1.2 equiv) to push the reaction to completion. The unreacted amines were scavenged by fluorous dichlorotriazine (F-DCT) in the presence of diisopropylethylamine (DIPEA) as the base.11 Upon completion of the scavenging reaction, each reaction mixture was treated with a macroporous polystyrene anion-exchange resin (MP-CO3) to free the DIPEA base. The free base in the product fraction was later removed with the solvent during vacuum concentration. The resin was removed by filtration, the filtrate was concentrated, dissolved in 0.5 mL of DMF and then queued up for RapidTrace F-SPE. MeOH-H2O (80:20) was used for fluorophobic elution for the products. The fluorous component retained on the cartridge was washed with THF to the waste bottle. Results summarized in Table 4 show that the yields of ureas are in a range between 48–96%, and thirteen out of eighteen products have purities greater than 90%.
Table 4.
Urea formation reactions using F-DCT as an amine scavenger
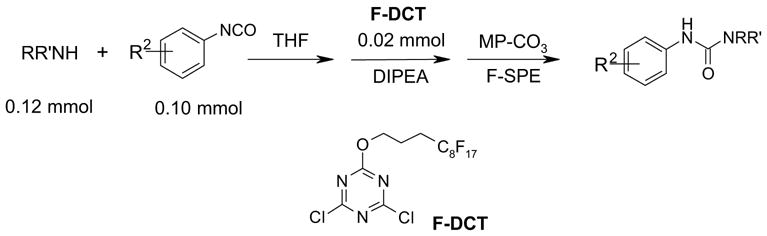
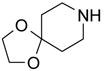
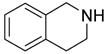
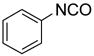
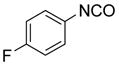
| BuNH2 |
|
|
|
|
|
|
|
47%(93%)* | 63%(94%) | 64%(94%) | 89%(96%) | 92%(90%) | 96%(80%) |
|
67%(96%) | 58%(96%) | 52%(96%) | 63%(95%) | 57%(94%) | 48%(97%) |
|
57%(77%) | 77%(98%) | 87%(90%) | 96%(86%) | 93%(80%) | 79%(87%) |
yield% (purity% by LCMS with UV254 detection)
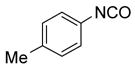
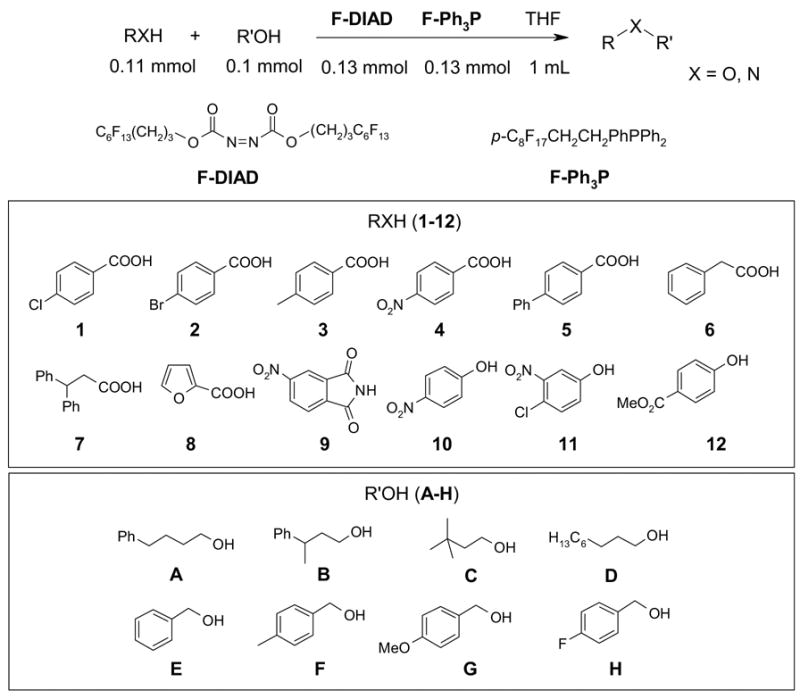
The promising preliminary results obtained from the small urea library encouraged us to conduct F-SPE purification of bigger libraries. Following the procedure reported by Curran and Dandapani,6f we performed an array of 8 x 12 Mitsunobu reactions in a 96-well plate at 0.05 mmole scale. Slight excesses of nucleophiles (1.1 equiv) and F-DIAD and F-PhPPh2 (1.2 equiv) were used. All the substrates and the reagents in THF stock solution (0.2-0.25 M) were distributed to a 96 well deep well plate using a 6-channel pipette. The reactions were completed in 1 h at room temperature. The reaction mixtures were transferred to a 96 well plate charged with silica gel-supported SAX ion exchange resin (OH counter ion, 0.25 meq/g, 0.2 g) to remove the unreacted nucleophiles. After rinsing the resin with THF, the combined filtrate collected in a plate was concentrated, re-dissolved in 0.5 mL of DMF and transferred to Eppendorf tubes for F-SPE. The product was collected in the 90:10 DMF-H2O (6 mL) fraction. The cartridge and the tubing were flushed with THF before reuse. The results are shown in Table 5 and the purity distribution is shown in Figure 2. Among 96 products, 87 have purities greater than 90% by LC-MS analysis with UV254 detection. Since two sets of products generated from acids 6 and 7 have low UV254 response, their purities were analyzed at UV230.
Table 5.
96-Parallel fluorous Mitsunobu reactions and RapidTrace F-SPE purifications
| 1 | 2 | 3 | 4 | 5 | 6c | 7c | 8 | 9 | 10 | 11 | 12 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 56a | 99b | 54 | 99 | 60 | 96 | 60 | 99 | 55 | 98 | 60 | 69 | 50 | 86 | 66 | 99 | 62 | 99 | 59 | 99 | 59 | 99 | 56 | 99 |
| B | 56 | 99 | 54 | 99 | 60 | 96 | 54 | 99 | 55 | 99 | 60 | 82 | 50 | 87 | 57 | 99 | 62 | 99 | 59 | 88 | 59 | 97 | 56 | 99 |
| C | 58 | 99 | 42 | 99 | 36 | 99 | 48 | 99 | 43 | 98 | 36 | 99 | 32 | 96 | 20 | 99 | 51 | 99 | 36 | 99 | 39 | 96 | 42 | 99 |
| D | 43 | 99 | 55 | 99 | 53 | 99 | 55 | 99 | 56 | 99 | 53 | 97 | 45 | 99 | 59 | 99 | 57 | 99 | 53 | 99 | 54 | 97 | 50 | 99 |
| E | 98 | 93 | 69 | 99 | 71 | 94 | 70 | 99 | 63 | 99 | 71 | 99 | 57 | 98 | 79 | 99 | 64 | 99 | 70 | 99 | 68 | 99 | 66 | 99 |
| F | 100 | 99 | 79 | 99 | 75 | 96 | 66 | 99 | 73 | 99 | 75 | 99 | 67 | 99 | 93 | 99 | 61 | 99 | 74 | 99 | 79 | 99 | 70 | 99 |
| G | 58 | 94 | 62 | 95 | 63 | 92 | 63 | 95 | 57 | 91 | 63 | 85 | 52 | 75 | 69 | 97 | 51 | 83 | 46 | 90 | 48 | 94 | 59 | 70 |
| H | 53 | 99 | 58 | 99 | 57 | 99 | 58 | 99 | 59 | 97 | 57 | 94 | 54 | 90 | 64 | 99 | 47 | 99 | 57 | 97 | 57 | 98 | 62 | 99 |
yield%,
purity% by LCMS with UV254 detection,
UV230 detection
Figure 2.
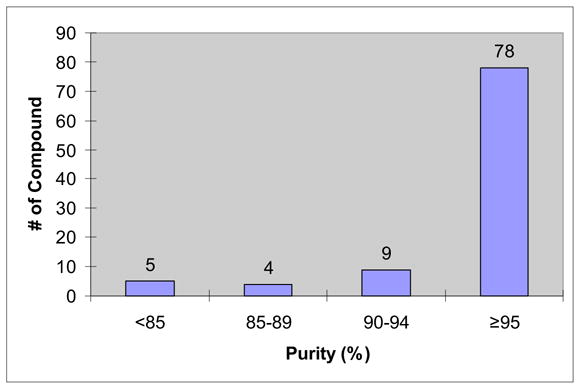
Purity distribution of 96-Mitsubonu reaction products
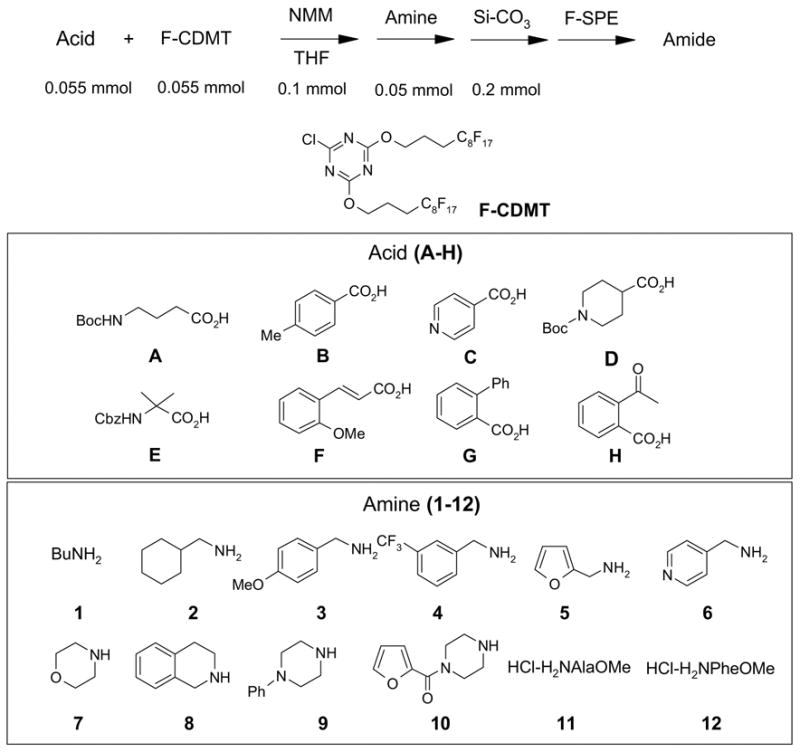
We also conducted 96 parallel reactions using 2-chloro-4,6-bis[(perfluorohexyl)propyloxy]-1,3,5-triazine (F-CDMT) as an amide coupling agent. A similar reagent has been developed by Dembinski and demonstrated in dipeptide synthesis.12 The selection of amines included six primary amines, four secondary amines, and two amino esters. The list of acids included aliphatic, aromatic and CBz-protected amino acids. All the substrates and the reagents in THF stock solution (0.275 - 0.55 M) were distributed to the plate by a 6-channel pipette. Equal amount of the acid and F-CDMT (0.055 mmole) and 0.1 mmole of N-methylmorpholine (NMM) were mixed and shaken for 30 min at 25° C before treatment with 0.05 mmole of amines. Upon the completion of reaction in 2 h, the reaction mixtures were transferred to a 96 well plate charged with silica gel-supported SAX ion exchange resin (CO3 counter ion, 0.8 meq/g, 0.25 g) to remove access acids and free the NMM base. After rinse the resin with THF, the combined filtrate collected in a plate was concentrated, re-dissolved in 1 mL of DMF and transferred to Eppendorf tubes for F-SPE. The product yields and purities for FPE are shown in Table 6 and the purity distribution is shown in Figure 3. Among 96 products, 87 have purities greater than 90% by LC-MS analysis at UV210.
Table 6.
96-Parallel F-CDMT amide coupling reactions and RapidTrace F-SPE purifications
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 64a | 99b | 74 | 99 | 75 | 99 | 85 | 99 | 78 | 92 | 68 | 99 | 74 | 94 | 75 | 98 | 69 | 96 | 71 | 99 | 73 | 99 | 66 | 99 |
| B | 49 | 99 | 69 | 99 | 71 | 99 | 75 | 99 | 84 | 99 | 62 | 99 | 29 | 99 | 64 | 86 | 29 | 99 | 60 | 99 | 60 | 99 | 74 | 99 |
| C | 17 | 99 | 83 | 93 | 91 | 96 | 67 | 99 | 71 | 96 | 66 | 94 | 42 | 87 | 76 | 98 | 75 | 99 | 77 | 96 | 36 | 80 | 14 | 62 |
| D | 71 | 93 | 80 | 99 | 75 | 99 | 78 | 99 | 65 | 97 | 69 | 99 | 67 | 99 | 64 | 73 | 70 | 99 | 77 | 99 | 73 | 99 | 72 | 99 |
| E | 23 | 99 | 72 | 99 | 73 | 99 | 71 | 99 | 57 | 99 | 61 | 99 | 33 | 99 | 40 | 73 | 10 | 93 | 25 | 99 | 77 | 99 | 75 | 99 |
| F | 76 | 99 | 59 | 99 | 67 | 98 | 60 | 99 | 54 | 99 | 37 | 96 | 73 | 98 | 48 | 85 | 68 | 99 | 47 | 99 | 48 | 98 | 59 | 94 |
| G | 65 | 99 | 82 | 98 | 76 | 98 | 73 | 99 | 58 | 97 | 49 | 56 | 60 | 99 | 70 | 88 | 64 | 93 | 56 | 99 | 81 | 99 | 84 | 99 |
| H | 65 | 99 | 77 | 99 | 64 | 99 | 75 | 99 | 66 | 99 | 71 | 99 | 52 | 99 | 72 | 99 | 71 | 99 | 67 | 99 | 61 | 95 | 80 | 73 |
yield%,
purity% by LCMS with UV210 detection
Figure 3.
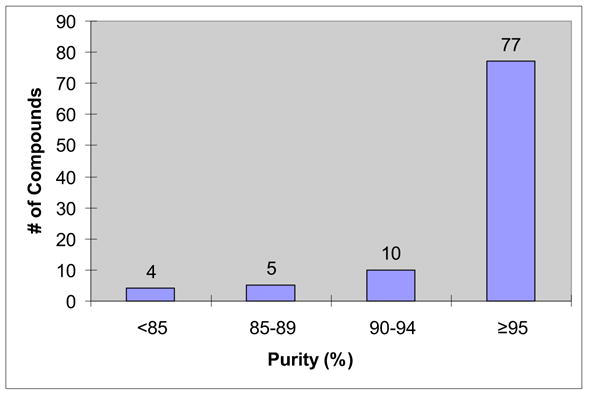
Purity distribution of 96 F-CDMT amide coupling products
Conclusion
In this study, we explored the utility of the RapidTrace workstation for automatic F-SPE. The 2 g fluorous SPE cartridge has the capability to purify up to 200 mg of crude sample. An important feature of the RapidTrace system is that it has the capability to deal with slurry samples. Important issues such as sample loading, elution, cartridge reuse and reproducibility were evaluated. The reaction mixtures generated from parallel reactions were subjected to simple workup and then dissolved in an appropriate loading solvent such as DMF for RapidTrace F-SPE. Product purities obtained from three demonstration libraries meet the general standard for parallel synthesis. This automated F-SPE technique enhances the capability of fluorous technologies in high-throughput solution-phase synthesis of compound libraries.
Experimental Section
General Methods
All fluorous reagents and silica gel (40–60 μm particle size) are available from Fluorous Technologies, Inc.13 Other reagents and solvents were obtained from commercial sources. The RapidTrace SPE system was purchased from Caliper Life Sciences.10 Whatman® 96-well plates were used for parallel reactions and post-reaction workup.14 LCMS spectra were obtained on an Agilent 1100 system. Genevac EZ-2 vacuum centrifuge was used for solvent evaporation. Products purities were determined by LCMS with a C18 column.
General Procedures for RapidTrace F-SPE
We have only two modular units in our lab. The workstation has the capability to control ten modular units. Each unit has ten 3 mL SPE cartridges charged with 2 g of fluorous silica gel. It can purify ten samples sequentially if only one fraction (either non-fluorous or fluorous) is collected. If two fractions are collected, each unit can purify five samples. The syringe pump can handle a flow rate up to 30 mL/min and back pressure up to 100 psi. The crude samples are usually dissolved in 0.5–1 mL of DMF and transferred to an Eppendorf centrifuge tube for sample loading. The Eppendorf tube reduces the residue volume after sample loading. Two standard test tubes (16x100 and 13x100 mm) are used for collection of elution fractions. The F-SPE on the RapidTrace usually has five steps: conditioning of the cartridge, loading sample, rinsing sample vial and loading, eluting and collecting, washing the cartridge and cannula. General procedures for purification of two 96 demonstration library samples are as follows: 1) condition the cartridge with 6 mL of DMF/H2O (12 mL/min); 2) load 1 mL of sample in DMF onto cartridge (15 mL/min); 3) add 1 mL of 90:10DMF/H2O to the sample vial, rinse twice, and load onto the cartridge (15 mL/min); 4) elute the cartridge with 4 mL of 90:10 DMF/H2O (12 mL/min); 5) wash the cannula with 6 mL of THF twice, 6 mL of DMF/H2O once (24 mL/min); 6) wash the cartridge with 6 mL of THF 3 times (18 mL/min). Fractions from step 2–4 were collected in the test tube. All other elution went to waste. The total run time for each cartridge is around 9 minutes.
General Procedures for Using F-DCT as an Amine Scavenger in Urea Formation Reactions.11
Each isocyanate was distributed into a rows of 6 vials placed in a 24-well plate (0.1 mmol in 0.1 mL of THF each vial). Each amine was distributed into a column of 3 vials placed in the same plate (0.12 mmol in 0.1 mL of THF each vial). The plate was shaken at 600 rpm for 1 h. DIPEA (0.05 mmol in 0.1 mL of THF) and F-DCT (0.02mmol in 0.2 mL of THF) were added to each vial and the plate were shaken at 600 rpm for 1 h at room temperature before MP-CO3 (0.2 mmol) was added. The reaction mixtures were shaken at 600 rpm for 1 h at room temperature, filtered through a 24-well filter plate and rinsed with THF (1 mL). After concentration of the filtrate, the residues were dissolved in DMF (0.5 mL) and transferred to 2 mL Eppendorf centrifuge tubes. RapidTrace F-SPEs were performed following the general procedures described above except that MeOH/H2O (80:20) was used instead of DMF/H2O. MeOH/H2O fractions were concentrated on a Genevac EZ-2 plus evaporator. The products were transferred to pre-weighed vials in CH2Cl2, concentrated on a Genevac EZ-2 plus evaporator and weighed. The purity of final products was determined by LC-MS analyses.
General Procedures for Fluorous Mitsunobu Reactions.6a,f
Each nucleophile was distributed into a column of a 96-well plate (0.055 mmol in 0.2 mL of THF each well). Each alcohol was distributed into a row of the same plate (0.05 mmol in 0.1 mL of THF each well). F-PhPPh2 (0.06 mmol in 0.1 mL of THF) and F-DIAD (0.06 mmol in 0.1 mL of THF) were added to each well sequentially. The plate was shaken at 600 rpm for 1 h at room temperature before transferred onto a 96-well plate charged with ion-exchange silica gel (SAX, OH counter ion, 0.05 mmol each well). The plate was washed with THF (0.75 mL) and the filtrates in the receiving plate were concentrated on a Genevac EZ-2 plus evaporator. The residues were dissolved in DMF (1 mL) and transferred to 2 mL Eppendorf centrifuge tubes. RapidTrace F-SPEs were performed following the general procedures described above. The DMF/H2O fractions were concentrated on a Genevac EZ-2 plus evaporator. The products were transferred to pre-weighed tubes in CH2Cl2, concentrated on a Genevac EZ-2 plus evaporator and weighed. The purity of final products was determined by LC-MS analyses.
General Procedures for Amide Coupling Reactions Using F-CDMT.9
Each carboxylic acid was distributed into a row of a 96-well plate (0.055 mmol in 0.1 mL of THF each well). NMM (0.1 mmol in 0.1 mL of THF) and F-CDMT (0.055 mmol in 0.2 mL of THF) was added to each well and the plate was shaken at 600 rpm for 30 min at room temperature. Each amine was distributed into a column of the same plate (0.05 mmol in 0.1 mL of THF each vial). The reaction mixtures were shaken at 600 rpm for 1 h at room temperature before transferred onto a 96-well plate charged with ion-exchange silica gel (SAX, CO3 counter ion, 0.2 mmol in each well). The plate was washed with THF (0.75 mL) and the filtrates in the receiving plate were concentrated on a Genevac EZ-2 plus evaporator. The residues were dissolved in DMF (1 mL) and transferred to 2 mL Eppendorf centrifuge tubes. RapidTrace F-SPEs were performed following the general procedures described above. The DMF/H2O fractions were concentrated on a Genevac EZ-2 plus evaporator. The products were transferred to pre-weighed vials in CH2Cl2, concentrated on a Genevac EZ-2 plus evaporator and weighed. The purity of final products was determined by LC-MS analyses.
Acknowledgments
This work was supported by National Institutes of General Medical Sciences SBIR Grant(2R44GM067326-02).
Footnotes
Supporting Information Available.
LC-MS of representative compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Curran DP. In: Handbook of Fluorous Chemistry. Gladysz JA, Curran DP, Horvath IT, editors. Wiley-VCH; Weinheim: 2004. pp. 101–127. [Google Scholar]; (b) Curran DP. Aldrichmica Acta. 2006;39:3–9. [Google Scholar]; (c) Zhang W. Chem Rev. 2004;104:2531–2556. doi: 10.1021/cr030600r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Fache F. New J Chem. 2004;28:1277–1283. [Google Scholar]; (e) Zhang W. Tetrahedron. 2003;59:4475–4489. [Google Scholar]; (f) Pozzi G, Shepperson I. Coord Chem Rev. 2003;242:115–124. [Google Scholar]; (g) Dobbs AP, Kimberley MR. J Fluorine Chem. 2002;118:3–17. [Google Scholar]; (h) Tzschucke CC, Markert C, Bannwarth W, Roller S, Hebel A. Angew Chem Int Ed. 2002;41:3964–4000. doi: 10.1002/1521-3773(20021104)41:21<3964::AID-ANIE3964>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 2.General reviews on fluorous silica gel-based separations: Curran DP. Synlett. 2001:1488–496.Curran DP. In: Handbook of Fluorous Chemistry. Gladysz JA, Curran DP, Horvath IT, editors. Wiley-VCH; Weinheim: 2004. pp. 101–127.Zhang W, Curran DP. Tetrahedron. 2006 in press.
- 3.Curran DP, Hadida S, He M. J Org Chem. 1997;62:6714–6715. [Google Scholar]
- 4.(a) Matsugi M, Curran DP. J Org Chem. 2005;70:1636–1642. doi: 10.1021/jo048001n. [DOI] [PubMed] [Google Scholar]; (b) Curran DP, Fischer K, Moura-Letts G. Synlett. 2004:1379–1382. [Google Scholar]; (c) Fawcett J, Hope EG, Stuart AM, West AJ. Green Chem. 2005;7:316–320. [Google Scholar]; (d) Beeler AB, Acquilano DE, Su Q, Yan F, Roth BL, Panek JS, Porco JA., Jr J Comb Chem. 2005;7:673–681. doi: 10.1021/cc050064b. [DOI] [PubMed] [Google Scholar]; (e) Simonelli B, Orlandi S, Benaglia M, Pozzi G. Eur J Org Chem. 2004:2669–2673. [Google Scholar]; (f) Dalicsek Z, Pollreisz F, Gomory A, Soos T. Org Lett. 2005;7:3243–3246. doi: 10.1021/ol051024j. [DOI] [PubMed] [Google Scholar]
- 5.(a) Zhang W, Curran DP, Chen CHT. Tetrahedron. 2002;58:3871–3875. [Google Scholar]; (b) Lindsley CW, Zhao Z, Leister WH. Tetrahedron Lett. 2002;43:4225–4228. [Google Scholar]; (c) Lindsley CW, Zhao Z, Leister WH, Strauss KA. Tetrahedron Lett. 2002;43:6319–6323. [Google Scholar]; (d) Zhang W, Chen CHT, Nagashima T. Tetrahedron Lett. 2003;44:2065–2068. [Google Scholar]; (e) Werner S, Curran DP. Org Lett. 2003;5:3293–3296. doi: 10.1021/ol035214a. [DOI] [PubMed] [Google Scholar]; (f) Zhang AS, Elmore CS, Egan MA, Melillo DG, Dean DC. J Labelled Comp Radiopharma. 2005;48:203–208. [Google Scholar]; (g) Lindsley CW, Zhao Z, Leister WH, Robinson RG, Barnett SF, Defeo-Jones D, Jones RE, Huber HE. Bioorg Med Chem Lett. 2005;15:761–764. doi: 10.1016/j.bmcl.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 6.(a) Dandapani S, Curran DP. Tetrahedron. 2002;58:3855–3864. [Google Scholar]; (b) Dobbs AP, McGregor-Johnson C. Tetrahedron Lett. 2002;43:2807–2810. [Google Scholar]; (c) Lindsley CW, Zhao Z, Newton RC, Leister WH, Strauss KA. Tetrahedron Lett. 2002;43:4467–4470. [Google Scholar]; (d) Zhang W, Chen CHT, Lu Y, Nagashima T. Org Lett. 2004;6:1473–1476. doi: 10.1021/ol0496428. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Christensen C, Clausen RP, Begtrup M, Kristensen JL. Tetrahedron Lett. 2004;45:7991–7993. [Google Scholar]; (f) Dandapani S, Curran DP. J Org Chem. 2004;69:8751–8757. doi: 10.1021/jo0488098. [DOI] [PubMed] [Google Scholar]; (g) Kaleta Z, Makowski BT, Soos T, Dembinski R. Org Lett. 2006;8:1625–1628. doi: 10.1021/ol060208a. [DOI] [PubMed] [Google Scholar]
- 7.(a) Zhang W. In: Handbook of Fluorous Chemistry. Gladysz JA, Curran DP, Horvath IT, editors. Wiley-VCH; Weinheim: 2004. pp. 222–236. [Google Scholar]; (b) Zhang W. Curr Opin Drug Disc Dev. 2004;7:784–797. [PMC free article] [PubMed] [Google Scholar]; (c) Luo ZY, Williams J, Read RW, Curran DP. J Org Chem. 2001;66:4261–4266. doi: 10.1021/jo010111w. [DOI] [PubMed] [Google Scholar]; (d) Curran DP, Amatore M, Campbell M, Go E, Guthrie D, Luo Z. J Org Chem. 2003;68:4643–4647. doi: 10.1021/jo0344283. [DOI] [PubMed] [Google Scholar]; (e) Cioffi CL, Berlin ML, Herr RJ. Synlett. 2003:841–845. [Google Scholar]; (f) Read R, Zhang C. Tetrahedron Lett. 2003;44:7045–7047. [Google Scholar]; (g) Zhang W, Lu Y. Org Lett. 2003;5:2555–2558. doi: 10.1021/ol034854a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Zhang W. Org Lett. 2003;5:1011–1014. doi: 10.1021/ol027469e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Chen CHT, Zhang W. Org Lett. 2003;5:1015–1017. doi: 10.1021/ol0274864. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Nagashima T, Zhang W. J Comb Chem. 2004;6:942–949. doi: 10.1021/cc049885r. [DOI] [PubMed] [Google Scholar]; (j) Zhang W, Chen CHT, Lu Y, Nagashima T. Org Lett. 2004;6:1473–1476. doi: 10.1021/ol0496428. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Zhang W, Tempest P. Tetrahedron Lett. 2004;45:6757–6760. [Google Scholar]; (l) Huang Y, Qing FL. Tetrahedron. 2004;65:8341–8349. [Google Scholar]; (m) Villard AL, Warrington BH, Ladlow M. J Comb Chem. 2004;6:611–622. doi: 10.1021/cc0499338. [DOI] [PubMed] [Google Scholar]; (n) McAllister LA, McCormick RA, Brand S, Procter DJ. Angew Chem Int Ed. 2005;44:452–455. doi: 10.1002/anie.200461930. [DOI] [PubMed] [Google Scholar]
- 8.(a) de Visser PC, van Helden M, Filtppov DV, van der Marel GA, Drijfhout JW, van Boom JH, Noort D, Overkleeft HS. Tetrahedron Lett. 2003;44:9013–906. [Google Scholar]; (b) Beller C, Bannwarth W. Helv Chim Acta. 2005;88:171–179. [Google Scholar]; (c) Brittain SM, Ficarro SB, Brock A, Peters EC. Nature Biotech. 2005;23:463–468. doi: 10.1038/nbt1076. [DOI] [PubMed] [Google Scholar]
- 9.Zhang W, Lu Y, Nagashima T. J Comb Chem. 2005;7:893–897. doi: 10.1021/cc050061z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caliper Life Sciences, www.caliperls.com. Pictures in Fig. 1 were provided by Mr. John de Kanel.
- 11.Lu Y, Zhang W. QSAR Comb Sci. 2006 doi: 10.1002/qsar.200640041. article asap. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Markowicz MW, Dembinski R. Synthesis. 2004:80–86. [Google Scholar]
- 13.For Fluorous Technologies, Inc. and FluoroFlash products, www.fluorous.com.
- 14.Whatman 96-well plate, www.whatman.com.


