Abstract
A number of studies reported that oxidative and nitrosative damage may be important in the pathogenesis of Alzheimer's disease (AD). However, whether oxidative damage precedes, contributes directly, or is secondary to AD pathogenesis is not known. Amnestic mild cognitive impairment (MCI) is a clinical condition that is a transition between normal aging and dementia and AD, characterized by a memory deficit without loss of general cognitive and functional abilities. Analysis of nitrosative stress in MCI could be important to determine whether nitrosative damage directly contributes to AD. In the present study, we measured the level of total protein nitration to determine if excess protein nitration occurs in brain samples from subjects with MCI compared to that in healthy controls. We demonstrated using slot blot that protein nitration is higher in the inferior parietal lobule (IPL) and hippocampus in MCI compared to those regions from control subjects. Immunohistochemistry analysis of hippocampus confirmed this result. These findings suggest that nitrosative damage occurs early in the course of MCI, and that protein nitration may be important for conversion of MCI to AD.
Keywords: Mild cognitive impairment, nitrosative stress, protein nitration, hippocampus, inferior parietal lobule, Alzheimer’s disease
1. Introduction
Oxidative and nitrosative stress result from an imbalance between oxidants and antioxidants. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) can lead to oxidative damage in the form of protein oxidation. Protein oxidation leads to protein function loss, cellular dysfunction, and ultimately cell death (Butterfield and Stadtman, 1997; Butterfield et al., 2006b; Castegna et al., 2002a; Sultana and Butterfield, 2004; Sultana et al., 2005). Among other indices, oxidative damage in a cell can be indexed by measuring the levels of protein carbonyls, tyrosine nitration, and protein adducts of alkenals such as acrolein and 4-hydroxynonenal, which are themselves reactive products of lipid peroxidation.
Tyrosine nitration is one specific form of protein oxidation that is associated with Alzheimer’s disease (AD) (Castegna et al., 2003; Gow et al., 1996; Smith et al., 1997; Sultana et al., 2006b). Nitric oxide (NO) reacting with the superoxide anion (O2.−) forms the product, peroxynitrite (ONOO−), known to lead to nitration of tyrosine (3-NT) residues (Butterfield and Kanski, 2001; Gow et al., 1996). Nitration of proteins results in the inactivation of several important mammalian proteins such as Mn superoxide dismutase (SOD), Cu/Zn SOD, actin, and tyrosine hydroxylase, and likely interfere with tyrosine phosphorylation-mediated cell signaling due to steric effects (Butterfield and Stadtman, 1997).
Mild cognitive impairment (MCI) is considered as a transition phase between normal aging and dementia (Petersen et al., 1999). Most patients experience slight memory loss as well; however, there is no evidence of dementia or alterations in the activities of daily living (Morris, 2005; Petersen et al., 1999). Conversion from amnestic MCI to dementia and AD occurs at a rate of 10% to 15% per year (Morris and Cummings, 2005) .
Previous studies from our laboratory showed elevated protein oxidation indexed by protein carbonyls in the hippocampus of AD and MCI subjects (Butterfield et al., 2006a; Castegna et al., 2002a; Castegna et al., 2002b; Sultana et al., 2006a). The current study was undertaken to compare the levels of protein nitration in MCI patients to that of control. The hypothesis of our study is that there is an elevation of nitrated proteins in the MCI brain, which therefore increases the overall protein oxidation level. The results are consistent with the notion that protein nitration is an early event in the progression of AD.
2. Results
Analysis of the overall level of nitrated proteins in brains from amnestic MCI patients demonstrates a ~25% higher level in the IPL (p<0.01) and ~41% higher level in the hippocampus region (p<0.01) compared to those regions in brains from controls (Fig. 1A). The amount of nitration in MCI IPL and hippocampus is estimated to be equivalent to the nitration of purified BSA achieved with approximately 9 mM and 27 mM peroxynitrite, respectively (Fig. 1D).
Figure 1.
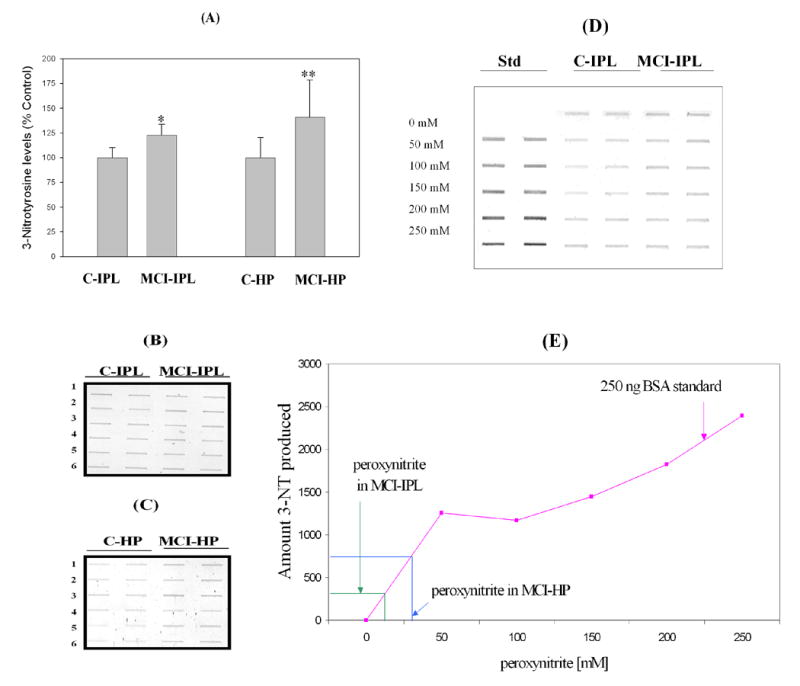
Tyrosine nitration as indexed by 3-NT immunoreactivity. ‘A’ represents the histogram obtained from Control and MCI hippocampus and IPL. ‘B’ is the slot blot results for IPL, while ‘C’ is the slot blot for hippocampus. (D) Slot blots showing standards that consist of BSA treated with defined concentrations of peroxynitrite along with control and MCI IPL *p<0.01. (E) Standard graph prepared with nitration of BSA with known concentration of peroxynitrite. The figure also shows the mean level of 3-NT in MCI hippocampus and IPL and associated concentration of peroxynitrite needed to produce these levels. Data are presented as the mean ± SEM. N=6.
Immunohistochemical staining performed with a polyclonal anti-3-nitrotyrosine antibody was far more intense in MCI (Fig. 2C) compared with that in control hippocampus (Fig. 2B). 3-nitrotyrosine was localized predominantly in neurons (indicated by arrows, Fig 2C). A negative control using rabbit IgG revealed no appreciable staining (Fig. 2A).
Figure 2.
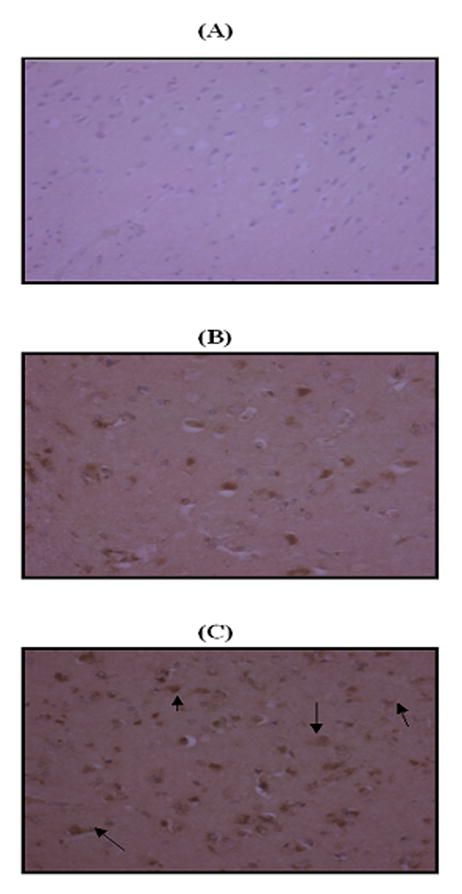
Immunohistochemical staining. ‘A’ represents the negative control using rabbit IgG. ‘B’ and ‘C’ are representative micrographs of immunohistochemistry obtained with a polyclonal antibody for 3-nitrotyrosine in control hippocampus and MCI hippocampus, respectively (×20 magnification). Intense nitrotyrosine staining is present in MCI hippocampus, whereas staining is far less prominent in control hippocampus. Nitrotyrosine is localized predominantly in neurons (arrows).
3. Discussion
A number of studies support the notion that nitrosative and oxidative injury contribute to neurodegeneration in AD (Castegna et al., 2003; Koppal et al., 1999; Smith et al., 1997; Sultana et al., 2006b; Varadarajan et al., 2000). Oxidative stress could also stimulate additional damage via the over expression of inducible (i) and neuronal (n) specific NO synthase (NOS: iNOS and nNOS) leading to increased levels of NO. Mitochondrial alterations in AD could facilitate the increased leakage of superoxide, which might also be involved in the deterioration observed in AD (Hensley et al., 1998; Koppal et al., 1999; Smith et al., 1997). NO and O2.− react at diffusion controlled rates to produce peroxynitrite, an extremely strong oxidant that causes oxidative damage to lipids, DNA, carbohydrates and proteins, particularly the amino acids cysteine, methionine, tryptophane, phenylalanine and especially tyrosine (Beckman, 1996; Koppal et al., 1999; Perry et al., 2000). Peroxynitrite can nitrate tyrosine (Halliwell, 1997) at the 3-position that, by steric effects, could prevent the phosphorylation of the OH moiety on tyrosine residues, thereby rendering that protein dysfunctional and potentially leading to cell death (Koppal et al., 1999; Yamakura et al., 1998). Peroxynitrite can also avidly react with thiols to form nitrosothiols, affecting the function of proteins (Halliwell, 1997). Nitration of proteins may lead to irreversible damage to the proteins (Beckman, 1996; Koppal et al., 1999; Perry et al., 2000; Yamakura et al., 1998) and also affect the energy status of neurons by inactivating key enzymes (Radi et al., 1994). This widespread occurrence of oxidative alterations not only decreases or eliminates the normal functions of these macromolecules (Hensley et al., 1995), but also may activate an inflammatory response (the complement cascade, cytokines, acute phase reactants and proteases) in AD brain.
Recent work demonstrates that ONOO− can induce α-synuclein oligomerization through covalent 3, 3’-dityrosine cross-linking and may facilitate the misfolding and deposition of select proteins through nitrosative and/or oxidative modification (Souza et al., 2000). Horiguchi et al. (2003) demonstrated the presence of nitrated tau in pretangles, neurofibrillary tangles, and tau inclusions in AD brain. The expression of nitration was robust in pretangles of early AD cases compared to that of more advanced cases, suggesting that tau nitration may be an early event in AD. In addition, proteins that are nitrated are more prone to proteosomal degradation than their counterparts (Gow et al., 1996). Ubiquitin carboxy-terminal hydrolase L-1 (UCH L-1), one of the components of the proteosomal pathway oxidized in the IPL and hippocampus of AD brain and known to be dysfunctional in AD (Butterfield, 2004), could be one of the reasons for the observed increased nitrated proteins in AD brain (Castegna et al., 2003; Sultana et al., 2006b).
In the present study, we observed an increase in the level of total protein nitration in both MCI hippocampus and IPL. The mean levels of the 3-NT are greater in hippocampus compared to that of IPL in subjects with amnestic MCI, consistent with the suggestion that the hippocampus is the more vulnerable region. The hippocampus is one of the most severely affected regions in AD brain, which could be due in part to functional isolation from the entorhinal cortex and subiculum, regions that convey information into and out of hippocampus, leading to loss of memory. The hippocampus is also the locus of the greatest change in levels of 3-NT and 3,3'-DT, showing increases of ~8- and 5-fold, respectively in AD, relative to age-matched controls (Smith et al., 1997).
In the current study, nitrotyrosine, as detected by immunohistochemistry, was more intense in MCI compared with control hippocampus. These findings are consistent with the quantitative data obtained from hippocampus homogenate using the slot blot method. Interestingly, nitrotyrosine staining was most intense in neurons. These cells are reported to have increased nitration in AD brain (Smith et al., 1997). It is possible that nitration of the proteins could be one of the causes of transition from MCI to AD, although this speculation awaits further studies.
Increased levels of protein carbonyls and protein-bound HNE were reported in IPL and hippocampus of subjects with MCI compared to that of controls (Butterfield et al., 2006a; Butterfield et al., 2006b), suggesting the build up of oxidative stress (Butterfield et al., 2006a; Butterfield et al., 2006b; Keller et al., 2005). A recent study from our laboratory reported the excess protein carbonylation (protein oxidation) of alpha-enolase, glutamine synthetase, pyruvate kinase M2 and peptidyl-prolyl cis/trans isomerase 1 (Pin1) in hippocampus of subjects with amnestic MCI using a redox proteomics approach (Butterfield et al., 2006a). Three of these proteins, i.e., Pin1, glutamine synthetase, and alpha enolase, were oxidatively modified in common with those in AD brain (Butterfield et al., 2006a). This approach conceivably can help better understand the progression from control to MCI to AD.
More recently, using a redox proteomics approach we reported specific nitration of alpha enolase, gamma-enolase, L-lactate dehydrogenase, triosephosphate isomerase, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), ATP synthase alpha chain, voltage dependent anion channel protein 1, and carbonic anhydrase 2 in AD brain (Castegna et al., 2003; Sultana et al., 2006b). These data support the notion that nitration of specific proteins perturbs energy metabolism, pH regulation, and mitochondrial functions, which could be involved in the mechanisms for neuronal loss and progression of AD.
The increase in protein nitration in MCI IPL and hippocampus suggest that protein nitration may be an early event in the progression from normal to AD, and further supports the hypothesis of oxidative stress as a mediator of synaptic loss and a presumed factor for the formation of neurofibrillary tangles and senile plaques (Aksenova et al., 1999; Butterfield et al., 2006b; Castegna et al., 2002a; Castegna et al., 2002b; Castegna et al., 2003; Choi et al., 2004; Sultana and Butterfield, 2004; Sultana et al., 2006a). The proteomics-mediated identification of the selected targets of brain protein nitration in subjects with MCI is in progress – studies that can help to provide insight into the mechanisms of disease progression and provide insight into development of pharmacological strategies to potentially modulate conversion of MCI to AD.
4. Materials and methods
4.1 Brain samples
The normal control subjects in this study were four females and two males, whose average age at death was 81 ± 6.4 years. The amnestic MCI patients were four females and two males, whose average age at death was 88 ± 3.8 years (Table 1). All subjects came from our longitudinally followed normal control group that has annual neuropsychological testing and neurological and physical examinations every two years. Control subjects had no cognitive complaints, normal cognitive test scores, normal objective memory test scores, and normal neurological examinations. MCI patients met the following criteria: a memory complaint that is corroborated by an associate, objective memory test impairment (age and education adjusted), general normal global intellectual function and Clinical Dementia Rating score of 0.0 to 0.5 (no dementia), and a clinical evaluation that revealed no other cause for memory decline (Morris, 2005). As also indicated in Table 1, the postmortem interval (PMI) prior to the acquisition of brain samples was approximately 3 h, with this short PMI an added advantage in studies of human brain.
Table 1.
Demographic data for subjects
| Samples (n=6) | Age (Yr) (Mean ± SD) | Gender (M/F) | Post Mortem Interval (h) (Mean ± SD) | Braak stage | Brain weight (g) |
|---|---|---|---|---|---|
| Control | 81±6.40 | 2/4 | 2.87±1.14 | I–II | 1260±120 |
| MCI | 88.0±3.8 | 2/4 | 3.125±1.033 | III–V | 1120±61 |
4.2. Slot blot
The hippocampal and inferior parietal lobule (IPL) samples were homogenized in a lysis buffer (10 mM HEPES, 137 mM NaCl, 4.6 mM KCl, 1.1 mM KH2PO4, 0.6 mM MgSO4) containing the protease inhibitors leupeptin (0.5 mg/mL), pepstatin (0.7 μg/mL), trypsin inhibitor (0.5 μg/mL), and PMSF (40 μg/mL). Homogenates were centrifuged at 15,800 x g for 10 min to remove debris. The supernatant was extracted to determine the total protein concentration by the BCA method (Pierce, Rockford, IL).
Levels of total 3-NT were determined immunochemically (Sultana et al., 2006b). Samples (5 μl) were incubated with an equal volume of modified Laemmli buffer containing 0.125 M Tris base pH 6.8, 4 % (v/v) SDS, and 20% (v/v) glycerol. The resulting sample (250 ng) was loaded per well in the slot blot apparatus. Samples were loaded onto a nitrocellulose membrane under vacuum pressure. The membrane was blocked with 3% (w/v) bovine serum albumin (BSA) in wash blot for 2 h and incubated with a 1:5000 dilution of anti-3-NT polyclonal antibody (Sigma, St. Louis, MO, USA) in wash blot for 2 h. Following completion of the primary antibody incubation, the membranes were washed three times in wash blot for 5 min each. An anti-rabbit IgG alkaline phosphatase secondary antibody (Sigma, St. Louis, MO, USA) was diluted 1:8000 in wash blot and added to the membrane for 1 h. The membrane was washed in wash blot three times for 5 min and developed using Sigmafast BCIP/NBT (5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium) tablets (Sigma, St. Louis, MO, USA). Blots were dried, scanned with Adobe Photoshop (San Jose, CA), and quantitated with Scion Image. The specificity of anti-3-nitrotyrosine antibody was confirmed by preincubation of the antibody with free 3-nitrotyrosine (10 mM) that revealed no nonspecific binding of the antibody, confirming our previous result (Sultana et al., 2004). The amount of nitration is calculated based upon the extent of nitration of bovine serum albumin (BSA) using peroxinitrite. Briefly, BSA (1mg/ml) was incubated with peroxynitrite over the concentration range of 0–250 mM at room temperature for 10 min, followed by addition of SDS and modified Laemmli buffer (mentioned above). Samples (250 ng) of nitrated-bovine serum albumin were loaded per lane in duplicate. The results are used as a standard for the calculation of the amount of nitration of hippocampus and IPL samples with respect to the quantity of peroxynitrite employed.
4.3. Immunohistochemistry
The hippocampus from MCI and control were placed in 4% formalin, processed, and embedded in paraffin. After removal of paraffin and rehydration, tissue sections were treated with 10 mM citric acid (pH 6). Sections were microwave heated (2 min, 3 times at 700 W) for antigen retrieval. Nonspecific binding was blocked with 10% normal goat serum in PBS (pH 7.4) for 30 min before incubation with polyclonal anti-nitrotyrosine antibody (1 μg/ml; Sigma-Aldrich) in PBS with 1% normal goat serum in PBS overnight at 4 °C. Tissue sections were then incubated for 30 min at room temperature with a biotinylated anti-rabbit IgG (1:800) secondary antibody by using the Vectastain ABC kit (Vector). Vector Red alkaline phosphatase substrate (Vector) was used to visualize 3-nitrotyrosine. The specificity of anti-3-nitrotyrosine antibodies was confirmed by using a nonimmune rabbit IgG (Vector) isotypic control.
4.4 Statistical analysis
The data were analyzed by Student's t tests. A value of p <0.05 was considered statistically significant.
Acknowledgments
The authors thank the University of Kentucky ADC Clinical and Neuropathology Cores for providing the brain specimens used for this study. This work was supported in part by grants from NIH to D.A.B. [AG-05119; AG-10836] and to W.R.M [AG-05119, AG-05144]. W.R.M. also thanks the Abercrombie Foundation for financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aksenova MV, Aksenov MY, Payne RM, Trojanowski JQ, Schmidt ML, Carney JM, Butterfield DA, Markesbery WR. Oxidation of cytosolic proteins and expression of creatine kinase BB in frontal lobe in different neurodegenerative disorders. Dement Geriatr Cogn Disord. 1999;10:158–65. doi: 10.1159/000017098. [DOI] [PubMed] [Google Scholar]
- Beckman JS. Oxidative damage and tyrosine nitration from peroxynitrite. Chem Res Toxicol. 1996;9:836–44. doi: 10.1021/tx9501445. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Stadtman ER. Protein oxidation processes in aging brain. Adv Cell Aging Gerontol. 1997;2:161–191. [Google Scholar]
- Butterfield DA, Kanski J. Brain protein oxidation in age-related neurodegenerative disorders that are associated with aggregated proteins. Mech Ageing Dev. 2001;122:945–62. doi: 10.1016/s0047-6374(01)00249-4. [DOI] [PubMed] [Google Scholar]
- Butterfield DA. Proteomics: a new approach to investigate oxidative stress in Alzheimer's disease brain. Brain Research. 2004;1000:1–7. doi: 10.1016/j.brainres.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Poon HF, St Clair D, Keller JN, Pierce WM, Klein JB, Markesbery WR. Redox proteomics identification of oxidatively modified hippocampal proteins in mild cognitive impairment: Insights into the development of Alzheimer's disease. Neurobiol Dis. 2006a;22:223–232. doi: 10.1016/j.nbd.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Reed T, Perluigi M, De Marco C, Coccia R, Cini C, Sultana R. Elevated protein-bound levels of the lipid peroxidation product, 4-hydroxy-2-nonenal, in brain from persons with mild cognitive impairment. Neurosci Lett. 2006b;397:170–3. doi: 10.1016/j.neulet.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Castegna A, Aksenov M, Aksenova M, Thongboonkerd V, Klein JB, Pierce WM, Booze R, Markesbery WR, Butterfield DA. Proteomic identification of oxidatively modified proteins in Alzheimer's disease brain. Part I: creatine kinase BB, glutamine synthase, and ubiquitin carboxy-terminal hydrolase L-1. Free Radic Biol Med. 2002a;33:562–71. doi: 10.1016/s0891-5849(02)00914-0. [DOI] [PubMed] [Google Scholar]
- Castegna A, Aksenov M, Thongboonkerd V, Klein JB, Pierce WM, Booze R, Markesbery WR, Butterfield DA. Proteomic identification of oxidatively modified proteins in Alzheimer's disease brain. Part II: dihydropyrimidinase-related protein 2, alpha-enolase and heat shock cognate 71. J Neurochem. 2002b;82:1524–32. doi: 10.1046/j.1471-4159.2002.01103.x. [DOI] [PubMed] [Google Scholar]
- Castegna A, Thongboonkerd V, Klein JB, Lynn B, Markesbery WR, Butterfield DA. Proteomic identification of nitrated proteins in Alzheimer's disease brain. J Neurochem. 2003;85:1394–401. doi: 10.1046/j.1471-4159.2003.01786.x. [DOI] [PubMed] [Google Scholar]
- Choi J, Levey AI, Weintraub ST, Rees HD, Gearing M, Chin LS, Li L. Oxidative modifications and down-regulation of ubiquitin carboxyl-terminal hydrolase L1 associated with idiopathic Parkinson's and Alzheimer's diseases. J Biol Chem. 2004;279:13256–64. doi: 10.1074/jbc.M314124200. [DOI] [PubMed] [Google Scholar]
- Gow AJ, Duran D, Malcolm S, Ischiropoulos H. Effects of peroxynitrite-induced protein modifications on tyrosine phosphorylation and degradation. FEBS Lett. 1996;385:63–6. doi: 10.1016/0014-5793(96)00347-x. [DOI] [PubMed] [Google Scholar]
- Halliwell B. What nitrates tyrosine? Is nitrotyrosine specific as a biomarker of peroxynitrite formation in vivo? FEBS Lett. 1997;411:157–60. doi: 10.1016/s0014-5793(97)00469-9. [DOI] [PubMed] [Google Scholar]
- Hensley K, Hall N, Subramaniam R, Cole P, Harris M, Aksenov M, Aksenova M, Gabbita SP, Wu JF, Carney JM, Lovell M, Markesbery WR, Butterfield DA. Brain regional correspondence between Alzheimer's disease histopathology and biomarkers of protein oxidation. J Neurochem. 1995;65:2146–56. doi: 10.1046/j.1471-4159.1995.65052146.x. [DOI] [PubMed] [Google Scholar]
- Hensley K, Maidt ML, Yu Z, Sang H, Markesbery WR, Floyd RA. Electrochemical analysis of protein nitrotyrosine and dityrosine in the Alzheimer brain indicates region-specific accumulation. J Neurosci. 1998;18:8126–32. doi: 10.1523/JNEUROSCI.18-20-08126.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksenova MV, Aksenov MY, Payne RM, Trojanowski JQ, Schmidt ML, Carney JM, Butterfield DA, Markesbery WR. Oxidation of cytosolic proteins and expression of creatine kinase BB in frontal lobe in different neurodegenerative disorders. Dement Geriatr Cogn Disord. 1999;10:158–65. doi: 10.1159/000017098. [DOI] [PubMed] [Google Scholar]
- Beckman JS. Oxidative damage and tyrosine nitration from peroxynitrite. Chem Res Toxicol. 1996;9:836–44. doi: 10.1021/tx9501445. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Stadtman ER. Protein oxidation processes in aging brain. Adv Cell Aging Gerontol. 1997;2:161–191. [Google Scholar]
- Butterfield DA, Kanski J. Brain protein oxidation in age-related neurodegenerative disorders that are associated with aggregated proteins. Mech Ageing Dev. 2001;122:945–62. doi: 10.1016/s0047-6374(01)00249-4. [DOI] [PubMed] [Google Scholar]
- Butterfield DA. Proteomics: a new approach to investigate oxidative stress in Alzheimer's disease brain. Brain Research. 2004;1000:1–7. doi: 10.1016/j.brainres.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Poon HF, St Clair D, Keller JN, Pierce WM, Klein JB, Markesbery WR. Redox proteomics identification of oxidatively modified hippocampal proteins in mild cognitive impairment: Insights into the development of Alzheimer's disease. Neurobiol Dis. 2006a;22:223–232. doi: 10.1016/j.nbd.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Reed T, Perluigi M, De Marco C, Coccia R, Cini C, Sultana R. Elevated protein-bound levels of the lipid peroxidation product, 4-hydroxy-2-nonenal, in brain from persons with mild cognitive impairment. Neurosci Lett. 2006b;397:170–3. doi: 10.1016/j.neulet.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Castegna A, Aksenov M, Aksenova M, Thongboonkerd V, Klein JB, Pierce WM, Booze R, Markesbery WR, Butterfield DA. Proteomic identification of oxidatively modified proteins in Alzheimer's disease brain. Part I: creatine kinase BB, glutamine synthase, and ubiquitin carboxy-terminal hydrolase L-1. Free Radic Biol Med. 2002a;33:562–71. doi: 10.1016/s0891-5849(02)00914-0. [DOI] [PubMed] [Google Scholar]
- Castegna A, Aksenov M, Thongboonkerd V, Klein JB, Pierce WM, Booze R, Markesbery WR, Butterfield DA. Proteomic identification of oxidatively modified proteins in Alzheimer's disease brain. Part II: dihydropyrimidinase-related protein 2, alpha-enolase and heat shock cognate 71. J Neurochem. 2002b;82:1524–32. doi: 10.1046/j.1471-4159.2002.01103.x. [DOI] [PubMed] [Google Scholar]
- Castegna A, Thongboonkerd V, Klein JB, Lynn B, Markesbery WR, Butterfield DA. Proteomic identification of nitrated proteins in Alzheimer's disease brain. J Neurochem. 2003;85:1394–401. doi: 10.1046/j.1471-4159.2003.01786.x. [DOI] [PubMed] [Google Scholar]
- Choi J, Levey AI, Weintraub ST, Rees HD, Gearing M, Chin LS, Li L. Oxidative modifications and down-regulation of ubiquitin carboxyl-terminal hydrolase L1 associated with idiopathic Parkinson's and Alzheimer's diseases. J Biol Chem. 2004;279:13256–64. doi: 10.1074/jbc.M314124200. [DOI] [PubMed] [Google Scholar]
- Gow AJ, Duran D, Malcolm S, Ischiropoulos H. Effects of peroxynitrite-induced protein modifications on tyrosine phosphorylation and degradation. FEBS Lett. 1996;385:63–6. doi: 10.1016/0014-5793(96)00347-x. [DOI] [PubMed] [Google Scholar]
- Halliwell B. What nitrates tyrosine? Is nitrotyrosine specific as a biomarker of peroxynitrite formation in vivo? FEBS Lett. 1997;411:157–60. doi: 10.1016/s0014-5793(97)00469-9. [DOI] [PubMed] [Google Scholar]
- Hensley K, Hall N, Subramaniam R, Cole P, Harris M, Aksenov M, Aksenova M, Gabbita SP, Wu JF, Carney JM, et al. Brain regional correspondence between Alzheimer's disease histopathology and biomarkers of protein oxidation. J Neurochem. 1995;65:2146–56. doi: 10.1046/j.1471-4159.1995.65052146.x. [DOI] [PubMed] [Google Scholar]
- Hensley K, Maidt ML, Yu Z, Sang H, Markesbery WR, Floyd RA. Electrochemical analysis of protein nitrotyrosine and dityrosine in the Alzheimer brain indicates region-specific accumulation. J Neurosci. 1998;18:8126–32. doi: 10.1523/JNEUROSCI.18-20-08126.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller JN, Schmitt FA, Scheff SW, Ding Q, Chen Q, Butterfield DA, Markesbery WR. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology. 2005;64:1152–6. doi: 10.1212/01.WNL.0000156156.13641.BA. [DOI] [PubMed] [Google Scholar]
- Koppal T, Drake J, Yatin S, Jordan B, Varadarajan S, Bettenhausen L, Butterfield DA. Peroxynitrite-induced alterations in synaptosomal membrane proteins: insight into oxidative stress in Alzheimer's disease. J Neurochem. 1999;72:310–7. doi: 10.1046/j.1471-4159.1999.0720310.x. [DOI] [PubMed] [Google Scholar]
- Morris JC. Mild cognitive impairment and preclinical Alzheimer's disease. Geriatrics. 2005;(Suppl):9–14. [PubMed] [Google Scholar]
- Morris JC, Cummings J. Mild cognitive impairment (MCI) represents early-stage Alzheimer's disease. J Alzheimers Dis. 2005;7:235–9. doi: 10.3233/jad-2005-7306. discussion 255–62. [DOI] [PubMed] [Google Scholar]
- Perry JM, Zhao Y, Marletta MA. Cu2+ and Zn2+ inhibit nitric-oxide synthase through an interaction with the reductase domain. J Biol Chem. 2000;275:14070–6. doi: 10.1074/jbc.275.19.14070. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–8. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Radi R, Rodriguez M, Castro L, Telleri R. Inhibition of mitochondrial electron transport by peroxynitrite. Arch Biochem Biophys. 1994;308:89–95. doi: 10.1006/abbi.1994.1013. [DOI] [PubMed] [Google Scholar]
- Smith MA, Richey Harris PL, Sayre LM, Beckman JS, Perry G. Widespread peroxynitrite-mediated damage in Alzheimer's disease. J Neurosci. 1997;17:2653–7. doi: 10.1523/JNEUROSCI.17-08-02653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza JM, Giasson BI, Chen Q, Lee VM, Ischiropoulos H. Dityrosine cross-linking promotes formation of stable alpha -synuclein polymers. Implication of nitrative and oxidative stress in the pathogenesis of neurodegenerative synucleinopathies. J Biol Chem. 2000;275:18344–9. doi: 10.1074/jbc.M000206200. [DOI] [PubMed] [Google Scholar]
- Sultana R, Butterfield DA. Oxidatively modified GST and MRP1 in alzheimer's disease brain: Implications for accumulation of reactive lipid peroxidation products. Neurochemical Research. 2004;29:2215–2220. doi: 10.1007/s11064-004-7028-0. [DOI] [PubMed] [Google Scholar]
- Sultana R, Newman S, Mohmmad-Abdul H, Keller JN, Butterfield DA. Protective effect of the xanthate, D609, on Alzheimer's amyloid beta-peptide (1–42)-induced oxidative stress in primary neuronal cells. Free Radic Res. 2004;38:449–58. doi: 10.1080/1071576042000206478. [DOI] [PubMed] [Google Scholar]
- Sultana R, Ravagna A, Mohmmad-Abdul H, Calabrese V, Butterfield DA. Ferulic acid ethyl ester protects neurons against amyloid beta- peptide(1–42)-induced oxidative stress and neurotoxicity: relationship to antioxidant activity. J Neurochem. 2005;92:749–58. doi: 10.1111/j.1471-4159.2004.02899.x. [DOI] [PubMed] [Google Scholar]
- Sultana R, Boyd-Kimball D, Poon HF, Cai J, Pierce WM, Klein JB, Merchant M, Markesbery WR, Butterfield DA. Redox proteomics identification of oxidized proteins in Alzheimer's disease hippocampus and cerebellum: An approach to understand pathological and biochemical alterations in AD. Neurobiol Aging. 2006a;27:1564–1576. doi: 10.1016/j.neurobiolaging.2005.09.021. [DOI] [PubMed] [Google Scholar]
- Sultana R, Poon HF, Cai J, Pierce WM, Merchant M, Klein JB, Markesbery WR, Butterfield DA. Identification of nitrated proteins in Alzheimer's disease brain using a redox proteomics approach. Neurobiol Dis. 2006b;22:76–87. doi: 10.1016/j.nbd.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Varadarajan S, Yatin S, Aksenova M, Butterfield DA. Review: Alzheimer's amyloid beta-peptide-associated free radical oxidative stress and neurotoxicity. J Struct Biol. 2000;130:184–208. doi: 10.1006/jsbi.2000.4274. [DOI] [PubMed] [Google Scholar]
- Yamakura F, Taka H, Fujimura T, Murayama K. Inactivation of human manganese-superoxide dismutase by peroxynitrite is caused by exclusive nitration of tyrosine 34 to 3-nitrotyrosine. J Biol Chem. 1998;273:14085–9. doi: 10.1074/jbc.273.23.14085. [DOI] [PubMed] [Google Scholar]
- Keller JN, Schmitt FA, Scheff SW, Ding Q, Chen Q, Butterfield DA, Markesbery WR. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology. 2005;64:1152–6. doi: 10.1212/01.WNL.0000156156.13641.BA. [DOI] [PubMed] [Google Scholar]
- Koppal T, Drake J, Yatin S, Jordan B, Varadarajan S, Bettenhausen L, Butterfield DA. Peroxynitrite-induced alterations in synaptosomal membrane proteins: insight into oxidative stress in Alzheimer's disease. J Neurochem. 1999;72:310–7. doi: 10.1046/j.1471-4159.1999.0720310.x. [DOI] [PubMed] [Google Scholar]
- Morris JC. Mild cognitive impairment and preclinical Alzheimer's disease. Geriatrics. 2005;(Suppl):9–14. [PubMed] [Google Scholar]
- Morris JC, Cummings J. Mild cognitive impairment (MCI) represents early-stage Alzheimer's disease. J Alzheimers Dis. 2005;7:235–9. doi: 10.3233/jad-2005-7306. discussion 255–62. [DOI] [PubMed] [Google Scholar]
- Perry JM, Zhao Y, Marletta MA. Cu2+ and Zn2+ inhibit nitric-oxide synthase through an interaction with the reductase domain. J Biol Chem. 2000;275:14070–6. doi: 10.1074/jbc.275.19.14070. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–8. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Radi R, Rodriguez M, Castro L, Telleri R. Inhibition of mitochondrial electron transport by peroxynitrite. Arch Biochem Biophys. 1994;308:89–95. doi: 10.1006/abbi.1994.1013. [DOI] [PubMed] [Google Scholar]
- Smith MA, Richey Harris PL, Sayre LM, Beckman JS, Perry G. Widespread peroxynitrite-mediated damage in Alzheimer's disease. J Neurosci. 1997;17:2653–7. doi: 10.1523/JNEUROSCI.17-08-02653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza JM, Giasson BI, Chen Q, Lee VM, Ischiropoulos H. Dityrosine cross-linking promotes formation of stable alpha -synuclein polymers. Implication of nitrative and oxidative stress in the pathogenesis of neurodegenerative synucleinopathies. J Biol Chem. 2000;275:18344–9. doi: 10.1074/jbc.M000206200. [DOI] [PubMed] [Google Scholar]
- Sultana R, Butterfield DA. Oxidatively modified GST and MRP1 in alzheimer's disease brain: Implications for accumulation of reactive lipid peroxidation products. Neurochemical Research. 2004;29:2215–2220. doi: 10.1007/s11064-004-7028-0. [DOI] [PubMed] [Google Scholar]
- Sultana R, Newman S, Mohmmad-Abdul H, Keller JN, Butterfield DA. Protective effect of the xanthate, D609, on Alzheimer's amyloid beta-peptide (1–42)-induced oxidative stress in primary neuronal cells. Free Radic Res. 2004;38:449–58. doi: 10.1080/1071576042000206478. [DOI] [PubMed] [Google Scholar]
- Sultana R, Ravagna A, Mohmmad-Abdul H, Calabrese V, Butterfield DA. Ferulic acid ethyl ester protects neurons against amyloid beta- peptide(1–42)-induced oxidative stress and neurotoxicity: relationship to antioxidant activity. J Neurochem. 2005;92:749–58. doi: 10.1111/j.1471-4159.2004.02899.x. [DOI] [PubMed] [Google Scholar]
- Sultana R, Boyd-Kimball D, Poon HF, Cai J, Pierce WM, Klein JB, Merchant M, Markesbery WR, Butterfield DA. Redox proteomics identification of oxidized proteins in Alzheimer's disease hippocampus and cerebellum: An approach to understand pathological and biochemical alterations in AD. Neurobiol Aging. 2006a;27:1564–1576. doi: 10.1016/j.neurobiolaging.2005.09.021. [DOI] [PubMed] [Google Scholar]
- Sultana R, Poon HF, Cai J, Pierce WM, Merchant M, Klein JB, Markesbery WR, Butterfield DA. Identification of nitrated proteins in Alzheimer's disease brain using a redox proteomics approach. Neurobiol Dis. 2006b;22:76–87. doi: 10.1016/j.nbd.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Varadarajan S, Yatin S, Aksenova M, Butterfield DA. Review: Alzheimer's amyloid beta-peptide-associated free radical oxidative stress and neurotoxicity. J Struct Biol. 2000;130:184–208. doi: 10.1006/jsbi.2000.4274. [DOI] [PubMed] [Google Scholar]
- Yamakura F, Taka H, Fujimura T, Murayama K. Inactivation of human manganese-superoxide dismutase by peroxynitrite is caused by exclusive nitration of tyrosine 34 to 3-nitrotyrosine. J Biol Chem. 1998;273:14085–9. doi: 10.1074/jbc.273.23.14085. [DOI] [PubMed] [Google Scholar]


