Abstract
Background
Pbx genes encode TALE class homeodomain transcription factors that pattern the developing neural tube, pancreas, and blood. Within the hindbrain, Pbx cooperates with Hox proteins to regulate rhombomere segment identity. Pbx cooperates with Eng to regulate midbrain-hindbrain boundary maintenance, and with MyoD to control fast muscle cell differentiation. Although previous results have demonstrated that Pbx is required for proper eye size, functions in regulating retinal cell identity and patterning have not yet been examined.
Results
Analysis of retinal ganglion cell axon pathfinding and outgrowth in pbx2/4 null embryos demonstrated a key role for pbx genes in regulating neural cell behavior. To identify Pbx-dependent genes involved in regulating retino-tectal pathfinding, we conducted a microarray screen for Pbx-dependent transcripts in zebrafish, and detected genes that are specifically expressed in the eye and tectum. A subset of Pbx-dependent retinal transcripts delineate specific domains in the dorso-temporal lobe of the developing retina. Furthermore, we determined that some Pbx-dependent transcripts also require Meis1 and Gdf6a function. Since gdf6a expression is also dependent on Pbx, we propose a model in which Pbx proteins regulate expression of the growth factor gdf6a, which in turn regulates patterning of the dorso-temporal lobe of the retina. This, in concert with aberrant tectal patterning in pbx2/4 null embryos, may lead to the observed defects in RGC outgrowth.
Conclusion
These data define a novel role for Pbx in patterning the vertebrate retina and tectum in a manner required for proper retinal ganglion cell axon outgrowth.
Background
Vertebrate embryos use a combination of transcription factors to specify position along the anterior-posterior (A-P) axis. Of particular importance is the Pbx (pre-B cell leukemia homeobox)-family of TALE (Three Amino acid Loop Extension)-class transcription factors, which are required globally to pattern the A-P axis of the developing vertebrate embryo. Using mouse knockout and zebrafish knockdown models, researchers have shown that Pbx proteins are required to specify cell fate in the midbrain, hindbrain, somites, pancreas, and blood [1-4]. In the hindbrain, trimeric DNA-binding complexes of Pbx, Hox and Meinox (Meis/Pknox) proteins specify rhombomere identity. In the midbrain, Pbx cooperates biochemically with Engrailed (Eng) proteins to maintain both midbrain-hindbrain and the diencephalic-mesencephalic boundaries [2,5].
Pbx clearly also plays a role in patterning regions outside of the midbrain and hindbrain. For example, mouse knockouts have demonstrated a critical role for Pbx during pancreatic development, in which interactions between Pbx and Insulin promoter factor 1 (Ipf1) are required for transcriptional activity, and subsequent expansion of pancreatic cell lineages [3]. Pbx also plays a role in the development of blood, as Pbx – Prep1 (also known as Pknox1) complexes are required for the production of normal populations of CD4 and CD8 T-lymphocytes [6]. Furthermore, Pbx – Meis complexes have been implicated in megakaryocyte differentiation in rats, through the ability to initiate transcription from the platelet factor 4 (PF4) promoter [4]. Recently, it has been shown during the development of skeletal muscle that Pbx is constitutively bound to the Myogenin promoter, can bind directly to the bHLH transcription factor MyoD, and is thus required for the development of muscle cell fates [7]. A zebrafish mutant, lazarus (lzr), that contains a null mutation in the pbx4 gene [8], displays global defects in embryonic patterning including hindbrain, muscle, blood, and midbrain tissues.
The Meinox (Meis/Pknox/Prep) family of TALE-class transcription factors are well characterized as DNA-binding cofactors for both Pbx and Hox proteins [9]. Zebrafish contain at least six meis genes that are expressed in dynamic and tissue specific manners [1,10-12]. In addition, zebrafish possess at least two pknox/prep genes that are both widely expressed [13]. meis genes are prominently expressed in the developing retina [1,10-12] suggesting a role for meis in regulating eye formation or patterning. In zebrafish, overexpression of hoxb2 throughout the embryo causes ectopic expression of hindbrain markers exclusively within the retina, demonstrating the existence of retinal specific Hoxb2 competency factors [14]. Pbx and Meinox proteins function as two of these retinal Hoxb2-competency factors, demonstrating that both Pbx and Meis can function in the retina [1,8,15,16]. The idea that Meinox proteins play a role in eye formation is supported by studies in Drosophila, where the pbx homologue extradenticle (exd) and the meis homologue homothorax (hth) inhibit eye formation [17], and in mice where the Pknox1 hypomorphic and Meis1 knockout phenotypes include defects in retinal development. [16,18]
Zebrafish make an excellent model for the study of retinal development. The optic primordium is distinct from surrounding tissues as early as 12 hours post fertilization (hpf), and by 24 hpf, eyecups have developed to include lens tissue and the surrounding neural retina [19]. By 30 hpf, the first post-mitotic neurons have differentiated [20] and by 50 hpf, lamination is evident across the retina. Two partially redundant pbx genes, pbx2 and pbx4, are expressed during early development when the optic primordium and optic cup are developing. The other zebrafish pbx genes, pbx1 and pbx3.1, are expressed more strongly starting at 24 hpf. Thus, inhibition of Pbx2 and Pbx4 in a homozygotic lazarus (lzr) (pbx4 mutant) embryo allows for analyses of development in the absence of Pbx function, prior to 24 hpf (see methods). The retina of lazarus (pbx4-/-) zebrafish embryos is noticeably smaller than wild type embryos, but no analysis of patterning has yet been undertaken. This manuscript provides an analysis of Pbx and Meis function in the developing zebrafish retina, as well as a role for Pbx in patterning the tectum, and includes a microarray screen for pbx dependent transcripts in zebrafish to identify gene regulatory networks downstream of this critical homeodomain protein. We propose a model in which Pbx functions to specify regional identity in the developing zebrafish retina via activation of the dorsal/temporal specific growth factor, gdf6a.
Results
Retinal laminar structure is normal but retinal ganglion cell axon outgrowth is aberrant in pbx2/4 null embryos
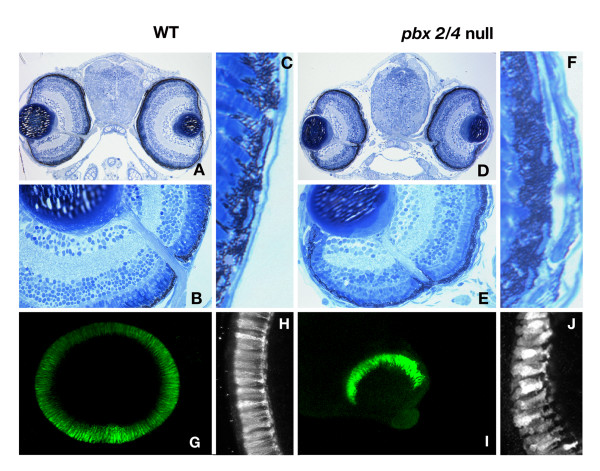
Previous studies have demonstrated a clear role for the homeodomain transcription factor Pbx in regulating cell fates in the hindbrain and at the midbrain-hindbrain boundary. The eyes of lazarus (pbx4-/-) embryos are distinctly smaller than their wild type counterparts, indicating that Pbx is required in this tissue as well [8]. As a first step towards dissecting the role of Pbx proteins in eye development, we compared eye morphology between wild type and pbx2/4 null (pbx4-/-; pbx2/4 MO) at 5 days post-fertilization (dpf). The laminar structure of the retina appears relatively normal in pbx2/4 null embryos (compare wild types in Figure 1A, B to pbx2/4 nulls in Figure 1D, E) and the optic nerve exits the eye in the correct location. All nuclear layers are clearly visible in pbx2/4 null eyes, including the inner most retinal ganglion cell layer, and the outermost nuclear layer containing the photoreceptor cells. The retinal pigmented epithelium (RPE) of pbx2/4 null embryos is thickened (9.14 +/- 1.9 % of total eye area, Figure 1F) in comparison to wild type controls (5.95 +/- 0.3 % of total eye area, p < 0.05, Figure 1C), indicating that Pbx has a role in regulating RPE differentiation. There is also a distinct change in layer morphology, at the interface of the RPE and photoreceptor layer. In addition to thickened RPE, pbx2/4 null embryos have a disorganized photoreceptor layer (compare Figure 1H with 1J), which likely contributes to the aberrant morphology seen at the interface. These data suggest that while pbx2/4 null eyes are smaller than their wild type counterparts, Pbx proteins are not required for establishing the laminar structure of the eye.
Figure 1.
The laminar structure of pbx2/4 null eyes is normal after 5 days of development. All laminar layers are present and in both wild type (A and B) and, and lzr mutants injected with pbx2/4 morpholinos (D and E). We note a consistent decrease in eye size in pbx2/4 null embryos, but the proportion of eye area occupied by the retinal pigmented epithelium in morphants is significantly increased (F verses C). The relative position of the optic nerve is similar in both wild type and morphant embryos. The retinal photoreceptor layer is absent in the ventral domain of the eye in lzr mutants injected with pbx2/4 morpholinos (I) when compared to wildtype (G) and is disorganized (compare H, wildtype, with J, lzr mutants injected with pbx2/4 morpholinos). Photoreceptor labeling was accomplished using the zpr-1 monoclonal antibody.
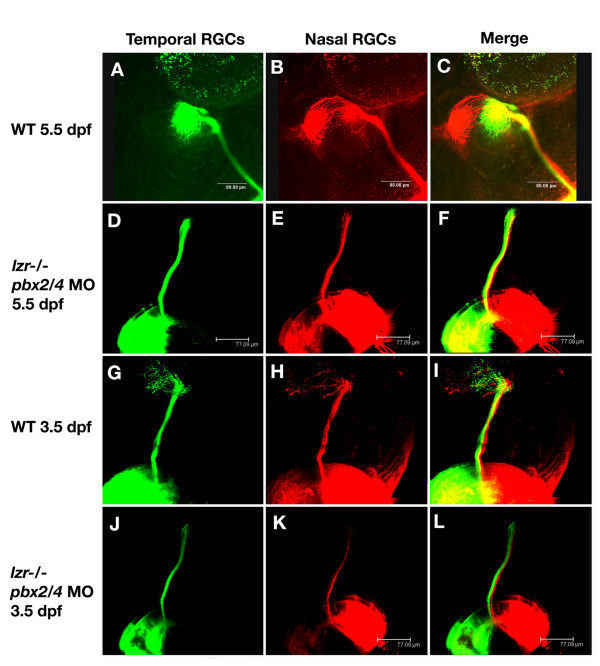
In wild type embryos, retinal ganglion cells innervate the optic tectum to form an inverse topographic map of the eye. Axons that originate in the temporal retina innervate the anterior tectum (Figure 2A, C), while axons that originate in the nasal retina innervate the posterior tectum (Figure 2B, C). In embryos lacking both pbx2 and pbx4, no topographic mapping of the RGC axons is observed at 3.5 or 5.5 dpf. In the majority of embryos (6/7 at 3.5dpf, 11/14 5.5dpf), the primary axon bundle projects toward the optic tectum, but axons appear stalled before entry to the tectum as no branching or outgrowth is observed (Figure 2D–F, 2J–L). These results suggest that Pbx proteins regulate transcriptional pathways that control RGC axonal outgrowth and topographic mapping.
Figure 2.
pbx2/4 null embryos exhibit RGC axonal outgrowth defects. In wild type embryos at 5.5 dpf, temporal RGC axons map to the anterior optic tectum (A) and (C), while nasal RGC axons map to the posterior region of the optic tectum (B) and (C). This is also seen at 3.5 dpf (G-I) although the connections on the tectum are more diffuse. In lzr-/- mutants injected with pbx2/4 morpholinos, both posterior and anterior RGC axons fail to map onto the optic tectum at both 3.5 dpf (J-L) and 5.5 dpf (D-F).
Whole embryo microarray analysis of Pbx mutants identifies known hindbrain patterning genes
In the presumptive hindbrain, Pbx2 and Pbx4 functions are critically required for initiation of early growth regulator 2b (egr2b, previously krox20), musculoaponeurotic fibrosarcoma oncogene family protein B (mafb, previously valentino), homeobox b1A (hoxb1a), hoxa2b, and hoxb2. To identify Pbx-dependent transcripts that might regulate retinotectal patterning, we conducted microarray comparisons between 18 hpf control embryos (pbx2 MO injected) and pbx2/4 null embryos (mz lzr; pbx2 MO). Each array contained 8448 cDNA probes, including 768 controls spots (e.g. negative, positive, and normalization controls) derived from a normalized mixed stage zebrafish cDNA library [21]. We identified 31 transcripts that were downregulated by at least 2 fold and 284 transcripts that were downregulated by at least 1.5 fold. We repeated these analyses using two independent biological replicates at 18 hpf, two biological replicates at 28 hpf, and using two biological replicates of 18 hpf zygotic lzr embryos injected with hoxb1a and hoxb1b morpholinos, as these phenotypically mimic the pbx2/4 null phenotype in the hindbrain [2]. Overall, we identified 366 ESTs that were downregulated by 1.5 fold in at least two datasets. Student T-tests were conducted on genes of interest based on annotation and known expression patterns. Focusing on clones that were affected at 18 hours, we generated antisense probes and sequence information for 132 EST clones. We examined 70 of these clones by in situ hybridization, to which 33 showed Pbx dependence at 18 hpf. Among these clones, we identified egr2b, hoxb3a, mafb, and engrailed 2b (eng2b, previously eng3) (Table 1). Each of these clones is expressed in either the midbrain-hindbrain boundary or the hindbrain at 18 hpf and were previously shown to be downregulated in pbx2/4 null embryos [2]. Given this validation, the microarray is clearly capable of identifying bona fide Pbx dependent transcripts.
Table 1.
Microarray identified Pbx-dependent transcripts expressed in the hindbrain, eye, and tectum
| gene | Expression pattern | Accession | Average fold change 18hpf | M | T |
| egr2b | rhombomere 3, 5 | NM_130997 | 7.98 | 3.00 | 35.71 |
| mafb | rhombomere 5 | AB006322 | 2.09 | 1.06 | 16.63 |
| meis3 | central nervous system, neural crest | NM_131778 | 1.88 | 0.91 | 5.54 |
| eng2b | midbrain/hindbrain boundary | NM_131040 | 2.94 | 1.33 | 3.17 |
| eng2a | mid/hindbrain boundary | NM_131044 | 1.78 | 0.83 | 3.14 |
| aldh1a2 | posterior retina, somites | NM_131850 | 1.80 | 0.83 | 3.46 |
| tbx5 | heart, pectoral fin, posterior retina | NM_130915 | 1.88 | 0.59 | 14.80 |
| hmx3b | lens, dorsal retina | NM_001045371 | 1.38 | 0.46 | 18.14 |
| fabp7 | eye, tectum, midbrain, hindbrain | NM_021272 | 2.10 | 1.07 | 16.63 |
| efna2 | tectum, midbrain, mid/hindbrain boundary | BC096783 | 1.37 | 0.46 | 2.87 |
| nat10 | eye, tectum, midbrain, somites | BC057462 | 1.39 | 0.48 | 6.53 |
M, log2 (WT/lzr, pbx mo)
T, ratio of mean difference/standard deviation
Microarray and whole mount in situ analysis demonstrates that Pbx proteins regulate gene expression in the developing retina
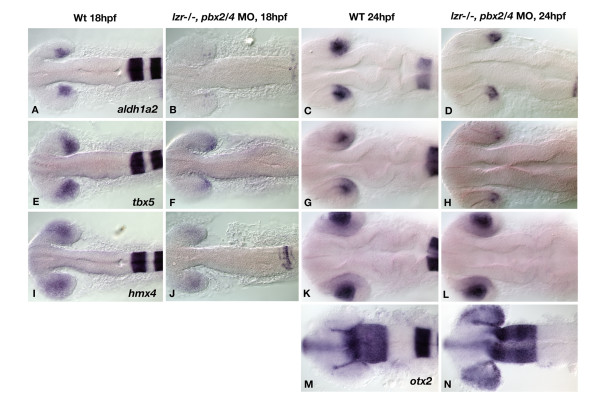
Microarray analysis also uncovered Pbx dependent transcripts expressed specifically in regions outside of the hindbrain, notably including genes expressed in the muscle [22] and in the retina and tectum (described in this manuscript). Of the 132 ESTs studied, 13 are specifically expressed in the retina, tectum, or both. Three genes, aldehyde dehydrogenase 1 family, member A2 (aldh1a2, previously raldh2), T-box 5 (tbx5), and hmx4 (ortholog of chick soho1) are expressed in the dorsal retina. The transcripts for aldh1a2, and tbx5 are expressed in the dorsal/temporal retina at both 18 and 24 hpf (Figure 3A and 3C, E and 3G), while hmx4 is specific to lens and dorsal retina (Figure 3I and 3K). To examine retinal patterning in pbx2/4 null embryos, we performed in situ hybridization with these three putative Pbx dependent genes as well as orthodenticle homolog 2 (otx2), a marker of retinal pigmented epithelium and the optic tectum at 24 hpf [23,24]. Three of these genes, aldh1a2, tbx5, and hmx4 are strongly downregulated in pbx2/4 null embryos at 18 hpf (Figure 3B, F, and 3J), thus supporting the microarray findings. At 24 hpf, the expression of aldh1a2 and tbx5 is downregulated (Figure 3D and 3H), while the expression of hmx4 is unaffected (Figure 3L). Although we do not observe any change in the expression of otx2 in the tectum of pbx2/4 null embryos at 24 hpf, there is a marked expansion of the domain of otx2 expression to include the entire eye in these embryos (Figure 3M and 3N). This demonstrates that Pbx proteins may function either directly or indirectly as a key repressor of otx2 expression. Other genes with known retinal gene expression were assayed but did not show Pbx dependence, including efnB2a and ephA4b (data not shown). Taken together, these data demonstrate a clear role for Pbx proteins in regulating gene expression within the vertebrate retina, especially within the dorsal region.
Figure 3.
Gene expression analysis via in situ hybridization shows altered expression of Pbx dependent transcripts in pbx2/4 null embryos. The expression of aldh1a2, tbx5, and hmx4 is reduced in pbx2/4 null embryos (B, F, and J), when compared to wild type (A, E, and I) at 18 hpf. At 24 hpf, aldh1a2 and tbx5 show reduced expression in pbx2/4 null embryos (D and H) when compared to wildtype (C and G), while the expression of hmx4 is unaffected (K and L). The expression of otx2 is limited to the RPE at the periphery of the eye in wild type embryos at 24 hpf (M), and is expanded to the posterior retina in pbx2/4 null embryos (N). All embryos were hybridized with the probe indicated, as well as egr2b, as an indicator of the level of Pbx function.
The role of Meis proteins in regulating retinal gene expression
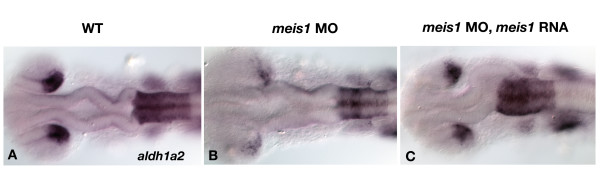
Within the hindbrain, Pbx cooperates with Hox and Meis/Pknox proteins to drive expression of target genes. Although hox genes are not expressed in the retina, meis/pknox genes are expressed strongly in this tissue [[1,10-12] and unpublished data]. Furthemore, pknox1 hypomorphs and Meis1 knockout mice have morphological defects in retinal formation [16,18]. As meis1.1 is specifically expressed in the retina during early eye patterning in zebrafish [1,25], we utilized a morpholino to knockdown Meis1.1 function. This treatment results in the reduction of aldh1a2 expression in the retina and hoxa2b expression in the hindbrain at 24 hpf (Figure 4B). Co-injection of MO-insensitive meis1.1 mRNA is sufficient to restore aldh1a2 and hoxa2b expression in meis1.1 morphants (Figure 4C), indicating the specificity of the morpholino phenotype. The reduction of retinal aldh1a2 expression in meis1.1 morphants is very similar to pbx2/4 null embryos and is thus consistent with the idea that Meis and Pbx proteins cooperate to regulate transcription of this particular gene.
Figure 4.
meis1.1 is required for expression of aldh1a2. The expression of aldh1a2 is reduced in meis1.1 morphants (B) when compared to wild type (A) at 24 hpf. As a control for morpholinos specificity, injection of meis 1.1 mRNA rescues this phenotype (compare B and C). All embryos were also hybridized with hoxa2b as well as aldh1a2.
Pbx is required for both early and late eye patterning genes
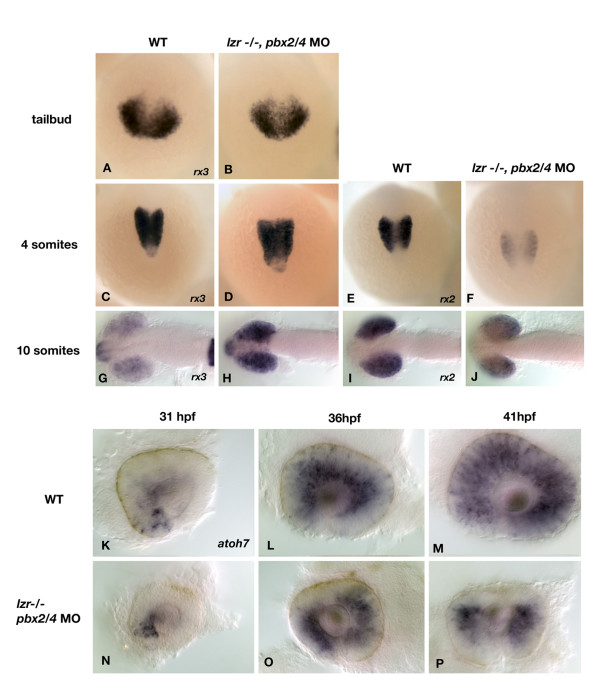
To determine when Pbx proteins are first required for eye patterning, we analyzed retinal homeobox (rx) gene expression in pbx2/4 null embryos at early stages of development. The expression of rx genes are limited to the eye fields during early development, and expression is required for proper eye development in zebrafish as over-expression leads to ectopic eye formation [26]. rx3 is expressed in the optic primordium at the tailbud stage (Figure 5A), while both rx2 and rx3 are expressed after somitogenesis (Figure 5C, G, E, and 5I). In pbx2/4 null embryos, the expression of rx3 appears unaffected during early eye specification, but has failed to be downregualted by 10 somites (21/28 embryos, Figure 5H). The expression of rx2, however, is reduced throughout somitogenesis in pbx2/4 null embryos (17/20 embryos at 4 somites, 18/23 at 10 somites, Figure 5F and 5J). Thus, pbx gene function plays a key role in the specification of early eye tissue.
Figure 5.
The expression of rx2, rx3, and atoh7 is aberrant in pbx2/4 null embryos. The expression of rx3, the earliest known eye field marker is unaffected at the tailbud and 4 somite stage (A-D) but is increased in expression at the 10 somite stage (H) when compared to wildtype (G). The expression of rx2 is reduced at both the 4 and 10 somite stage in pbx2/4 null embryos (F and J), when compared to wildtype (E and I). No expression of rx2 is observed at the tailbud stage. The expression of atoh7 was analyzed as a marker of differentiating retinal ganglion cells. In wild type embryos, expression begins in the ventral nasal domain of the retina at about 31 hpf (K). Expression proceeds in a wave-like fashion to include the dorsal retina by 36 hpf (L), and has filled the entire retina by 41 hpf (M). In pbx2/4 null embryos, expression is initiated correctly at 31 hpf (N). Expression proceeds in a wave-like fashion but is excluded from the dorsal domain of the retina at both 36 hpf (O) and 41 hpf (P).
It is likely that pbx2/4 function is also needed later in eye development as processes such as retinal ganglion cell outgrowth are also affected when Pbx function is inhibited. We therefore analyzed the expression of atonal homolog 7 (atoh7), which is expressed in a wave-like fashion (Figure 5K–M) in the inner retina prior to retinal ganglion cell differentiation [27]. There is a marked reduction in the expression of atoh7 in the dorsal retina at both 36 hpf (18/27 embryos) and 41 hpf (22/32 embryos) (Figure 5O and 5P), while expression in the rest of the inner retinal tissue remains unaffected. This indicates that a lack of atoh7 in the dorsal retina may affect retinal ganglion cell differentiation in this domain, which may contribute to the observed defects in retinal ganglion cell outgrowth.
Pbx proteins regulate polarized gene expression in the optic tectum
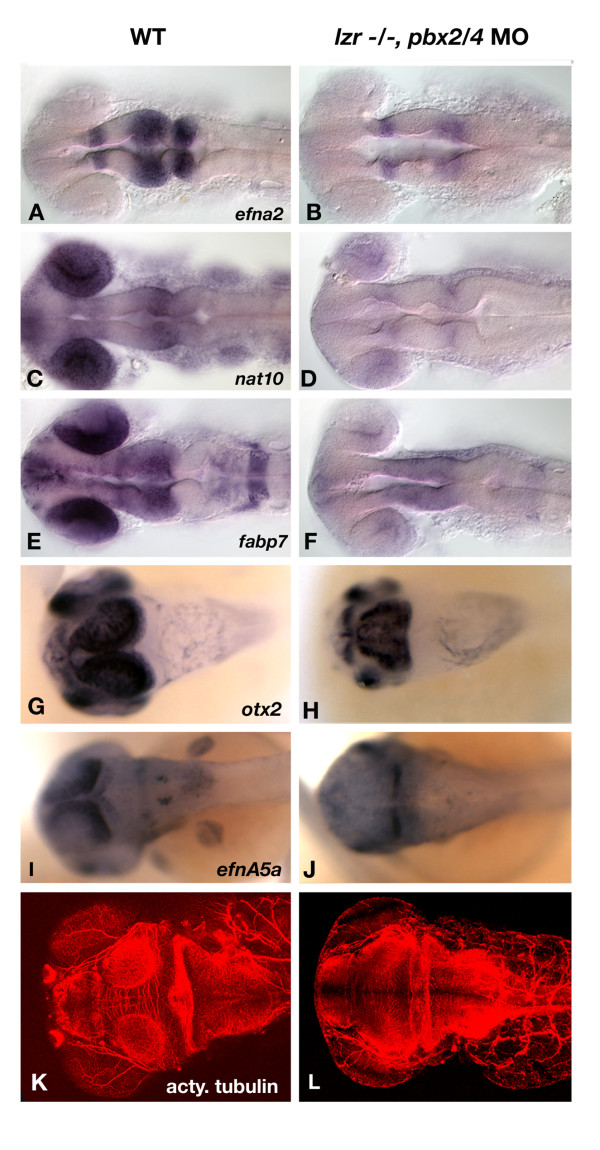
Previous research has demonstrated a role for Pbx in patterning both the midbrain-hindbrain and diencephalic-mesencephalic boundaries. Consistent with this role, our microarray identified three putative Pbx dependent transcripts are expressed within the tectum. These genes, ephrin A2 (efna2), fatty acid binding protein 7a (fabp7a), N-acetyltransferase 10 (nat10) were examined by whole mount in situ hybridization to confirm the results of the microarray experiment. efna2 is expressed in the diencephalon and tectum (Figure 6A). The other two genes, fabp7a and nat10, have significant expression in the retina as well as the tectum (Figure 6C and 6E). Each transcript is downregulated in pbx2/4 null embryos with residual expression of each gene found at the midbrain-hindbrain boundary (Figure 6B, D, F). This is consistent with previous findings showing that Pbx proteins are required for the maintenance of gene expression in the mesencephalon.
Figure 6.
Aberrant tectal patterning is observed in pbx2/4 null embryos. efna2 expression includes the posterior forebrain, the tectum, and the anterior hindbrain in wild type embryos (A), and is reduced in pbx2/4 null embryos (B) at 24 hpf. The expression of both nat10 and fabp7a includes both the eye and tectum in wild type embryos (C) and (E), and is almost completely absent in both regions in pbx2/4 null embryos (D and F). otx2 is expressed in the optic tectum at 48 hpf, specifically toward the periphery of the tectum with lower levels observed immediately adjacent to the eye (G). In pbx2/4 null embryos, there is a distinct change in expression domain in which the highest levels of expression are observed immediately adjacent to the eye (H). The gradient of efnA5a expression, which decreases toward the anterior (I), is attenuated at 48 hpf in pbx2/4 null embryos (J). The acetylated tubulin antibody marks the axons of the optic tectum at 3 dpf (K), which is smaller and disorganized in pbx2/4 null embryos (L).
As development proceeds, the tectum becomes highly organized and is characterized by the polarization of gene expression. At 48 hpf, the expression of otx2 and ephrinA5a (efnA5a) is strongest at the periphery of the tectum, with the highest expression observed distal to the eye while decreasing proximally (Figure 6G and 6I). These gradients are disrupted in pbx2/4 null embryos, whereby expression of otx2 is greatest proximal to the eye (Figure 6H), and expression of efnnA5a is confined to the posterior tectum (Figure 6J). By 3 dpf, the optic tectum is smaller and disorganized in lzr-/-, pbx2/4 morphant embryos (Figure 6L), as shown by visualization of acetylated tubulin-positive axons. These data clearly indicate a requirement for Pbx proteins in patterning the optic tectum.
Pbx is critically required for expression of gdf6a, itself a regulator of dorsal retina patterning
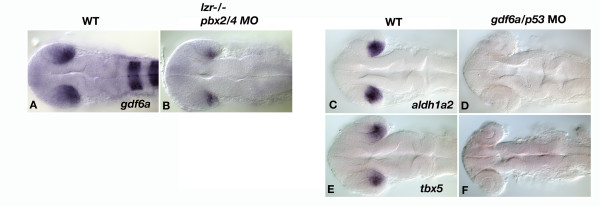
Gdf6a is a growth factor of the BMP family, whose retinal expression is restricted to the temporal/dorsal domain. Knockdown of gdf6a results in smaller eyes, as well as reduced expression of eye markers in the dorsal/temporal retina [28]. As such, we analyzed whether pbx and gdf6a function in the same pathway to regulate eye patterning, and further analyzed the expression of eye markers in gdf6a morphants. A knockdown of gdf6a results in the elimination of aldh1a2 and tbx5 expression (Figure 7D and 7F), markers of the dorsal/temporal retina. As the expression of genes in the dorsal/temporal retina is similar in gdf6a and pbx2/4 null embryos, we tested whether gdf6a functions in the same genetic pathway as pbx. When Pbx2/4 function is eliminated, the expression of gdf6a is strongly reduced (Figure 7B), indicating that gdf6a functions genetically downstream of pbx. The expression of pbx4 is not affected in gdf6a morphants (data not shown), further supporting the idea that gdf6a functions genetically downstream of pbx.
Figure 7.
gdf6a is genetically downstream of pbx2/4 and is necessary for expression of genes in the dorsal/temporal retina. Expression of gdf6a is strongly reduced in pbx2/4 null embryos (B), when compared with wildtype (A) at 24 hpf. Knockdown of gdf6a results in a loss of aldh1a2 and tbx5 expression (D and F) when compared with wildtype (C and E). pbx2/4 null embryos were also hybridized with egr2b as well as gdf6a, as a marker of pbx function.
Discussion
In this paper, we present evidence that pbx2/4 gene function is essential for proper retinal ganglion cell axon pathfinding. This phenotype likely results from aberrant Pbx mediated gene transcription in both the eye and tectum of pbx2/4 null embryos. Although the eye laminar structure is relatively normal after 5 days of development, connections between the RGC axons and the optic tectum are not made. Through microarray and in situ analysis, we have identified numerous candidate genes that may contribute to RGC axon guidance phenotype seen in embryos with reduced Pbx function. Abnormalities in eye patterning begin during early somitogenesis, and persist throughout development. Defects in the differentiation of retinal ganglion cells may also contribute to this phenotype, as the expression of atoh7 is eliminated in the dorsal region of the neural retina in pbx2/4 null embryos. The data presented here builds on current knowledge of Pbx proteins in embryonic patterning, and provides a novel role for Pbx in patterning the eye and tectum, and subsequently, the regulation of RGC axon outgrowth and pathfinding.
An absence of Pbx function results in reduced expression of genes involved in eye patterning. In pbx2/4 null embryos, defects are seen in the expression of rx2 during early somitogenesis, and at 10 somites, rx3 expression has not been downregulated as seen in wild type embryos [26]. This could lead to defects in retinal ganglion cell and photoreceptor development, as rx2 and rx3 are also expressed in these subsets of cells later in development. We have identified aldh1a2, hmx4, and tbx5 as Pbx-dependent transcripts at 18 hpf. Each of these genes has the potential to play a strong role in regulating retinal patterning. For example, aldh1a2, which is involved in the synthesis of retinoic acid, is likely to affect development of the visual system in zebrafish as retinoic acid is required for development of photoreceptor cells and pigmented epithelium in the developing zebrafish eye [29]. By 3 dpf, the photoreceptor layer is highly disorganized in fish with reduced pbx gene function, indicating a possible effect of aldh1a2 misexpression. Moreover, we also observed a downregulation of tbx5 via both microarray and in situ analysis, a downstream effector of retinoic acid signaling that is essential for establishment of the dorsoventral boundary of the eye [30,31].
It has been documented that the homeodomain transcription factor soho1 represses the expression of epha3 in chicks, and is necessary for eye development. The zebrafish Pbx dependent gene hmx4 is likely the functional ortholog of chick soho1, since it is expressed in the retina and shares extensive sequence similarity. misexpression of soho1 in chicks affects epha3 expression [32], and loss of epha3 leads to premature arborization by RGC axons that originate in the nasal retina, while those that originate in the posterior retina appear unaffected [33]. In pbx2/4 null embryos, hmx4 is down regulated during early eye patterning, and thus may also play a role in phenotypes observed in eyes with reduced pbx gene function, such as a lack of RGC axon outgrowth.
Given the profound changes in gene expression, it is surprising that laminar structure and positioning of the optic nerve is relatively normal without Pbx2/4 function. As such, there is no evidence for overt dorsal/ventral patterning defects or improper cell differentiation. We believe a partial answer to this paradox lies in the data from the 28 hpf microarray, in which each of these Pbx-dependent transcripts has more normal levels of expression (data not shown). We confirmed these results with in situ analysis, showing that changes in aldh1a2 and tbx5 expression are more subtle, while the expression of hmx4 is near wild-type levels at 24 hpf, indicating that Pbx2/4 are essential for proper initiation of these transcripts, but that other factors are capable of regulating their transcription in the absence of Pbx2/4. At 24 hpf, other pbx genes, including pbx1 and pbx3.1, are expressed in the zebrafish embryo. As such, these Pbx proteins or other transcription factors might also regulate eye patterning genes at 24 hpf.
Although not identified in our microarray screen for Pbx dependent transcripts, we analyzed the expression of otx2, which in wild type embryos, is expressed throughout the optic vessicle early in development [23], but is restricted to the RPE between 22–24 hpf. otx2 is also strongly expressed in the optic tectum, and could therefore be responsible for axon guidance through either eye or tectal patterning. We noticed an expansion of expression to include the entire eye in pbx2/4 null embryos at 24 hpf, indicating that these embryos are unable to restrict the expression of otx2 as development proceeds. A loss of retinoid signaling in mice leads to reduced expression of tbx5 and expanded expression otx2 throughout the retina, placing both genes downstream of aldh1a2 [34]. This provides a possible signaling mechanism for the expansion of otx2 in pbx2/4 null zebrafish whereby Pbx is required for expression of aldh1a2, which, in turn, is required for the restriction of otx2 to the RPE. As restriction of otx2 expression is required for specification of the RPE layer, it is logical that pbx2/4 null embryos would have defects in the specification of RPE cells. In both lzr and pbx2/4 null embryos, the RPE is thickened in a subtle, but reproducible manner. Thus it is likely that the ectopic expression of otx2 in pbx2/4 null embryos leads to the thickening of the RPE, but aberrant expression of other retinal transcripts may also be involved.
Many Pbx functions, such as hindbrain patterning and blood development, are known to involve Meinox binding partners. Meinox proteins can stabilize Pbx proteins, and are often essential for Pbx function through cooperative DNA binding. [1,9]. Meis1 knockout mice and Prep/Pknox mutants have morphological defects in retinal formation including an inappropriately positioned optic nerve [16]. The position of the optic nerve implies that Meis1 is required for proper dorsal-ventral patterning of the retina, although marker analysis has yet been performed. To create zebrafish with reduced Meis1.1 function, we injected embryos with meis1.1 translation blocking morpholinos. As with pbx2/4 null embryos, knockdown of Meis1.1 function reduced or eliminated aldh1a2 expression. Thus, it is likely that Pbx2/4 and Meis1.1 act cooperatively to regulate at least some aspects of eye patterning. This is in concert with previously published reports that indicate that both proteins are needed to pattern tissues such as the hindbrain [1,9].
Using our microarray approach, we have also identified reduced tectal expression of fabp7a, nat10, and efna2 in pbx2/4 null embryos. Interestingly, fabp7a and nat10 also show decreased expression in the eye in pbx2/4 null embryos, and thus may be important in patterning both structures in a Pbx dependent fashion. FABP7 has been identified as a downstream target of Pbx/Meinox signaling in humans, whereby increased expression of PKNOX1 (human prep1 homologue) leads to overexpression of FABP7 in fetal trisomy leading to Down's Syndrome [35].
Further in development, the optic tectum becomes highly polarized in wild type embryos, which is required for proper retinal ganglion cell axon outgrowth and mapping. By 48 hpf, tectal gradients of otx2 and ephrinA5a expression have been disrupted in embryos with reduced Pbx function, indicating its vital role for patterning this tissue. Given these results, it is possible that RGC axon pathfinding defects may result in part from pbx dependent patterning defects in the tectum in concert with pbx patterning defects in the retina. An alternative hypothesis would indicate that forebrain patterning, specifically via sonic hedgehog (shh) signaling could lead to the observed axon outgrowth defect, as shh has been shown to negatively regulate growth cone movement [36]. This is not the case in pbx2/4 null embryos as no changes in shh expression, nor two of its downstream target genes titf1a and titf1b, are observed (data not shown).
It has recently been reported that a knockdown of gdf6a, a growth factor of the BMP family, results in smaller eyes in both zebrafish and frogs [28,37]. Furthermore, the expression of marker genes in the dorsal/temporal domain of the retina is dependent on Gdf6a function. We have expanded on the marker analysis in gdf6a morphants to include aldh1a2 and tbx5, which are both eliminated in gdf6a morphants. Moreover, gdf6a expression is strongly reduced in pbx2/4 null embryos, indicating that gdf6a acts genetically downstream of both pbx, and is essential for patterning the dorsal/temporal retina. As similar phenotypes are seen with ectopic expression of FGF3/8 in the temporal retina [38], it is likely that FGFs and GDFs represent opposing signals to pattern the nasal and temporal domains of the retina, respectively. Given that our data suggest that Pbx proteins are genetically upstream of gdf6a expression in the retina, it appears that Pbx may play an important role in regulating the Fgf-Gdf antagonism that leads to correct retinal patterning.
Conclusion
The results presented support a model where aberrant eye and tectal patterning in pbx2/4 null embryos leads to RGC axon outgrowth errors. Eye patterning in the dorsal-temporal domain of the retina involve the function of meis1.1 and a downstream growth factor, gdf6a.
Methods
Zebrafish strains, morpholinos, and in situ hybridization
The mutant pbx4 allele (b557), also known as lazarus, (lzr), was identified by altered expression patterns of egr2b (krox20) in the developing hindbrain, and has been described previously [8]. For the microarray experiments, pbx2/4 null embryos were created by injecting pbx2 (CCGTTGCCTGTGATGGGCTGCTGCG) translation blocking morpholinos into one-cell maternally and zygotically mutant lzr embryos (mz lzr). Germ line transplantation was used to create mz lzr embryos and has been described previously [2]. For all other experiments, pbx2/4 null embryos were created by injecting pbx2 and pbx4 (AATACTTTTGAGCCGAATCTCTCCG) translation blocking morpholinos into one-cell zygotically mutant lzr embryos. These embryos are phenotypically indistinguishable from mz lzr embryos injected with pbx2 morpholinos. Approximately 3 nl of pbx2/4 morpholino was injected at a concentration of 1 mg/ml. To create a similarly mispatterned embryo for microarray studies, we injected lzr embryos with morpholinos directed against hoxb1a (GGAACTGTCCATACGCAATTAA) and hoxb1b (AATTCATTGTTGACTGACCAAGCAA and ACCAAGCAAAATTGATTAAGCAGGG).
For gdf6a knockdown, a splice blocking morpholino (GCAATACAAACCTTTTCCCTTGTCC) was utilized. This morpholino results in the production of gdf6a messenger RNA containing its lone intron. This allows for normal maternal gdf6a function, as gdf6a is required for patterning the early embryo, but inhibits zygotic gdf6a function prior to eye development. As high levels of necrosis are observed in gdf6a morphants, gdf6a morpholinos were co-injected with a p53 translation blocking morpholino (GCGCCATTGCTTTGCAAGAATTG) [39]. To block meis1.1 function, we injected a translation-blocking morpholinos (GTATATCTTCGTACCTCTGCGCCAT) into 1-cell zebrafish embryos. To rescue the meis1.1MO phenotype, we injected approximately 200 pg of 6X-myc tagged meis1.1 mRNA synthesized in vitro using the Ambion SP6 mMessage machine kit.
Whole mount in situ hybridization analysis was performed essentially as previously described [40], with the following modifications: probes were not hydrolyzed; Proteinase K treatment (10 ug/ml) was performed for 30 seconds (tailbud- 10 somite embryos), 3 minutes (18–24 hpf embryos), and 20 minutes (embryos between 24–48 hpf). Embryos were manually deyolked, and photographed using a Zeiss AxioImager Z1 compound microscope and an Axiocam HR digital camera or on an Olympus steroscope with a Qimaging micropublisher camera. Embryos were raised at 25–31°C and staged according to published staging hallmarks [41].
cDNA microarray analysis
For each microarray hybridization, target cDNA was synthesized from either mz lzr, or lzr embryos injected with either pbx2 or hox-1 morpholinos, respectively. cDNA was generated from RNA templates isolated at both 18 hpf and 28 hpf. cDNA was labeled with either Cy3 or Cy5, and hybridized to microarrays containing 8448 cDNA probes, including 768 controls spots (e.g. negative, positive, and normalization controls). An Axon scanner (Molecular Devices) and Genepix software (Molecular Devices) were used to determine the intensities of each spot and normalize the data. All experiments were conducted in biological duplicate while simultaneously switching fluorescent labeling compounds.
Identification of hmx4
A previously uncharacterized EST clone was identified by microarray analysis as having reduced expression in pbx2/4 null embryos. in situ analysis identified expression in the lens and dorsal retina. The clone was sequenced and genomic location identified in zebrafish, and compared to sequences in other organisms. The EST (DY55561.1), is highly similar to that of hmx3 in zebrafish (55% amino acid identity), and is also highly similar to chick soho1 (59% amino acid identity). Based on the homology of the homeodomain and genomic organization, we have named this gene hmx4.
Eye cross sections
Wild type and pbx2/4 morpholino injected embryos were grown to 5 dpf, then fixed in 4% PFA. Embryos were embedded in Spurr's Resin for 1 μm ultramicrotome sectioning with a diamond blade, and stained with Richardson's blue for visualization of retinal layers. Sections were visualized using Zeiss Axioimager Z1 coumpound microscope and photographed with Zeiss Axiocam HR. Eye retinal pigmented epithelium and total eye area were quantified using ImageJ software.
DiI and DiO RGC labeling
Two lipophylic dyes, DiI and DiO (Molecular Probes), were used to label retinal ganglion cell axons. DiI was dissolved in dimethylformamide (DMF), at a concentration of 12.5 mg/ml. DiO was dissolved in chloroform at a concentration of 12.5 mg/ml. Embryos were raised in 0.0015% phenylthiourea (PTU) to inhibit pigment formation and fixed in 4% paraformaldhyde at 3.5 and 5.5 days. Embryos were mounted dorsal side up in 1% in low melting point agrose for injections. DiI was injected into the nasal domain of the right eye, while DiO was injected into the temporal domain of the right eye. Embryos were removed form the agrose and incubated in PBS 0.01% tween20 overnight at 28°C. Embryos were flat mounted in 70% glycerol, dorsal side up. Imaging was accomplished with a SP2 confocal microscope (Leica) using 20X objective.
Immunohistochemistry
The acetylated tubulin antibody (Sigma-Aldrich) staining protocol has been published previously [42]. Briefly, zebrafish embryos (3 dpf) were fixed in Dent's fix (80% methanol/20%DMSO), and primary antibody incubation was completed overnight at 4°C (1:500) after blocking in PBS-DBT (1% DMSO/1% BSA/0.5% tween-20, 10% goat serum). After 5 washes in PBS 0.5% tween, a secondary antibody (anti-mouse Alexaflour 546, Molecular Probes, 1:1000), was added and incubated overnight at 4°C.
For zpr-1 (1:200) staining, embryos were fixed in 4% PFA overnight at 4°C. Embryos were blocked in PBS 2% goat serum, 2 mg/ml BSA, 0.1% triton X-100. After washing in PBSDTT, a secondary antibody (anti-mouse Alexaflour 488, Molecular Probes) was diluted 1:1000 and incubated overnight at 4°C. Embryos were mounted in 70% glycerol for imaging on a SP2 confocal microscope (Leica) using both 20X and 40X (oil) objective.
Authors' contributions
CRF was responsible for RGC labeling assays and in situ analysis in pbx2/4 null embryos, gdf6 morphants, immunohistochemistry, and writing of the manuscript. TE was responsible for meis1.1 morphant analysis, and editing the manuscript. DC and RK were responsible for eye cross sections in pbx2/4 null embryos. KB was involved in in situ analysis in pbx2/4 null embryos and was involved in the microarray studies. DH, JS, KWS, JN, CBM, and AJW were involved in the microarray analysis and subsequent in situ hybridization verifications. AJW is the principal investigator.
Acknowledgments
Acknowledgements
We would like to thank Chi-Bin Chien who kindly provided his protocol for retinal axon labeling. We would also like to thank Stephen Ekker for sharing his unpublished data regarding the use of p53 morpholinos to reduce morpholinos induced cell death. This work was supported by grants from Alberta Ingenuity (TE, AJW), NSERC (CF, TE, and AJW), NIH (JN and CBM), HHMI (CBM), and CIHR (AJW). Andrew Waskiewicz is a Canada Research Chair and Alberta Ingenuity New Faculty.
Contributor Information
Curtis R French, Email: crfrench@ualberta.ca.
Timothy Erickson, Email: timothye@ualberta.ca.
Davon Callander, Email: dccallan@ucalgary.ca.
Karyn M Berry, Email: karynberry@hotmail.com.
Ron Koss, Email: ron.koss@ualberta.ca.
Daniel W Hagey, Email: dhagey@ualberta.ca.
Jennifer Stout, Email: jstout@fhcrc.org.
Katrin Wuennenberg-Stapleton, Email: Katrin_Stapleton@affymetrix.com.
John Ngai, Email: jngai@socrates.Berkeley.EDU.
Cecilia B Moens, Email: cmoens@fhcrc.org.
Andrew J Waskiewicz, Email: aw@ualberta.ca.
References
- Waskiewicz AJ, Rikhof HA, Hernandez RE, Moens CB. Zebrafish Meis functions to stabilize Pbx proteins and regulate hindbrain patterning. Development. 2001;128:4139–4151. doi: 10.1242/dev.128.21.4139. [DOI] [PubMed] [Google Scholar]
- Waskiewicz AJ, Rikhof HA, Moens CB. Eliminating zebrafish pbx proteins reveals a hindbrain ground state. Dev Cell. 2002;3:723–733. doi: 10.1016/S1534-5807(02)00319-2. [DOI] [PubMed] [Google Scholar]
- Dutta S, Gannon M, Peers B, Wright C, Bonner-Weir S, Montminy M. PDX:PBX complexes are required for normal proliferation of pancreatic cells during development. Proc Natl Acad Sci U S A. 2001;98:1065–1070. doi: 10.1073/pnas.031561298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Nagai R, Sato T, Matsuura E, Minami T, Morita I, Doi T. Homeodomain proteins MEIS1 and PBXs regulate the lineage-specific transcription of the platelet factor 4 gene. Blood. 2003;101:4748–4756. doi: 10.1182/blood-2002-02-0380. [DOI] [PubMed] [Google Scholar]
- Erickson T, Scholpp S, Brand M, Moens CB, Waskiewicz AJ. Pbx proteins cooperate with Engrailed to pattern the midbrain-hindbrain and diencephalic-mesencephalic boundaries. Developmetal Biology. 2007;301:504–517. doi: 10.1016/j.ydbio.2006.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penkov D, Di Rosa P, Fernandez Diaz L, Basso V, Ferretti E, Grassi F, Mondino A, Blasi F. Involvement of Prep1 in the alphabeta T-cell receptor T-lymphocytic potential of hematopoietic precursors. Mol Cell Biol. 2005;25:10768–10781. doi: 10.1128/MCB.25.24.10768-10781.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkes CA, Bergstrom DA, Penn BH, Seaver KJ, Knoepfler PS, Tapscott SJ. Pbx marks genes for activation by MyoD indicating a role for a homeodomain protein in establishing myogenic potential. Mol Cell. 2004;14:465–477. doi: 10.1016/S1097-2765(04)00260-6. [DOI] [PubMed] [Google Scholar]
- Popperl H, Rikhof H, Chang H, Haffter P, Kimmel CB, Moens CB. lazarus is a novel pbx gene that globally mediates hox gene function in zebrafish. Mol Cell. 2000;6:255–267. doi: 10.1016/S1097-2765(00)00027-7. [DOI] [PubMed] [Google Scholar]
- Moens CB, Selleri L. Hox cofactors in vertebrate development. Dev Biol. 2006;291:193–206. doi: 10.1016/j.ydbio.2005.10.032. [DOI] [PubMed] [Google Scholar]
- Biemar F, Devos N, Martial JA, Driever W, Peers B. Cloning and expression of the TALE superclass homeobox Meis2 gene during zebrafish embryonic development. Mech Dev. 2001;109:427–431. doi: 10.1016/S0925-4773(01)00554-8. [DOI] [PubMed] [Google Scholar]
- Vlachakis N, Ellstrom DR, Sagerstrom CG. A novel pbx family member expressed during early zebrafish embryogenesis forms trimeric complexes with Meis3 and Hoxb1b. Dev Dyn. 2000;217:109–119. doi: 10.1002/(SICI)1097-0177(200001)217:1<109::AID-DVDY10>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Zerucha T, Prince VE. Cloning and developmental expression of a zebrafish meis2 homeobox gene. Mech Dev. 2001;102:247–250. doi: 10.1016/S0925-4773(01)00299-4. [DOI] [PubMed] [Google Scholar]
- Deflorian G, Tiso N, Ferretti E, Meyer D, Blasi F, Bortolussi M, Argenton F. Prep1.1 has essential genetic functions in hindbrain development and cranial neural crest cell differentiation. Development. 2004;131:613–627. doi: 10.1242/dev.00948. [DOI] [PubMed] [Google Scholar]
- Yan YL, Jowett T, Postlethwait JH. Ectopic expression of hoxb2 after retinoic acid treatment or mRNA injection: disruption of hindbrain and craniofacial morphogenesis in zebrafish embryos. Dev Dyn. 1998;213:370–385. doi: 10.1002/(SICI)1097-0177(199812)213:4<370::AID-AJA3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Cooper KL, Leisenring WM, Moens CB. Autonomous and nonautonomous functions for Hox/Pbx in branchiomotor neuron development. Dev Biol. 2003;253:200–213. doi: 10.1016/S0012-1606(02)00018-0. [DOI] [PubMed] [Google Scholar]
- Hisa T, Spence SE, Rachel RA, Fujita M, Nakamura T, Ward JM, Devor-Henneman DE, Saiki Y, Kutsuna H, Tessarollo L, Jenkins NA, Copeland NG. Hematopoietic, angiogenic and eye defects in Meis1 mutant animals. Embo J. 2004;23:450–459. doi: 10.1038/sj.emboj.7600038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai CY, Kuo TS, Jaw TJ, Kurant E, Chen CT, Bessarab DA, Salzberg A, Sun YH. The Homothorax homeoprotein activates the nuclear localization of another homeoprotein, extradenticle, and suppresses eye development in Drosophila. Genes Dev. 1998;12:435–446. doi: 10.1101/gad.12.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti E, Villaescusa JC, Di Rosa P, Fernandez-Diaz LC, Longobardi E, Mazzieri R, Miccio A, Micali N, Selleri L, Ferrari G, Blasi F. Hypomorphic mutation of the TALE gene Prep1 (pKnox1) causes a major reduction of Pbx and Meis proteins and a pleiotropic embryonic phenotype. Mol Cell Biol. 2006;26:5650–5662. doi: 10.1128/MCB.00313-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt EA, Dowling JE. Early eye morphogenesis in the zebrafish, Brachydanio rerio. J Comp Neurol. 1994;344:532–542. doi: 10.1002/cne.903440404. [DOI] [PubMed] [Google Scholar]
- Burrill JD, Easter SS., Jr. The first retinal axons and their microenvironment in zebrafish: cryptic pioneers and the pretract. J Neurosci. 1995;15:2935–2947. doi: 10.1523/JNEUROSCI.15-04-02935.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MD, Hennig S, Herwig R, Clifton SW, Marra MA, Lehrach H, Johnson SL, Group t W. An oligonucleotide fingerprint normalized and expressed sequence tag characterized zebrafish cDNA library. Genome Res. 2001;11:1594–1602. doi: 10.1101/gr.186901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maves L, Waskiewicz AJ, Biswajit P, Moens CB, Tapscott SJ. Pbx Homeodomain Proteins Direct Myod Activity to Promote Fast Muscle Differentiation. submitted. [DOI] [PubMed]
- Martinez-Morales JR, Signore M, Acampora D, Simeone A, Bovolenta P. Otx genes are required for tissue specification in the developing eye. Development. 2001;128:2019–2030. doi: 10.1242/dev.128.11.2019. [DOI] [PubMed] [Google Scholar]
- Hever AM, Williamson KA, van Heyningen V. Developmental malformations of the eye: the role of PAX6, SOX2 and OTX2. Clin Genet. 2006;69:459–470. doi: 10.1111/j.1399-0004.2006.00619.x. [DOI] [PubMed] [Google Scholar]
- Wu S, Page L, Sherwood NM. A role for GnRH in early brain regionalization and eye development in zebrafish. Mol Cell Endocrinol. 2006;257-258:47–64. doi: 10.1016/j.mce.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Chuang JC, Mathers PH, Raymond PA. Expression of three Rx homeobox genes in embryonic and adult zebrafish. Mech Dev. 1999;84:195–198. doi: 10.1016/S0925-4773(99)00077-5. [DOI] [PubMed] [Google Scholar]
- Kay JN, Link BA, Baier H. Staggered cell-intrinsic timing of ath5 expression underlies the wave of ganglion cell neurogenesis in the zebrafish retina. Development. 2005;132:2573–2585. doi: 10.1242/dev.01831. [DOI] [PubMed] [Google Scholar]
- Asai-Coakwell M, French CR, Berry KM, Ye M, Koss R, Somerville M, Mueller R, van Heyningen V, Waskiewicz AJ, Lehmann OJ. GDF6, a novel locus for a spectrum of ocular developmental anomalies. Am J Hum Genet. 2007;80:306–315. doi: 10.1086/511280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhudesai SN, Cameron DA, Stenkamp DL. Targeted effects of retinoic acid signaling upon photoreceptor development in zebrafish. Dev Biol. 2005;287:157–167. doi: 10.1016/j.ydbio.2005.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golz S, Lantin C, Mey J. Retinoic acid-dependent regulation of BMP4 and Tbx5 in the embryonic chick retina. Neuroreport. 2004;15:2751–2755. [PubMed] [Google Scholar]
- Leconte L, Lecoin L, Martin P, Saule S. Pax6 interacts with cVax and Tbx5 to establish the dorsoventral boundary of the developing eye. J Biol Chem. 2004;279:47272–47277. doi: 10.1074/jbc.M406624200. [DOI] [PubMed] [Google Scholar]
- Schulte D, Cepko CL. Two homeobox genes define the domain of EphA3 expression in the developing chick retina. Development. 2000;127:5033–5045. doi: 10.1242/dev.127.23.5033. [DOI] [PubMed] [Google Scholar]
- Feldheim DA, Nakamoto M, Osterfield M, Gale NW, DeChiara TM, Rohatgi R, Yancopoulos GD, Flanagan JG. Loss-of-function analysis of EphA receptors in retinotectal mapping. J Neurosci. 2004;24:2542–2550. doi: 10.1523/JNEUROSCI.0239-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halilagic A, Ribes V, Ghyselinck NB, Zile MH, Dolle P, Studer M. Retinoids control anterior and dorsal properties in the developing forebrain. Dev Biol. 2007;303:362–375. doi: 10.1016/j.ydbio.2006.11.021. [DOI] [PubMed] [Google Scholar]
- Sanchez-Font MF, Bosch-Comas A, Gonzalez-Duarte R, Marfany G. Overexpression of FABP7 in Down syndrome fetal brains is associated with PKNOX1 gene-dosage imbalance. Nucleic Acids Res. 2003;31:2769–2777. doi: 10.1093/nar/gkg396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trousse F, Marti E, Gruss P, Torres M, Bovolenta P. Control of retinal ganglion cell axon growth: a new role for Sonic hedgehog. Development. 2001;128:3927–3936. doi: 10.1242/dev.128.20.3927. [DOI] [PubMed] [Google Scholar]
- Hanel ML, Hensey C. Eye and neural defects associated with loss of GDF6. BMC Dev Biol. 2006;6:43. doi: 10.1186/1471-213X-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker A, Brand M. Fgf signals from a novel signaling center determine axial patterning of the prospective neural retina. Development. 2005;132:4951–4962. doi: 10.1242/dev.02071. [DOI] [PubMed] [Google Scholar]
- Robu ME, Larson JD, Nasevicius A, Beiraghi S, Brenner C, Farber SA, Ekker SC. p53 activation by knockdown technologies. PLoS Genet. 2007;3:e78. doi: 10.1371/journal.pgen.0030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince VE, Price AL, Ho RK. Hox gene expression reveals regionalization along the anteroposterior axis of the zebrafish notochord. Dev Genes Evol. 1998;208:517–522. doi: 10.1007/s004270050210. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kramer-Zucker AG, Olale F, Haycraft CJ, Yoder BK, Schier AF, Drummond IA. Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer's vesicle is required for normal organogenesis. Development. 2005;132:1907–1921. doi: 10.1242/dev.01772. [DOI] [PubMed] [Google Scholar]