Abstract
Light adaptation is a process that enables photoreceptor cells to operate over a wide range of light intensities without saturation. In invertebrate photoreceptors, fast adaptation is mediated by a Ca2+-dependent negative-feedback mechanism, which mainly affects the terminal steps of the cascade. Therefore, the response to each photon is smaller as light intensity increases, accommodating both high sensitivity and a vast dynamic range. Here, we describe a novel type of adaptation, which is mediated by one of the first steps in the phototransduction cascade affecting the sensitivity to absorbed photons. Long exposure to light resulted in dramatic reduction in the probability of each absorbed photon to elicit a response, whereas the size and shape of each single photon response did not change. To dissect the molecular mechanism underlying this form of adaptation we used a series of Drosophila mutants. Genetic dissection showed a pivotal role for light-induced translocation of Gqα between the signaling membrane and the cytosol. Biochemical studies revealed that the sensitivity to light depends on membrane Gqα concentration, which was modulated either by light or by mutations that impaired its targeting to the membrane. We conclude that long-term adaptation is mediated by the movement of Gqα from the signaling membrane to the cytosol, thereby reducing the probability of each photon to elicit a response. The slow time scale of this adaptation fits well with day/night light intensity changes, because there is no need to maintain single photon sensitivity during daytime.
Keywords: heterotrimeric Gq protein, quantum bumps, phototransduction, light adaptation, Drosophila mutants, protein translocation
Introduction
Light and dark adaptation in Drosophila photoreceptors include fast Ca2+-dependent (<1 min) adaptation and long-term adaptation, which operates on a time scale of many minutes and arises from diverse processes. Examples of long-term adaptation processes are the light-dependent translocation of arrestin 2 (Lee et al., 2003) and of light-sensitive channel transient receptor potential like (TRPL) out of the signaling membrane (Bähner et al., 2002; Cronin et al., 2006).
In invertebrate photoreceptors, heterotrimeric Gq-protein is an essential component of phototransduction, connecting photon absorption by rhodopsin to phospholipase C (PLC) activation and is mainly localized to the plasma membrane domain. The two major determinants of membrane Gqα localization are the dynamic lipid modification of G-proteins by palmitoylation (Wedegaertner et al., 1993) and anchoring of the Gβγ subunits to the membrane (Elia et al., 2005). Light-driven translocation of the vertebrate (Sokolov et al., 2002) and invertebrate G-proteins was found in photoreceptor cells [invertebrates: crayfish (Terakita et al., 1996), squid (Suzuki et al., 1995), Drosophila (Kosloff et al., 2003; Cronin et al., 2004)]. In Drosophila photoreceptors, the rhodopsin-activated Gqα translocates to the cytosol during illumination and subsequently returns to the membrane (Kosloff et al., 2003; Cronin et al., 2004). Despite previous studies showing that a large reduction in the concentration of Gqα in Drosophila mutants reduces the sensitivity to light (Scott et al., 1995), these studies have not experimentally examined whether light-dependent dynamic changes of membrane Gqα concentration mediate long-term adaptation under physiological conditions.
Drosophila photoreceptors exhibit the ultimate sensitivity to light because they are able to detect the absorption of single photons. This property allows measuring of the physiological implication of Gqα movements in vivo at the highest resolution. Photon absorption by rhodopsin in fly photoreceptors activates either one (Scott et al., 1995) or few Gqα protein molecules (Minke and Stephenson, 1985; Hardie et al., 2002), which subsequently activates PLC (Devary et al., 1987; Bloomquist et al., 1988) and leads, in a still unclear way, to the generation of a single-photon response called a quantum bump (Yeandle and Spiegler, 1973). The bumps sum to produce the macroscopic response to light (Dodge et al., 1968; Barash and Minke, 1994). Fast light adaptation is known to reduce the amplitude, but not the frequency of the bump production (Wong et al., 1982) by a still unclear Ca2+-dependent mechanism (Lisman and Brown, 1972; Henderson et al., 2000).
In the present study, we identified a novel mechanism of long-term adaptation, which was manifested in a large (approximately fivefold) reduction of bump frequency. Genetic dissection using the phototransduction mutants Gβe2 and myosin III ninaCP235 revealed that both long-term adaptation and the targeting of Gqα to the signaling membrane were markedly impaired in these mutants. In addition, a reduction in rhabdomeric Gqα by light or by G-protein mutations was correlated with a reduction in sensitivity to light during long-term adaptation. Therefore, we conclude that dynamic changes of membrane Gqα, which translocates in and out of the signaling membrane in a light-dependent manner, constitute a novel mechanism of long-term adaptation.
Materials and Methods
Fly stocks and light protocols.
Drosophila melanogaster of the following strains were used: wild-type Oregon-R w, Gqα1 and heterozygote Gqα1, Gβe1 and heterozygote Gβe1, Gβe2, arr23, ninaCP235, Rh1Δ356, and trpl302. Unless otherwise stated, flies were raised at 24°C in a 12 h light/dark cycle and the pupae were placed 13 cm from white fluorescent light (L 36, 20 W; OSRAM, Munich, Germany) 4–6 h before eclosion (light adapted), and then the vials were also transferred into darkness for 2 h before eclosion (dark adapted).
Electrophysiology.
To measure light responses, red (RG 610 edge filter; Schott, Mainz, Germany), orange (OG 590 Schott edge filter) or white lights were used. The light source was a xenon high-pressure lamp (75 W) and the light stimuli were delivered to isolated ommatidia via the objective lens (40×; Zeiss, Oberkochen, Germany) and attenuated up to approximately six orders of magnitude by neutral density filters as indicated. The relative intensity of red and white lights is expressed in terms of equivalent intensity of orange lights (Schott OG 590 edge filter) that elicited the same frequency of quantum bumps. The maximal luminous intensity, I0, of the orange light at the level of the ommatidia was 3.2 mW/cm2. This absolute light intensity was 3.3 log units higher than the light intensity that induced the half-maximal voltage response of dark adapted trpl302 mutant flies during current-clamp measurements. Dissociated ommatidia were prepared from newly eclosed adult flies (<1 h posteclosion). In all cases, ∼20 min of very dim red light was applied during the dissection of the eye before the beginning of the experiments. Whole-cell voltage-clamp recordings were performed as described previously (Hardie and Minke, 1992; Peretz et al., 1994). In short, recordings were made at 21°C using patch pipettes of 8–12 MΩ pulled from fiber-filled borosilicate glass capillaries. Series resistance was below 25 MΩ and was carefully compensated (>75%) for most of the experiments. Signals were amplified using Axopatch-200B (Molecular Devices, Sunnyvale, CA) patch-clamp amplifier and the current was sampled at 2 kHz and filtered below 1 kHz. For bump analysis, the membrane potential was kept at −70 mV and signals were filtered below 100 Hz. Currents were sampled using the Digidata card and analyzed by the pClamp 9.0 software (Molecular Devices). For recordings in the current-clamp mode, a small negative current of <50 pA was applied to recorded cells to fix the resting potential of the photoreceptor at −60 ± 2 mV. Before application of this current, the resting potential of all of the recorded cells in our solutions (see below) was in the range of 40–55 mV and there was no significant difference in the resting potentials of dark and light raised flies. The bath solution contained (in mm) 120 NaCl, 5 KCl, 10 N-Tris-(hydroxymethyl)-methyl-2-amino-ethanesulphonic acid, pH 7.15 (TES buffer), 4 MgSO4, and 1.5 CaCl2. For all experiments, the whole-cell recording pipette contained (in mm) 140 K-gluconate, 2 MgSO4, 10 TES buffer, pH 7.15, 4 MgATP, 0.4 Na2GTP, and 1 nicotinamide adenine dinucleotide.
Assay of light-dependent Gαq localization.
Assay for light-dependent localization of Gqα was performed as described previously (Kosloff et al., 2003). In short, dark adapted flies were subjected to illumination with either blue or white light (18 W white light lamp, 13 cm away from the flies, with or without a blue Schott BG 28 broad-band filter) for various durations at 22°C. Control experiments showed that white and blue illuminations give similar results. Termination was performed by transferring the flies to 4°C in the dark and promptly separating the fly heads. Ten flies were used for each time point. The fly heads were homogenized in 1 ml of hypotonic homogenization buffer (20 mm HEPES, pH 7.6, 20 μg/ml leupeptin, 1 μg/ml pepstatin A, 0.35 mg/ml o-phenantroline, 15 mm N-ethylmaleamide). Membranes and cytosol were separated by centrifugation (15,800 × g for 15 min at 4°C). The pellet was washed, centrifuged again, and the supernatants were combined. Ultracentrifugation at 150,000 × g for 30 min did not precipitate additional Gαq-proteins or change the distribution between the fractions. The proteins were precipitated by 5% TCA, run on 10% SDS-PAGE, and subjected to quantification. After separation by SDS-PAGE, Western blotting was performed using an anti-Gqα polyclonal antibody (Kosloff et al., 2003). Relative protein amounts on the same gel were determined by quantification of the ECL signal using the Fuji (Tokyo, Japan) LAS-1000 system. To reduce the variance caused by the experimental procedure, the amounts of Gqα in each fraction were calculated as a percentage of the total Gqα in both the pellet and supernatant of each treatment.
Results
Prolonged illumination of dark-raised flies revealed a novel type of adaptation
To investigate the effects of prolonged illumination on the sensitivity to light in the most sensitive manner, we used whole-cell voltage-clamp recordings from isolated Drosophila ommatidia and measured the response to absorption of single photons (quantum bumps). Wild-type (WT) flies were raised in a 12 h dark/light cycle and then were either illuminated (light raised) or kept in the dark (dark raised) for ∼12 h before eclosion. To examine the effect of previous illumination on the response to light at the quantum bump level, we applied very dim red lights of increasing intensities and measured the effect of previous illumination during the raising period on the properties of the quantum bums. Surprisingly, the only parameter that was affected by the previous illumination conditions was the bump frequency and not the bump shape and amplitude. A relatively high rate of bump frequency was observed in photoreceptors of dark-raised flies relative to the bump frequency of light-raised flies in response to the same light stimuli (Fig. 1A, compare −log I = 5.5). Importantly, a lower sensitivity to light, as manifested by lower bump frequency in light-raised flies was not accompanied by changes in bump-shape parameters relative to dark-raised flies (Fig. 1B). The measured bump-shape parameters included the average peak amplitude (Fig. 1B, amplitude, left columns) and shape. The shape was characterized by average bump duration at half-maximal amplitude (Fig. 1B, half width, middle columns) and the total charge of the bump (Fig. 1B, area, right columns).
Figure 1.
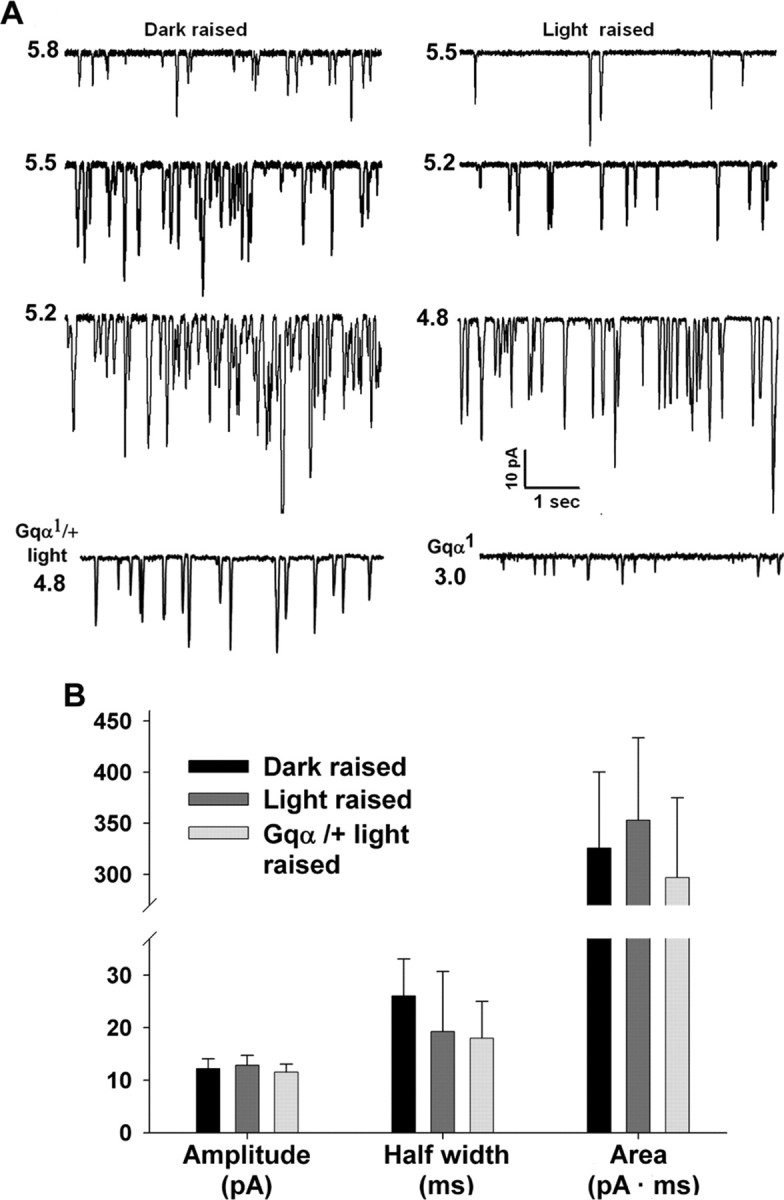
Prolonged illumination reduces quantum bump frequency without affecting bump shape and amplitude. A, Bumps elicited in response to different intensities of dim red lights in dark- and light-raised WT Drosophila (3 top lines) and in light-raised Gqα1/+ and dark-raised Gqa1 mutants (bottom line). The left column shows bumps recorded from dark-raised and the right from light-raised WT flies. The relative light intensity I is presented in −log units on the left of each trace. The relative intensity of the dim red lights is expressed in terms of equivalent intensity of orange lights (Schott OG 590 edge filter) that elicited the same frequency of quantum bumps in all figures. Notice that light intensity is different for the left and right columns. B, A histogram showing that despite a large difference in bump frequency between the two populations of dark- and light-raised WT flies and in the light-raised Gqα1/+ mutant, no significant difference in bump shape parameters is observed. In contrast, in the homozygote Gqα1 mutant, in which Gqα is drastically reduced, the bump amplitude is highly reduced. Error bars are SEM in all figures. There is no significant difference among the various columns (p > 0.05; n = 5–9).
Because prolonged illumination, which reduced membrane Gqα to 30% of maximum (Kosloff et al., 2003), did not change the bump shape and amplitude (Fig. 1B), we also examined whether the shape and amplitude of the quantum bumps are modified when the Gq level is further reduced by mutations in Gqα. In Figure 1A (bottom line), we examined the effects of reduction in membrane Gαq concentration to ∼15 and ∼1% by prolonged illumination of the heterozygote Gqα1/+ and in homozygote Gqα1 mutants, respectively (for measurements of Gqα level in these mutants, see Figs. 6, 7). Figure 1, A (bottom line, left) and B, shows that there is no significant difference between the bump shape and amplitude of WT flies (either dark or light raised) and light-raised heterozygote Gqα1/+ flies. However, when the Gqα level was drastically reduced by the homozygote Gqα1 mutation to ∼1%, which is much below the physiological range of Gq modulations, the bump amplitude was significantly reduced (Fig. 1A, bottom line, right) as described previously (Hardie et al., 2002).
Figure 6.
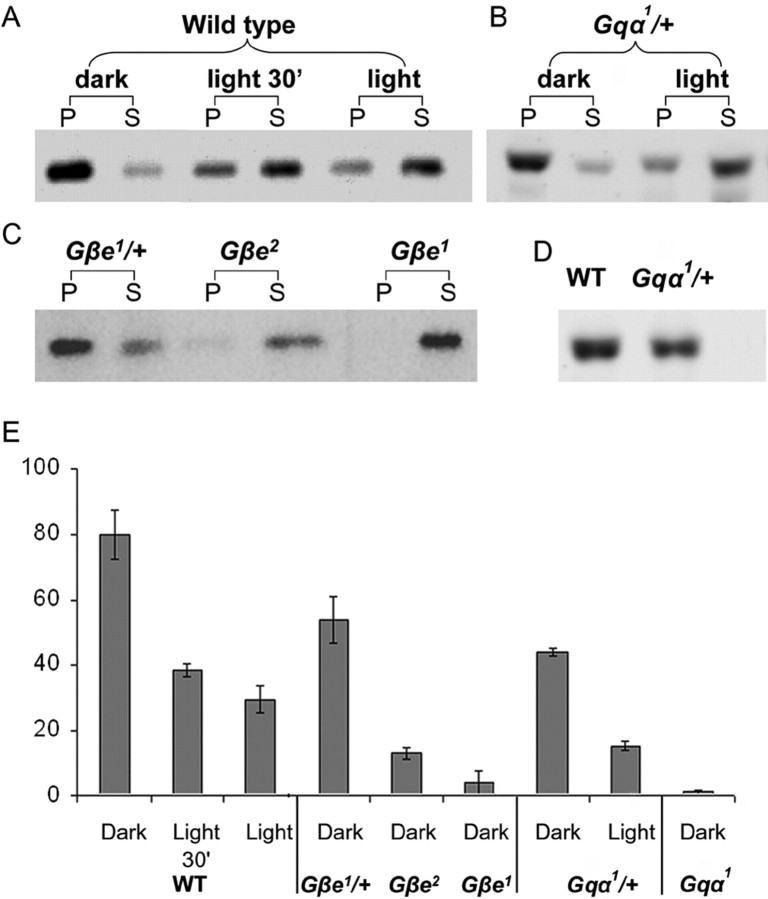
Relative concentration of membrane Gqα in WT and various Gq mutants. A–C, Western blot analysis shows by fractionation analysis the localization of Gqα in the membrane [pellet (P)] and in cytosol [supernatant (S)] in dark- (2 h) and light-adapted (4 h) WT flies (A), dark- (2 h) and light-adapted (4 h) heterozygote Gqα1 (B), and dark-adapted Gβe mutants (C). D, Total amounts of Gqα in WT and heterozygote Gqα1. E, Gqα concentration in the membrane presented as percentage of total Gqα of WT flies.
Figure 7.
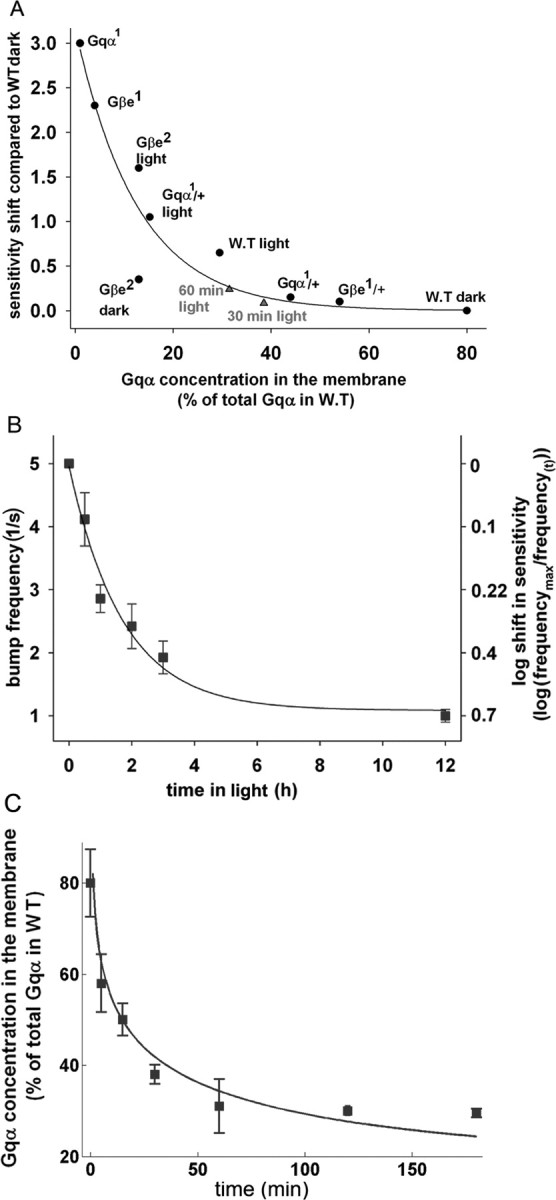
A correlation between long-term adaptation and membrane Gqα concentration in WT and Gq mutants. A, The shift required to fit the R-log-I curve of the various mutants at steady-state dark/light conditions (as indicated) to that of dark-raised WT flies was plotted against the steady-state concentration of membrane Gqα measured by Western blot analysis in the various Gq protein mutants (Fig. 6). The shift of Gqα1 was taken from Scott et al. (1995). B, The triangles and WT light were derived from kinetic measurements of changes in bump frequency. The smooth curve is an exponential function [sensitivity shift, 3.168 × exp (−0.0786 × Gqα)] that fits the experimental points, excluding the kinetic data (triangles). This function shows a good correlation (R2 = 0.89) between the shift in sensitivity to light and the membrane concentration of Gqα at steady-state conditions. The kinetic of the change in bump frequency during illumination is shown. Bump frequency was measured in flies raised in darkness and transferred to light for the specified time. The figure shows that the decrease in bump frequency elicited in response to dim orange light (−log I = 5.5) follows a first-order exponential function with an apparent time constant of ∼196 min [sensitivity shift, 0.7125(1 − exp(0.0051 × t(min))]. C, A comparison between the direct biochemical measurements of membrane Gqα concentration (squares) (Kosloff et al., 2003) and membrane Gqα concentration calculated from the shift in sensitivity as a function of time during illumination. The smooth curve was calculated from the kinetic of the shift in sensitivity (B, right scale) together with the nonlinear function describing the relationship between membrane Gqα concentration and the shift in sensitivity (A). The biochemical measurements of light-induced translocation of Gqα fit well with the reduction in membrane Gqα calculated from the shift in sensitivity to light during times <2 h.
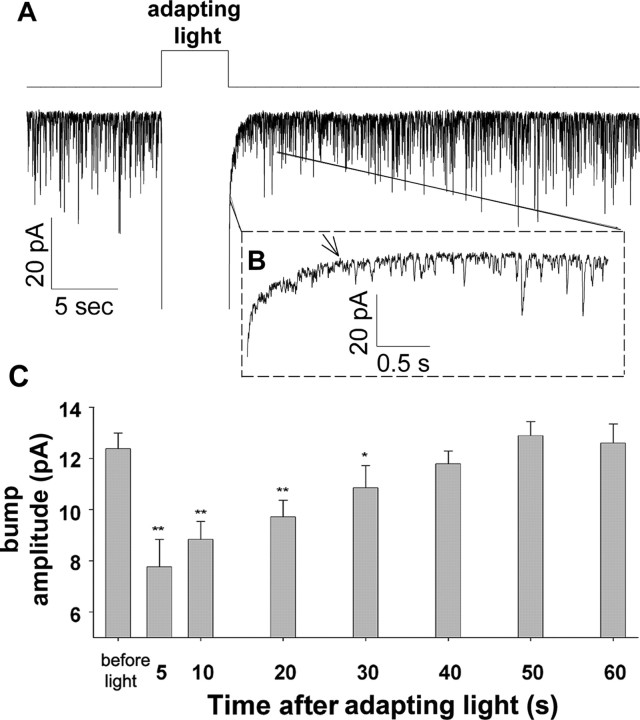
To distinguish between the reduction in sensitivity to light after prolonged illumination (Fig. 1) and the known Ca2+-dependent fast light adaptation (Henderson et al., 2000), we elicited bumps by application of prolonged dim red background light to dark adapted flies and then superimposed a short (5 s) intense orange adapting light on the background illumination (Fig. 2A). During the initial 1 s after the cessation of the adapting light, the bump amplitude was reduced below the noise level (Fig. 2B, arrow). The bump amplitude increased quickly with time, reaching ∼70% of the averaged dark adapted level within 10 s (Fig. 2C). The average reduction in bump amplitude fully recovered to the original dark adapted level in <1 min (Fig. 2C). We also found that the bump shape parameters, which were significantly reduced after the adapting light, returned to their previous level in <1 min (data not shown). Together, the data in Figure 2 demonstrate that fast adaptation is characterized by a large reduction in bump amplitude, which recovered in <1 min.
Figure 2.
Fast adaptation affects bump amplitude. A, Dim red light (−log I = 5) elicited a continuous production of bumps, which was interrupted by application of intense orange light (adapting light, −log I = 1.0) for 5 s that elicited LIC with amplitude out of scale. B, The initial ∼4 s of A after the cessation of the adapting light is displayed in an expanded time scale as indicated. The arrow indicates 1 s after the cessation of the adapting light. C, Histogram plotting the average peak amplitude of the bumps before and after application of the adapting light as a function of time after the cessation of the adapting light as indicated. **p < 0.01, *p < 0.05, t test (n = 5).
Figure 1 reveals a new mechanism of adaptation solely affecting bump frequency. We designate this mechanism long-term adaptation throughout this work. Together, Figures 1 and 2 indicate that fast and long-term adaptation represent entirely separate yet complementary adaptation processes: fast adaptation affects bump shape and amplitude, whereas long-term adaptation affects only bump frequency.
Both the changes in bump frequency and in peak amplitude of the light-induced current can be used to characterize long-term adaptation
To quantify long-term adaptation in terms of bump frequency, we plotted bump frequency as a function of relative light intensity of prolonged stimuli (R-log-I curve) for dark- or light-raised WT flies, as indicated (Fig. 1A). Figure 3A shows an increase in the bump frequency in dark-raised flies relative to light-raised flies as manifested by an ∼0.6 log shift of the R-log-I curve (Fig. 3A, dotted line). We designated this shift in sensitivity to light “sensitivity shift” throughout this study.
Figure 3.
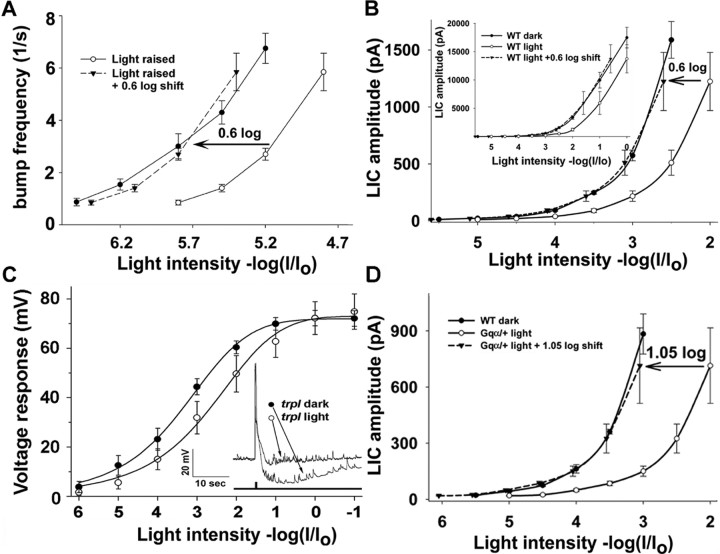
Both the changes in bump frequency and in peak amplitude of the LIC can be used to characterize long-term adaptation. A, Average bump frequency (see Fig. 1) is plotted as a function of relative orange light intensity for light-raised and dark-raised flies (n = 4). To measure the sensitivity shift, the R-log-I curve of the light-raised flies was shifted by 0.6 log units (dashed line, triangles). Note that at higher light intensities, the bump frequency is underestimated because of superposition of the bumps. B, The peak amplitude of the LIC of dark- (filled circles) and light-raised (empty circles) flies is plotted against the relative orange light intensity at dim and medium range. The dashed line (triangles) shows a 0.6 log unit shift of the light-adapted curve, which fits the dark-adapted curve. Inset, The data in B are presented at the entire range of light intensities. Note that at the maximal light intensity, the peak LIC reached ∼20 nA inward current although no sign of saturation is observed. C, The average peak amplitude of the voltage responses, measured in the current-clamp mode from the trpl302 mutant, is plotted against the relative orange light intensity in a paradigm identical to that of B. The relative intensity of the intense white lights (−log I/I0 = −1) at saturated voltage responses is expressed in terms of equivalent intensity of orange lights (Schott OG 590 edge filter) that elicited the same light response. A significant shift toward more intense lights of 0.7 log units (p < 0.007; n = 8) was observed when dark-raised flies were exposed to prolonged illumination. C, Inset, The inset is sample responses to maximal orange light intensity. The afterhyperpolarization of the voltage responses of dark-raised flies (right arrow) and its absence in the responses of light-raised flies (left arrow) demonstrates the complexity of the voltage response to light. D, The R-log-I curve of light-adapted heterozygote Gqα1 (Gqα1/+, empty circles) is compared with the R-log-I curve of dark-adapted WT flies (filled circles). A shift of 1.05 log units of the heterozygote Gqα1 curve (dashed line) is required to fit the R-log-I curve of the mutant to that of dark adapted WT flies.
Bump frequency can be measured reliably only at a limited range of very dim light intensities because the bumps readily superimpose to form a macroscopic light-induced current (LIC) even in dim red lights (Fig. 1A). Bump frequency could not be measured reliably in some of the mutants used in this work because the mutant bumps were too small to be reliably detected (e.g., in the trp mutant) (Henderson et al., 2000), or because a large frequency of spontaneous bumps masked the detection of light-induced bumps (e.g., the Gβe1/+ mutant) (Elia et al., 2005). Thus, the use of various Drosophila mutants for analyzing the molecular mechanism underlying long-term adaptation required extending the measurements to a wider range of light intensities. To accomplish this, we derived the R-log-I curves from measurements of the macroscopic LIC.
To determine the sensitivity shift from the macroscopic LIC, we plotted the peak amplitude of the LIC as a function of increasing light intensities (the R-log-I curve) in light-raised (Fig. 3B, empty circles) and dark-raised (Fig. 3B, filled circles) WT flies. The sensitivity shift was determined by shifting the position of the R-log-I curve of the light-raised flies to achieve the best fit to the R-log-I curve of dark-raised WT flies that was measured under the same experimental conditions. This resulted in a shift of 0.6 log units (Fig. 3B, dotted line), similar to the shift obtained by measuring bump frequency. The R-log-I curve was measured in the range of dim to medium light intensities (peak LIC, <2000 pA). Increasing the intensity range to include intense lights (approximately six orders of magnitudes) did not lead to saturation of the LIC, nor did it affect the magnitude of the shift of the R-log-I curve and, thus, did not arise from response compression (Fig. 3B, inset). However, at the high-intensity range, the response amplitude already elicited currents larger than 15 nA, which are too large to allow reliable whole-cell patch-clamp recordings (Fig. 3B inset). Therefore, we limited our measurements to the dim-medium light intensity range (Fig. 3B). Figure 3, A and B, further shows that a similar sensitivity shift was obtained from the R-log-I curves measured from either the macroscopic LIC or bump frequency under similar previous illumination conditions.
The effect of illumination during rearing conditions on the voltage response to light is by far more complex than its effect on the LIC. One aspect of this complexity is demonstrated by the large afterhyperpolarization of voltage response in dark-raised flies and its absence in light-raised flies in response to the same saturating stimulus (Fig. 3C, inset) (Wolfram and Juusola, 2004). The complexity of the voltage response to light was the main reason to use voltage-clamp rather than current-clamp recordings in our study. Nevertheless, to further support our conclusion that the sensitivity shift that we measured under the voltage-clamp condition did not arise from response compression, we plotted R-log-I curves using data from the current-clamp recording mode from isolated ommatidia of the trpl302 mutant, which expresses only TRP channels (Niemeyer et al., 1996). We used this mutant to maintain a constant composition of the light-sensitive channels in the rhabdomere and, thus, to ensure a single reversal potential in dark- and light-raised flies (Bähner et al., 2002). Figure 3C presents R-log-I curves measured from the peak amplitude of the voltage responses to light of increasing intensities of dark-raised (filled circles) and light-raised trpl302 mutant flies. The R-log-I curves measured under current-clamp conditions reached saturation at intense lights as expected from voltage responses (Fig. 3C). Figure 3C shows a 0.7 log unit shift of the R-log-I curve measured from dark-raised flies after prolonged illumination. Voltage responses recorded in current-clamp mode are known to simulate the physiological response to light. Therefore, the similarity between the sensitivity shifts obtained in voltage- and current-clamp modes indicates that the shift obtained in voltage-clamp mode is reliable and represents physiological phenomenon.
An example of the analysis of the effect of a mutation on long-term adaptation is illustrated in Figure 3D. First, the R-log-I curves of illuminated and dark-adapted WT and G-protein mutant flies were measured. Then, the positions of the R-log-I curve of the dark- and light-adapted mutant and light-adapted WT flies were shifted to achieve best fit to the R-log-I curve of dark-adapted WT flies that was measured under the same experimental conditions. The shift of the R-log-I curve (defined as the sensitivity shift) demonstrates how the R-log-I curve of light-adapted heterozygote Gqα1 (Gqα1/+) flies was best fitted to the R-log-I curve of dark-adapted WT flies by a shift of 1.05 log units toward less intense light (Fig. 3D, dotted line)
Strong inhibition of long-term adaptation induced by mutations that affect the targeting of Gqα to the plasma membrane
The most convenient and reliable way to characterize long-term adaptation in our study was to plot the R-log-I curve by measuring the peak amplitude of the LIC in response to increasing intensities of short light pulses in illuminated flies (Fig. 4A, empty circles) and then measure this curve again in other flies of the same vial after 2 h of dark adaptation (Fig. 4A, filled circles). Fig. 4A shows that dark-adapted flies (for 2 h) are more sensitive to light (by ∼0.45 log units) than light-adapted flies. When the dark adaptation period was extended to 12 h, dark-adapted WT flies were more sensitive to light by ∼0.6 log units (Fig. 3B).
Figure 4.
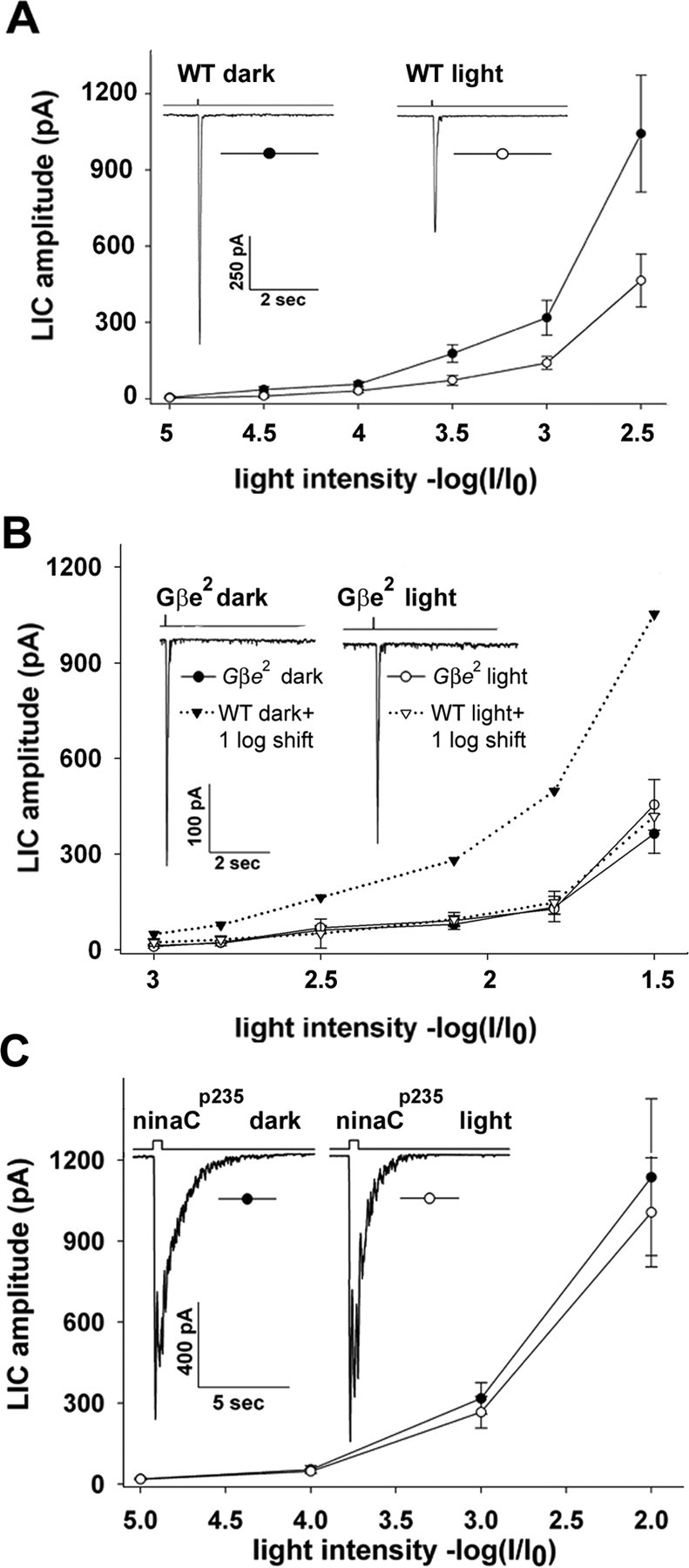
Two different mutations, which impaired the targeting of Gqα to the membrane, strongly inhibit long-term adaptation. The average peak amplitude of the light-induced current is plotted against the relative orange light intensity. A, Intensity–response relationship of WT Drosophila photoreceptors measured in illuminated flies (4 h; WT light; empty circles) followed by dark adaptation (2 h; WT dark; filled circles). There is a clear shift toward dimmer light intensities of the R-log-I curve after 2 h of dark adaptation. The inset shows sample responses to test light of the highest intensity (i.e., log = −2.5) from illuminated (right) and dark-adapted (left) flies (n = 6). B, The measurements in A were repeated in the Gβe2 mutant in which the shift of the R-log-I curve after 2 h of dark adaptation was completely blocked. For comparison, the R-log-I curve of WT flies from A (dotted lines, triangles) were shifted by ∼1 log unit toward more intense lights to fit the R-log-I curve of the mutant. The figure shows that whereas 2 h of dark adaptation was sufficient to reveal significant shift of the R-log-I curve in WT flies, no shift was observed in the Gβe2. C, The measurements in A and B were repeated in the ninaCP235 mutant in which the shift of the R-log-I curve measured after 2 h of dark adaptation was completely blocked. The insets are sample responses to the highest light intensity of the presented R-log-I curves.
Previous studies have shown that prolonged illumination of Drosophila Gqα mutants induces translocation of Gqα from the signaling membrane to the cytosol, followed by translocation of the cytosolic Gqα back to the rhabdomere in the dark after the cessation of light (Kosloff et al., 2003; Cronin et al., 2004). It was also demonstrated that a large reduction of Gqα concentration in Drosophila photoreceptor reduces the sensitivity to light (Scott et al., 1995). If light-dependent modulation of rhabdomeric Gqα underlies long-term adaptation, it is expected that specific mutations that impair the targeting of cytosolic Gqα to the signaling membrane should also impair long-term adaptation.
To critically test this hypothesis, we measured the R-log-I curves of the macroscopic LIC in illuminated mutants in which the targeting of Gqα to the signaling membrane is impaired. We then measured this curve again in flies from the same vial after 2 h of dark adaptation to determine the effect of specific mutations on long-term adaptation.
Mutations that reduce Gβe concentration markedly affect both the targeting of Gqα to the membrane and the recovery of sensitivity in the dark after prolonged illumination
It has been shown previously that in Gβe mutants, Gqα is mainly localized to the cytosol (see Fig. 6) (Elia et al., 2005) and its membrane association is defective because it is anchored to the membrane by binding to the βγ dimmer (Kosloff et al., 2003). Figure 4B compares the R-log-I curve measured in the light adapted Gβe2 mutant, which has only 5–14% Gβe (Dolph et al., 1994; Elia et al., 2005), with the R-log-I curve of flies from the same vial measured after 2 h in the dark. Strikingly, no significant shift toward dim lights of the R-log-I curve was observed in the Gβe2 mutant after 2 h darkness. This result is in sharp contrast to WT flies in which the R-log-I curve was significantly shifted (Fig. 4A). When a longer (12 h) dark period was allowed for the Gβe2 mutant, a significant shift of the R-log-I curve was observed (data not shown), indicating that after 2 h darkness, the Gβe mutant was still in the light adapted state. To allow comparing the effect of 2 h dark adaptation between illuminated WT flies and the Gβe2 mutant, the R-log-I curves of dark- and light-adapted WT flies were shifted to higher levels of light intensities by ∼1 log unit (Fig. 4B, dotted lines). After the shift, the light-adapted curves of WT and the mutant overlapped whereas their dark-adapted curves revealed a large difference. Thus, Figure 4B demonstrates that long-term adaptation is markedly suppressed in a Gβe2 mutant in which the targeting of Gqα to the membrane is defective.
The ninaCP235 myosin III mutation, which slows down the return of Gqα to the signaling membrane, also slows down the recovery of sensitivity in the dark after prolonged illumination
The ninaC mutant, which lacks the NINAC myosin III proteins (Montell and Rubin, 1988) displays a significantly reduced rate of Gqα transport from the cell body to the rhabdomere after illumination (Cronin et al., 2004). Accordingly, NINAC determines Gqα concentration in the rhabdomere after illumination. Figure 4C compares the R-log-I curves measured in light- and dark-adapted ninaCP235 null mutants. Similar to the Gβe2 mutant, no significant shift toward dim lights of the R-log-I curve was observed in the mutant after 2 h of darkness. When a longer (12 h) dark period was allowed, a significant shift of the R-log-I curve was observed in this mutant, indicating that, after 2 h of darkness, the ninaCP235 mutant was still in the light-adapted state, similar to the Gβe2 mutant.
Figure 4, B and C, demonstrate that the recovery of sensitivity to light during a 2 h dark period after prolonged illumination is markedly and similarly suppressed in mutants that affect the targeting of Gqα to the signaling membrane by two entirely different mechanisms. The result of Figure 4 strongly supports the hypothesis that long-term adaptation is mediated by light-activated Gqα translocation.
Light-regulated translocation of TRPL does not account for the recovery of sensitivity to light in the dark after prolonged illumination
To test the specificity of impairing Gqα targeting to the membrane on long-term adaptation, we repeated the experiments of Figure 4 in a variety of mutants. These mutants are known to reduce the concentration of signaling proteins that in the WT flies translocate between the signaling membrane and the cell body after illumination.
It has been found previously that the Drosophila TRPL channel translocates back and forth between the signaling membrane and the cytosol by a light-regulated mechanism (Bähner et al., 2002; Cronin et al., 2006). Because TRPL translocation was accompanied by changes in sensitivity to light during very dim background illumination and this effect was blocked in the null trpl mutant, we examined whether TRPL translocation underlies long-term adaptation by measuring the R-log-I curves in the trpl null mutant. Figure 5A, which repeats the experiments of Figure 4 using the null trpl mutant (trpl302) (Niemeyer et al., 1996), shows a clear shift of the R-log-I curve of the light-raised fly after 2 h of darkness, as found in WT flies.
Figure 5.
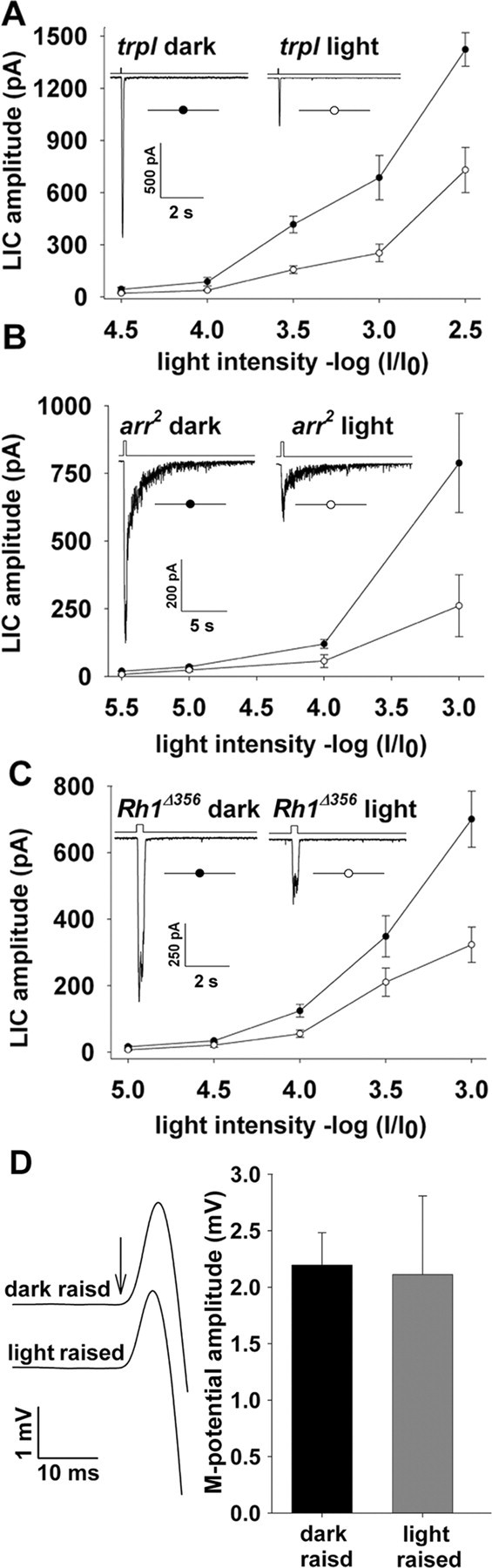
Impairing the targeting of Gqα to the signaling membrane by mutations is a specific effect. The average peak amplitude of the LIC of various mutants is plotted against the relative orange light intensity in a paradigm identical to that in Figure 4. A–C, The insets are sample responses to the highest light intensity of the presented R-log-I curves. The null trpl (trpl302) mutant (A), the hypomorph Arr2 (arr23) mutant (B), and a mutant rhodopsin having Rh1 without the phosphorylation sites at the C terminus (Rh1Δ356; C) had no significant effects on long-term adaptation. D, There is no significant reduction in the photopigment level between light-raised and dark-raised WT flies. The photopigment level was measured by eliciting the M-potential, which is a linear electrical manifestation of the photopigment level in the fly eye. Flies <2 d old were illuminated with maximal intensity blue (BG28; Schott broad-band blue filter) for 20 s and after 10 s in the dark a maximal intensity white flash (arrow; stroboscopic photographic flash lamp of 70 Joules) elicited the M-potential (left traces). The histogram compares the average amplitude of the M-potential in dark-raised (left column) and light-raised (right column) flies. No significant difference between the averaged M-potential amplitudes under the two adaptation conditions was observed (t test, p > 0.05).
Figure 5A shows that translocation of the TRPL channel plays no significant role in recovery of sensitivity in the dark after prolonged illumination.
Light regulated translocation of arrestin 2 does not account for long-term adaptation
Inactivation of the physiologically active photopigment is achieved by the binding of the arrestin 2 (Arr2) to the phosphorylated metarhodopsin, which prevents additional association between metarhodopsin and the Gqα protein (Yamada et al., 1990; Byk et al., 1993; Dolph et al., 1993). Therefore, the lifetime of active metarhodopsin is rather short (<100 ms) as measured directly in the Limulus median eye (Richard and Lisman, 1992) and depends on the concentration of arrestin (Ranganathan and Stevens, 1995). Several studies have shown that Arr2 translocates into the rhabdomere after illumination (Byk et al., 1993; Lee et al., 2003) and thereby may contribute to long-term adaptation (Lee et al., 2003). To test this hypothesis, we measured the R-log-I curves in an Arr2 mutant (arr23), which has a highly reduced level of Arr2 (Alloway et al., 2000).
Figure 5B compares the R-log-I curves measured in the illuminated (4 h) arr23 mutant and in the same mutant after 2 h of darkness (empty and filled circles, respectively). Figure 5B shows a significant shift of the R-log-I curve toward dim lights after 2 h of darkness similar to that of WT flies, thus indicating that Arr2 is not an essential molecule for the type of long-term adaptation found in this study.
The photopigment cycle is not involved in long-term adaptation
Several studies have shown that, under pathological conditions, light induces a stable association between metarhodopsin and Arr2, which is followed by phosphorylation-dependent internalization of this complex (Alloway and Dolph, 1999; Alloway et al., 2000; Kiselev et al., 2000) (but see Satoh and Ready, 2005). Furthermore, a previous study has shown that the constitutively active rhodopsin mutant ninaEpp100 causes a stable association of the photopigment to Arr2 and thereby reduces the sensitivity to light by >10-fold (Iakhine et al., 2004). To examine the involvement of photopigment internalization in long-term adaptation, we examined whether photopigment phosphorylation plays a role in long-term adaptation. To this end we measured the R-log-I curve in transgenic flies in which the C-terminal portion of the major rhodopsin (Rh1) was deleted (Vinos et al., 1997) and internalization of the photopigment was prevented (Alloway et al., 2000; Satoh and Ready, 2005). Figure 5C compares the R-log-I curves measured in illuminated (4 h) and dark-adapted Rh1Δ356 mutants. The Figure shows that the shift of the R-log-I curve toward dim lights after 2 h of darkness was similar to that of WT flies, indicating that phosphorylation of metarhodopsin and the subsequent internalization of the photopigment are not essential for long-term adaptation. Together, the observation that long-term adaptation is preserved in either the arr2 (Fig. 5B) or in the Rh1Δ356 mutants (Fig. 5C) suggests that the photopigment cycle or the light-induced turnover of the photopigment molecules are not essential for long-term adaptation.
To further eliminate the possibility that light-induced modulations in the photopigment level underlie long-term adaptation under our experimental conditions, we measured the photopigment level in dark- and light-raised WT flies. The photopigment level was measured by eliciting the metarhodopsin potential (M-potential), which is a linear electrical manifestation of the photopigment level in the fly (Pak and Lidington, 1974; Minke and Kirschfeld, 1980). Figure 5D compares the amplitude of the M-potential in dark- and light-raised WT flies. The figure shows that there is no significant difference between the average amplitude of the M-potentials of illuminated and dark-adapted flies, thus ruling out the possibility that modulations in the photopigment levels underlie long-term adaptation.
Together, Figures 4 and 5 reveal that suppression of long-term adaptation by mutations, which affect targeting of Gqα to the membrane, is a specific phenomenon and strongly suggest that translocation of Gqα mediates long-term adaptation in Drosophila photoreceptors.
The sensitivity to light depends on membrane Gqα concentration in WT and G-protein mutants
It has been well established using transgenic Drosophila Gqα mutants, which express variable amounts of normal Gqα, that the sensitivity of the photoreceptor cell to light critically depends on the concentration of Gqα (Scott et al., 1995). In addition, other studies show that the membrane concentration of Gqα changes with prolonged illumination (Kosloff et al., 2003; Cronin et al., 2004). Although these studies suggested that Gqα translocation contributes to light adaptation they did not actually measure the physiological consequences of Gqα translocation.
To study the effect of changes in membrane Gqα concentration on the magnitude of the sensitivity shift, we analyzed the relationship between the concentration of membrane Gqα in mutants with variable concentration of Gqα and the sensitivity shift. To this end we measured by Western blot analysis the membrane concentration of Gqα in homozygote Gqα1, Gβe1, and Gβe2 mutants and heterozygote Gqα1 and Gβe1 flies (Fig. 6). All of these mutants show reduced Gqα concentration in the signaling membrane relative to WT flies (Fig. 6).
Figure 7A plots the sensitivity shift (as measured in Fig. 3D) as a function of membrane Gqα concentration of the various mutants relative to dark-raised WT flies. The extreme upper point represents measurements from the strong hypomorph Gαq1 mutant, showing maximal shift at minimal membrane Gqα concentration. The extreme lower point shows dark-raised WT flies that by definition have no shift at maximal membrane Gqα concentration. The intermediate points that were obtained from the various mutants show that reduction in membrane Gqα concentration resulted in an increased shift of the R-log-I curve relative to that of dark-raised WT. The smooth curve is an exponential function that fits the experimental points. Figure 7A shows a good correlation (R2 = 0.89) between the sensitivity shift and the membrane Gqα concentration.
The kinetics of long-term adaptation and its relationship to the kinetics of Gqα translocation
To further test the hypothesis that the movement of Gqα in and out of the signaling membrane underlies long-term adaptation, the kinetics of Gqα translocation from the membrane to the cytosol was compared with the kinetics of long-term adaptation.
The kinetics of Gqα movement from the membrane to the cytosol during light has been measured previously (Kosloff et al., 2003). To measure the kinetics of long-term adaptation, we measured the reduction of bump frequency of dark-raised WT flies at various time points during continuous illumination (Fig. 7B). The reduction of bump frequency as a function of time fits a first-order reaction described by an exponential function with an apparent time constant of ∼196 min (Fig. 7B, smooth curve). The reduction in bump frequency as a function of time during illumination can also be expressed as a shift in the sensitivity to light toward more intense lights (sensitivity shift) (Fig. 7B, right scale). Indeed, the difference between the sensitivity to light of dark- and light-raised WT flies measured by a shift in the R-log-I curve was similar to the shift in sensitivity calculated from the reduction in bump frequency as a function of time during prolonged illumination [i.e., ∼0.6 log (Fig. 3A) and 0.7 log (Fig. 7B, right scale)].
Previous data (Kosloff et al., 2003) and Figure 7B show that the kinetics of both long-term adaptation and Gqα translocation fit a first-order reaction described by an exponential function, but the time constant of the Gqα translocation is significantly shorter. However, there is a difficulty in correlating the kinetics of these two processes. Fig. 7A shows that the relationship between the sensitivity shift and the change in membrane Gqα concentration is highly nonlinear. This nonlinearity is reflected in the almost recessive phenotype of the strong hypomorph Gqα1 (Scott et al., 1995) and most likely arises from the very high expression of Gqα in the photoreceptor cell (see below). Accordingly, ∼50% reduction in Gqα concentration in the Gqα1/+ mutant has a very small effect on the sensitivity shift (Fig. 7A). To overcome this difficulty and to estimate the correlation between the kinetics of long-term adaptation and Gqα translocation, we used the function obtained from the data of Figure 7A (smooth curve) to calculate the membrane Gqα concentration, which is predicted from the sensitivity shift (Fig. 7B, right scale). As a first step, we translated the bump frequency at particular times to a shift in sensitivity relative to dark-raised WT flies and plotted the relative shift as a function of time (Fig. 7B, right scale). We then used the function described by the smooth curve (Fig. 7A) to calculate the predicted membrane Gqα concentration from the measured shift in sensitivity as a function of time during illumination (Fig. 7C, smooth curve). The squares in Figure 7C are direct biochemical measurements of membrane Gqα concentration (Kosloff et al., 2003). Fig. 7C shows a good fit between the direct biochemical measurements of Gqα concentration as a function of time during illumination and the Gqα concentration calculated from the shift in sensitivity. There is a relatively small discrepancy between the measured Gqα membrane concentration and that calculated from the shift in sensitivity at long (>2 h) illumination. This is because the measured Gqα membrane concentration reaches saturation after ∼1 h (Kosloff et al., 2003) (see Discussion).
Consistent with the data of Figure 7C, measurements of the shift in the R-log-I curve in WT flies illuminated for 30 and 60 min relative to dark-raised WT flies fit well to the smooth curve of Figure 7A that was constructed without using any of the kinetic measurements (Fig. 7A, triangles).
Together, the data in Figure 7 reveal a good fit between the predicted membrane Gqα concentrations calculated from the reduction in sensitivity to light during prolonged illumination and the membrane Gqα concentration measured directly. The fact that the reduction in membrane Gqα concentration during illumination can be predicted from the shift in sensitivity to light that reflects long term adaptation (Fig. 7C) gives additional support to the notion that light-induced translocation of Gqα underlies long-term adaptation.
Prolonged illumination increases the latency of the single photon responses
The results of Figure 7 imply that in Drosophila photoreceptors the functional consequences of depleting membrane Gqα only manifest when the reduction in membrane Gqα is severe. To reconcile this observation with the high concentrations of rhodopsin and Gq in the microvilli, we examined theoretically the effect of a reduction in the concentration of Gqα (and hence in the available Gq protein) in the microvillar membrane on its ability to encounter an active rhodopsin molecule.
Drosophila rhabdomeres have very high amount (∼3 × 107) of rhodopsin molecules and ∼10-fold less Gq-protein molecules. The rhabdomere contains ∼30,000 microvilli and each microvillus contains ∼1000 rhodopsin molecules and ∼80 Gq molecules (Hardie and Raghu, 2001). According to the Einstein diffusion equation D = x2/2t, where t is the lifetime of an activated rhodopsin molecule (which is ∼0.1 s) (Richard and Lisman, 1992), x2 reflects the area covered by the Brownian motion of a Gq molecule, and D is the diffusion coefficient of Gq in tissue culture cells (0.10 μm2/s) (Perez et al., 2006). The calculated area of the Brownian motion of heterotrimeric Gq in 0.1 s is, therefore, 0.02 μm2.
The calculated membrane area of a single microvillus, assuming a microvillus diameter of 60 nm and length of 1.5 μm (Hardie and Raghu, 2001) is 0.28 μm2. Because there are ∼1000 rhodopsin molecules in this area, the Brownian motion area of each Gq during the lifetime of activated rhodopsin molecule includes ∼70 rhodopsin molecules in dark-adapted WT cells.
During dim lights, only one rhodopsin molecule is activated in a single microvillus. The Brownian motion of the 80 available Gq molecules can theoretically interact with 5600 rhodopsin molecules in an ideal situation, which is much more than the 1000 rhodopsin molecules in a microvillus. This excessive amount of Gq molecules available for excitation should lead to a nonlinear relationship between the reduction in Gq and the ability of an activated rhodopsin to encounter Gq. The data of Figure 7A and previous studies (Scott et al., 1995) show that a 50% reduction in the available Gq molecules led to a minor reduction in the sensitivity to light, whereas a reduction to 30% resulted in a significant reduction in sensitivity. These findings suggest that the area covered by the Brownian motion of the available Gq molecules (and hence the diffusion coefficient of Gq) in the microvilli should be smaller than that of the tissue culture cells. Because Gq is the only signaling molecule that moves in the microvillar membrane (Hardie and Raghu, 2001), its movement must be slowed down by the large concentration of the other membrane proteins of the microvillus such as the rhodopsin and the TRP and TRPL channels.
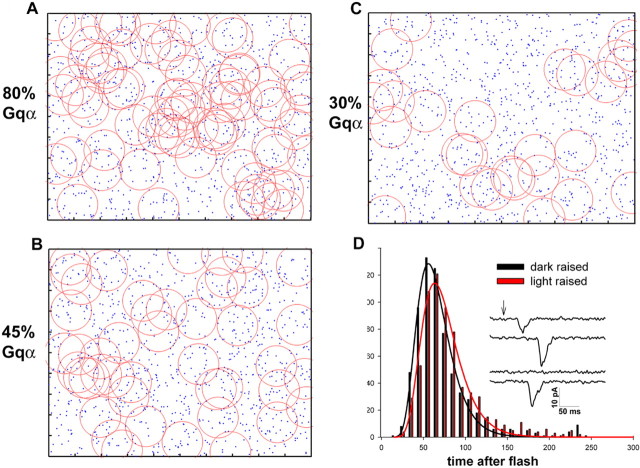
In Figure 8A–C we simulated a planar microvillus area containing 1000 randomly distributed rhodopsin molecules (blue points) and randomly distributed Gq molecules at three different amounts: 80 (representing 80% Gq level at dark-adapted state of WT flies) (Fig. 8A), 45 (representing dark-adapted Gαq/+ mutant) (B), and 30 (representing light-adapted state of WT flies) (C). The average area of the Brownian motion of Gαq molecules in 0.1 s is represented by red circles, arbitrarily assuming that diffusion coefficient, D, of Gq in the microvilli is threefold smaller than that of the tissue culture cells (i.e., 0.03 μm2/s). Figure 8A–C shows that for the simulated cases of dark-adapted WT, Gqα/+, and light-adapted WT flies, the Brownian motion area of all Gq molecules covers ∼80, ∼70, and ∼35% of the microvillus area, respectively (Fig. 8A–C, respectively). The relative area of the microvillus covered by the Brownian motion of Gq can be used to calculate the shift is sensitivity to light [e.g., for the light-raised WT fly, log(80%/35%)], yielding a 0.06 log and 0.35 log shift for the dark-raised Gqα1/+ and light-raised WT fly, respectively. Thus, Figure 8 demonstrates the nonlinearity of the relationship between Gqα concentration and sensitivity to light by showing that a reduction in membrane Gqα significantly reduces the ability of Gq molecules to encounter an activated rhodopsin only when the reduction in membrane Gqα is severe.
Figure 8.
Model simulation portraying the ability of activated rhodopsin to encounter Gq molecules when Gq concentration in a microvillus is reduced. A–C, Three examples of random generated images (Matlab 6.5) representing the area of a single microvillus that contains 1000 rhodopsin molecules (blue) and different amounts of heterotrimeric Gq molecules. The red circles represent the area covered by the Brownian motion of Gq molecules in 0.1 s, assuming D of 0.03 μm2/s. A, 80% of maximal level of membrane Gq. B, Forty-five percent of Gq molecules. C, Thirty percent of Gq molecules. D, Prolonged illumination that decreases membrane Gqα concentration increases the averaged latency of the single photon responses. The histograms plot bump-latency distribution as a function of time in bins of 10 ms for dark-raised (black) and light-raised (red) WT flies. The smooth curve is the log-normal function that best fits the data. There is a highly significant difference between the two histograms (p < 0.01; n = 6). D shows that the peak amplitude of the bump latency distribution is shifted by 7.5 ms after prolonged illumination that reduces Gqα concentration. The inset presents an example of responses to repeated stimulations with constant intensity dim red light (−log I = 4.1) of light-raised WT flies that elicited, on average, 0.4 bumps/stimulus.
Together, the model simulation shows that it is possible to explain the nonlinear relationship between the reduction in sensitivity to light and the reduction in the available Gq molecules during prolonged illumination (Fig. 8A–C).
An interesting prediction of the above model is that the average latency of the quantum bumps is expected to increase when the available concentration of Gq molecules is reduced by prolonged illumination or by mutation. In Figure 8D, we tested whether a reduction of Gqα to ∼30% by illumination changes the bump latency distribution. To measure the bump latency distribution, we repeatedly applied very dim test flashes that elicited ∼0.4 bumps per flash on the average (Fig. 8D, inset). The plot of the bump latency distribution (Fig. 8D) shows that a reduction of membrane Gqα to ∼30% by prolonged illumination resulted in a highly significant (p < 0.01) increase of 7.5 ms of the peak bump latency distribution that well fits the log-normal distribution. A slightly larger increase in the peak of the bump latency distribution (of 10 ms) was observed in the illuminated heterozygote Gqα1/+ mutant in which membrane Gqα was reduced to ∼ 15% (data not shown).
Reduction in Gqα level has only minor effect on the kinetics of response to light (Fig. 8D) in contrast to reduction in PLC level that has a strong effect on response kinetics (see Discussion). This demonstrates that the reduction in concentration of Gqα has only minor influence on the dwell time before a newly activated Gqα finds PLC, even when Gqα level is reduced (see Discussion).
Together, theoretical considerations reveal that diffusion of Gq in the microvillar membrane is sufficient to account for rhodopsin-Gq interaction in a microvillus during very dim light as long as the Gq concentration is large in dark adapted cells. However, when membrane Gqα concentration is largely reduced after prolonged illumination, the Gq molecules available for interaction with the active rhodopsin can easily become the limiting factor of excitation if the diffusion coefficient of the microvillar Gq is only threefold slower than that of tissue culture cells. The increase in bump latency distribution after prolonged illumination (Fig. 8D) is fully consistent with this notion, thus, strongly supporting the hypothesis that the translocation of Gqα underlies long-term adaptation.
Discussion
Genetic dissection of long-term adaptation
Multiple mechanisms have been shown to reduce the sensitivity to light during or after prolonged illumination in Drosophila photoreceptors: translocation of the TRPL channel is involved in adaptation to dim background light (Bähner et al., 2002). Arr2 translocates from the cell body to the rhabdomere after illumination and it is necessary for fast termination of the light response (Fig. 5B, inset) (Yamada et al., 1990; Byk et al., 1993; Dolph et al., 1993). In addition, recycling of the phosphorylated photopigment reduces the photopigment level (Alloway and Dolph, 1999; Alloway et al., 2000; Kiselev et al., 2000) and, consequently, the probability of quantum catch. However, our data show that none of these mechanisms can account for the long-term adaptation described in the present study. Additional studies have found that prolonged intense illumination reduced the photopigment level (Lee and Montell, 2004). However, when we measured the photopigment levels, no significant difference was found under the two illumination conditions (Fig. 5D). Because of the linear relationship between the photopigment level and the sensitivity to light that characterizes invertebrate photoreceptors (Hamdorf, 1979), a fivefold reduction in the photopigment level is required to account for the fivefold reduction in sensitivity to light after prolonged illumination, making such a mechanism unsuitable to explain long-term adaptation.
It may be argued that a reduction in the PLC levels underlies long-term adaptation. However, a previous study that measured the PLC level of the fly microvilli before and during prolonged illumination did not find any reduction in the PLC level after illumination (Bähner et al., 2002). Moreover, other studies have shown that a reduction of PLC level to ∼20% has negligible effect on the sensitivity to light and only increased the bump latency (Pearn et al., 1996; Scott and Zuker, 1998; Cook et al., 2000). This is explained by the dual function of the light activated PLC as an activator and a negative regulator of the transduction cascade because of its GTPase activating protein (GAP) function (Cook et al., 2000). Accordingly, at low PLC levels, Gqα stays active for a long time until it encounters a PLC. In contrast, active rhodopsin has a short lifetime during which it must encounter the G-protein to induce excitation. This is why a reduction in Gqα level but not in PLC has a strong effect on the sensitivity to light (Scott and Zuker, 1998; Cook et al., 2000).
The experiments using flies with mutations that interfere with the targeting of Gqα to the membrane provide conclusive evidence that light-regulated translocation of Gqα underlies long-term adaptation. Strikingly, the Gβe and ninaCP235 mutations (Kosloff et al., 2003; Cronin et al., 2004) concomitantly slowed down the recovery of sensitivity to light in the dark after prolonged illumination and inhibited long-term adaptation (Fig. 4). The Gβe mutation most likely inhibits the association of Gqα to the membrane, whereas the ninaCP235 mutation affects the actin cytoskeleton and hence Gqα movement. The fact that impairments of the retune of Gqα to the signaling membrane is the common denominator between these two completely different mutations strongly supports the hypothesis that the hitherto unexplored physiological role of light-induced translocation of Gqα (Kosloff et al., 2003; Cronin et al., 2004) is to regulate the sensitivity to light during or after prolonged illuminations.
Long-term adaptation and its relationship to the kinetics of Gqα translocation
To further support the notion that translocation of Gqα in and out of the signaling membrane underlies long-term adaptation, the kinetics of light-induced Gqα translocation (Kosloff et al., 2003) was compared with the kinetics of long-term adaptation in WT flies (Fig. 7B,C). This comparison was complicated by the fact that changes in membrane concentration of Gqα affect the sensitivity to light in a highly nonlinear manner (Fig. 7A). When the nonlinear relationship between the reduction in sensitivity to light during prolonged illumination and membrane Gqα concentration was taken into account, the kinetics of Gqα translocation was similar to that of long-term adaptation (Fig. 7C). The relatively small discrepancy at long time periods may be explained by the incorporation of some Gqα molecules into membranes other than the signaling membrane (e.g., endoplasmic reticulum) before their incorporation into the signaling membranes. Indeed, the study of Cronin et al. (2004) did show that in the dark the return of Gqα back to the rhabdomere took >2.5 h to be completed. Future studies will have to address this issue.
To explain the nonlinear dependence of the sensitivity to light on Gqα concentration, we examined theoretically the feasibility that heterotrimeric Gq will encounter an activated rhodopsin when the ratio of Gq/rhodopsin of 1:10 is reduced by fourfold and the diffusion coefficient of Gq in the microvilli is slower than that of tissue culture cells because of the condensed packing of signaling proteins in the microvilli. The outcome of these calculations suggests that a reduction in Gqα level in the signaling membrane can lead to the observed reduction in the sensitivity to light caused by light-induced translocation of Gqα. The calculations of the diffusion of Gq in a microvillus suggest that the nonlinear dependence of the sensitivity shift on membrane Gqα concentration is caused by the large amount of Gq protein in the microvilli, which is presumably required to achieve the high sensitivity of the photoreceptors to light in dark-raised flies.
A shift of the bump latency distribution after prolonged illumination
The latency distribution of the bumps in WT flies is relatively narrow (∼50 ms width at half maximum) (Fig. 8D) and reflects the short lifetime of active rhodopsin. If a light-induced reduction in the available microvillar Gq molecules makes Gq the limiting factor of excitation, it is expected that a large reduction in membrane Gqα will increase the average bump latency as found in the present study. This finding is consistent with previous measurements of the bump-latency distribution of WT and the homozygote Gqα1, which showed that the average bump latency of ∼60 ms in WT flies increased to ∼100 ms in the mutant (Scott and Zuker, 1998).
The bump latency distribution is sensitive to a reduction in PLC level (Scott and Zuker, 1998). However, a relatively modest reduction in PLC level causes a dramatic increase in both the averaged bump latency and the width of the bump latency distribution (to several hundreds milliseconds), in contrast to the relative small effect on these parameters when Gqα is reduced (Fig. 8D) (Scott and Zuker, 1998). Therefore, it is unlikely that the modest increase in bump latency after prolonged illumination arises from a reduction in the PLC level.
The fact that light-induced translocation of Gqα also significantly increased the averaged bump latency (Fig. 8D) constitutes additional strong support for the hypothesis that translocation of Gqα underlies long-term adaptation.
We found similar average bump amplitudes in dark- and light-raised flies despite large changes in membrane Gqα concentration (Fig. 1). The similar average bump amplitudes at different membrane Gqα concentrations may arise from compensatory mechanisms that keep the average bump size constant even when Gqα is reduced to 15%. Possible compensatory mechanisms are the change in the Gqα/Gqβ ratio (Elia et al., 2005) and the involvement of regulators of G-protein signaling (RGSs) (De Vries and Gist, 1999) in fly photoreceptors (Elmore et al., 1998). Both Gβ and RGSs have been shown to have strong effects on GAP activity in vertebrate rods (Keresztes et al., 2004) and they may have similar roles in the determination of the lifetime of Gqα and, hence, the lifetime of PLC molecules when Gqα concentration is reduced. This issue requires additional investigation.
Physiological implications of long-term adaptation through Gqα translocation
The transformation of each absorbed photon into a bump requires vast amounts of mobile membrane Gqα protein (Hardie and Raghu, 2001). Although such high concentrations of membrane Gqα have an obvious advantage during dim lights, they pose an unnecessary load on a highly demanding transduction cascade during daylight, when the sensitivity to each absorbed photon is not required. Translocation of Gqα out of the signaling membrane during prolonged illumination typical for day light is an efficient mechanism to reduce the load on the transduction machinery at an early stage of the transduction cascade.
A mechanism similar to that described in the present study was previously found in vertebrate rods. In vertebrates, a massive light-dependent translocation of the photoreceptor-specific G-protein transducin occurs between the functional compartments of rods. Up to 90% of transducin translocates from rod outer segments to other cellular compartments on the time scale of tens of minutes. The reduction in the transducin content of the rod outer segments is accompanied by a corresponding reduction in the amplification of the rod photoresponse, allowing rods to operate in illumination up to 10-fold higher than would otherwise be possible (Sokolov et al., 2002). The similarity in the physiological effect of light-induced G-protein translocation between vertebrate and invertebrate photoreceptors suggests that this mechanism of long-term adaptation is a common theme in the animal kingdom.
Footnotes
This work was supported by grants from the National Institutes of Health (EY 03529), the Israel Science Foundation, the German Israeli Foundation, the Israel Korea scientific cooperation program, the Moscona Foundation, and the Minerva Foundation. We thank Drs. Boaz Cook, Mickey Kosloff, and Ariela Gordon-Shaag for very useful comments and critical reading of this manuscript. We also thank Ben Katz, Moshe Parnas, and Shaya Lev for advice during the performance of this study and for critical reading of this manuscript.
References
- Alloway PG, Dolph PJ. A role for the light-dependent phosphorylation of visual arrestin. Proc Natl Acad Sci USA. 1999;96:6072–6077. doi: 10.1073/pnas.96.11.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloway PG, Howard L, Dolph PJ. The formation of stable rhodopsin-arrestin complexes induces apoptosis and photoreceptor cell degeneration. Neuron. 2000;28:129–138. doi: 10.1016/s0896-6273(00)00091-x. [DOI] [PubMed] [Google Scholar]
- Bähner M, Frechter S, Da Silva N, Minke B, Paulsen R, Huber A. Light-regulated subcellular translocation of Drosophila TRPL channels induces long-term adaptation and modifies the light-induced current. Neuron. 2002;34:83–93. doi: 10.1016/s0896-6273(02)00630-x. [DOI] [PubMed] [Google Scholar]
- Barash S, Minke B. Is the receptor potential of fly photoreceptors a summation of single-photon responses? Comments Theor Biol. 1994;3:229–263. [Google Scholar]
- Bloomquist BT, Shortridge RD, Schneuwly S, Perdew M, Montell C, Steller H, Rubin G, Pak WL. Isolation of a putative phospholipase C gene of Drosophila, norpA, and its role in phototransduction. Cell. 1988;54:723–733. doi: 10.1016/s0092-8674(88)80017-5. [DOI] [PubMed] [Google Scholar]
- Byk T, Bar Yaacov M, Doza YN, Minke B, Selinger Z. Regulatory arrestin cycle secures the fidelity and maintenance of the fly photoreceptor cell. Proc Natl Acad Sci USA. 1993;90:1907–1911. doi: 10.1073/pnas.90.5.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook B, Bar YM, Cohen-Ben AH, Goldstein RE, Paroush Z, Selinger Z, Minke B. Phospholipase C and termination of G-protein-mediated signalling in vivo. Nat Cell Biol. 2000;2:296–301. doi: 10.1038/35010571. [DOI] [PubMed] [Google Scholar]
- Cronin MA, Diao F, Tsunoda S. Light-dependent subcellular translocation of Gqα in Drosophila photoreceptors is facilitated by the photoreceptor-specific myosin III NINAC. J Cell Sci. 2004;117:4797–4806. doi: 10.1242/jcs.01371. [DOI] [PubMed] [Google Scholar]
- Cronin MA, Lieu MH, Tsunoda S. Two stages of light-dependent TRPL-channel translocation in Drosophila photoreceptors. J Cell Sci. 2006;119:2935–2944. doi: 10.1242/jcs.03049. [DOI] [PubMed] [Google Scholar]
- De Vries L, Gist FM. RGS proteins: more than just GAPs for heterotrimeric G proteins. Trends Cell Biol. 1999;9:138–144. doi: 10.1016/s0962-8924(99)01515-9. [DOI] [PubMed] [Google Scholar]
- Devary O, Heichal O, Blumenfeld A, Cassel D, Suss E, Barash S, Rubinstein CT, Minke B, Selinger Z. Coupling of photoexcited rhodopsin to inositol phospholipid hydrolysis in fly photoreceptors. Proc Natl Acad Sci USA. 1987;84:6939–6943. doi: 10.1073/pnas.84.19.6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge FA, Jr, Knight BW, Toyoda J. Voltage noise in Limulus visual cells. Science. 1968;160:88–90. doi: 10.1126/science.160.3823.88. [DOI] [PubMed] [Google Scholar]
- Dolph PJ, Ranganathan R, Colley NJ, Hardy RW, Socolich M, Zuker CS. Arrestin function in inactivation of G protein-coupled receptor rhodopsin in vivo. Science. 1993;260:1910–1916. doi: 10.1126/science.8316831. [DOI] [PubMed] [Google Scholar]
- Dolph PJ, Man Son Hing H, Yarfitz S, Colley NJ, Deer JR, Spencer M, Hurley JB, Zuker CS. An eye-specific Gβ subunit essential for termination of the phototransduction cascade. Nature. 1994;370:59–61. doi: 10.1038/370059a0. [DOI] [PubMed] [Google Scholar]
- Elia N, Frechter S, Gedi Y, Minke B, Selinger Z. Excess of Gβe over Gqα in vivo prevents dark, spontaneous activity of Drosophila photoreceptors. J Cell Biol. 2005;171:517–526. doi: 10.1083/jcb.200506082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore T, Rodriguez A, Smith DP. dRGS7 encodes a Drosophila homolog of EGL-10 and vertebrate RGS7. DNA Cell Biol. 1998;17:983–989. doi: 10.1089/dna.1998.17.983. [DOI] [PubMed] [Google Scholar]
- Hamdorf K. The physiology of invertebrate visual pigments. In: Autrum H, editor. Handbook of sensory physiology. Comparative physiology and evolution of vision in invertebrates. Berlin: Springer; 1979. pp. 145–224. [Google Scholar]
- Hardie RC, Minke B. The trp gene is essential for a light-activated Ca2+ channel is Drosophila photoreceptors. Neuron. 1992;8:643–651. doi: 10.1016/0896-6273(92)90086-s. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Raghu P. Visual transduction in Drosophila. Nature. 1992;413:186–193. doi: 10.1038/35093002. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Martin F, Cochrane GW, Juusola M, Georgiev P, Raghu P. Molecular basis of amplification in Drosophila phototransduction. Roles for G protein, phospholipase C, and diacylglycerol kinase. Neuron. 2002;36:689–701. doi: 10.1016/s0896-6273(02)01048-6. [DOI] [PubMed] [Google Scholar]
- Henderson SR, Reuss H, Hardie RC. Single photon responses in Drosophila photoreceptors and their regulation by Ca2+ J Physiol (Lond) 2000;524:179–194. doi: 10.1111/j.1469-7793.2000.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iakhine R, Chorna-Ornan I, Zars T, Elia N, Cheng Y, Selinger Z, Minke B, Hyde DR. Novel dominant rhodopsin mutation triggers two mechanisms of retinal degeneration and photoreceptor desensitization. J Neurosci. 2004;24:2516–2526. doi: 10.1523/JNEUROSCI.5426-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keresztes G, Martemyanov KA, Krispel CM, Mutai H, Yoo PJ, Maison SF, Burns ME, Arshavsky VY, Heller S. Absence of the RGS9.Gβ5 GTPase-activating complex in photoreceptors of the R9AP knockout mouse. J Biol Chem. 2004;279:1581–1584. doi: 10.1074/jbc.C300456200. [DOI] [PubMed] [Google Scholar]
- Kiselev A, Socolich M, Vinos J, Hardy RW, Zuker CS, Ranganathan R. A molecular pathway for light-dependent photorecptor apoptosis in Drosophila. Neuron. 2000;28:139–152. doi: 10.1016/s0896-6273(00)00092-1. [DOI] [PubMed] [Google Scholar]
- Kosloff M, Elia N, Joel-Almagor T, Timberg R, Zars TD, Hyde DR, Minke B, Selinger Z. Regulation of light-dependent Gqα translocation and morphological changes in fly photoreceptors. EMBO J. 2003;22:459–468. doi: 10.1093/emboj/cdg054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Montell C. Suppression of constant-light-induced blindness but not retinal degeneration by inhibition of the rhodopsin degradation pathway. Curr Biol. 2004;14:2076–2085. doi: 10.1016/j.cub.2004.11.054. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Xu H, Kang LW, Amzel LM, Montell C. Light adaptation through phosphoinositide-regulated translocation of Drosophila visual arrestin. Neuron. 2003;39:121–132. doi: 10.1016/s0896-6273(03)00390-8. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Brown JE. The effects of intracellular iontophoretic injection of calcium and sodium ions on the light response of Limulus ventral photoreceptors. J Gen Physiol. 1972;59:701–719. doi: 10.1085/jgp.59.6.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minke B, Kirschfeld K. Fast electrical potentials arising from activation of metarhodopsin in the fly. J Gen Physiol. 1980;75:381–402. doi: 10.1085/jgp.75.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minke B, Stephenson RS. The characteristics of chemically induced noise in Musca photoreceptors. J Comp Physiol. 1985;156:339–356. [Google Scholar]
- Montell C, Rubin GM. The Drosophila ninaC locus encodes two photoreceptor cell specific proteins with domains homologous to protein kinases and the myosin heavy chain head. Cell. 1988;52:722–757. doi: 10.1016/0092-8674(88)90413-8. [DOI] [PubMed] [Google Scholar]
- Niemeyer BA, Suzuki E, Scott K, Jalink K, Zuker CS. The Drosophila light-activated conductance is composed of the two channels TRP and TRPL. Cell. 1996;85:651–659. doi: 10.1016/s0092-8674(00)81232-5. [DOI] [PubMed] [Google Scholar]
- Pak WL, Lidington KJ. Fast electrical potential from a long-lived, long-wavelength photoproduct of fly visual pigment. J Gen Physiol. 1974;63:740–756. doi: 10.1085/jgp.63.6.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearn MT, Randall LL, Shortridge RD, Burg MG, Pak WL. Molecular, biochemical, and electrophysiological characterization of Drosohpila norpA mutants. J Biol Chem. 1996;271:4937–4945. doi: 10.1074/jbc.271.9.4937. [DOI] [PubMed] [Google Scholar]
- Peretz A, Suss-Toby E, Rom-Glas A, Arnon A, Payne R, Minke B. The light response of Drosophila photoreceptors is accompanied by an increase in cellular calcium: effects of specific mutations. Neuron. 1994;12:1257–1267. doi: 10.1016/0896-6273(94)90442-1. [DOI] [PubMed] [Google Scholar]
- Perez JB, Segura JM, Abankwa D, Piguet J, Martinez KL, Vogel H. Monitoring the diffusion of single heterotrimeric G proteins in supported cell-membrane sheets reveals their partitioning into microdomains. J Mol Biol. 2006;363:918–930. doi: 10.1016/j.jmb.2006.08.036. [DOI] [PubMed] [Google Scholar]
- Ranganathan R, Stevens CF. Arrestin binding determines the rate of inactivation of the G protein-coupled receptor rhodopsin in vivo. Cell. 1995;81:841–848. doi: 10.1016/0092-8674(95)90004-7. [DOI] [PubMed] [Google Scholar]
- Richard EA, Lisman JE. Rhodopsin inactivation is a modulated process in Limulus photoreceptors. Nature. 1992;356:336–338. doi: 10.1038/356336a0. [DOI] [PubMed] [Google Scholar]
- Satoh AK, Ready DF. Arrestin1 mediates light-dependent rhodopsin endocytosis and cell survival. Curr Biol. 2005;15:1722–1733. doi: 10.1016/j.cub.2005.08.064. [DOI] [PubMed] [Google Scholar]
- Scott K, Zuker CS. Assembly of the Drosophila phototransduction cascade into a signalling complex shapes elementary responses. Nature. 1998;395:805–808. doi: 10.1038/27448. [DOI] [PubMed] [Google Scholar]
- Scott K, Becker A, Sun Y, Hardy R, Zuker C. Gqα protein function in vivo: genetic dissection of its role in photoreceptor cell physiology. Neuron. 1995;15:919–927. doi: 10.1016/0896-6273(95)90182-5. [DOI] [PubMed] [Google Scholar]
- Sokolov M, Lyubarsky AL, Strissel KJ, Savchenko AB, Govardovskii VI, Pugh EN, Jr, Arshavsky VY. Massive light-driven translocation of transducin between the two major compartments of rod cells: a novel mechanism of light adaptation. Neuron. 2002;34:95–106. doi: 10.1016/s0896-6273(02)00636-0. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Terakita A, Narita K, Nagai K, Tsukahara Y, Kito Y. Squid photoreceptor phospholipase C is stimulated by membrane Gq α but not by soluble Gq α. FEBS Lett. 1995;377:333–337. doi: 10.1016/0014-5793(95)01364-4. [DOI] [PubMed] [Google Scholar]
- Terakita A, Takahama H, Tamotsu S, Suzuki T, Hariyama T, Tsukahara Y. Light-modulated subcellular localization of the α-subunit of GTP-binding protein Gq in crayfish photoreceptors. Vis Neurosci. 1996;13:539–547. doi: 10.1017/s095252380000821x. [DOI] [PubMed] [Google Scholar]
- Vinos J, Jalink K, Hardy RW, Britt SG, Zuker CS. A G protein-coupled receptor phosphatase required for rhodopsin function. Science. 1997;277:687–690. doi: 10.1126/science.277.5326.687. [DOI] [PubMed] [Google Scholar]
- Wedegaertner PB, Chu DH, Wilson PT, Levis MJ, Bourne HR. Palmitoylation is required for signaling functions and membrane attachment of Gq α and Gs α. J Biol Chem. 1993;268:25001–25008. [PubMed] [Google Scholar]
- Wolfram V, Juusola M. Impact of rearing conditions and short-term light exposure on signaling performance in Drosophila photoreceptors. J Neurophysiol. 2004;92:1918–1927. doi: 10.1152/jn.00201.2004. [DOI] [PubMed] [Google Scholar]
- Wong F, Knight BW, Dodge FA. Adapting bump model for ventral photoreceptors of Limulus. J Gen Physiol. 1982;79:1089–1113. doi: 10.1085/jgp.79.6.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Takeuchi Y, Komori N, Kobayashi H, Sakai Y, Hotta Y, Matsumoto H. A 49-kilodalton phosphoprotein in the Drosophila photoreceptor is an arrestin homolog. Science. 1990;248:483–486. doi: 10.1126/science.2158671. [DOI] [PubMed] [Google Scholar]
- Yeandle S, Spiegler JB. Light-evoked and spontaneous discrete waves in the ventral nerve photoreceptor of Limulus. J Gen Physiol. 1973;61:552–571. doi: 10.1085/jgp.61.5.552. [DOI] [PMC free article] [PubMed] [Google Scholar]