Abstract
Objective: The functional relationship between calculated alpha band spectral power and inter-/intra-hemispheric coherence during a three-level working memory task of patients with mild cognitive impairment (MCI) was investigated. Methods: Subjects included 35 MCI patients according to the DSM-IV criteria (mean age: 62.3, SD: 6.5) and 34 healthy controls (mean age: 57.4, SD: 4.0) were selected from the community at large. All subjects performed a simple calculation and recall task with three levels of working memory load while electroencephalograph (EEG) signal was recorded. The spectral EEG power was computed over alpha1 (8.0~10.0 Hz) and alpha2 (10.5~13.0 Hz) frequency bands and was compared between rest stage and working memory processing stage by two-way ANOVA. Post hoc testing analyzed the differences between each two levels of working memory load during task processing. The inter-hemisphere EEG coherence of frontal (F3-F4), central (C3-C4), parietal (P3-P4), temporal (T5-T6) as well as occipital (O1-O2) was compared between MCI patients and normal controls. The EEG signals from F3-C3, F4-C4, C3-P3, C4-P4, P3-O1, P4-O2, T5-C3, T6-C4, T5-P3 and T6-P4 electrode pairs resulted from the intra-hemispheric action for alpha1 and alpha2 frequency bands. Result: There was significantly higher EEG power from MCI patients than from normal controls both at rest and during working memory processing. Significant differences existed between rest condition and three-level working memory tasks (P<0.001). The inter- and intra-hemispheric coherence during working memory tasks showed a “drop to rise” tendency compared to that at rest condition. There was significantly higher coherence in MCI patients than in the controls. When task difficulties increased, the cortical connectivity of intra-hemispheric diminished while the inter-hemispheric connectivity dominantly maintained the cognitive processing in MCI patients. Conclusion: The results of the present study indicate that the alpha frequency band may be the characteristic band in distinguishing MCI patients from normal controls during working memory tasks. MCI patients exhibit greater inter-hemispheric connectivity than intra-hemispheric connectivity when memory demands increase. MCI patients mobilize a compensatory mechanism to maintain the processing effectiveness while the processing efficiency is reduced.
Keywords: Spectral power, Coherence, Mild cognitive impairment, Working memory
INTRODUCTION
Mild cognitive impairment (MCI) is characterized by selective memory impairment insufficient to meet criteria for a diagnosis of dementia (Petersen et al., 1999; Hogan et al., 2003). This condition is considered as a prodromic stage of AD (Alzheimer’s disease) (Petersen et al., 2001). The annual conversion rate from normality to AD is estimated at about 0.2%~4%, whereas that from MCI to AD is about 6%~25% (Petersen et al., 2001; Hogan et al., 2003). These data suggest that, in several (but not all) cases, MCI is a transition state on a linear progression towards AD. Therefore, studying the cognitive impairment in MCI may provide diagnostic clues and early symptoms for AD.
Working memory has been a major topic of cognitive research during the last 25 years. Many studies proved that working memory plays an important role in learning, calculating, reasoning and verb comprehension of cognitive processing. Working memory tasks are known to change functional connectivity (Hogan et al., 2003). The human electroencephalograph (EEG) has proven to be a useful tool in the examination of the brain’s functioning. Quantitative electroencephalography (qEEG) and coherence provide additional sources of information about the topography of synchronous oscillation activity and potential cortico-cortical interactions during cognitive testing (Gevins and Smith, 2000; Schürmann and Başar, 2001; Weiss and Rappelsberger, 2000).
It has been suggested that alpha activity is generated by cortico-cortical and thalamocortical networks. The responses of the alpha frequency band have been found to reflect alertness, expectancy and attention demands (6~10 Hz), as well as semantic processing (10~12 Hz) (Jensen et al., 2002; Klimesch et al., 1997; 2001). The lower alpha band is believed to reflect attention processes, whereas the upper alpha band may reflect long-term semantic memory (Klimesch, 1999; Stam, 2000).
As a cognitive impairment state between normal aging and dementia, MCI can serve as a probe in AD diagnosing and early intervention. Because of the key role in complex cognitive processing and evaluating, studying working memory in MCI patients is very important. In the present study, the EEG and working memory tasks were combined to investigate the brain function during cognitive activity in MCI patients. The goal of our study was to investigate alpha frequency band spectral power and coherence during a three-level working memory task between MCI and normal controls.
MATERIALS AND METHODS
Subjects
The patient group consisted of 35 patients (18 females and 17 males) selected from the community and were diagnosed by psychiatrists in the Department of Psychiatry, the Second Affiliated Hospital, Zhejiang University, China. All patients accorded with DSM-IV criteria (American Psychiatric Association, 1994) for the study diagnosis of MCI, and the following criteria: (1) memory loss complaints, (2) normal activities of daily living, (3) normal general cognitive function, (4) abnormal memory for aging, (5) not demented, (6) the course of memory damage was over three months. Their mean age (±SD) was (62.3±6.5) years, range 52~71 years. Each patient was evaluated by the mini-mental state examination (MMSE) (Folstein et al., 1975), clinical dementia rating (CDR) (Hughes et al., 1982), functional assessment staging (FAST) (Reisberg, 1988), and activities of daily living scale (ADL) (Lawton and Brody, 1969). The mean MMSE score (±SD) was 26.6±2.0, range 25~30; the CDR score was 0.5; the FAST result was 3; the ADL score was <22. None of the patients were receiving psychoactive medications such as antipsychotic drugs or cerebral vasodilators. Moreover, in order to rule out other organic brain diseases, e.g. multiple sub-cortical infarctions with MCI, magnetic resonance imagining (MRI) and/or computed tomography (CT) examinations were performed on the patient group.
The control group consisted of 34 healthy volunteers (17 females and 17 males) without personal or family history of psychiatric or neurological abnormality. Their mean age was (57.4±4.0) years, range 51~63 years. The normal controls were also obtained from the general community population. They functioned normally in daily life with no cognitive impairment. The mean MMSE score (±SD) was 29.38±0.88, range 29~30.
The MCI patients had no significant differences with controls in age, gender, or education (P>0.05). The MCI patients had significantly lower MMSE score than normal controls (t=−6.521, P<0.001). All subjects were right-handed and agreed to participate in this study with full knowledge of the experimental nature of the research.
EEG recording and analysis
During EEG recording the subjects were in a resting state with eyes closed, sitting in a semi-darkened, electrically shielded, sound attenuated room. According to the international 10~20 system, original EEG signals were recorded from scalp electrodes and separate ear electrodes A1 and A2, with electrodes referenced to linked ear lobes. Impedance of electrodes/skin was kept below 5 000 Ω. The signals were amplified and filtered by a 128-channel EEG (EEG-NATION 918, Shanghai, China) with an upper frequency cut-off 60 Hz and 0.1 time constant. EEGs were recorded at 16 electrode sites: Fp1, Fp2, F3, F4, F7, F8, C3, C4, T3, T4, T5, T6, P3, P4, O1 and O2 electrodes for 10 min for each subject with eyes closed. Names of electrode sites are defined by the rule of the international 10~20 system. Selection of segments recorded when subjects’ eyes were closed while awake was based on visual inspection of EEG and electro-oculographic (EOG) recordings. Segments containing eye movements, blinks, or muscle activity were excluded from the analysis.
EEG coherence was calculated by the fast Fourier transform (FFT) method. One epoch consisted of 2 s, and 20 artifact-free epochs per subject were processed with a spectral resolution of 0.5 Hz. Coherence between two waveforms x and y was calculated spectrally as:
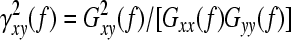 , ,
|
where γxy(f) is the coherence on band f, Gxy(f) is the mean cross-power density and Gxx(f) and Gyy(f) are the respective mean auto-power spectral densities. The details of the method for calculating the coherence have previously been published (Jiang, 2004; 2005). In this study, inter-hemispheric EEG coherence was measured between the following 5 homologous electrode pairs: frontal (F3-F4), left-right central (C3-C4), left-right parietal (P3-P4), left-right temporal (T5-T6), left-right occipital (O1-O2); intra-hemispheric EEG was measured between the following 10 electrode pairs: left-right fronto-central (F3-C3, F4-C4), left-right centro-parietal (C3-P3, C4-P4), left-right parieto-occipital (P3-O1, P4-O2), left-right temporo-central (T5-C3, T6-C4), and left-right temporo-pareietal (T5-P3, T6-P4). The coherence coefficients were calculated and banded into alpha1 band (8.0~10.0 Hz), alpha2 band (10.5~13.0 Hz).
Working memory task
After recording in the rest condition a three-level working memory task was performed. Studies related to working memory task have been reported by deToledo-Morrell et al.(1991) involving Sternberg (1969)-type memory scanning task.
The task design was a replication of sums (arithmetic) with three levels working memory loads. Recitation of three-digit numbers and mental calculation were based on Salthouse and Babcock (1991). The working memory task was two simple unit numerals addition and the result was a simple numeral. Level 1 was one arithmetic addition (WM1); level 2 was two arithmetic additions (WM2) and level 3 was three arithmetic additions (WM3). The subjects were asked to remember the answer during each level of the working memory task, while at the same time, the EEG was recording from the time the question was asked until the answer was recalled.
Statistics
In the present study, a logarithmic transformation of absolute power and Fisher’s Z transformation of coherence value of each band in each derivation were implemented to normalize the distribution of power and coherence values, respectively. Factors between groups (MCI vs control) and within subjects (electrode position) were analyzed by two-way analysis of variance (ANOVA). A Post hoc test analyzed the differences between each two levels of working memory load. As the EEG recording method for analysis of inter-hemispheric coherence within subject factors, electrode pair involved five levels, for analysis of intra-hemispheric coherence within subject factor, electrode pair involved ten levels. Separate ANOVAs were conducted for alpha1 and alpha2 frequency bands in order to test inter- and intra-hemispheric EEG coherence, respectively. Statistical significance was defined as P<0.05.
RESULTS
Spectral power
Table 1 shows the EEG spectral power at rest and during working memory task for the controls and MCI on alpha1 and alpha2 frequency bands. There was significantly higher EEG power for MCI patients than for the normal controls both at rest and during working memory processing. The power at rest condition was significantly higher than that in working memory condition. Post hoc analyses showed there was no significant difference found between each two working memory levels (WM1-WM2, WM2-WM3, WM1-WM3).
Table 1.
Alpha1 and Alpha2 bands EEG power values (mean±SD) at rest and during working memory task
| Alpha1 |
Alpha2 |
||||||||
| Rest | WM1 | WM2 | WM3 | Rest | WM1 | WM2 | WM3 | ||
| F3 | MCI | 0.83±0.40*# | 0.52±0.32* | 0.70±0.26* | 0.66±0.26* | 0.63±0.27*# | 0.41±0.34* | 0.59±0.24* | 0.54±0.26* |
| CON | 0.63±0.27# | 0.40±0.21 | 0.48±0.19 | 0.47±0.24 | 0.45±0.40# | 0.28±0.23 | 0.32±0.25 | 0.37±0.27 | |
| F4 | MCI | 0.86±0.39*# | 0.52±0.27* | 0.68±0.27* | 0.66±0.28* | 0.66±0.27*# | 0.44±0.33* | 0.56±0.28* | 0.52±0.28* |
| CON | 0.66±0.27# | 0.38±0.19 | 0.45±0.29 | 0.47±0.26 | 0.43±0.42# | 0.29±0.25 | 0.33±0.29 | 0.37±0.29 | |
| C3 | MCI | 0.65±0.42*# | 0.36±0.27* | 0.50±0.28* | 0.44±0.30* | 0.55±0.29*# | 0.31±0.30* | 0.46±0.28* | 0.38±0.29* |
| CON | 0.55±0.30# | 0.24±0.20 | 0.27±0.22 | 0.30±0.25 | 0.43±0.36# | 0.15±0.14 | 0.18±0.17 | 0.26±0.24 | |
| C4 | MCI | 0.76±0.39*# | 0.41±0.26* | 0.55±0.27* | 0.50±0.21* | 0.64±0.27*# | 0.38±0.28* | 0.53±0.26* | 0.47±0.23* |
| CON | 0.64±0.27# | 0.22±0.21 | 0.32±0.28 | 0.31±0.28 | 0.45±0.36# | 0.19±0.12 | 0.28±0.24 | 0.29±0.28 | |
| P3 | MCI | 0.95±0.52*# | 0.50±0.30* | 0.62±0.37* | 0.58±0.33* | 0.92±0.44*# | 0.56±0.36* | 0.69±0.40* | 0.66±0.35* |
| CON | 0.92±0.44# | 0.30±0.27 | 0.31±0.24 | 0.36±0.34 | 0.55±0.53# | 0.29±0.27 | 0.39±0.32 | 0.43±0.37 | |
| P4 | MCI | 1.14±0.61*# | 0.61±0.32* | 0.75±0.34* | 0.73±0.28* | 1.03±0.47*# | 0.69±0.41* | 0.83±0.41* | 0.82±0.36* |
| CON | 1.03±0.47# | 0.29±0.26 | 0.41±0.34 | 0.39±0.36 | 0.60±0.56# | 0.36±0.35 | 0.44±0.38 | 0.42±0.34 | |
| T5 | MCI | 0.98±0.69*# | 0.49±0.39* | 0.60±0.43* | 0.63±0.37* | 0.84±0.54*# | 0.54±0.41* | 0.65±0.41* | 0.68±0.39* |
| CON | 0.84±0.54# | 0.30±0.27 | 0.30±0.28 | 0.44±0.36 | 0.56±0.53# | 0.34±0.31 | 0.40±0.38 | 0.48±0.44 | |
| T6 | MCI | 1.13±0.61*# | 0.57±0.44* | 0.72±0.44* | 0.73±0.42* | 1.00±0.55*# | 0.71±0.48* | 0.81±0.51* | 0.80±0.43* |
| CON | 1.00±0.55# | 0.28±0.24 | 0.36±0.35 | 0.50±0.44 | 0.57±0.52# | 0.39±0.33 | 0.43±0.37 | 0.52±0.45 | |
| O1 | MCI | 0.58±0.56*# | 0.30±0.20* | 0.35±0.33* | 0.28±0.25* | 0.53±0.48*# | 0.24±0.23* | 0.36±0.32* | 0.30±0.23* |
| CON | 0.53±0.48# | 0.08±0.05 | 0.13±0.12 | 0.23±0.19 | 0.28±0.25# | 0.09±0.07 | 0.10±0.10 | 0.16±0.11 | |
| O2 | MCI | 0.91±0.58*# | 0.40±0.35* | 0.54±0.41* | 0.53±0.40* | 0.75±0.49*# | 0.47±0.43* | 0.61±0.47* | 0.58±0.43* |
| CON | 0.75±0.49# | 0.09±0.07 | 0.20±0.19 | 0.23±0.21 | 0.32±0.23# | 0.15±0.12 | 0.23±0.20 | 0.26±0.19 | |
CON: Normal controls;
Spectral power in MCI patients was significantly higher than that in normal controls, P<0.05;
Spectral power in rest condition was significantly higher than that in working memory condition, P<0.05
Inter-hemispheric coherence
According to the inter-hemispheric coherence in rest condition, there was no significant difference between MCI and controls both on alpha1 and alpha2 frequency bands at different electrode pairs (P>0.05).
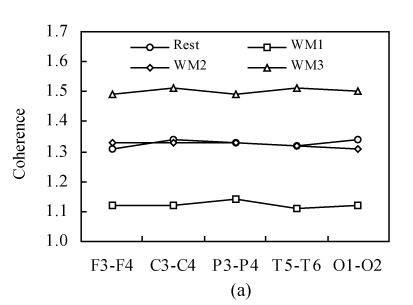
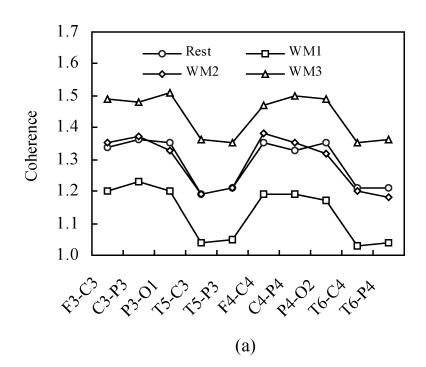
Fig.1 shows the inter-hemispheric coherence values at rest and during three-level working memory processing for all subjects on alpha1 and alpha2 frequency bands. For alpha1 and alpha2 bands, a two-way ANOVA analysis was run. Significant differences existed among rest and three working memory levels [F α1(1,3)=1030.75, P<0.001; F α2(1,3)=730.19, P<0.001]. Post hoc analysis showed there was significantly higher inter-hemispheric coherence at rest than that in WM1 level (P<0.001); there was significantly higher inter-hemispheric coherence in WM2 than that in WM1 level (P<0.001); there was significantly higher inter-hemispheric coherence in WM3 than that in WM2 level (P<0.001) and there was no significant difference between WM2 and rest condition (P>0.05). The coherence during working memory processing showed a “drop to rise” tendency compare to that at rest condition.
Fig. 1.
Inter-hemispheric coherence values at rest and during three-level working memory (WM1, WM2, WM3) processing. (a) Inter-hemispheric coherence on alpha1 frequency band; (b) Inter-hemispheric coherence on alpha2 frequency band
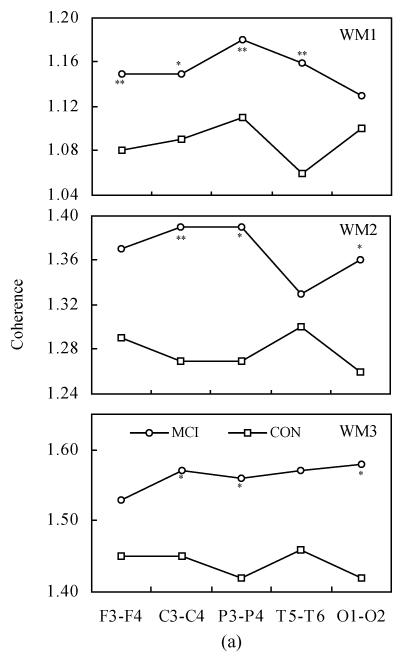
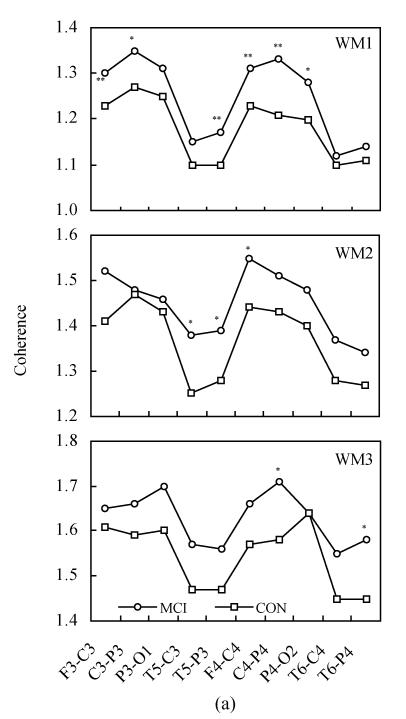
Fig.2 shows the inter-hemispheric coherence values at rest and during three-level working memory processing in MCI and controls. Two-way ANOVA revealed the differences between MCI and normal controls, listed as follows:
Fig. 2.
Inter-hemispheric coherence values at rest and during three-level working memory processing in MCI and controls (CON). (a) Inter-hemispheric coherence on alpha1 frequency band during WM1, WM2 and WM3; (b) Inter-hemispheric coherence on alpha2 frequency band during WM1, WM2 and WM3
Compared to controls: * P<0.05, ** P<0.01
On the alpha1 frequency band, the inter-hemispheric coherence of MCI patients was significantly higher than that of controls at F3-F4, C3-C4, P3-P4 and T5-T6 electrode pairs [F(1,68)=10.331, P<0.01; F(1,68)=7.013, P<0.05; F(1,68)=16.321, P<0.01; F(1,68)=25.060, P<0.01] during WM1 task. The inter-hemispheric coherence of MCI patients was significantly higher than that of controls at C3-C4, P3-P4 and O1-O2 electrode pairs [F(1,68)=7.634, P<0.01; F(1,68)=6.766, P<0.05; F(1,68)=4.721, P<0.05] during WM2 task. The inter-hemispheric coherence of MCI patients was significantly higher than that of controls at C3-C4, P3-P4 and O1-O2 electrode pairs [F(1,68)=3.939, P<0.05; F(1,68)=4.778, P<0.05; F(1,68)=7.706, P<0.05] during WM3 task.
On the alpha2 frequency band, the inter-hemispheric coherence of MCI patients was significantly higher than that of controls at F3-F4, C3-C4, P3-P4 and T5-T6 electrode pairs [F(1,68)=10.267, P<0.01; F(1,68)=8.771, P<0.05; F(1,68)=13.125, P<0.01; F(1,68)=16.884, P<0.01] during WM1 task. The inter-hemispheric coherence of MCI patients was significantly higher than that of controls at C3-C4, P3-P4 and O1-O2 electrode pairs [F(1,68)=7.809, P<0.01; F(1,68)=5.749, P<0.05; F(1,68)=4.275, P<0.05] during WM2 task. The inter-hemispheric coherence of MCI patients was significantly higher than that of controls at C3-C4, P3-P4 and O1-O2 electrode pairs [F(1,68)=4.183, P<0.05; F(1,68)=4.817, P<0.05; F(1,68)=8.553, P<0.01] during the WM3 task.
Intra-hemispheric coherence
The intra-hemispheric coherence in rest condition at different electrode pairs had no significant difference between MCI and controls.
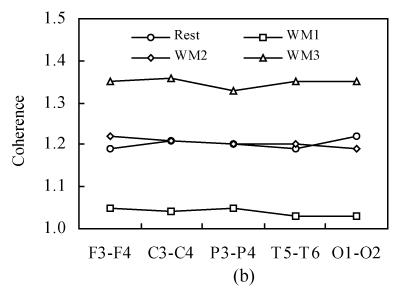
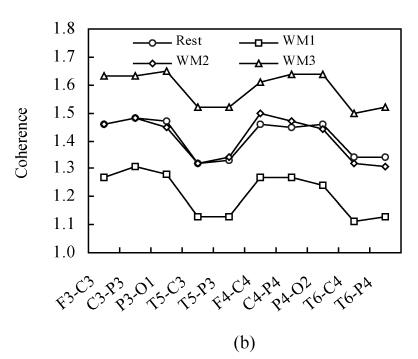
Fig.3 shows the intra-hemispheric coherence values at rest and during three-level working memory processing for all subjects on alpha1 and alpha2 frequency bands. For alpha1 and alpha2 bands, a two-way ANOVA analysis was run. There was a significant difference among rest and three working memory levels [F α1(1,3)=687.32, P<0.001; F α2(1,3)=528.21, P<0.001]. Post hoc analysis showed there was significantly higher intra-hemispheric coherence at rest than in WM1 level (P<0.001); there was significantly higher intra-hemispheric coherence in WM2 than in WM1 level (P<0.001); there was significantly higher intra-hemispheric coherence in WM3 than in WM2 level (P<0.001) and there was no significant difference between WM2 and rest condition (P>0.05). The intra-hemispheric coherence exhibits the same “drop to rise” tendency as the inter-hemispheric coherence during the working memory task.
Fig. 3.
Intra-hemispheric coherence values at rest and during three-level working memory (WM1, WM2, WM3) processing. (a) Intra-hemispheric coherence on alpha1 frequency band; (b) Intra-hemispheric coherence on alpha2 frequency band
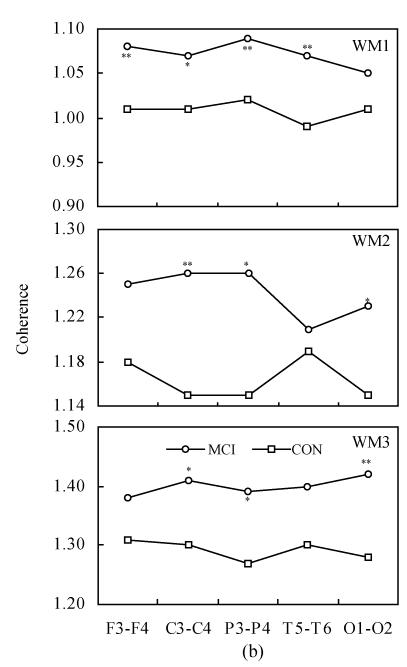
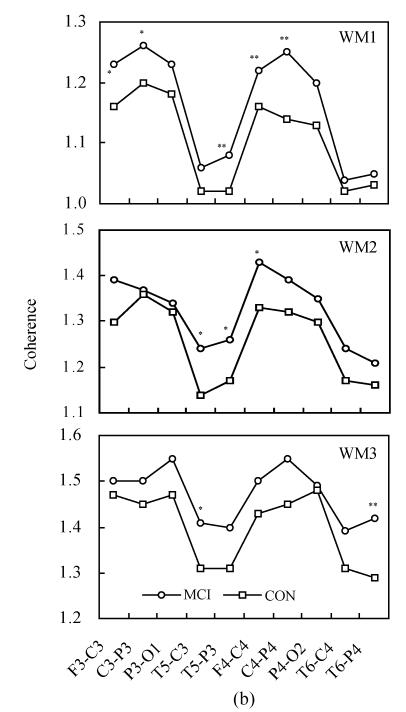
Fig.4 shows the intra-hemispheric coherence values at rest and during three-level working memory processing in MCI and controls. Two-way ANOVA revealed the differences between MCI and normal controls, listed as follows:
Fig. 4.
Intra-hemispheric coherence values at rest and during three-level working memory processing in MCI and controls (CON). (a) Intra-hemispheric coherence on alpha1 frequency band during WM1, WM2 and WM3; (b) Intra-hemispheric coherence on alpha2 frequency band during WM1, WM2 and WM3
Compared to controls: * P<0.05, ** P<0.01
On the alpha1 frequency band, the intra-hemispheric coherence of MCI patients was significantly higher than that of controls at F3-C3, C3-P3, T5-P3, F4-C4, C4-P4 and P4-O2 electrode pairs [F(1,68)=7.705, P<0.01; F(1,68)=7.755, P<0.05; F(1,68)=11.075, P<0.01; F(1,68)=10.824, P<0.01; F(1,68)=23.450, P<0.01; F(1,68)=5.217, P<0.05] during WM1 task. The intra-hemispheric coherence of MCI patients was significantly higher than that of controls at T5-C3, T5-P3 and F4-C4 electrode pairs [F(1,68)=6.806, P<0.05; F(1,68)=4.508, P<0.05; F(1,68)=4.194, P<0.05] during WM2 task. The intra-hemispheric coherence of MCI patients was significantly higher than that of controls at C4-P4 and T6-P4 electrode pairs [F(1,68)=4.784, P<0.05; F(1,68)=4.986, P<0.05] during WM3 task.
On alpha2 frequency band, the intra-hemispheric coherence of MCI patients was significantly higher than that of controls at F3-C3, C3-P3, T5-P3, F4-C4 and C4-P4 electrode pairs [F(1,68)=6.810, P<0.05; F(1,68)=5.624, P<0.05; F(1,68)=10.856, P<0.01; F(1,68)=7.598, P<0.01; F(1,68)=21.116, P<0.01] during the WM1 task. The inter-hemispheric coherence of MCI patients was significantly higher than that of controls at T5-C3, T5-P3 and F4-C4 electrode pairs [F(1,68)=5.802, P<0.05; F(1,68)=4.142, P<0.05; F(1,68)=5.353, P<0.05] during the WM2 task. The inter-hemispheric coherence of MCI patients was significantly higher than that of controls at T5-C3 and T6-P4 electrode pairs [F(1,68)=4.015, P<0.05; F(1,68)=7.218, P<0.01] during the WM3 task.
DISCUSSION
In the present study we examined the EEG spectral power and inter-/intra-hemispheric coherence on alpha frequency bands in 35 MCI patients and 34 normal controls.
Spectral power on alpha1 and alpha2 bands was significantly higher in MCI than that in controls both during rest and working memory effort, as shown in Table 1. The results also showed that the power in rest condition was significantly higher than that during all three levels working memory tasks. EEG alpha (around 10 Hz) is the dominant rhythm in the human brain during conditions of mental inactivity. High amplitudes as observed at rest usually diminish during cognitive effort, which is consistent with the current study. The results of the quantitative EEG analysis could be interpreted as reflecting generally enhanced effort in MCI patients. Additionally, the spectral power differences between each two levels of working memory difficulty had no statistical significance, indicating that the mobilization of cognitive resources would not be enhanced by the increasing of task difficulties.
In all subjects, the EEG inter- and intra-hemispheric coherence exhibited a “drop to rise” tendency, as shown in Fig.1 and Fig.3. The coherence values compared to those at rest condition dropped at WM1 stage and then rose higher and higher from WM2 to WM3 stages. The phenomena known as event-related desynchronization (ERD) and event-related synchronization (ERS) exist on alpha frequency bands. The ERD is related to the event-stimulus and the ERS is related to the working memory task, reflecting the different aspects of message processing (Suffczynski et al., 2001). The ERS and ERD on alpha frequency bands occur simultaneously during message processing, in a phemenon known as “focal ERD/surround ERS”. The results of the current study indicate that the ERD/ERS also exists in inter- and intra-hemispheric coherence at rest and during working memory tasks.
In contrast to the spectral power, the inter-/intra-hemispheric coherence had no significant difference between MCI and controls during rest. While during working memory task, the inter- and intra-hemispheric coherence was significantly higher in MCI patients than that in normal controls, as shown in Fig.2 and Fig.4. The inter-hemispheric coherence values in MCI patients on left-right centrals (C3-C4) and left-right parietal (P3-P4) were significantly higher than that in controls during all three working memory levels. The alpha frequency band is known as the cognitive band (Fink et al., 2005; Hebert et al., 2005) and the coherence on alpha band is comparatively stronger than that on other frequency bands (Jing and Takigawa, 2000). The present results could be interpreted as reflecting generally enhanced effort in MCI patients. The high synchronization represents a compensational mechanism in MCI during cognitive performance (Pijnenburg et al., 2004). Our finding is consisted with previous studies.
Interestingly, we found that the number of electrode pairs of significantly higher intra-hemispheric coherence in MCI patients decreased from WM1 to WM3 levels, while the number of significant electrode pairs of inter-hemispheric coherence had almost no change, as shown in Fig.2 and Fig.4. This indicates that by the increasing of task difficulties, the cortical connectivity of intra-hemispheric coherence diminished while the inter-hemispheric connectivity dominantly maintained the cognitive processing in MCI patients. By the increasing of task difficulties the MCI patients mobilized more cognitive resources of inter-hemispheric than that of intra-hemispheric.
Working memory tasks are known to change the cerebral function connectivity. Measurement of spectral power and coherence may offer insight into underlying cortical disruption and/or cortical reorganization (Hogan et al., 2003). The findings of this study suggest that both the increasing alpha power and the enhanced alpha coherence are part of the signature that distinguishes cortical processing of MCI and controls during memory activity.
The early detection of subjects with MCI, who are at high risk to progress to AD, is crucial for currently available treatment strategies. Multiple brain areas, including the lateral prefrontal cortex, mediotemporal areas and posterior association cortex are functionally involved in working memory. When studying retarded memory, damage related to the temporal lobe, hippocampus, parietal lobe and other cortexes would occur and result in change of EEG coherence (Jiang and Zheng, 2006). In previous study, we found impaired theta-band activity during working memory task in MCI patients (Jiang and Zheng, 2006). The results of the present study suggest that alpha frequency band may be the characteristic band in distinguishing MCI patients from normal controls during working memory tasks, with MCI showing more inter-connectivity than intra-connectivity. When memory demands increase, MCI patients mobilize a compensatory mechanism to maintain the processing effectiveness while the processing efficiency is reduced.
Acknowledgments
We would like to thank the members of Clinic EEG Laboratory, the Second Affiliated Hospital, School of Medicine, Zhejiang University, for their invaluable help with this study.
Footnotes
Project (No. 2003B070) supported by the Science and Technology Program of Zhejiang Province, China
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Ed. Washington, DC: APA Press; 1994. (DSM-IV) [Google Scholar]
- 2.deToledo-Morrell L, Evers S, Hoeppner TJ, Morrell F, Garron DC, Fox JH. A ‘stress’ test for memory dysfunction. Electrophysiogical manifestation of early Alzheimer’s disease. Arch Neurol. 1991;48(6):605–609. doi: 10.1001/archneur.1991.00530180061018. [DOI] [PubMed] [Google Scholar]
- 3.Fink A, Grabner RH, Neuper C, Neubauer AC. EEG alpha band dissociation with increasing task demands. Cogn Brain Res. 2005;24(2):252–259. doi: 10.1016/j.cogbrainres.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 5.Gevins A, Smith ME. Neurophysiological measures of working memory and individual differences in cognitive ability and cognitive style. Cereb Cortex. 2000;10(9):829–839. doi: 10.1093/cercor/10.9.829. [DOI] [PubMed] [Google Scholar]
- 6.Hebert R, Lehmann D, Tan G. Enhanced EEG alpha time domain phase synchrony during transcendental meditation: implications for cortical integration theory. Signal Processing. 2005;85(11):2213–2232. doi: 10.1016/j.sigpro.2005.07.009. [DOI] [Google Scholar]
- 7.Hogan MJ, Swanwick GRJ, Kaiser J, Rowman M, Lawlor B. Memory-related EEG power and coherence reduction in mild Alzheimer’s disease. Int J Psychophysiol. 2003;49(2):147–163. doi: 10.1016/S0167-8760(03)00118-1. [DOI] [PubMed] [Google Scholar]
- 8.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RA. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 9.Jensen O, Gelfand J, Kounios J, Lisman JE. Oscillations in the alpha band (9~12 Hz) increase with memory load during retention in a short-term memory task. Cereb Cortex. 2002;12(8):877–882. doi: 10.1093/cecor/12.8.877. [DOI] [PubMed] [Google Scholar]
- 10.Jiang ZY. Research of diagnosis of Alzheimer’s disease based on coherence analysis of EEG signal. Chinese Journal of Sensor and Actuator. 2004;17(3):363–366. (in Chinese) [Google Scholar]
- 11.Jiang ZY. Abnormal cortical functional connections in Alzheimer’s disease: analysis of inter- and intra-hemispheric EEG coherence. J Zhejiang Univ Sci. 2005;6B(4):259–264. doi: 10.1631/jzus.2005.B0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang ZY, Zheng LL. Inter- and intra-hemipheric EEG coherence in patients with mild cognitive impairement at rest and during working memory task. J Zhejiang Univ Sci. 2006;7B(5):357–364. doi: 10.1631/jzus.2006.B0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jing H, Takigawa M. Observation of EEG coherernce after repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2000;111(9):1620–1631. doi: 10.1016/S1388-2457(00)00357-6. [DOI] [PubMed] [Google Scholar]
- 14.Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Rev. 1999;29(2-3):169–195. doi: 10.1016/S0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- 15.Klimesch W, Doppelmayr M, Pachinger T, Ripper B. Brain oscillations and human memory: EEG correlates in the upper alpha and theta band. Neurosci Lett. 1997;238(1-2):9–12. doi: 10.1016/S0304-3940(97)00771-4. [DOI] [PubMed] [Google Scholar]
- 16.Klimesch W, Doppelmayr M, Stadler W, Pöllhuber D, Sauseng P, Röhm D. Episodic retrieval is reflected by a process specific increase in human electro-encephalographic theta activity. Neurosci Lett. 2001;302(1):49–52. doi: 10.1016/S0304-3940(01)01656-1. [DOI] [PubMed] [Google Scholar]
- 17.Lawton WP, Brody MP. Assessment of older people self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:176–186. [PubMed] [Google Scholar]
- 18.Petersen RC, Smith GE, Waring SC, Ivnic RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 19.Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B. Current concepts in mildcognitive impairment. Arch Neurol. 2001;58(12):1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 20.Pijnenburg YAL, Made Y, Knol DL, van Cappellen van Walsum AM, Knol DL, Scheltens P, Stam CJ. EEG synchronization likelihood in mild cognitive impairment and Alzheimer’s disease during a working memory task. Clin Neurophysiol. 2004;115(6):1332–1339. doi: 10.1016/j.clinph.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 21.Reisberg B. Functional assessment staging (FAST). Psychopharmacal. Bull. 1988;24(4):653–659. [PubMed] [Google Scholar]
- 22.Salthouse TA, Babcock RL. Decomposing adult age difference in working memory. Developmental Psychology. 1991;27(5):763–776. doi: 10.1037/0012-1649.27.5.763. [DOI] [Google Scholar]
- 23.Schürmann M, Başar E. Functional aspects of alpha oscillations in the EEG. Int J Psychophysiol. 2001;39(2-3):151–158. doi: 10.1016/S0167-8760(00)00138-0. [DOI] [PubMed] [Google Scholar]
- 24.Stam CJ. Brain dynamics in theta and alpha frequency bands and working memory performance in humans. Neurosci Lett. 2000;286(2):115–118. doi: 10.1016/S0304-3940(00)01109-5. [DOI] [PubMed] [Google Scholar]
- 25.Sternberg S. Memory-scanning: mental processes revealed by reaction-time experiments. Am Sci. 1969;57(4):421–457. [PubMed] [Google Scholar]
- 26.Suffczynski P, Kalitzin S, Pfurtscheller G, Lopes da Silva FH. Computational model of thalamo-cortical networks: dynamical control of alpha rhythms in relation to focal attention. Int J Psychophysiol. 2001;43(1):25–40. doi: 10.1016/S0167-8760(01)00177-5. [DOI] [PubMed] [Google Scholar]
- 27.Weiss S, Rappelsberger P. Long-range EEG synchronization during word encoding correlates with successful memory performance. Cogn Brain Res. 2000;9(3):299–312. doi: 10.1016/S0926-6410(00)00011-2. [DOI] [PubMed] [Google Scholar]