Abstract
Since the future role of Laboratory Medicine is strongly and equally challenged by economic and new technological pressures, it is essential to take a broad view of the discipline and present to the administrators and other decision-makers the full spectrum of activities and benefits Laboratory Medicine can provide. In particular, the importance and the true impact of Laboratory Medicine can only be achieved by adding value to laboratory tests, represented by their effectiveness in influencing the management of patients and related clinical outcomes.
Introduction
Clinical laboratories represent an area of healthcare that has always undergone major changes because of technological advances and external economic pressures.1 In the recent past, many new diagnostic techniques and laboratory tests have been introduced as a result of both research on the fundamental pathogenesis of diseases and the development of new methods in themselves.
The two Nobel prizes awarded respectively to the inventors of monoclonal antibodies (G. Koehler and C. Milstein, 1984) and the polymerase chain reaction (K.B. Mullis, 1993) are only the more visible tips of a huge iceberg of innovation in the field. Without these techniques, many immunoassays and methods of molecular genetic testing that are currently taken for granted would simply have been impossible. On the other hand, in recent years, significant changes have been made to health care systems and care policy, largely because governments have had to address extremely complex economic issues.2
Experiencing a Paradigm Shift
Reaction on the part of administrators and decision makers to decreased availability of funds has begun on several fronts, and the funding position of clinical laboratories throughout the world is becoming critical. Laboratories are indeed an easy target for economic restrictions and limitations due to their technological characteristics.2 Furthermore, laboratory testing on hospital inpatients usually is reimbursed under a diagnostic-related group (DRG). Under this arrangement, the hospital is paid a fixed rate for a DRG regardless of how many (or how few) tests actually are performed. Reducing laboratory costs will therefore improve the profit margin of the hospital.3
In clinical laboratories, cost savings have frequently been realised by consolidation of laboratory sections with the creation of central core laboratories. Further economies of scale have been sought through regionalisation of laboratory services with the creation of individual laboratories serving different health care facilities.4 In some situations, supposed savings have also been achieved by the addition of automated pre-analytical specimen handling using robotic systems.5 Unfortunately, this “technological” approach to lowering costs per assay has frequently been used to undermine the influence of laboratory professionals and to further isolate them from clinical problems.1 On the other hand, laboratory professionals are usually trained to concentrate on the technical performance and on the achievement and maintenance of the highest quality test results generated in laboratories. Often forgotten is the value of clinical information associated with clinical laboratory testing. But it is clearly not enough to report the right results if such data are not used for patient care. From the patient’s point of view the conversion of data into useful information is the only thing that counts.6 The entire picture requires a general knowledge model that moves from laboratory data to information, into new knowledge to facilitate medical decisions by care givers and, ultimately, the intervention and outcome.7 This integration and understanding is the real challenge faced by laboratory pathologists and scientists in an era when the number of available test parameters have increased enormously and the available funds have significantly decreased. Thus, the survival of Laboratory Medicine in such an environment ultimately depends on the ability to add value to the care of patients. The key to appreciating the importance and the true impact of diagnostic testing can only be achieved if the cost aspects are considered in the wider overall context of health economics and not within the more blinkered area of pure laboratory economics where, almost by definition, every test represents a cost, and its value is outside the scope of the laboratory practice.8
Measuring the Outcome of Laboratory Practice
How can this thinking be applied in Laboratory Medicine? It is clear that the “raison d’être” of laboratories should be assessed only in the context of the impact of their output on clinical services, and the other benefits from the laboratory service. In other words, clinical laboratories have to use outcomes research to be competitive in a changed health-care landscape that is characterised by financial problems, and in the use of a wide variety of medical procedures and technologies.9 Laboratory professionals must now think more globally and perform studies that demonstrate the impact of laboratory tests on overall patient health, the cost of patient care, and other less tangible utilitarian measures, such as quality of life and patient satisfaction.10 Understanding laboratory-related outcomes enables the clinical laboratory to become involved with institutional process improvement, including practice guideline development, redesign of laboratory services, and application of patient satisfaction measures within the organisation.11
Assessment of clinical outcomes in relation to clinical diagnostics is, however, difficult.12 Typical measures in outcomes include morbidity, mortality, quality of life, satisfaction with care, and cost of care, but there are many problems performing outcome studies in Laboratory Medicine, such as the gap between the outcome measures and the biochemical testing.1 Frequently, there is a role for surrogate markers to be used to assess the clinical impact of laboratory practice (Table 1).10 In fact, it is easier and quicker to measure changes in utilisation of resources, such as the length of hospital stay or the number of clinic visits, than it is to assess the years of life gained. These outcomes may not be traditional, but they are valuable, and we should start using them. One of the best examples of a surrogate outcome is glycated haemoglobin (HbA1c), which can be used as a surrogate marker of glycaemic control and for assessing compliance with therapy in diabetic patients.
Table 1.
Types of outcome measures.
| Clinical outcome | Surrogate outcome |
|---|---|
| Mortality | Length of stay |
| Morbidity | Number of clinic visits |
| Quality of life, e.g. quality-adjusted life year (QALY) | Disease markers, e.g. HbA1c, LDL cholesterol |
| Cost of episode | Complication rate |
| Cost of treatment | Readmission rate |
Three levels of laboratory-related patient outcomes have been defined.11 The first-order laboratory outcome is simply the performance of a given test result, in terms of sensitivity and specificity in actual practice. Thus every test has at least four sets of outcomes associated with it; namely, the consequences of a true positive, a true negative, a false positive, and a false negative result. The second-order laboratory outcome is the probability of disease in the patient as estimated by the caregiver receiving the laboratory result; namely, the predictive value of the test as determined using Bayes’ theorem. The third-order laboratory outcome is the actual probability of a change in health status of the patient resulting from any therapeutic interventions either instituted or foregone based on the test result. In the end, all healthcare measures, including laboratory tests, should be judged with respect to their ability to maintain or restore a patient’s health.
Presently, there are good examples of situations where the judicious choice and use of diagnostic testing can significantly reduce the overall costs of treating the patient, accompanied frequently by a better overall clinical outcome for the patient. In certain clinical situations the introduction of new and more effective laboratory tests has influenced the management of patients and related clinical outcomes directly. One example of this is the introduction of cardiac troponin for the diagnosis and treatment of patients with diseases in the spectrum of acute coronary syndrome.13 Cardiac troponin could be the paradigm of the new role of Laboratory Medicine in many diseases.14 As yet, no other clinical information or any other diagnostic test can replace the information provided by the measurement of troponin. Cardiac troponins are presently regarded as the most specific and sensitive of the currently available diagnostic techniques for myocardial damage, and the redefined criteria used to classify acute coronary syndrome patients presenting with ischaemic symptoms as myocardial infarction patients are heavily predicated on an increased concentration of these markers in blood.15 Troponins also are the only markers identifying high-risk coronary patients who should be treated with anti-thrombotic agents, such as glycoprotein IIb/IIIa antagonists, and referred for invasive evaluation at the earliest convenience.16 When compared with the traditional diagnostic approach (elevated CK-MB), troponin is markedly effective in altering patient management by enabling early discharge of patients, resulting in significant cost savings and increasing bed availability. In a British study conducted over six months, the introduction of troponin led to a saving of more than £20,000 to the hospital from fewer bed days and reduced patient episode cost.17 In another study of more than 850 consecutive patients presenting to the emergency department with suspected myocardial infarction who were randomised to receive a standard evaluation with serial electrocardiograms and CK-MB tests (control group) with or without a serial cardiac troponin evaluation, the length of stay was significantly shorter and hospital charges were less for patients who had troponin measurements, with an impressive potential annual saving of about US$4 million.18 Collinson et al. recently showed that 5% of all admissions in their hospital for suspected acute coronary syndrome were incorrectly classified as myocardial infarction using the traditional WHO criteria.19 The potential annual drug cost for treatment of these patients as infarction patients was approximately £56,000, with a 10-year estimated cost close to half a million pounds in wasted resources.19
Another example is represented by the use of B-type natriuretic peptide (BNP) in screening symptomatic patients for left ventricular dysfunction. In a recently published analysis, screening of high risk individuals by BNP before echocardiogram appeared to be more cost-effective than referring all subjects for echocardiography, with a reduction in the cost of screening per detected case of left ventricular systolic dysfunction by 21%.20
In addition to diagnostic problems, clinical laboratories are now increasingly becoming involved in assisting physicians to make therapeutic decisions. For instance, the recently updated guidelines of the U.S. National Cholesterol Education Program for treatment of hypercholesterolaemia in adults are based on well-defined low-density lipoprotein (LDL) cholesterol values, indicating when drug therapy should be initiated and what the treatment goals will be.21 Another example is represented by HbA1c. The clinical use of this marker as a target for more aggressive therapy in order to reduce the development and the progression of retinopathy, nephropathy, and neuropathy in diabetes mellitus patients is now well recognised. But it has recently been reported that HbA1c also predicts mortality in non-diabetic men, with an increasing risk as the concentration increases, even below the commonly used upper reference limit.22 A last example is a recently published study, demonstrating that procalcitonin-guided treatment of lower respiratory tract infections is able to significantly reduce antibiotic use in this type of disease without any compromise in outcome.23 Low serum procalcitonin concentrations identified patients without clinically relevant bacterial infections, in whom antimicrobial therapy can be safely withheld. Thus, in view of the current overuse of antibiotics in acute respiratory tract infections, treatment based on procalcitonin measurement may have important financial and clinical implications. In addition to lower costs, a reduction of antibiotic use also results in fewer side effects and, in the long-term, leads to diminishing drug resistance.
Changing Role for Medical Laboratory Professionals
In order to meet the changing testing needs, the role of the laboratory in patient management should therefore be improved by adding value to laboratory tests derived from appropriate test request and utilisation. This brings us to what the laboratory scientist actually does within his own laboratory. Although it is fundamental that he takes responsibility for how laboratory tests are used for patient care, many people still emphasise the development of analytical expertise at the expense of the application of laboratory science to Medicine. Some reasons can be enumerated to explain this situation: reluctance by laboratory scientists to involve themselves in test structuring and requesting and in the inspection of work as it arrives because it is assumed that all requests are clinically necessary (it is a fact that once blood has been taken and the request has reached the laboratory, it is easier to perform the test than to discuss its suitability with the referrers); poor communication and integration between wards and laboratory, due in part to the uncommunicative attitude of some clinicians to the “service” departments; and, last but not least, the need for an excellent cultural and scientific background for implementing outcome research. This requires the laboratory scientists to have knowledge in a diverse group of medical specialties and organisational and leadership skills that are necessary for functioning successfully in inter-departmental multidisciplinary teams.
On the other hand, physicians who frequently request laboratory tests outside of their field of expertise lack the knowledge base to order the optimal sequence of tests and to correctly interpret the results.24 Conversely, medical laboratory professionals, combining clinical knowledge with experience in the performance of laboratory assays, have the unique expertise to advise their clinical colleagues in regard to the appropriate test selection and interpretation of laboratory results.25 Knowledge of analytical and biological variation and the influence of physiological status and co-morbidities are critical in the interpretation of laboratory results, but many clinicians are unaware of these. For example, the reliability of information derived from a laboratory test may heavily depend on the quality of the analytical performance of the assay being used for the corresponding measurement.
It is well demonstrated that the use of the more sensitive cardiac troponin instead of the traditional criteria for the diagnosis of myocardial infarction leads to an average increase in the number of infarcts diagnosed, from 20 to 30%, in patients admitted with suspected acute coronary syndrome.26 However, the percentage of patients re-categorised from angina to myocardial infarction is also critically dependent on the performance of the troponin assay used.27 Since experimental data indicates that various commercial methods have significantly different sensitivities for detection of cardiac troponin in blood samples with very low concentrations of this biomarker, the selection of the troponin assay by the clinical laboratory represents one of the major factors influencing the clinical performance of this important biomarker.28
Biological variation is frequently the most important source of variability in laboratory measurements. Knowledge of the biological variability is critical to understanding the significance of a laboratory result (Table 2). The importance of the critical difference, also called “reference change value”, is to determine whether changes in an individual’s serial results are really significant. Only by knowing analytical and biological variability is it possible to calculate this figure.29 Laboratories need to put these tools into everyday practice, ensuring that consumers of our services actually use these aids to test interpretation. Recent studies have provided information on the biological variation of BNP and N-terminal proBNP, showing broad fluctuations of their concentrations in the blood of healthy subjects.30 The critical difference for these markers has been calculated as being approximately 70–90%. Therefore, caution should be exercised in interpreting concentration changes of BNP of less than 80% on average as being related to medical therapy. Minor changes could simply be due only to the random fluctuation of the biomarker around the homeostatic set point of the individual and not to the effect of a given therapeutic regimen.31
Table 2.
Practical significance of biological variation: knowledge of the biological variation for the analyte is required in order to answer the following questions.
| What is the significance of this result?
When should I measure it again? Has this result changed significantly over time? Is the performance of the analytical assay appropriate (imprecision, bias)? |
A demonstration of the possible influence of the physiological situation on the clinical value of laboratory tests can be derived from the behaviour of pancreatic amylase in infants and children. Due to the slow development and maturation of some functions of the exocrine pancreas, pancreatic amylase reaches adult concentrations only after the fifth year of life.32 As a consequence, the use of this enzyme for the diagnosis of acute pancreatitis in young children should be avoided, and be replaced with the measurement of pancreatic lipase. Nevertheless, some paediatricians are unaware of this and continue to request an amylase determination in children with acute abdominal pain and suspected acute pancreatitis.33
Co-morbidities are also critical in test interpretation, as in the case of the influence of a reduction in the glomerular filtration rate on blood concentrations of C-telopeptide of type I collagen (CTx), a biomarker of bone resorption.34 Thus, in patients with impaired renal function, measurement of serum CTx needs to be interpreted with great caution. In this type of patient, other serum markers of bone resorption, such as tartrate-resistant acid phosphatase 5b isoform, which is not influenced by renal function, should be considered.35
It is clear from my personal experience that physicians are greatly confused by the amount of information and make many errors in the selection and interpretation of laboratory tests. As an example, Figure 1 displays the results of an audit on the reasons for the request of measurement of bone turnover markers in different clinical departments done in my hospital some years ago. When asked to explain the reason for the test request at the time of ordering, orthopaedic surgeons were unable to formulate sound reasons in all but one case, and the number of profiles decreased from 49 in the three month period before the introduction of the specific request, to only one in the same period the year after the introduction of the justification process. Clearly, the exercise helped to identify misconceptions and ignorance on the use of these types of tests. Other authors have shown that the involvement of laboratory professionals in test selection and interpretation can significantly decrease the likelihood of some types of medical errors.24
Figure 1.
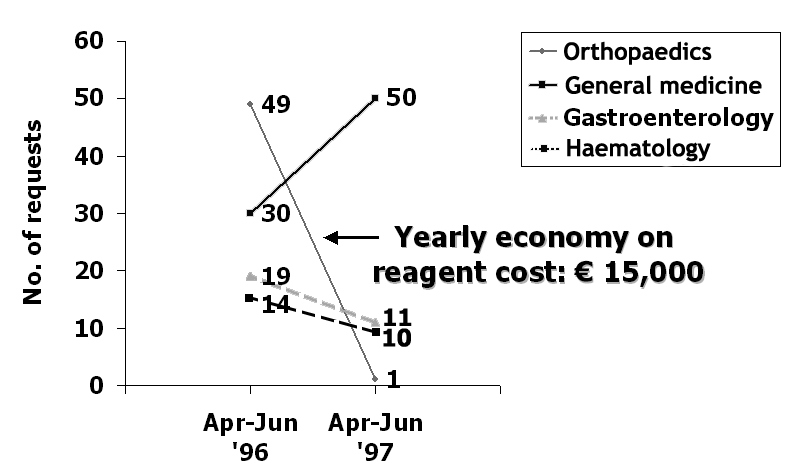
Results of an audit on the reasons of the request for measurement of bone turnover markers. Apr–Jun ’96: number of profiles before the introduction of the specific request; Apr–Jun ’97: number of profiles after the introduction of the specific request.
Promoting the Laboratory-Clinic Interface
The laboratory-clinic interface is, therefore, of fundamental importance to ensure that the patient is given high quality care, because it provides the boundary for the multidisciplinary activities which result in the improvement of the appropriateness of test requests and in the exchange of information on test results.36,37
In order to fill the need for better quality health care, avoidance of medical errors, and cost reduction, three strategies have been recommended for supporting and promoting clinical consultancy in Laboratory Medicine: 1. use of reflex testing and algorithms; 2. providing interpretative comments; and 3. organisation of clinical audits.1
Many examples demonstrate the effectiveness of reflex testing and algorithms for shortening the time of diagnosis and rationalising the use of laboratory testing. The most common example where a cascade of tests is performed based on an abnormal (frequently chance) biochemical finding, is in the case where monoclonal gammopathy is suspected. In this case, an abnormal band found on protein electrophoresis might trigger the performance of immunofixation and monoclonal protein quantitation to confirm the presence of this abnormality. Figure 2 shows another example related to an algorithm proposed for the interpretation of hyperamylasaemia.38 This work-up begins with the measurement of amylase in serum. A high value leads to reflexive testing for pancreatic lipase, followed by serum creatinine or isoamylase assays. The algorithm is able to determine, with a high degree of confidence, if the underlying pathophysiology is due to the presence of acute pancreatitis or of other causes of hyperamylasaemia, such as extra-pancreatic abdominal disorders or renal insufficiency.38
Figure 2.
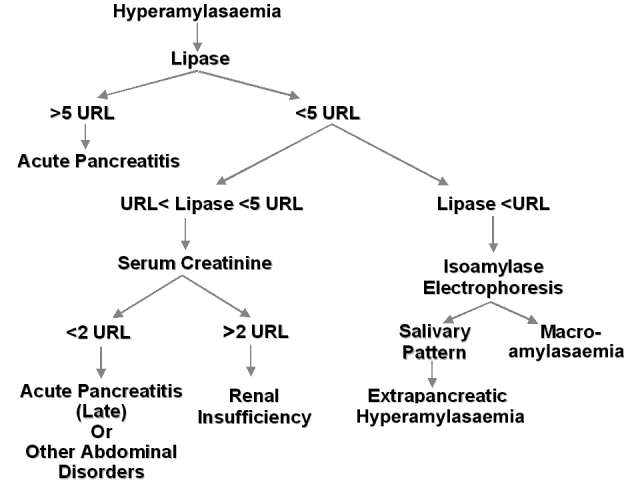
Proposed algorithm for the interpretation of hyperamylasaemia. Adapted from ref. 38. URL, upper reference limit.
The second recommended strategy is to provide a patient-specific comment and, if necessary, graphical interpretation of complex test results in order to allow a more objective utilisation of the data.39 Adding an interpretative comment to the patient’s results and, eventually, giving advice on any action that should be undertaken represents an essential tool for adding value to laboratory reports. An audit of this type of activity in our institution demonstrated the impact of the availability of laboratory-generated interpretative comments on clinical decision making.40 Our investigation showed that comments appeared to be useful to better classify patients with suspected acute coronary syndrome in 70% of cases out of a total of 60 requests of cardiac marker tests. Only in less than 15% of these cases were the laboratory comments fully ignored by the clinicians.40 Similar findings were recently obtained at the Massachusetts General Hospital in Boston.41 We may pose questions on the responsibility of and accountability for these actions, and on potential pitfalls of making a judgement on clinical issues based on the knowledge of biochemical pattern recognition, without necessarily having an insight into the clinical process of patient management.42 However, if we consider that in many cases laboratory investigations should aim to identify a pathophysiological process rather to confirm a diagnosis, I don’t see any problems in a laboratory comment reporting, for instance, “a significant increase of specific cardiac markers consistent with the presence of myocardial necrosis” or “a significant increase of bone resorption markers consistent with increased osteoclast activity”. As laboratory specialists, while assuming responsibility to guarantee reliable laboratory information, we have to educate physicians to accept laboratory results as information describing a pathophysiological process, not a morphological diagnosis.43 Using bone disorders as an example, Jabor and Palicka have well illustrated the issue of the rational and non-rational use of laboratory tests.44 If the clinical question is to make the diagnosis of osteoporosis, the correct test is bone densitometry, which can provide a morphological diagnosis. Conversely, biochemical markers should be used if clinicians need to ascertain any modifications in the activity of osteoblasts and osteoclasts in order to identify alterations of bone turnover, including the effect of appropriate therapies.44
Although the practice of commenting varies among countries, audit findings show that still too few laboratories regularly add interpretative comments to their reports. In a recent national survey performed in the field of cardiac biomarkers, only 9% of participants declared that they performed this type of activity, even when, as in 46% of cases, clinicians required advice from the laboratory, especially for interpretative doubts, or when test results were not consistent with clinical and analytical information.45 The largest barrier to the wide implementation of a program to generate narrative interpretations in the clinical laboratory is probably the lack of a sufficient number of specialists in one laboratory to provide adequate interpretations. A recent report clearly shows the potential negative consequences of using laboratory staff with inadequate expertise for commenting.46
The third pillar of the model system of clinical consulting is clinical audit. Audit in Laboratory Medicine may be defined as a process of review and assessment of laboratory performance.47 It is important that laboratories find out whether they are providing a useful service for the clinicians they serve, in order to ensure that they provide the optimum service to the patient. Once again, this activity requires co-operation with functional areas outside the laboratory, reflecting the real world of medicine: a co-operative venture among medical specialty fields.48 As an example, biochemical protocols for diagnosing and monitoring patients with acute coronary syndrome in our hospital are subjected to constant refinement and, if necessary, to changes in parallel with analytical innovations and new recommendations coming from expert groups.49 The continuous availability of new tests in this field is forcing laboratory professionals and clinicians to revise and compare diagnostic strategies and different protocols to evaluate whether the new tests are to be used in addition to, or instead of, other more traditional tests.50 Our experience shows that the collaboration and co-operation between those with expertise in Cardiology and Laboratory Medicine working in the hospital may permit us to achieve a significant delay reduction through a continuous improvement of the processes, as well as introduction of changes aimed at further improving the results, thus ensuring better patient triage.49
Conclusions
Some years ago, presidents of European Societies of Laboratory Medicine were asked what they considered to be the most relevant issues for the future development of their profession.51 The implementation of request strategies, the diagnostic validation of tests and knowledge of test interpretation were indeed ranked as the most important issues. Today, the complexity of the health-care environment and the availability of an ever expanding array of laboratory tests have further increased the need for more integration between clinical information and laboratory data.6 This is especially true in genetic testing, because it should be performed as an adjunct to the management of the individual and must be used in conjunction with the total information concerning the patient. The impact of the clinical laboratory on the medical environment of the future will be not only to maintain the highest quality generated data and to improve the total quality of the process of providing laboratory information, but also to maximise the influence of the laboratory results on the management of patients. Advances in science and technology will continue to result in the introduction of more complex, expensive, and difficult-to-interpret tests. By integrating pathophysiologic rationale and preferences of the clinicians responsible for the care of the patient with valid and up-to-date clinical research evidence, Laboratory Medicine, supported by computerised information and expert systems, will promote the use of this new knowledge in a timely and responsible manner, contributing to the provision of better care more economically. It is undoubtedly impossible to predict the future, but that does not mean that it is impossible to prepare for it, keeping the best interest of the patient first in mind. As laboratory professionals, we will remain viable only if we build our own future and educate others about the contribution that Laboratory Medicine can and does make to health care.
References
- 1.Plebani M. Charting the course of medical laboratories in a changing environment. Clin Chim Acta. 2002;319:87–100. doi: 10.1016/s0009-8981(02)00028-1. [DOI] [PubMed] [Google Scholar]
- 2.Pansini N. The national health system: future possibilities for the clinical laboratory. Clin Chim Acta. 2002;319:101–5. doi: 10.1016/s0009-8981(02)00029-3. [DOI] [PubMed] [Google Scholar]
- 3.Young DS, Sachais BS, Jefferies LC. Laboratory costs in the context of disease. Clin Chem. 2000;46:967–75. [PubMed] [Google Scholar]
- 4.Burke MD. Laboratory medicine in the 21th century. Am J Clin Pathol. 2000;114:841–6. doi: 10.1309/TH8P-1CAL-9K3G-VFTM. [DOI] [PubMed] [Google Scholar]
- 5.Boyd JC, Felder RA, Savory J. Robotics and the changing face of the clinical laboratory. Clin Chem. 1996;42:1901–10. [PubMed] [Google Scholar]
- 6.Marques MB, McDonald JM. Defining/measuring the value of clinical information. Clin Leadersh Manag Rev. 2000;14:275–9. [PubMed] [Google Scholar]
- 7.Goldschmidt HMJ. Postanalytical factors and their influence on analytical quality specifications. Scand J Clin Lab Invest. 1999;59:551–4. doi: 10.1080/00365519950185337. [DOI] [PubMed] [Google Scholar]
- 8.Marshall DA, O’Brien BJ. Economic evaluation of diagnostic tests. In: Price CP, Christenson RH editors. Evidence-based laboratory medicine. From principles to outcomes, AACC Press, Washington; 2003. pp. 159–86.
- 9.Lundberg GD. The need for an outcomes research agenda for clinical laboratory testing. JAMA. 1998;280:565–6. doi: 10.1001/jama.280.6.565. [DOI] [PubMed] [Google Scholar]
- 10.St. John A, Price CP. Measures of outcome. In: Price CP, Christenson RH editors. Evidence-based laboratory medicine. From principles to outcomes, AACC Press, Washington; 2003. pp. 55–74.
- 11.Bissell MG. Introduction: what’s in a laboratory outcome? In: Bissell MG editor. Laboratory-related measures of patient outcomes: An introduction, AACC Press, Washington; 2000. pp. 3–10.
- 12.Bruns DE. Laboratory-related outcomes in healthcare. Clin Chem. 2001;47:1547–52. [PubMed] [Google Scholar]
- 13.Panteghini M. Acute coronary syndrome. Biochemical strategies in the troponin era. Chest. 2002;122:1428–35. doi: 10.1378/chest.122.4.1428. [DOI] [PubMed] [Google Scholar]
- 14.Panteghini M. Role and importance of biochemical markers in clinical cardiology. Eur Heart J. 2004;25:1187–96. doi: 10.1016/j.ehj.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 15.Jaffe AS, Ravkilde J, Roberts R, et al. It’s time for a change to a troponin standard. Circulation. 2000;102:1216–20. doi: 10.1161/01.cir.102.11.1216. [DOI] [PubMed] [Google Scholar]
- 16.Braunwald E, Antman EM, Beasley JW, et al. ACC/ AHA guidelines for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee on the management of patients with unstable angina) J Am Coll Cardiol. 2000;36:970–1062. doi: 10.1016/s0735-1097(00)00889-5. [DOI] [PubMed] [Google Scholar]
- 17.Owen A, Khan W, Griffiths KD. Troponin T: role in altering patient management and enabling earlier discharge from a district general hospital. Ann Clin Biochem. 2001;38:135–9. doi: 10.1258/0004563011900443. [DOI] [PubMed] [Google Scholar]
- 18.Zarich S, Bradley K, Seymour J, et al. Impact of troponin T determinations on hospital resource utilization and costs in the evaluation of patients with suspected myocardial ischemia. Am J Cardiol. 2001;88:732–6. doi: 10.1016/s0002-9149(01)01842-2. [DOI] [PubMed] [Google Scholar]
- 19.Collinson PO, Rao AC, Canepa-Anson R, Joseph S. Impact of European Society of Cardiology/ American College of Cardiology guidelines on diagnostic classification of patients with suspected acute coronary syndromes. Ann Clin Biochem. 2003;40:156–60. doi: 10.1258/000456303763046085. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen OW, McDonagh TA, Robb SD, Dargie HJ. Retrospective analysis of the cost-effectiveness of using plasma brain natriuretic peptide in screening for left ventricular systolic dysfunction in the general population. J Am Coll Cardiol. 2003;41:113–20. doi: 10.1016/s0735-1097(02)02625-6. [DOI] [PubMed] [Google Scholar]
- 21.Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP). Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult treatment panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 22.Khaw KT, Wareham N, Luben R, et al. Glycated haemoglobin, diabetes, and mortality in men in Norfolk cohort of European Prospective Investigation of Cancer and nutrition (EPIC-Norfolk) Br Med J. 2001;322:15–8. doi: 10.1136/bmj.322.7277.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chist-Crain M, Jaccard-Stolz D, Bingisser R, et al. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster-randomized, single-blinded intervention trial. Lancet. 2004;363:600–7. doi: 10.1016/S0140-6736(04)15591-8. [DOI] [PubMed] [Google Scholar]
- 24.Kratz A, Laposata M. Enhanced clinical consulting – moving toward the core competencies of laboratory professionals. Clin Chim Acta. 2002;319:117–25. doi: 10.1016/s0009-8981(02)00032-3. [DOI] [PubMed] [Google Scholar]
- 25.Price CP, Christenson RH. Teaching evidence-based laboratory medicine: a cultural experience. In: Price CP, Christenson RH editors. Evidence-based laboratory medicine. From principles to outcomes, AACC Press, Washington; 2003. pp. 225–45.
- 26.Koukkunen H, Penttilä K, Kemppainen A, et al. Differences in the diagnosis of myocardial infarction by troponin T compared with clinical and epidemiologic criteria. Am J Cardiol. 2001;88:727–31. doi: 10.1016/s0002-9149(01)01841-0. [DOI] [PubMed] [Google Scholar]
- 27.Ferguson JL, Beckett GJ, Stoddart M, Walker SW, Fox KAA. Myocardial infarction redefined: the new ACC/ESC definition, based on cardiac troponin, increases the apparent incidence of infarction. Heart. 2002;88:343–7. doi: 10.1136/heart.88.4.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panteghini M, Pagani F, Yeo KTJ, et al. Evaluation of imprecision for cardiac troponin assays at low-range concentrations. Clin Chem. 2004;50:327–32. doi: 10.1373/clinchem.2003.026815. [DOI] [PubMed] [Google Scholar]
- 29.Fraser CG, Hyltoft Petersen P. The importance of imprecision. Ann Clin Biochem. 1991;28:207–11. doi: 10.1177/000456329102800301. [DOI] [PubMed] [Google Scholar]
- 30.Wu AHB, Smith A, Wieczorek S, et al. Biological variation for N-terminal pro- and B-type natriuretic peptides and implications for therapeutic monitoring of patients with congestive heart failure. Am J Cardiol. 2003;92:628–31. doi: 10.1016/s0002-9149(03)00741-0. [DOI] [PubMed] [Google Scholar]
- 31.Panteghini M, Clerico A. Understanding the clinical biochemistry of N-terminal pro-B-type natriuretic peptide: the prerequisite for its optimal clinical use. Clin Lab. 2004;50:325–31. [PubMed] [Google Scholar]
- 32.Gillard BK, Simbala JA, Goodglick L. Reference intervals for amylase isoenzymes in serum and plasma of infants and children. Clin Chem. 1983;29:1119–23. [PubMed] [Google Scholar]
- 33.Tikanoja T, Rautiainen P, Lijala M, Svens E, Tikanoja S. Hyperamylasemia after cardiac surgery in infants and children. Intensive Care Med. 1996;22:959–63. doi: 10.1007/BF02044123. [DOI] [PubMed] [Google Scholar]
- 34.Pagani F, Bonetti G, Stefini F, Panteghini M. Evaluation of a fully automated assay to measure C-telopeptide of type I collagen in serum. Clin Chem Lab Med. 2000;38:1111–3. doi: 10.1515/CCLM.2000.166. [DOI] [PubMed] [Google Scholar]
- 35.Pagani F, Boselli C, Panteghini M. Evaluation of an immunoassay specific for serum tartrate-resistant acid phosphatase (sTRAP) 5b isoform, a novel marker of bone turnover. Clin Chem Lab Med. 2003;41(suppl):S87. [Google Scholar]
- 36.Büttner J. Good laboratory practice: the medical aspects. Eur J Clin Chem Clin Biochem. 1997;35:251–6. [PubMed] [Google Scholar]
- 37.Plebani M. The clinical importance of laboratory reasoning. Clin Chim Acta. 1999;280:35–45. doi: 10.1016/s0009-8981(98)00196-x. [DOI] [PubMed] [Google Scholar]
- 38.Panteghini M, Pagani F. Clinical evaluation of an algorithm for the interpretation of hyperamylasemia. Arch Path Lab Med. 1991;115:355–8. [PubMed] [Google Scholar]
- 39.Dighe AS, Soderberg BL, Laposata M. Narrative interpretations for clinical laboratory evaluations. Am J Clin Pathol. 2001;116:S123–8. doi: 10.1309/M6T4-91H2-2KYB-EEK5. [DOI] [PubMed] [Google Scholar]
- 40.Panteghini M, Cuccia C, Pagani F, Bonetti G. Gli “enzimi cardiaci” nell’era delle troponine: cosa salvare. Biochim Clin. 1999;23:378–85. [Google Scholar]
- 41.Laposata M. Patient-specific narrative interpretations of complex clinical laboratory evaluations: who is competent to provide them? Clin Chem. 2004;50:471–2. doi: 10.1373/clinchem.2003.028951. [DOI] [PubMed] [Google Scholar]
- 42.Waise A, Plebani M. Which surrogate marker can be used to assess the effectiveness of the laboratory and its contribution to clinical outcome? Ann Clin Biochem. 2001;38:589–95. doi: 10.1258/0004563011900885. [DOI] [PubMed] [Google Scholar]
- 43.Plebani M. The changing face of clinical laboratories. Clin Chem Lab Med. 1999;37:711–7. doi: 10.1515/CCLM.1999.109. [DOI] [PubMed] [Google Scholar]
- 44.Jabor A, Palicka V. Rational use of clinical chemistry investigations: from diagnoses to processes. Ann Clin Biochem. 1998;35:351–3. doi: 10.1177/000456329803500302. [DOI] [PubMed] [Google Scholar]
- 45.Sciacovelli L, Zardo L, Secchiero S, Zaninotto M, Plebani M. Interpretative comments and reference ranges in EQA programs as a tool for improving laboratory appropriateness and effectiveness. Clin Chim Acta. 2003;333:209–19. doi: 10.1016/s0009-8981(03)00188-8. [DOI] [PubMed] [Google Scholar]
- 46.Lim EM, Sikaris KA, Gill J, et al. Quality assessment of interpretative commenting in clinical chemistry. Clin Chem. 2004;50:632–7. doi: 10.1373/clinchem.2003.024877. [DOI] [PubMed] [Google Scholar]
- 47.Plebani M, Chiozza ML. Audit in laboratory medicine. Eur J Clin Chem Clin Biochem. 1996;34:655–7. [PubMed] [Google Scholar]
- 48.Lewandrowski K. Managing utilization of new diagnostic tests. Clin Leadersh Manag Rev. 2003;17:318–24. [PubMed] [Google Scholar]
- 49.Panteghini M, Pagani F, Bonetti G, Cuccia C. Biochemical algorithms in the troponin era: audit of some care maps one year after their introduction. Biochim Clin. 2000;24:469–75. [Google Scholar]
- 50.Panteghini M. Biochemical markers of cardiac damage: what is current, what is redundant? Biochim Clin. 2000;24:431–8. [Google Scholar]
- 51.Guder WG, Büttner J. Clinical chemistry in laboratory medicine in Europe – Past, present and future challenges. Eur J Clin Chem Clin Biochem. 1997;35:487–94. [PubMed] [Google Scholar]


