Abstract
Ceramide, a sphingosine-based lipid molecule, has emerged as a key regulator of a wide spectrum of biological processes such as cellular differentiation, proliferation, apoptosis and senescence. Sphingomyelinase-dependent hydrolysis of sphingomyelin, and de novo synthesis involving the coordinated action of serinepalmitoyl transferase and ceramide synthase, are the two major pathways involved in ceramide synthesis. Clustering of plasma membrane rafts into ceramide enriched platforms serves as an important transmembrane signaling mechanism for cell surface receptors. Ceramides have been implicated in apoptosis, stress-signaling cascades as well as ion channels. There is accumulating evidence that targeted manipulation of ceramide metabolism pathway has immense therapeutic potential and may eventually prove to be a boon in the design of novel strategies and development of innovative treatments for diverse conditions including cardiovascular diseases, cancer and Alzheimer’s disease. As yet uncharacterized natural ceramide analogs and novel inhibitors of ceramide metabolism might prove to be potent drugs. In this review, we discuss significant advances that continue to provide intriguing insights into the complex cellular and molecular mechanisms underlying ceramide-mediated signaling cascades.
Keywords: Apoptosis, Atherosclerosis, Cancer, Cardiovascular diseases, Ceramide, Ion channels, Neurodegeneration, Raft, Sphingomyelinase
Introduction
Deciphering the complexities of ceramide-mediated transmembrane signaling has been a primary objective of numerous experimental studies in life sciences in recent years. Ceramides have emerged as critically important bioactive messengers playing significant roles in cellular differentiation, proliferation, apoptosis and senescence. Ceramide biosynthesis occurs via sphingomyelinase-dependent catabolism of sphingomyelins as well as by de novo synthesis (Hannun et al., 2001; Mathias et al., 1998). Ceramide-enriched membrane microdomains facilitate receptor reorganization and clustering, thereby amplifying the signaling, sometimes almost 100-fold as in the case of CD95(Kolesnick et al., 2000; Grassme et al., 2003). Ceramide has been implicated in diverse signaling cascades that involve protein kinases viz. SAPK, JNK (Verheij et al., 1996), PKC ζ (Bourbon et al., 1992), kinase suppressor of Ras (KSR) (Zhang et al., 1997), Raf (Huwiler et al., 1996), double stranded RNA-dependent protein kinase (PKR) as well as protein phosphatases such as PP2A and PP1 (Ruvolo et al., 2001; Chalfant et al., 1999). Ceramide-induced apoptosis usually involves the SAPK/JNK signaling pathway (Verheij et al., 1996). Ceramide production is enhanced in response to diverse stress stimuli such as cytokines, environmental stress and chemotherapeutic or anti-cancer drugs (Hannun and Obeid, 2002; Senchenkov et al., 2001; Spiegel and Milstein, 2002) (Figure 1). Numerous experimental and clinical studies reveal the significance of ceramides in pathophysiology of diverse disease conditions. The past few years have witnessed an upsurge of interest in the exploration of complex cellular and molecular mechanisms underlying ceramide-mediated signaling cascades. Identification and comprehensive characterization of ceramide analogs and inhibitors of ceramide metabolism as logical therapeutic targets may yield opportunities for pharmacological interventions and design of novel strategies that will undoubtedly lead to spectacular gains in our ability to fully understand the pathophysiology of diverse human diseases.
Figure 1.
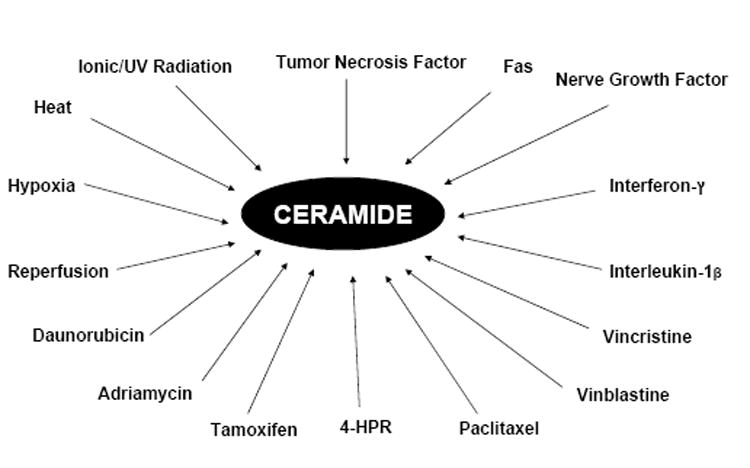
Ceramide production in response to diverse stress stimuli. Ceramide production is enhanced in response to environmental sress, ionic/Ultra Violet radiation, heat, hypoxia, reperfusion, cytokines and growth factors, tumor necrosis factor, interferon-gamma and interleukin-1-beta as well as chemotherapeutic agents/anticancer drugs, Daunorubicin, Adriamycin, Tamoxifen, Paclitaxel, 4-(N-hydroxyphenyl)retinamide (HPR), Vincristine and Vinblastine.
Ceramide metabolism
Sphingomyelinase-dependent hydrolysis of sphingomyelin and the de novo synthesis are the two major pathways of ceramide biosynthesis (Mathias et al., 1998; Hannun and Luberto, 2000; Riboni et al., 1997; Hannun, 1994) (Figure 2). Sphingomyelinases (SMases) catalyze the hydrolysis of sphingomyelin (ceramidephosphorylcholine) into ceramide and phosphorylcholine (Levade and Jaffrezou, 1999). SMases have been characterized as acid SMase, secretory SMase, neutral Mg2+ dependent SMase, Mg2+ independent neutral SMase, alkaline SMase and bacterial SMase-phospholipase C. Acid SMase deficiency has been implicated in the Niemann-Pick syndrome, secretory SMase in atherogenesis, neutral Mg2+ dependent SMase in atherosclerosis and apoptosis and alkaline SMase in colon carcinogenesis (Samet and Barenholz, 1999; Tabas, 1999; Chatterjee, 1999; Ghosh et al., 1998; Nilsson and Duan, 1999; Vazquez-Boland et al., 2001). Ceramide is generated from sphingomyelin located in the outer layer of plasma membrane by neutral Mg2+ dependent sphingomyelinase while same ceramide are formed from sphingomyelin in endo/lysosomes by acid SMase. Ceramidase catabolizes ceramide to form sphingosine. Ceramide can also be converted to sphingomyelin, glucosyl ceramide, galactosyl ceramide, ceramide-1-phosphate, and ceramide phosphoethanolamine (Hannun and Luberto, 2000; Riboni et al., 1997). De novo ceramide synthesis begins in the endoplasmic reticulum with the serine palmitoyltransferase-catalyzed condensation of serine and palmitoyl-CoA and a subsequent series of reactions produce ceramide on the cytosolic side of the ER ( Mandon et al., 1992; Hirschberg et al., 1993;Michael and Van Echten Deckert G, 1997; Cuvillier et al., 1996). Lip 1p, a novel subunit of the enzyme ceramide synthase, the active form of which has been recently purified from yeast, is a single-span ER membrane protein required for ceramide synthase activity (Vallee and Riezman, 2005).
Figure 2.
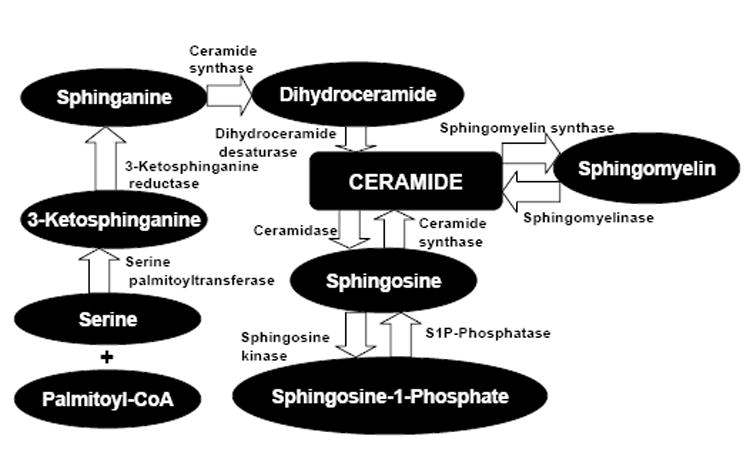
Schematic representation of ceramide metabolism.
Sphingomyelin synthesis is dependent on the translocation of ceramide from the cytoplasmic to the luminal surface of the Golgi membranes (Futerman et al., 1990). Transport of ceramide by vesicular (COP II-dependent) and non-vesicular (CERT-dependent) mechanisms from its site of synthesis in the ER to the Golgi is a critically important step in sphingolipid biosynthesis (Perry and Ridgway, 2005).
The recent identification of Good-pasture antigen binding protein (GPBP) as the ceramide transporter protein CERT will certainly provide a better understanding of the molecular mechanisms involved in the regulation of ceramide transport (Raya et al., 1999). CERT, a cytoplasmic protein with a phosphatidylinositol-4-monophosphate-binding domain and a domain for catalyzing lipid transfer, is involved in the non-vesicular transport of ceramide from the ER to Golgi apparatus for sphingomyelin synthesis (Kumagai et al., 2005; Fukasawa et al., 1999).
The non-vesicular transport involves the simultaneous binding of CERT, at ER-Golgi membrane contact sites, to both vesicle-associated membrane protein (VAMP)-associated protein (VAP) in the ER and PhosphatidylInositol-4-Phosphate at the Golgi apparatus via the FFAT (two phenylalanyl in an acidic tract) and PH (pleckstrin homology) domains, respectively. Ceramide is bound and transferred from the ER to the trans-Golgi via the START (steroidogenic acute regulatory protein-related lipid transfer) domain. Recruitment of CERT to ER occurs through interaction with VAP via the FFAT domain. At the ER, the CERT START domain extracts ceramide from the membrane bilayer, is released from the ER and transports ceramide across the cytoplasmic compartment to the cis/medial/trans-elements of the Golgi apparatus through interaction with phosphatidylinositol 4-phosphate via the PH domain. At the Golgi apparatus, ceramide is transferred into the membrane bilayer and CERT is released and recruited back to the ER to continue the cycle. Ceramide transport to the Golgi apparatus is coupled to sphingomyelin production by sphingomyelin synthase 1 on the luminal surface of the Golgi apparatus. Ceramide transport between the ER and the Golgi apparatus also occurs by COP II-dependent vesicular trafficking and utilizes glucosylceramide transferase and sphingomyelin synthase-1 for production of glucosylceramide and sphingomyelin respectively (Hanada et al., 2003; Wyles et al., 2002; Lowen and Levine, 2005; Tsujishita and Hurley, 2000).
Ceramide-dependent raft system
Transbilayer lipid motion (flip-flop) and clustering of plasma membrane rafts into ceramide-enriched platforms serves as a transmembrane signaling mechanism for cell surface receptors and probably constitutes a central element in the initiation of receptor signaling. Experimental studies have demonstrated CD95 signaling via ceramide-rich membrane rafts; acid-sphingomyelinase (ASM)-released ceramide is essential for CD95 clustering (Grassme et al., 2001). Extracellularly oriented ceramide, released upon CD95-triggered translocation of ASM to the plasma membrane outer surface, enables clustering of CD95 in sphingolipid-rich membrane rafts as well as apoptosis induction; ASM deficiency, raft destruction or neutralization of surface ceramide prevented CD95 clustering and apoptosis, suggesting that CD95-mediated clustering by ceramide is a prerequisite to signaling and explained the importance of death.
Recent studies (Gulbins and Kolesnick, 2003) suggest that following relevant stimuli, ASM translocates into distinct plasma membrane sphingolipid-enriched microdomains termed rafts where it generates ceramide. Ceramide displays the unique biophysical property to self-associate through hydrogen-bonding, thereby providing the driving force for coalescence of microscopic rafts into large membrane macrodomains that serve as platforms for protein concentration and oligomerization in transmitting signals across the plasma membrane (Figure 3). Ceramide-rich membrane rafts have been shown to mediate CD40 clustering, which appears a critical event in CD40-initiated cell signaling (Grassme et al., 2002). Pseudomonas aeruginosa, Rhinovirus, UV light, Cisplatin, CD20 and limitation of neutrophil life span by reactive oxygen species-mediated activation of death receptor signaling have been implicated in the induction of ceramide-enriched membrane platforms (Grassme et al., 2003; Charruyer et al., 2005; Lacour et al., 2004; Grassme et al., 2005; Scheel-Toellner et al., 2004; Abdel Shakor et al., 2004).
Figure 3.
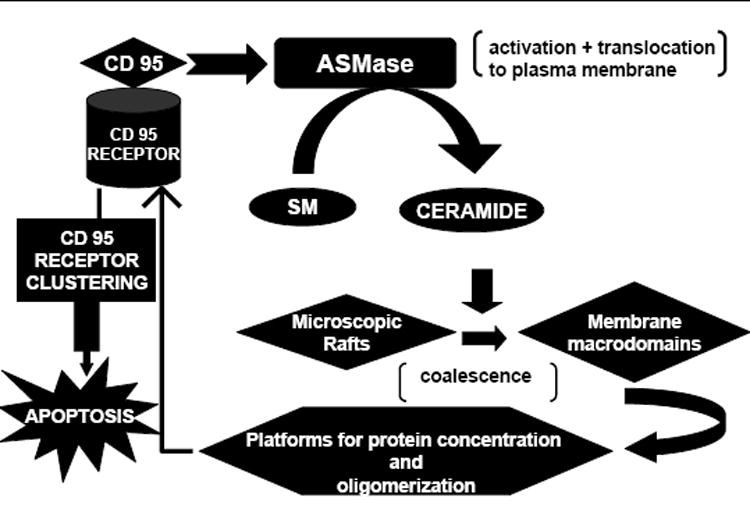
Ceramide-dependent raft system. SM, Sphingomyelin.
The first direct visual evidence for sphingomyelinase-induced formation of ceramide-enriched domains in sphingomyelin monolayer under precise control of the surface intermolecular packing has been provided recently (Fanani et al., 2002). Furthermore, it has been indicated that lateral enzyme-specific out-of equilibrium organization of lipid domains represents a new level of signal transduction from local (nm) to long-range (μm) scales (Hartel et al., 2005). Cross-talk between lateral domain structures and dipolar electrostatic fields certainly adds new dimensions to the mechanisms of SMase-mediated signal transduction in biological membranes.
Immunobiological and immunopathological role of ceramide-mediated signaling
Numerous experimental as well as clinical studies have recently focused on dissecting the complexities associated with ceramide-mediated transmembrane signaling and continue to provide substantial evidence regarding the role of ceramide as a key mediator in diverse cellular events in health and disease.
Ceramide and apoptosis
Ceramide-induced apoptosis often involves stress signaling cascades including protein kinases as well as protein phosphatases (Figure 4). It has been suggested that ceramide activates JNK via Rac-1, PKC ζ and TAK-1 (Brenner et al., 1997; Lozano et al., 1994; Shirakabe et al., 1997). Blockade of apoptosis in myeloid and lymphoid cells by using a dominant negative c-jun mutant suggests a role for c-jun activation by JNK in ceramide-mediated cell death in these cells (Verheij et al., 1996). Ceramide activation of PKC ζ appears to be essential for SAPK pathways in some cell types since a dominant negative PKC ζ protein can block SAPK activation and inhibit anti-proliferative responses when cells are treated with ceramide. Direct activation of PKC ζ, involves ceramide binding to the cysteine-rich domain (Bourbon et al., 1992). Kinase suppressor of RAS (KSR) has been implicated as a mediator of the effects of ceramide on Ras, Raf and extracellular signal-regulated kinases (ERKs). TNF-alpha or ceramide analogs significantly increased KSR autophosphorylation and its ability to activate Raf-1. Low concentrations of natural ceramide also had similar effects in vitro. Threonine269, the Raf-1 site phosphorylated by ceramide-activated protein (CAP) kinase, is also recognized by KSR (Zhang et al., 1997). TNFα and ceramide induced activation of ERK1/2 and involves KSR and Raf-1. BAD enables ceramide-mediated apoptotic signaling via Ras and Raf-1 (Huwiler et al., 1996; Yan and Polk, 2001; Basu et al., 1998). Bax has been demonstrated to be a critical regulator of ceramide-induced apoptotic pathway, upstream of cytochrome c release. Antisense bax was found to inhibit cytochrome c release, poly (ADP-ribose) (PARP) cleavage and cell death in HL-60 cells; furthermore, ceramide induced mitochondrial translocation of Bax, and increased the ratio of Bax to Bcl-xl (Kim et al., 2001). Recent studies suggest that TNF-alpha-mediated mitochondrial generation of ceramide is involved in translocation of Bax to mitochondria and subsequent release of cytochrome c and cell death (Birbes et al., 2005). Moreover, ultraviolet light-induced acid sphingomyelinase activation is involved in conformational change of Bax at the mitochondrial membrane and subsequent release of cytochrome c (Kashkar et al., 2005). Activation of double-stranded RNA-dependent protein kinase (PKR) by ceramide involves the phosphorylation of RAX, the cellular PKR activator; overexpression of RAX was found to sensitize cells to ceramide-induced apoptosis. Ceramide promoted eukaryotic translation initiation factor 2 alpha subunit (eIF-2α) phosphorylation and was found to inhibit protein synthesis in a dose-dependent manner (Ruvolo et al., 2001). Ceramide can activate the p38 SAPK resulting in the phosphorylation of the transcription factors CREB and ATF-1 (Scheid et al., 1999). Guanine nucleotide-exchange factor Vav activation of the Rho GTPases has also been implicated in ceramide-mediated apoptosis. This process is p53 independent, but can be inhibited by Bcl2. Rac-1-mediated signaling cascades may be involved since dominant negative N17Rac1 prevents SAPK activation and suppresses apoptosis (Brenner et al., 1997; Gulbins et al., 1994). Ceramide-activated protein phosphatases PP1 and PP2A have been reported to mediate the actions of ceramide in diverse cell types. Phosphatase inhibitors inhibit the ability of ceramide to cause dephosphorylation of PKCα, Akt/PKB, c-Jun and Bcl-2. Inhibition of PP1 with phosphatidic acid blocks ceramide-induced dephosphorylation of retinoblastoma protein (Rb) resulting in cell cycle arrest and apoptosis (Kishikawa et al., 1999). Ceramide also activates lysosomal protease Cathepsin D which is a ceramide-binding protein; cathepsin D participates in caspase-3 activation after translocation from the lysosomes (De Stefanis et al., 2002). Phospholipase A2 (PLA2) has been identified as a direct target of ceramide; binding of ceramide to the calcium-dependent lipid binding (CaLB) domain of PLA2 facilitates membrane docking of PLA2 and release of arachidonic acid and prostaglandin synthesis (Huwiler et al., 2001).
Figure 4.
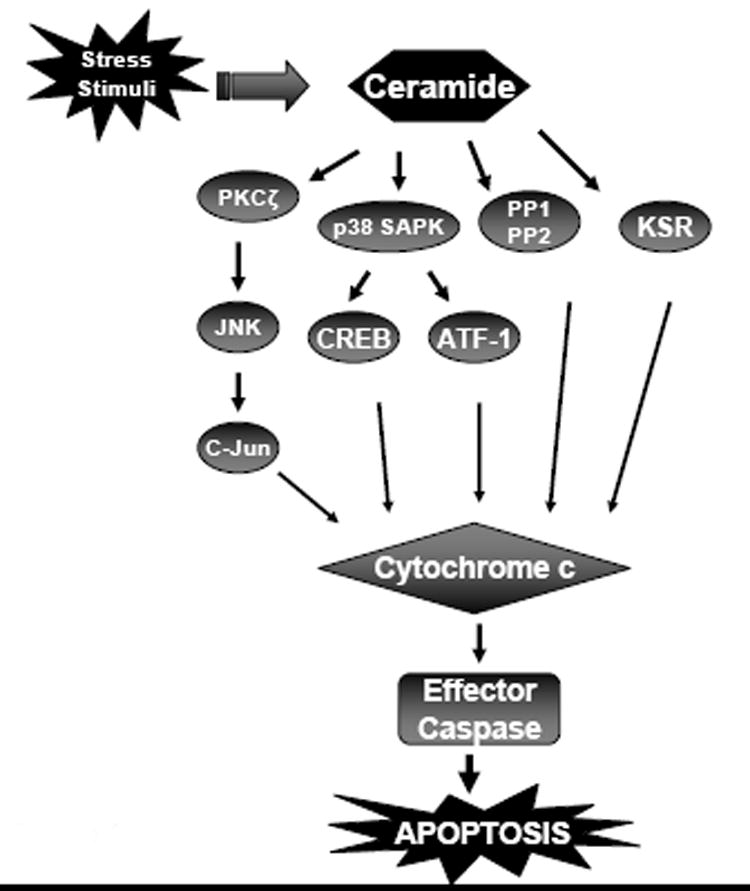
Schematic depiction of various components involved in ceramide-induced apoptosis. PKC ζ, Protein Kinase C zeta; JNK, c-Jun N-terminal Kinase; PP1, 2, Protein Phosphatase 1, 2; KSR, Kinase Suppressor of Ras; SAPK, Stress Activated Protein Kinase; CREB, cAMP Response Element Binding protein; ATF-1, Activating Transcription Factor.
Ceramide acts as a second messenger in regulating the apoptotic cascade. Many studies show that ceramide is a central player in CD95-triggered apoptosis. Acid sphingomyelinase-deficient cells fail to release ceramide upon CD95 stimulation and resist apoptosis, which is restored by addition of ceramide. CD95 stimulated hepatocytes of acid sphingomyelinase-deficient mice showed a significant reduction of apoptosis (Grassme et al., 2001; Kirschneik et al., 2000). Ceramide-enriched membrane platforms facilitate clustering of dispersed CD95 receptor trimers with subsequent activation of Caspase 8 and thus the induction of apoptosis (Grassme et al., 2001). Moreover, endothelial apoptosis, induced by inhibition of integrins αVβ3 and αVβ5, involves ceramide metabolic pathways (Erdreich-Epstein et al., 2005). Genetic deficiency of A-SMase delays and prevents developmental apoptosis of oocytes in mice, resulting in oocyte hyperplasia at birth. Also, cumulus cells surrounding the oocytes produce ceramide (Morita et al., 2000; Perez et al, 2005). Ceramide has also been implicated in the developmental death of neutrophils (Scheel-Toellner et al., 2004).
Recent studies implicate endogenous sphingosine kinase-1 (SK-1) as an important survival enzyme in MCF-7 breast cancer cells and link the biological consequences of knocking down the enzyme to its biological role as a regulator of sphingolipid metabolism. SK-1 knockdown by small interfering RNA caused cell cycle arrest and induced apoptosis (Taha et al., 2006). Cell death involved effector caspase activation, cytochrome-c release and Bax oligomerization in the mitochondrial membrane, thus, placing SK-1 knockdown upstream of the mitochondrial pathway of apoptosis. SK-1 knockdown induced significant increases in ceramide levels in whole cells and in mitochondria-enriched fractions of cells. Inhibition of de novo sphingolipid biosynthesis with myriocin significantly attenuated Bax oligomerization and downstream caspase activation after SK1 loss. A recent study demonstrates decreased levels of ceramide in lesional epidermis of psoriasis patients and this was associated with the downregulation of apoptotic signaling molecules such as PKC-α and JNK, suggesting that decreased ceramide levels downregulate apoptotic pathway, leading to epidermal proliferation in psoriasis (Lew et al., 2006).
The effects of C2-ceramide and C6-ceramide on apoptosis in human HaCaT keratinocytes were examined and it was found that C6-ceramide induced apoptosis easily but C2-ceramide did not, indicating that these epidermal cells exhibit selective responses to the carbon length of the fatty acid of ceramide (Takeda et al., 2006). These studies revealed that the ceramide recycling pathway contributes to the C6-ceramide-induced apoptosis in HaCaT cells. Ceramide derivatives containing a lauroyl group, N-lauroyl-d-erythrosphingosine and N-lauroyl-d-erythro-C-20-sphingosine, may inhibit production of interleukin-4 in the activated T cells, via the downregulation of AP-1/NF-AT activation and PKC activity (Park et al., 2006). De novo ceramide biosynthesis has been reported to be involved in ATP-induced macrophage death in a caspase-dependent manner, thereby indicating a novel role for ceramide in P2X7-regulated cell death. Benzoylbenzoyl ATP, a potent P2X7 agonist, was found to mimic the effects of ATP on ceramide production in macrophages while ATP has the opposite effect. Ceramide accumulation was blocked by de novo ceramide biosynthesis inhibitors. ATP-induced caspase-3/7 activation is dependent on ceramide generation (Raymond and Le Stunff, 2006). It has been recently shown that endogenous ceramides are key second messengers in interleukin-1β-induced apoptosis in pig thyroid cells through inhibition of adenylyl cyclase and ERK1/2 activation (El Btaouri et al., 2006). Dihydrotestosterone-dependent suppression of neutral sphingomyelinase activity has been found to reduce the concentrations of ceramide, increase sensitivity to alpha (1)-adrenoreceptor-mediated mitogenic signaling and facilitate norepinephrine-dependent smooth muscle growth in prepubertal and adult guinea pigs (Durham and Mawhinns, 2006).
Ceramide mediates tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis via a redox mechanism (Dumitru and Gulbins, 2006). Splenocytes and other tumor cell lines respond to TRAIL treatment with activation of acid-sphingomyelinase, release of ceramide, formation of ceramide-enriched membrane platforms, and apoptosis. Acid-sphingomyelinase-deficient splenocytes do not respond to TRAIL and addition of natural C16-ceramide restores death receptor DR5-clustering and apoptosis in the splenocytes. Ceramide-mediated activation of mitochondrial p38 MAPK has been proposed as a potential mechanism for loss of mitochondrial transmembrane potential and apoptosis (Kong et al., 2005). Ceramide-induced apoptosis depends on mitochondrial respiratory function, cytochrome c release and caspase-3 activation in Hep-G2 cells. Ceramide-induced cell death has been attributed to hydrogen peroxide generation at the ubiquinone site of the mitochondrial respiratory chain and rapid decline of oxidation in the mitochondrial electron transport chain (Garcia-Ruiz, et al., 1997; Gudz et al., 1997; Quillet-Mary et al., 1997; Gentil et al., 2003). The interactions of ceramide with nitric oxide generated by endothelial nitric oxide synthase (NOS III) have also been implicated in cell death. NOS III is localized at both the plasma membrane and the Golgi complex through its ability to be myristoylated and palmitoylated (Voelkel-Johnson et al., 2005). Ceramide generation has been shown to lead to NOS III activation in numerous studies in the past few years. NOS III activation can involve ceramide generation by either acid or neutral sphinomyelinases, stimulated either by basic fibroblast growth factor (bFGF) in CHO-K1 cells (Goldkorn et al., 1998). It can also involve tumor necrosis factor-α (TNF-α) in HeLa cell clones transfected with NOS III under a tetracycline-responsive element (Barsacchi et al., 2003; Bulotta et al., 2001). NOS III activation by TNF-α requires stimulation of the phosphatidylinositol 3 kinase (PI3K)/Akt pathway while bFGF activated NOS III is independent of PI3/Akt activation. The neutral SMase-dependent activation of NOS III negatively regulates TNF-α induced apoptosis, while the acid SMase-dependent NO generation upregulates the mitogenic effect of bFGF. Many studies indicate that low physiological concentrations of NO reduce the generation of ceramide and are implicated in cell survival and differentiation. High levels of NO increase ceramide levels and contribute to cell death (Sciorati et al., 1999; Florio et al., 2003; Huwiler et al., 1999; Takeda et al., 1999).
Ceramide and ion channels
There is growing interest in the implications of a relationship between ceramide and ion channels. Studies in recent times have shown the involvement of ceramide, produced through sphingomyelinase-mediated catalysis, in airway anion secretion of polarized Calu-3 cells (Ito et al., 2004). Short-circuit current measurement revealed that 0.1 μM isoproterenol-induced anion secretion is prevented by pretreatment with 0.3 U/ml SMase for 30 min from the basolateral but not the apical side, although basal and 1-ethyl-2-benzimidazolinone (a Ca2+-activated K+ channel opener)-induced short circuit current is unaffected. Forskolin and 8-bromo-cAMP were found to reproduce the effects of SMase. Furthermore, C2-ceramide, a cell-permeable analog, also represses the 8-bromo-cAMP-induced responses. Nystatin permeabilization studies confirmed that the SMase- and C2-ceramide-induced repressions are due to hindrance of augmentation of cystic fibrosis transmembrane conductance regulator (CFTR)-mediated conductance across the apical membrane. These data suggest that the ceramide originating from basolateral sphingomyelin acts on activated CFTR from the cytosolic side, thereby hindering and resulting in reduction of transepithelial airway anion secretion. Ceramide interferes with the activities of maxi-K+ and voltage-gated K+ channels in various cells. Ceramide inhibits large-conductance Ca2+ activated potassium channels in vascular smooth muscle cells from small bovine coronary arteries, thereby exerting vasoconstrictory effect in coronary circulation (Li et al., 1999). Blockade of inwardly rectifying potassium current in rat pituitary GH(3) cells has also been reported (Wu et al., 2001).
Ceramide-induced oligodendrocyte depolarization involves inhibition of inwardly rectifying potassium currents via a Ras- and Raf-1-dependent pathway, which results in the phosphorylation of the inward rectifier K+ channel protein (Hida et al., 1998). A possible role for galactocerebroside in transmembrane signaling, in modulation of voltage-sensitive Ca2+ channels in the U-87 MG human glioma cell line has also been suggested (Joshi and Mishra, 1992). Inhibition of voltage-gated potassium channel Kv1.3 in Jurkat T lymphocytes by ceramide has been demonstrated recently (Gulbins et al., 1997); this effect of ceramide is mediated by tyrosine kinases. However, the precise biochemical mechanisms governing the regulation of ion channels by ceramide are still unknown.
Ceramide in disease pathophysiology and therapeutics
There has been an upsurge of interest in unraveling the roles of ceramide in the pathophysiology of human diseases including cardiovascular disorders, cancer and neurodegenerative disorders, chiefly Alzheimer’s disease. Targeted manipulation of ceramide metabolic pathway as well as specific components in the ceramide-mediated signaling cascades may prove to be a promising strategy in disease treatment and therapy. Currently, numerous experimental studies are aimed at the identification of ceramide-based analogs and inhibitors of ceramide metabolism as possible drug targets.
Ceramide and cardiovascular disorders
Ceramide-mediated transmembrane signaling has been strongly implicated in vascular function by many researchers (Figure 5). Auge and colleagues demonstrated the critical role of the sphingomyelin-ceramide pathway in the oxidized low density lipoprotein (ox-LDL)-induced smooth muscle cell proliferation and atherogenesis (Auge et al., 1996). Lactosylceramide (LacCer)-mediated plaque formation involves aortic smooth muscle cell proliferation (Chatterjee et al., 1997). In vitro cell proliferation of aortic smooth muscle cells by LacCer involves complex signaling mechanisms that involve the activation of NADPH oxidase and a mitogen activated protein kinase signaling cascade. There is evidence regarding significant role of Ras-GTP loading, MEK, Raf, p44MAPK cascade activation and c-fos expression as a potential mechanism in LacCer-mediated proliferation of aortic smooth muscle cells (Bhunia et al., 1996). Further studies to elucidate the precise role of LacCer in the pathogenesis of atherosclerosis revealed that specific activation of membrane-associated NADPH oxidase by LacCer generates endogenous superoxide which mediates aortic smooth muscle cell proliferation via activation of kinase cascade (Bhunia et al., 1997). Galactosyl transfer from UDP-galactose to Glucosylceramide is catalyzed by lactosylceramide synthase (UDP-Gal:GlcCer, β(1-4)-galactosyltransferase; GalT-2). LacCer has also been implicated in the TNF-α-induced expression of Nuclear Factor-κB and intercellular adhesion molecule (ICAM-1) in vascular endothelial cells by increasing the activity of GalT-2. LacCer was found to mediate laminar shear-induced superoxide production and ICAM-1 expression in human umbilical vein vascular smooth muscle cells. Preincubation of cells with the antioxidant N-acetylcysteine (NAC) was found to completely abolish the shear-induced superoxide production and significantly inhibit the ICAM-1 expression. Also, D-1-phenyl-2-decanoylamino-3-morpholino-1-propanol (D-PDMP), attenuates the shear-induced activation of GalT-2, superoxide production and ICAM-1 expression (Yeh et al., 2001; Chatterjee, 1998). The triggering of an inflammatory reaction following activation of transcription factors NF-κB and AP-1 causes phenotypic modulation of medial vascular smooth muscle cells and their migration and subsequent proliferation in the intimal layer; increased extracellular matrix production by these vascular smooth muscle cells increases the volume of intimal tissue causing neointimal hyperplasia, ultimately leading to restenosis (Krueger et al., 2006; Mitra and Agrawal, 2006). The surface expression of Mac-1 or CD11/CD8 on human neutrophils is also stimulated by LacCer, ultimately leading to atherosclerosis. Ceramides have also been linked with vessel wall thickening, plaque formation as well as erosion and thrombosis (Auge et al., 1999; Mallat and Tedgui, 2001). Plasminogen activator inhibitor-1 release in thrombosis is also regulated by ceramide. Sphingomyelinase-mediated hydrolysis results in an increased efflux of cholesterol from LDL. Exogenous cell-permeable ceramide induces cardiomyocyte apoptosis in vitro, which contributes to myocardial ischemia/reperfusion injury (Soeda et al., 1995; Galle et al, 1991; Leventhal et al., 2001; Schissel et al., 1998; Bielawska et al., 1997). Induction of endothelial dysfunction in small coronary arteries by ceramide occurs via NADPH oxidase-mediated superoxide production and subsequent increase in peroxynitrite. NADPH oxidase activation by ceramide was associated with the translocation of p47 (phox) subunit of NADPH oxidase to the cytoplasmic membrane (Zhang et al., 2003). Recent studies to determine whether sulfatides (3-sulfated galactosyl ceramides), the native ligands of L- and P- selectin, affect the development of intimal hyperplasia suggest that neutrophil accumulation on the subendothelial matrix or adherence of platelets mediated by adhesive interactions between L- or P-selectin with sulfatides may contribute to the development of intimal hyperplasia (Shimazawa et al., 2005). Neutrophil accumulation may be mediated by an increase in Mac-1 expression caused by the agonistic effects of sulfatides on the neutrophil membrane surface, or by an increase in L- and P-selectin ligands, resulting from the binding of sulfatides to the exposed subendothelial matrix. The proatherogenic properties of serine palmitoyl-CoA transferase (SPT) have been demonstrated recently (Hojjati et al., 2005). Intraperitoneal administration of myriocin in apoE-deficient mice inhibited SPT and decreased the levels of plasma sphingomyelin, ceramide, sphingosine and sphingosine-1-phosphate. Strategies aimed at inhibiting the activity of SPT could be an alternative treatment for atherosclerosis. Such findings provide considerable evidence for the role of ceramides in vascular biology.
Figure 5.
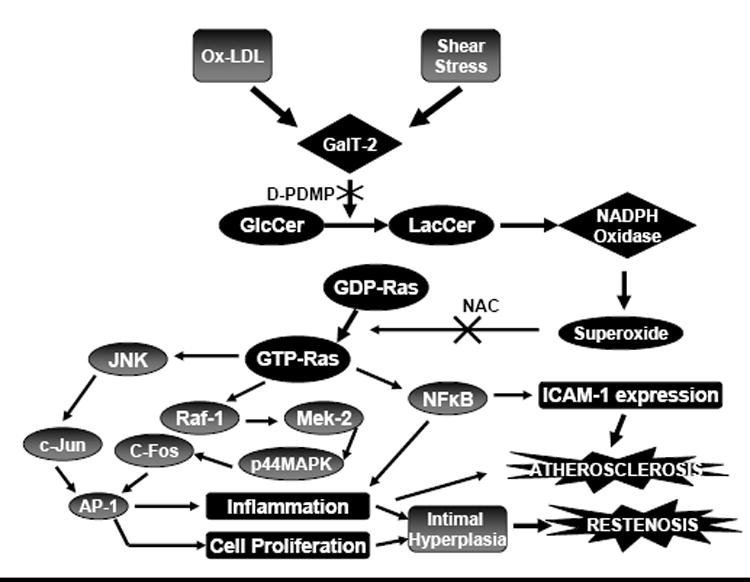
Implications of ceramide-mediated signaling in atherosclerosis. Ox-LDL, oxidized low density lipoprotein; GalT-2, UDP-Gal:GlcCer,β(1-4)-galactosyltransferase; D-PDMP, D-1-phenyl-2-decanoylamino-3-morpholino-1-propanol; GlcCer, Glucosylceramide; LacCer, Lactosylceramide; NAC, N-acetylcysteine, NF-κB, Nuclear Factor Kappa B; MAPK, Mitogen Activated Protein Kinase; ICAM, Intercellular Adhesion Molecule.
Ceramide and cancer
Various clinically important cytotoxic agents have been found to activate ceramide-mediated pathways in cancer cells. The anthracyline daunorubicin stimulates ceramide synthase activity and thus, promotes ceramide formation and apoptosis. Fuminosin B1, an inhibitor of ceramide synthase, inhibits daunorubicin-induced apoptosis. Daunorubicin increases ceramide levels by stimulating ceramide synthase in Jurkat E6.1 lymphoblastomic leukemia cells (Bose et al., 1995; Wang et al., 1991; Turnbull et al., 1999). Doxorubicin exposure increases ceramide levels in drug-sensitive MCF-7 breast cancer cells (Lucci et al., 1999). Cytotoxic properties of Vincristine and Vinblastine, vinca alkaloids, frequently used in the treatment of leukemia, have been suggested to be due to their ability to increase levels of cellular ceramide. Exposure of ALL-697 leukemia cells to vincristine causes apoptosis after a sustained increase in ceramide (Zhang et al., 1996). Paclitaxel, a drug that inhibits microtubule depolymerization and is effective against a number of solid tumors including ovarian and breast cancers, upon coadministration with exogenous ceramide, substantially inhibits cell proliferation and synergistically elicits apoptosis in Jurkat T cells as well as Tu 138 head and neck squamous cell cancer. The effects of Paclitaxel are linked to the de novo synthesis of ceramide in MDA-MB-468 and MCF-7 breast cancer cells. Furthermore, Paclitaxel-dependent cytotoxicity is abrogated by blocking ceramide production with L-cycloserine, an inhibitor of ceramide synthesis (Mc Closkey et al., 1996; Mehta et al., 2000). Tamoxifen, a triphenylethylene antiestrogen, blocks the conversion of ceramide to glucosylceramide, thereby promoting an increase in cellular ceramide concentration (Cabot et al., 1996; Lavie et al., 1997). The synthetic retinoid, 4-(N-hydroxyphenyl) retinamide (4-HPR) increases the level of intracellular ceramide in drug-resistant neuroblastoma cell lines and the LNCaP prostate cancer cell line (Maurer et al., 1999).
Ceramide and neurodegeneration
Many workers have emphasized the role of ceramide in neurodegenerative diseases especially Alzheimer’s disease. A recent study indicates a possible role of nuclear sphingomyelinase/sphingomyelin-synthase balance in serum deprivation-induced apoptosis in HN9 10e embryonal hippocampal cell line (Albi et al., 2006). Treatment of cultured hippocampal neurons with ceramide causes death-associated protein kinase (DAP) activation and neuronal cell death (Pelled et al., 2002). Cultured neurons isolated from DAP kinase deficient mice were found to be quite resistant to ceramide-induced cell death, thereby strongly suggesting that DAP kinase might be a pivotal player in ceramide-mediated cell death in cultured neurons. Several studies have suggested that alterations in the trafficking and function of the endocytic pathway may cause a redistribution of several lysosomal hydrolases into early endosomes, leading to the overproduction of neurotoxic amyloid peptide. Abnormal endocytosis in post mitotic neurons can, in part, be attributed to alterations in sphingomyelin/ceramide metabolism, resulting in the intracellular accumulation of ceramide (Soreghan et al., 2003). O-methylserine dodecylamide (MSD), a lysosomotropic agent, disrupts the neuronal lysosomal proton gradient, leading to intra-neuronal ceramide accumulation. Perturbations in the intracellular transport of cholesterol and sphingolipids have been proposed to play a significant role in Alzheimer’s disease. Recently, N-SMase and A-SMase have been strongly implicated in soluble oligomeric amyloid-β peptide (Aβ)-induced apoptosis. Enzymatic activity measurements, inhibition studies and antisense oligonucleotide strategy strongly suggest that soluble oligomers of Aβ (1-40) and Aβ (1-42) induce neuronal apoptosis via a redox-sensitive cytosolic calcium-dependent phospholipase A2–arachidonic acid-dependent pathway, predominantly through the activation of both N-SMase and A-SMase (Malaplate-Armand et al., 2006). Ceramide has been implicated as a key mediator in photoreceptor apoptosis in rat retina neuronal cultures (German et al., 2006). Such studies strongly highlight the involvement of ceramide in neurodegeneration.
Ceramide-based therapeutics
Considerable interest persists in the identification and comprehensive characterization of ceramide analogs and novel inhibitors of ceramide metabolism as potential drugs. Ceramide analogs, applied directly to damaged arteries, can be strongly antiproliferative. In vivo, C6-ceramide-coated balloon catheters prevent stretch-induced neointimal hyperplasia in rabbit carotid arteries by inactivating ERK and AKT signaling and thereby inducing cell cycle arrest (Charles et al., 2000; Bourbon et al., 2001). Ceramidase inhibition seems to offer a promising therapeutic strategy for selective toxicity towards malignant cells; B 13, a potent ceramidase inhibitor, increases the ceramide content of tumor cells and induces tumor cell apoptosis in metastatic human colon cancer, without affecting the ceramide level or survival of normal liver cells (Selzner et al., 2001). The recent identification of a novel CERK (ceramide kinase) inhibitor, F-12509A olefin isomer K1, demonstrates the therapeutic potential of CERK inhibitors in the treatment of allergic diseases (Kim et al., 2005). Ceramide-mediated signaling has been implicated in tumor necrosis factor (TNF)-alpha-induced impairment of endothelin-dependent vasorelaxation in coronary arteries. Pretreatment of isolated bovine small coronary arteries with desipramine (10 μM), an inhibitor of acid sphingomyelinase, restored the inhibitory effect of TNF-alpha on bradykinin- and A-23187-induced vasorelaxation. Furthermore, activation of acid sphingomyelinase by TNF-alpha enhances ceramide levels in coronary endothelium (Zhang et al., 2002). Endostatin-mediated increase in intracellular ceramide levels in bovine coronary endothelial cells leads to enhanced superoxide production, suggesting a critical role of ceramide-mediated signaling in endostatin-induced endothelial dysfunction (Zhang et al., 2005). Recent studies with Fuminosin B1 and Myriocin in mesangial cells revealed that these inhibitors of the de novo synthesis pathway of ceramide prevent L-homocysteine-induced ceramide formation and attenuate hyperhomocysteinemia-associated glomerular injury and proteinuria (Yi et al., 2004). Ceramide analogs and inhibitors of ceramide metabolism appear to be attractive targets for future therapy.
Future perspectives
The emergence of ceramide as a critically important bioactive messenger in diverse biological processes undoubtedly offers new opportunities to fully dissect the intricacies involved in ceramide-mediated signaling cascade. It is indeed intriguing how ceramide, a simple structural molecule, is able to regulate a wide spectrum of cellular events. The precise biochemical mechanisms involved in ceramide-enriched membrane platforms, receptor clustering and subsequent signal transduction need to be further analyzed. Innovative strategies targeting the components of ceramide metabolism may provide a better understanding of the role of ceramide in disease pathophysiology. Future research in the area of ceramide signaling may lead to the development of clinically useful diagnostic markers.
Acknowledgments
This work was supported by a grant from the Nebraska Cancer and Smoking Related Disease Program, Department of Health, Nebraska (to D.K.A.), and by NIH grants R01HL070885 and R01HL073349 (both to D.K.A.) and the Carpenter Chair (to R.F.M.) of Creighton University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel Shakor AB, Kwiatkowska K, Sobota A. Cell surface ceramide generation precedes and controls Fas clustering and phosphorylation in rafts. J Biol Chem. 2004;279:36778–36787. doi: 10.1074/jbc.M402170200. [DOI] [PubMed] [Google Scholar]
- Albi E, Cataldi S, Bartoccini E, Magni MV, Marini F, Mazzoni F, Rainaldi G, Evangelista M, Garcia-Gil M. Nuclear sphingomyelin pathway in serum deprivation-induced apoptosis of embryonic hippocampal cells. J Cell Physiol. 2006;206:189–95. doi: 10.1002/jcp.20448. [DOI] [PubMed] [Google Scholar]
- Auge N, Andrieu N, Negre-Salvayre A, Thiers JC, Levade T, Salvayre R. The sphingomyelin-ceramide signaling pathway is involved in oxidized low density lipoprotein-induced cell proliferation. J Biol Chem. 1996;271:19251–19255. doi: 10.1074/jbc.271.32.19251. [DOI] [PubMed] [Google Scholar]
- Auge N, Nikolova-Karakashian M, Carpentier S, Parthasarathy S, Negre-Salvayre A, Salvayre R, Merril AH, Jr, Levade T. Role of sphingosine 1-phosphate in the mitogenesis induced by oxidized low density lipoprotein in smooth muscle cells via activation of sphingomyelinase, ceramidase, and sphingosine kinase. J Biol Chem. 1999;274:21533–21538. doi: 10.1074/jbc.274.31.21533. [DOI] [PubMed] [Google Scholar]
- Barsacchi R, Perrotta C, Bulotta S, Moncada S, Borgese N, Clementi E. Activation of endothelial nitric-oxide synthase by tumor necrosis factor-alpha: a novel pathway involving sequential activation of neutral sphingomyelinase, phosphatidylinositol-3′ kinase, and Akt. Mol Pharmacol. 2003;63:886–895. doi: 10.1124/mol.63.4.886. [DOI] [PubMed] [Google Scholar]
- Basu S, Bayoumy S, Zhang Y, Lozano J, Kolesnick R. BAD enables ceramide to signal apoptosis via Ras and Raf-1. J Biol Chem. 1998;273:30419–26. doi: 10.1074/jbc.273.46.30419. [DOI] [PubMed] [Google Scholar]
- Bhunia AK, Han H, Snowden A, Chatterjee S. Lactosylceramide stimulates Ras-GTP loading kinases (MEK, Raf), p44-mitogen activated protein kinase and c-fos expression in human aortic smooth muscles. J Biol Chem. 1996;27:10660–10666. doi: 10.1074/jbc.271.18.10660. [DOI] [PubMed] [Google Scholar]
- Bhunia AK, Han H, Snowden A, Chatterjee S. Redox-regulated signaling by lactosylceramide in the proliferation of human aortic smooth muscle cells. J Biol Chem. 1997;272:15642–15649. doi: 10.1074/jbc.272.25.15642. [DOI] [PubMed] [Google Scholar]
- Bielawska AE, Shapiro JP, Jiang L, Melkonyan HS, Piot C, Wolfe CL, Tomei LD, Hannun YA, Umansky SR. Ceramide is involved in triggering of cardiomyocyte apoptosis induced by ischemia and reperfusion. Am J Pathol. 1997;151:1257–1263. [PMC free article] [PubMed] [Google Scholar]
- Birbes H, Luberto C, Hsu YT, El Bawab S, Hannun YA, Obeid LM. A mitochondrial pool of sphingomyelin is involved in TNF-alpha-induced Bax translocation to mitochondria. Biochem J. 2005;386:445–451. doi: 10.1042/BJ20041627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose R, Verheij M, Haimovitz-Friedman A, Scotto K, Fuks Z, Kolesnick R. Ceramide synthase mediates daunorubicin-induced apoptosis: an alternative mechanism for generating death signals. Cell. 1995;82:405–414. doi: 10.1016/0092-8674(95)90429-8. [DOI] [PubMed] [Google Scholar]
- Bourbon NA, Yun J, Kester M. Ceramide directly activates protein kinase C ζ to regulate a stress-activated protein signaling complex. J Biol Chem. 1992;267:5048–51. doi: 10.1074/jbc.M007346200. [DOI] [PubMed] [Google Scholar]
- Bourbon NA, Yun J, Berkey D, Wang Y, Kester M. Inhibitory actions of ceramide upon PKC-epsilon/ERK interactions. Am J Physiol Cell Physiol. 2001;280:c1403–1411. doi: 10.1152/ajpcell.2001.280.6.C1403. [DOI] [PubMed] [Google Scholar]
- Brenner B, Koppenhoefer U, Weinstock C, Lindercamp O, Lang F, Gulbins E. Fas- or ceramide-induced apoptosis is mediated by a Rac-1 regulated activation of Jun N-terminal kinase/p38 kinases and GADD 153. J Biol Chem. 1997;272:22173–22181. doi: 10.1074/jbc.272.35.22173. [DOI] [PubMed] [Google Scholar]
- Bulotta S, Barsacchi R, Rotiroti D, Borgese N, Clementi E. Activation of the endothelial nitric-oxide synthase by tumor necrosis factor-alpha. A novel feedback mechanism regulating cell death. J Biol Chem. 2001;276:6529–66536. doi: 10.1074/jbc.M006535200. [DOI] [PubMed] [Google Scholar]
- Cabot MC, Guiliano AE, Volner A, Han TY. Tamoxifen retards glycosphingolipid metabolism in human cancer cells. FEBS Lett. 1996;394:129–131. doi: 10.1016/0014-5793(96)00942-8. [DOI] [PubMed] [Google Scholar]
- Chalfant CE, Kishikawa K, Mumby MC, Kamibaya C, Bielawska A, Hannun YA. Long chain ceramides activate protein phosphatase-1 and protein phosphatase-2A: Activation is stereospecific and regulated by phosphatidic acid. J Biol Chem. 1999;274:20313–7. doi: 10.1074/jbc.274.29.20313. [DOI] [PubMed] [Google Scholar]
- Charles R, Sandirasegarane L, Yun J, Bourbon N, Wilson R, Rothstein RP, Levison SW, Kester M. Ceramide-coated balloon catheters limit neointimal hyperplasia after stretch injury in carotid arteries. Circ Res. 2000;87:282–288. doi: 10.1161/01.res.87.4.282. [DOI] [PubMed] [Google Scholar]
- Charruyer A, Grazide S, Benzombes C, Muller S, Laurent G, Jaffrezou JP. UV-C induces raft-associated acid sphingomyelinase and JNK activation and translocation independently on a nuclear signal. J Biol Chem. 2005;280:19196–19204. doi: 10.1074/jbc.M412867200. [DOI] [PubMed] [Google Scholar]
- Chatterjee SB, Dey S, Shi WY, Thomas K, Hutchins GM. Accumulation of glycosphingolipids in human atherosclerotic plaque and unaffected aorta tissues. Glycobiology. 1997;7:57–65. doi: 10.1093/glycob/7.1.57. [DOI] [PubMed] [Google Scholar]
- Chatterjee S. Sphingolipids in atherosclerosis and vascular biology. Arterioscler Thromb. 1998;18:1523–1533. doi: 10.1161/01.atv.18.10.1523. [DOI] [PubMed] [Google Scholar]
- Chatterjee S. Neutral sphingomyelinase: past, present and future. Chem Phys Lipids. 1999;102:79–96. doi: 10.1016/s0009-3084(99)00077-8. [DOI] [PubMed] [Google Scholar]
- Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind S, Spiegel S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381:800–803. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- De Stefanis D, Reffo P, Bonelli G, Baccino FM, Sala G, Ghidoni R, Codogno P, Isidoro C. Increase in ceramide level alters the lysosomal targeting of Cathepsin D prior to onset of apoptosis in HT-29 colon cancer cells. Biol Chem. 2002;383:989–99. doi: 10.1515/BC.2002.106. [DOI] [PubMed] [Google Scholar]
- Dumitru CA, Gulbins E. TRAIL activates acid sphingomelinase via a redox mechanism and releases ceramide to trigger apoptosis. Oncogene. 2006:1–14. doi: 10.1038/sj.onc.1209568. [DOI] [PubMed] [Google Scholar]
- Durham JP, Mawhinns MG. Downregulation of neutral sphingomyelinase in androgen-dependent smooth muscle. J Surg Res. 2006;130:136–139. doi: 10.1016/j.jss.2005.06.035. [DOI] [PubMed] [Google Scholar]
- El Btaouri H, Rath G, Morjani H, Schneider C, Petitfrere C, Antonicelli F, Martiny L. Interleukin-1-beta-induced apoptosis through adenylyl cyclase and ERK1/2 inhibition in primary cultured thyroid cells. Biochem Biophys Res Commun. 2006;339:469–476. doi: 10.1016/j.bbrc.2005.10.213. [DOI] [PubMed] [Google Scholar]
- Erdreich-Epstein A, Tran LB, Cox OT, Huang EY, Laug WE, Shimada H, Millard M. Endothelial apoptosis induced by inhibition of integrins αVβ3and αVβ5 involves ceramide metabolic pathways. Blood. 2005;105:4353–4361. doi: 10.1182/blood-2004-08-3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanani ML, Harte S, Olivera RG, Maggio B. Bidirectional control of sphingomyelinase activity and surface topography in lipid monolayers. Biophys J. 2002;83:3416–3424. doi: 10.1016/S0006-3495(02)75341-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florio T, Arena S, Pattarozzi A, Thellung S, Corsaro A, Villa V, Massa A, Diana F, Spoto G, Forcella S, Damonte G, Filocamo M, Benatti U, Schettini G. Basic fibroblast growth factor activates endothelial nitric oxide synthase in CHO-CK1 cells via the activation of ceramide synthesis. Mol Pharmacol. 2003;63:297–310. doi: 10.1124/mol.63.2.297. [DOI] [PubMed] [Google Scholar]
- Fukasawa M, Nishijima M, Hanada K. Genetic evidence for ATP-dependent endoplasmic reticulum-to-Golgi apparatus trafficking of ceramide for sphingomyelin synthesis in Chinese hamster ovary cells. J Cell Biol. 1999;144:673–85. doi: 10.1083/jcb.144.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futerman AH, Stieger B, Hubbard AL, Pagano RE. Sphingomyelin synthesis in rat liver occurs predominantly at the cis and medial cisternae of the Golgi apparatus. J Biol Chem. 1990;265:8650–57. [PubMed] [Google Scholar]
- Galle J, Mulsch A, Busse R, Bassenge E. Effects of native and oxidized low density lipoproteins on formation and inactivation of endothelium derived relaxing factor. Arterioscler Thromb. 1991;11:198–203. doi: 10.1161/01.atv.11.1.198. [DOI] [PubMed] [Google Scholar]
- Garcia-Ruiz C, Collel A, Mari M, Morales A, Fernandez-Checa JC. Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species: role of mitochondrial glutathione. J Biol Chem. 1997;272:11369–11377. doi: 10.1074/jbc.272.17.11369. [DOI] [PubMed] [Google Scholar]
- Gentil B, Grimot F, Riva C. Commitment to apoptosis by ceramides depends on mitochondrial respiratory function, cytochrome c release and caspase-3 activation in Hep-G2 cells. Mol Cell Biochem. 2003;254:203–210. doi: 10.1023/a:1027359832177. [DOI] [PubMed] [Google Scholar]
- German OL, Miranda GE, Abraham CE, Rotstein NP. Ceramide is a mediator of apoptosis in retina photoreceptors. Invest Opthalmol Vis Sci. 2006;47:1658–1668. doi: 10.1167/iovs.05-1310. [DOI] [PubMed] [Google Scholar]
- German OL, Miranda GE, Abraham CE, Rotstein NP. Ceramide is a mediator of apoptosis in retina photoreceptors. Invest Opthalmol Vis Sci. 2006;47:1658–1668. doi: 10.1167/iovs.05-1310. [DOI] [PubMed] [Google Scholar]
- Goldkorn T, Balaban N, Shannon M, Chea V, Matsukuma K, Gilchrist D, Wang H, Chan C. H2O2 acts on cellular membranes to generate ceramide signaling and initiate apoptosis in tracheobronchial epithelial cells. J Cell Sci. 1998;111:3209–3220. doi: 10.1242/jcs.111.21.3209. [DOI] [PubMed] [Google Scholar]
- Grassme H, Jekle A, Riehle A, Schwarz H, Berger J, Sandhoff K, Kolesnick R, Gulbins E. CD95-Signaling via ceramide-rich membrane rafts. J Biol Chem. 2001;276:20589–20596. doi: 10.1074/jbc.M101207200. [DOI] [PubMed] [Google Scholar]
- Grassme H, Jendrossek V, Bock J, Riehle A, Gulbins E. Ceramide-rich membrane rafts mediate CD 40 clustering. J Immunol. 2002;168:298–307. doi: 10.4049/jimmunol.168.1.298. [DOI] [PubMed] [Google Scholar]
- Grassme H, Cremesti A, Kolesnick RN, Gulbins E. Ceramide mediated clustering is required for CD95-DISC formation. Oncogene. 2003;22:5457–5470. doi: 10.1038/sj.onc.1206540. [DOI] [PubMed] [Google Scholar]
- Grassme H, Jendrossek V, Riehle A, von Kurthy G, Berger J, Schwarz H, Weller M, Kolesnick R, Gulbins E. Host defence against Pseudomonas aeruginosa requires ceramide-rich membrane rafts. Nat Med. 2003;9:322–30. doi: 10.1038/nm823. [DOI] [PubMed] [Google Scholar]
- Grassme H, Riehle A, Wilker B, Gulbins E. Rhinoviruses infect human epithelial cells via ceramide-enriched membrane platforms. J Biol Chem. 2005;80:26256–26262. doi: 10.1074/jbc.M500835200. [DOI] [PubMed] [Google Scholar]
- Gudz TI, Tseng KY, Hoppel CL. Direct inhibition of mitochondrial respiratory chain complex III by cell-permeable ceramide. J Biol Chem. 1997;272:24154–58. doi: 10.1074/jbc.272.39.24154. [DOI] [PubMed] [Google Scholar]
- Gulbins E, Coggeshall KM, Baier G, Telford D, Langlet C, Baier-Bitterlich G, Bonnefoy-Berard N, Burn P, Wittinghofer A, Altman A. Direct stimulation of Vav guanine nucleotide exchange activity for Ras by phorbol esters and diglycerides. Mol Cell Biol. 1994;14:4749–58. doi: 10.1128/mcb.14.7.4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulbins E, Szabo I, Baltzer K, Lang F. Ceramide-induced inhibition of T lymphocyte voltage-gated potassium channel is mediated by tyrosine kinases. Proc Natl Acad Sci USA. 1997;94:7661–7666. doi: 10.1073/pnas.94.14.7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada K, Kumagai K, Yasuda S, Miura Y, Kawano M, Fukasawa M, Nishijima M. Molecular machinery for non-vesicular trafficking of ceramide. Nature. 2003;426:803–809. doi: 10.1038/nature02188. [DOI] [PubMed] [Google Scholar]
- Hannun YA. The sphingomyelin cycle and the second messenger function of ceramide. J Biol Chem. 1994;26:3125–3128. [PubMed] [Google Scholar]
- Hannun YA, Luberto C. Ceramide in the eukaryotic stress response. Trends Cell Biol. 2000;10:73–80. doi: 10.1016/s0962-8924(99)01694-3. [DOI] [PubMed] [Google Scholar]
- Hannun YA, Luberto C, Argraves KM. Enzymes of sphingolipid metabolism: from modular to integrative signaling. Biochemistry. 2001;40:4893–4903. doi: 10.1021/bi002836k. [DOI] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM. The ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J Biol Chem. 2002;277:25847–25850. doi: 10.1074/jbc.R200008200. [DOI] [PubMed] [Google Scholar]
- Hartel S, Fanani ML, Maggio B. Shape transitions and lattice structuring of ceramide-enriched domains generated by sphingomyelinase in lipid monolayers. Biophys J. 2005;88:287–304. doi: 10.1529/biophysj.104.048959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hida H, Takeda M, Soliven B. Ceramide inhibits inwardly rectifying K+ currents via a Ras- and Raf-1-dependent pathway in cultured oligodendrocytes. J Neurosci. 1998;18:8712–8719. doi: 10.1523/JNEUROSCI.18-21-08712.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg K, Rodger J, Futerman AH. The long chain sphingoid base of sphingolipids is acylated at the cytosolic surface of the endoplasmic reticulum in rat liver. Biochem J. 1993;290:751–57. doi: 10.1042/bj2900751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojjati MR, Li Z, Zhou H, Tang S, Huan C, Ooi E, Lu S, Jiang XC. Effect of Myriocin on plasma sphingolipid metabolism and atherosclerosis in apo-E-deficient mice. J Biol Chem. 2005;280:10284–10289. doi: 10.1074/jbc.M412348200. [DOI] [PubMed] [Google Scholar]
- Huwiler A, Brunner J, Hummel R, Vervoordeldonk M, Stabel S, van den Bosch H, Pfeilschifter J. Ceramide binding and activation defines protein kinase c-Raf as a ceramide-activated protein kinase. Proc Natl Acad Sci USA. 1996;93:6959–63. doi: 10.1073/pnas.93.14.6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huwiler A, Pfeilschifter J, van den Bosch H. Nitric oxide donors induce stress signaling via ceramide formation in rat renal mesangial cells. J Biol Chem. 1999;274:7190–7195. doi: 10.1074/jbc.274.11.7190. [DOI] [PubMed] [Google Scholar]
- Huwiler A, Johansen B, Skarstad A, Pfeilschifter J. Ceramide binds to the CaLB domain of phospholipase A2 and facilitates its membrane docking and arachidonic acid release. FASEB J. 2001;15:7–9. doi: 10.1096/fj.00-0370fje. [DOI] [PubMed] [Google Scholar]
- Ito Y, Sato S, Ohashi T, Nakayama S, Shimokata K, Kume H. Reduction of airway anion secretion via CFTR in sphingomyelin pathway. Biochem Biophys Res Commun. 2004;324:901–908. doi: 10.1016/j.bbrc.2004.09.134. [DOI] [PubMed] [Google Scholar]
- Joshi PG, Mishra S. Galactocerebroside mediates Ca2+ signalling in cultured glioma cells. Brain Res. 1992;597:108–13. doi: 10.1016/0006-8993(92)91511-c. [DOI] [PubMed] [Google Scholar]
- Kashkar H, Wiegmann K, Yazdanpanah B, Haubert D, Kronke M. Acid sphingomyelinase is indispensable for UV light-induced Bax conformational change at the mitochondrial membrane. J Biol Chem. 2005;280:20804–20813. doi: 10.1074/jbc.M410869200. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Mun JY, Chun YJ, Choi KH, Kim MY. Bax-dependent apoptosis is induced by ceramide in HL-60 cells. FEBS Lett. 2001;505:264–268. doi: 10.1016/s0014-5793(01)02836-8. [DOI] [PubMed] [Google Scholar]
- Kim JW, Inagaki Y, Mitsutake S. Suppression of mast cell degranulation by a novel ceramide kinase inhibitor, the F-12509 A olefin isomer K1. Biochim Biophys Acta. 2005;1738:82–90. doi: 10.1016/j.bbalip.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Kirschnek S, Paris F, Weller M, Grassme H, Ferlinz K, Riehle A, Fuks Z, Kolesnick R, Gulbins E. CD 95-mediated apoptosis in vivo involves acid sphingomyelinase. J Biol Chem. 2000;275:27316–23. doi: 10.1074/jbc.M002957200. [DOI] [PubMed] [Google Scholar]
- Kishikawa K, Chalfant CE, Perry DK, Bielawska A, Hannun YA. Phosphatidic acid is a potent and selective inhibitor of protein phosphatase 1 and an inhibitor of ceramide-mediated responses. J Biol Chem. 1999;274:21335–21341. doi: 10.1074/jbc.274.30.21335. [DOI] [PubMed] [Google Scholar]
- Kolesnick RN, Goni FM, Alonso A. Compartmentalization of ceramide signaling: physical foundations and biological effects. J Cell Physiol. 2000;184:285–300. doi: 10.1002/1097-4652(200009)184:3<285::AID-JCP2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Kong JY, Klassen SS, Rabkin SW. Ceramide activates a mitochondrial p38-mitogen activated protein kinase: a potential mechanism for loss of mitochondrial transmembrane potential and apoptosis. Mol Cell Biochem. 2005;278:39–51. doi: 10.1007/s11010-005-1979-6. [DOI] [PubMed] [Google Scholar]
- Krueger KD, Mitra AK, Del Core MD, Hunter WJ, Agrawal DK. A comparison of stent-induced stenosis in coronary and peripheral arteries. J Clin Pathol. 2006;59:575–579. doi: 10.1136/jcp.2004.025643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai K, Ysuda S, Okemoto K, Nishimija M, Kobayashi S, Hanada K. CERT mediates intermembrane transfer of various molecular species of ceramides. J Biol Chem. 2005;280:6488–6495. doi: 10.1074/jbc.M409290200. [DOI] [PubMed] [Google Scholar]
- Lacour S, Hammann A, Grazide S, Lagadic-Gossmann D, Athias A, Sergent O, Laurent G, Gambert P, Solary E, Dimanche-Boitrel MJ. Cisplatin-induced CD 95 redistribution into membrane lipid rafts of HT 29 human colon cancer cells. Cancer Res. 2004;64:3593–3598. doi: 10.1158/0008-5472.CAN-03-2787. [DOI] [PubMed] [Google Scholar]
- Lavie Y, Cao HT, Volner A, Lucci A, Han TY, Geffen V, Giuliano AE, Cabot MC. Agents that reverse multidrug resistance, tamoxifen, verapamil, and cyclosporin A, block glycosphingolipid metabolism by inhibiting ceramide glycosylation in human cancer cells. J Biol Chem. 1997;272:1682–1687. doi: 10.1074/jbc.272.3.1682. [DOI] [PubMed] [Google Scholar]
- Levade T, Jaffrezou JP. Signaling sphingomyelinases: which, where, how, and why? Biochim Biophys Acta. 1999;1438:1–17. doi: 10.1016/s1388-1981(99)00038-4. [DOI] [PubMed] [Google Scholar]
- Leventhal AR, Chen W, Tall AR, Tabas I. Acid Sphingomyelinase-deficient Macrophages Have Defective Cholesterol Trafficking and Efflux. J Biol Chem. 2001;276:44976–83. doi: 10.1074/jbc.M106455200. [DOI] [PubMed] [Google Scholar]
- Lew BL, Cho Y, Kim J, Sim WY, Kim NI. Ceramides and cell signaling molecules in psoriatic epidermis: reduced levels of ceramides, PKC-alpha and JNK. J Korean Med Sci. 2006;21:95–99. doi: 10.3346/jkms.2006.21.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li PL, Zhang DX, Zou AP, Campbell WB. Effect of ceramide on KCa channel activity and vascular tone in coronary arteries. Hypertension. 1999;33:1441–1446. doi: 10.1161/01.hyp.33.6.1441. [DOI] [PubMed] [Google Scholar]
- Loewen CJ, Levine TP. A highly conserved binding site in VAP for the FFAT motif of lipid binding proteins. J Biol Chem. 2005;280:14097–14104. doi: 10.1074/jbc.M500147200. [DOI] [PubMed] [Google Scholar]
- Lozano J, Berra E, Municio MM. Protein kinase C zeta isoform is critical for kappaB dependent promoter activation by sphingomyelinase. J Biol Chem. 1994;269:19200–19202. [PubMed] [Google Scholar]
- Lucci A, Han TY, Liu YY, Giuliano AE, Cabot MC. Modification of ceramide metabolism increases sensitivity to cytotoxics. Int J Oncol. 1999;15:541–546. doi: 10.3892/ijo.15.3.541. [DOI] [PubMed] [Google Scholar]
- Malaplate-Armand C, Florent-Bechard S, Youssef I, Koziel V, Sponne I, Kriem B, Leininqer-Muller B, Olivier JL, Oster T, Pillot T. Soluble oligomers of amyloid-β peptide induce neuronal apoptosis by activating a cPLA-dependent sphingomyelinase-ceramide pathway. Neurobiol Dis. 2006;23:178–189. doi: 10.1016/j.nbd.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Mallat Z, Tedgui A. Current Perspective on the Role of Apoptosis in Atherothrombotic Disease. Circ Res. 2001;88:998–1003. doi: 10.1161/hh1001.090571. [DOI] [PubMed] [Google Scholar]
- Mandon EC, Ehses I, Rother J, van Echten G, Sandhoff K. Subcellular localization and membrane topology of serine palmitoyltransferase 3-dehydrosphinganine reductase and sphinganine N-acyltransferase in mouse liver. J Biol Chem. 1992;267:11144–111448. [PubMed] [Google Scholar]
- Mathias S, Pena LA, Kolesnick RN. Signal transduction of stress via ceramide. Biochem J. 1998;335:465–480. doi: 10.1042/bj3350465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer BJ, Metelitsa LS, Seeger RC, Cabot MC, Reynolds CP. Increase of ceramide and induction of mixed apoptosis/necrosis by N-(4-hydroxyphenyl)-retinamide in neuroblastoma cell lines. J Natl Cancer Inst. 1999;91:1138–1146. doi: 10.1093/jnci/91.13.1138. [DOI] [PubMed] [Google Scholar]
- Mc Closkey DE, Kaufmann SH, Prestigiacomo LJ, Davidson NE. Paclitaxel induces programmed cell death in MDA-MB-468 human breast cancer cells. Clin Cancer Res. 1996;2:847–854. [PubMed] [Google Scholar]
- Mehta S, Blackinton D, Omar I, Kouttab N, Myrick D, Klostergaard J, Wanebo H. Combined cytotoxic action of paclitaxel and ceramide against the human Tu138 head and neck squamous carcinoma cell line. Cancer Chemother Pharmacol. 2000;48:85–92. doi: 10.1007/s002800000140. [DOI] [PubMed] [Google Scholar]
- Michael C, van Echten Deckert G. Conversion of dihydroceramide to ceramide occurs at the cytosolic face of the endoplasmic reticulum. FEBS Lett. 1997;416:153–55. doi: 10.1016/s0014-5793(97)01187-3. [DOI] [PubMed] [Google Scholar]
- Mitra AK, Agrawal DK. In stent restenosis: bane of the stent era. J Clin Pathol. 2006;59:232–239. doi: 10.1136/jcp.2005.025742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita Y, Perez GI, Paris F, Miranda SR, Ehleiter D, Haimovitz-Friedman A. Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate pathway. Nat Med. 2000;6:1109–1114. doi: 10.1038/80442. [DOI] [PubMed] [Google Scholar]
- Nilsson A, Duan RD. Alkaline sphingomyelinases and ceramidases of the gastrointestinal tract. Chem Phys Lipids. 1999;102:97–105. doi: 10.1016/s0009-3084(99)00078-x. [DOI] [PubMed] [Google Scholar]
- Park J, Kim SH, Li Q, Chang YT, Kim TS. Inhibition of interleukin-4 production in activated T cells via the downregulation of AP-1/NF-AT activation by N-lauroyl-D-erythro-sphingosine and N-lauroyl-D-erythro-C20-sphingosine. Biochem Pharmacol. 2006;71:1229–1239. doi: 10.1016/j.bcp.2005.12.036. [DOI] [PubMed] [Google Scholar]
- Pelled D, Raveh T, Riebeling C, Fridkin M, Berissi H, Futerman AH, Kimchi A. Death-associated protein (DAP) kinase plays a central role in ceramide-induced apoptosis in cultured hippocampal neurons. J Biol Chem. 2002;2002;277:1957–1961. doi: 10.1074/jbc.M104677200. [DOI] [PubMed] [Google Scholar]
- Perez GI, Jurisicova A, Matikainen T, Moriyama T, Kim MR, Takai Y, Pru JK, Kolesnick RN, Tilly JL. A central role for ceramide in the age-related acceleration of apoptosis in the female germline. FASEB J. 2005;19:860–862. doi: 10.1096/fj.04-2903fje. [DOI] [PubMed] [Google Scholar]
- Quillet-Mary A, Jaffrezou JP, Mansat V, Bordier C, Naval J, Laurent G. Implication of mitochondrial hydrogen peroxide generation in ceramide-induced apoptosis. J Biol Chem. 1997;272:21388–21395. doi: 10.1074/jbc.272.34.21388. [DOI] [PubMed] [Google Scholar]
- Raya A, Revert F, Navarro S, Saus J. Characterization of a novel type of serine/threonine kinase that specifically phosphorylates the human good pasture antigen. J Biol Chem. 1999;274:12642–12649. doi: 10.1074/jbc.274.18.12642. [DOI] [PubMed] [Google Scholar]
- Raymond MN, Le Stunff H. Involvement of de novo ceramide biosynthesis in macrophage death induced by activation of ATP-sensitive P2X7 receptor. FEBS Lett. 2006;580:131–136. doi: 10.1016/j.febslet.2005.11.066. [DOI] [PubMed] [Google Scholar]
- Riboni L, Viani P, Bassi R, Prinetti A, Tettamanti G. The role of sphingolipids in the process of signal transduction. Prog Lipid Res. 1997;36:153–195. doi: 10.1016/s0163-7827(97)00008-8. [DOI] [PubMed] [Google Scholar]
- Ruvolo PP, Gao FG, Blalock WL, Deng X, Stratford May W. Ceramide regulates protein synthesis by a novel mechanism involving the cellular PKR activator RAX. J Biol Chem. 2001;276:11754–65. doi: 10.1074/jbc.M011400200. [DOI] [PubMed] [Google Scholar]
- Raya A, Revert F, Navarro S, Saus J. Characterization of a novel type of serine/threonine kinase that specifically phosphorylates the human good pasture antigen. J Biol Chem. 1999;274:12642–12649. doi: 10.1074/jbc.274.18.12642. [DOI] [PubMed] [Google Scholar]
- Samet D, Barenholz Y. Characterization of acid and neutral sphingomyelinase activities in crude extracts of HL-60 cells. Chem Phys Lipids. 1999;102:65–77. doi: 10.1016/s0009-3084(99)00076-6. [DOI] [PubMed] [Google Scholar]
- Scheel-Toellner D, Wang K, Craddock R, Webb PR, Mc Gettrick HM, Assi LK, Parkes N, Clough LE, Gulbins E, Salmon M, Lord JM. Reactive oxygen species limit neutrophil life span by activating death receptor signaling. Blood. 2004;104:2557–64. doi: 10.1182/blood-2004-01-0191. [DOI] [PubMed] [Google Scholar]
- Scheid MP, Foltz IN, Young PR, Schrader JW, Duronio V. Ceramide and cyclic adenosine monophosphate (c AMP) induce cAMP response element binding protein phosphorylation via distinct signaling pathways while having opposite effects on myeloid cell survival. Blood. 1999;93:217–25. [PubMed] [Google Scholar]
- Schissel SL, Jiang X, Tweedie-Hardman J, Jeong T, Camejo EH, Najib J, Rapp JH, Williams KJ, Tabas I. Secretory sphingomyelinase, a product of the acid sphingomyelinase gene, can hydrolyze atherogenic lipoproteins at neutral pH. J Biol Chem. 1998;273:2738–2746. doi: 10.1074/jbc.273.5.2738. [DOI] [PubMed] [Google Scholar]
- Sciorati C, Rovere P, Ferrarini M, Paulucci C, Heltai S, Vaiani R, Clementi E, Manfredi M. Generation of nitric oxide by the inducible nitric oxide synthase protects gamma delta T cells from Mycobacterium tuberculosis-induced apoptosis. J Immunol. 1999;163:1570–1576. [PubMed] [Google Scholar]
- Selzner M, Bielawska A, Morse MA, Rudiger HA, Sindram D, Hannun YA, Clavien PA. Induction of apoptotic cell death and prevention of tumor growth by ceramide analogues in metastatic human colon cancer. Cancer Res. 2001;61:1233–1240. [PubMed] [Google Scholar]
- Senchenkov A, Litvak DA, Cabot MC. Targeting ceramide metabolism- a strategy overcoming drug resistance. J Natl Cancer Institute. 2001;93:347–57. doi: 10.1093/jnci/93.5.347. [DOI] [PubMed] [Google Scholar]
- Shimazawa M, Kondo K, Hara H, Nakashima M, Umemura K. Sulfatides, L- and P-selectin ligands, exacerbate the intimal hyperplasia occurring after endothelial injury. Eur J Pharmacol. 2005;520:118–126. doi: 10.1016/j.ejphar.2005.06.039. [DOI] [PubMed] [Google Scholar]
- Shirakabe K, Yamaguchi K, Shibuya H, Irie K, Matsuda S, Moriguchi T, Gotoh Y, Matsumoto K, Nishida E. TAK 1 mediates the ceramide signaling to stress-activated protein kinase/c-Jun N-trminal kinase. J Biol Chem 1997. 1997;272:8141–8144. doi: 10.1074/jbc.272.13.8141. [DOI] [PubMed] [Google Scholar]
- Soeda S, Honda O, Shimeno H, Nagamatsu A. Sphingomyelinase and cell-permeable ceramide analogs increase the release of plasminogen activator inhibitor-1 from cultured endothelial cells. Thromb Res. 1995;80:509–518. doi: 10.1016/0049-3848(95)00206-5. [DOI] [PubMed] [Google Scholar]
- Soreghan B, Thomas SN, Yang AJ. Aberrant sphingomyelin/ceramide metabolic-induced neuronal endosomal/lysosomal dysfunction: potential pathological consequences in age-related neurodegeneration. Adv Drug Delivery Reviews. 2003;55:1515–1524. doi: 10.1016/j.addr.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstein S. Sphingosine 1-Phosphate, a key cell signaling molecule. J Biol Chem. 2002;277:25851–25854. doi: 10.1074/jbc.R200007200. [DOI] [PubMed] [Google Scholar]
- Tabas I. Secretory sphingomyelinase. Chem Phys Lipids. 1999;102:123–30. doi: 10.1016/s0009-3084(99)00080-8. [DOI] [PubMed] [Google Scholar]
- Taha TA, Kitatani K, El-Alwani M, Bielawski J, Hannun YA, Obeid LM. Loss of sphingosine kinase-1 activates the intrinsic pathway of programmed cell death: modulation of sphingolipid levels and the induction of apoptosis. FASEB J. 2006;20:482–484. doi: 10.1096/fj.05-4412fje. [DOI] [PubMed] [Google Scholar]
- Takeda Y, Tashima M, Takahashi A, Uchiyama T, Ukazaki T. Ceramide generation in nitric oxide-induced apoptosis. Activation of magnesium-dependent neutral sphingomyelinase via caspase-3. J Biol Chem. 1999;274:10654–10660. doi: 10.1074/jbc.274.15.10654. [DOI] [PubMed] [Google Scholar]
- Takeda S, Mitsutake S, Tsuji K, Igarashi Y. Apoptosis occurs via the ceramide recycling pathway in human HaCaT keratinocytes. J Biochem. 2006;139:255–62. doi: 10.1093/jb/mvj026. [DOI] [PubMed] [Google Scholar]
- Tsujishita Y, Hurley JH. Structure and lipid transport mechanism of a StAR-related domain. Nat Struct Biol. 2000;7:408–414. doi: 10.1038/75192. [DOI] [PubMed] [Google Scholar]
- Turnbull KJ, Brown BL, Dobson PR. Caspase-3-like activity is necessary but not sufficient for daunorubicin-induced apoptosis in Jurkat human lymphoblastic leukemia cells. Leukemia. 1999;13:1056–1061. doi: 10.1038/sj.leu.2401438. [DOI] [PubMed] [Google Scholar]
- Vallee B, Riezman H. Lip 1p: a novel subunit of Acyl-Co A ceramide synthase. EMBO J. 2005;24:730–41. doi: 10.1038/sj.emboj.7600562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Boland JA, Kuhn M, Berche P, Chakraborty T, Dominguez-Bernal G, Goebel W, Gonzalez-Zorn B, Wehland J, Kreft J. Listeria pathogenesis and molecular virulence determinants. Clin Microbiol Rev. 2001;14:584–640. doi: 10.1128/CMR.14.3.584-640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheij M, Bose R, Lin XH, Yao B, Jarvis WD, Grant S, Birrer MJ, Szabo E, Zon LI, Kyriakis JM, Haimovitz-Friedman A, Fuks Z, Kolesnick R. Requirement for ceramide-initiated SAPK/JNK signaling in stress-induced apoptosis. Nature. 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- Voelkel Johnson C, Hannan YA, El-Zawahry A. Resistance to TRAIL is associated with defects in ceramide signaling that can be overcome by exogenous C6-ceramide without requiring down-regulation of cellular FLICE inhibitory protein. Molecular Cancer Therapeutics. 2005;4:1320–27. doi: 10.1158/1535-7163.MCT-05-0086. [DOI] [PubMed] [Google Scholar]
- Wang E, Norred WP, Bacon CW, Riley RT, Merrill AH. Inhibition of sphingolipid biosynthesis by fumonisins. Implications for diseases associated with Fusarium moniliforme. J Biol Chem. 1991;266:14486–90. [PubMed] [Google Scholar]
- Wu SN, Lo YK, Kuo BI, Chiang HT. Ceramide inhibits the inwardly rectifying potassium current in GH(3) lactotrophs. Endocrinology. 2001;142:4785–4794. doi: 10.1210/endo.142.11.8508. [DOI] [PubMed] [Google Scholar]
- Wyles JP, Mc Master CR, Ridgway ND. Vesicle-associated membrane protein-associated protein-A (VAP-A) interacts with oxysterol-binding protein to modify export from the endoplasmic reticulum. J Biol Chem. 2002;277:29908–29918. doi: 10.1074/jbc.M201191200. [DOI] [PubMed] [Google Scholar]
- Yan F, Polk DB. Kinase suppressor of ras is necessary for tumor necrosis factor alpha activation of extracellular ligand-regulated kinase/mitogen-activated protein kinase in intestinal epithelial cells. Cancer Res. 2001;61:963–69. [PubMed] [Google Scholar]
- Yeh LH, Kiney AM, Chatterjee S, Alevriadou BR. Lactosylceramide mediates shear-induced endothelial superoxide production and intercellular adhesion molecule-1 expression. J Vasc Res. 2001;38:551–559. doi: 10.1159/000051091. [DOI] [PubMed] [Google Scholar]
- Yi F, Zhang AY, Janscha JL, Li PL, Zou AP. Homocysteine activates NADH/NADPH oxidase through ceramide-stimulated Rac GTPase activity in rat mesangial cells. Kidney Int. 2004;66:1977–1987. doi: 10.1111/j.1523-1755.2004.00968.x. [DOI] [PubMed] [Google Scholar]
- Zhang J, Alter N, Reed JC, Borner C, Obeid LM, Hannun YA. Bcl-2 interrupts the ceramide-mediated pathway of cell death. Proc Natl Acad Sci USA. 1996;93:5325–5328. doi: 10.1073/pnas.93.11.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yao B, Delikat S, Bayoumy S, Lin XH, Basu S, Mc Ginley M, Chan-Hui PY, Linchenstein H, Kolesnick R. Kinase suppressor of Ras is ceramide-activated protein kinase. Cell. 1997;89:63–72. doi: 10.1016/s0092-8674(00)80183-x. [DOI] [PubMed] [Google Scholar]
- Zhang DX, Yi FX, Zou AP, Li PL. Role of ceramide in TNF-alpha-induced impairment of endothelium-dependent vasorelaxation in coronary arteries. Am J Physiol Heart Circ Physiol. 2002;283:H1785–94. doi: 10.1152/ajpheart.00318.2002. [DOI] [PubMed] [Google Scholar]
- Zhang AY, Teggatz EG, Zoua AP, Campbell WB, Li PL. Endostatin uncouples NO and Ca2+ response to bradykinin through enhanced O2* production in intact coronary endothelium. Am J Physiol Heart Circ Physiol. 2005;288:H686–694. doi: 10.1152/ajpheart.00174.2004. [DOI] [PubMed] [Google Scholar]


