Abstract
The synthesis of phospholipids in mammalian cells is regulated by the availability of three critical precursor pools: those of choline, cytidine triphosphate and diacylglycerol. Diacylglycerols containing polyunsaturated fatty acids (PUFAs) apparently are preferentially utilized for phosphatide synthesis. PUFAs are known to play an important role in the development and function of mammalian brains. We therefore studied the effects of unsaturated, monounsaturated and polyunsaturated fatty acids on the overall rates of phospholipid biosynthesis in PC12 rat pheochromocytoma cells. Docosahexaenoic acid (DHA, 22:6n-3), eicosapentaenoic acid (EPA, 20:5n-3) and arachidonic acid (AA, 20:4n-6) all significantly stimulated the incorporation of 14C-choline into total cellular phospholipids. In contrast, monounsaturated oleic acid (OA) and the unsaturated palmitic (PA) and stearic (SA) acids did not have this effect. The action of DHA was concentration-dependent between 5 and 50 μM; it became statistically significant by 3 hrs after DHA treatment and then increased over the ensuing 3 hours. DHA was preferentially incorporated into phosphatidylethanolamine (PE) and phosphatidylserine (PS), while AA predominated in phosphatidylcholine (PC).
Keywords: membrane metabolism, phospholipids, docosahexaenoic acid, eicosapentaenoic acid, arachidonic acid, PC12 cells
1. Introduction
Polyunsaturated fatty acids (PUFAs) are constituents of the phospholipids of mammalian cell membranes, and as such are vitally important in the normal development and optimal functioning of the brain (for reviews, see [1–4]). Vertebrates cannot synthesize PUFAs de novo but rather derive them from metabolic precursor molecules of plant origin. Alternatively, long chain PUFAs (LCPUFAs) with 20 or more carbons can be obtained directly from the animal, and particularly the fish, components of the diet. The membrane phospholipids in brain contain high concentrations of the LCPUFA docosahexaenoic acid (DHA) [5], with highest levels observed in synaptic membranes [6,7], the growth cones of neurites [8,9] and rod outer segments of the retina [10,11]. Clinical and laboratory studies indicate that a number of neurodegenerative diseases involve cell membrane abnormalities which are associated with DHA deficiency [12–15], and that supplementation with DHA may offer protection against neuronal damage and cell death [16–20].
We recently reported in vivo studies showing that repeated administration of DHA can increase brain levels of phosphatides and of certain synaptic proteins, and that these responses are enhanced if animals also receive choline and uridine monophosphate, which raises brain CTP concentrations [21]. In order to characterize the effects of various PUFAs on phospholipid biosynthesis we have used in the present studies undifferentiated, non-transfected PC12 cells. These cells are derived from a rat pheochromocytoma [22] and have been widely used as a model system for studies of neuronal cell metabolism [23–25].
2. Materials and Methods
Cell culture
PC12 cells were obtained from the American Type Culture Collection (Rockville, MD, USA). Powdered nutrient medium DMEM and fetal bovine serum were purchased from Invitrogen (Grand Island, NY, USA) and tissue culture plastic ware from Becton Dickinson (Franklin Lakes, NJ, USA). Fatty acids were obtained from Matreya (Pleasant Gap, PA, USA). Stock solutions (10–300 mM) were prepared in ethanol, aliquoted into brown glass vials, flushed with N2, capped, and frozen at −20°C. Each aliquot was used only once.
Cells were maintained as monolayer cultures at 37°C in a humidified atmosphere of 95% air, 5% CO2 in DMEM supplemented with 10% fetal bovine serum; the medium was replaced at 2-day intervals. For experiments, cells from a homogeneous pool were plated on replicate 35-mm diameter dishes and grown to subconfluence before use. At the start of an experiment nutrient medium was aspirated and the cells were washed twice with warm, sterile PBS. The cells were then incubated for 4 hrs, unless otherwise stated, in 1.5 ml/dish of serum-free DMEM which contained fatty acids at the concentrations indicated as a 4:1 molar complex with fatty acid-free BSA (100 mg/ml). Control cells were exposed to BSA only.
Phospholipid measurements
Phosphatidylcholine synthesis was measured as the incorporation into cell phospholipids of 14C-choline during the final 2 hrs of the standard 4 hr incubation. At the start of this interval the medium was changed to serum- and choline-free DMEM supplemented with 10μM choline, 3 μCi/ml 14C-choline (GE Healthcare, Piscataway, NJ, USA), and the fatty acids being tested. At the end of incubation the medium was aspirated, the cells were washed twice with cold PBS, and scraped into 1 ml of cold methanol. The cells were then sonically disrupted; 100 μl aliquots of the cell suspension were removed for measurement of DNA by the fluorimetric method of Labarca and Paigen [26] using calf thymus DNA as the standard. Phospholipids in the remainder of the cell sonicates were extracted by the sequential addition, with vortexing, of 1.8 ml of cold chloroform and 0.9 ml cold water [27]. The aqueous and organic phases were separated by centrifugation for 15 min. at 3000 rpm. The lower, organic phases were transferred quantitatively to borosilicate tubes, and a 50 μl aliquot of each was counted by liquid scintillation spectrophotometry as a measure of the synthesis of choline-containing phospholipids. The remainder was dried in a rotary evaporator. Individual phospholipids in the dried organic phase were separated by thin layer chromatography on silica gel G plates (Alltech, Deerfield, IL) with a mobile phase of chloroform/ethanol/triethylamine/water (30:34:30:8, by vol.). They were then identified by comparison with authentic standard preparations after spraying the plate with diphenylhexatriene in petroleum ether, and were visualized by long-wave UV light. Individual spots were scraped from the plate and quantitated by phosphate assay [28]. During a 2-hr 14C-choline pulse >95% of the radioactive phospholipid fraction was PC, Sphingomyelin contributed < 0.5%. We therefore took the incorporation of 14C-choline into cell PL as a measure of PC synthesis.
Incorporation of DHA and AA into various phospholipid species was studied by incubating separate groups of dishes in serum-free DMEM containing 30 μM choline and 14C-DHA (American Radiolabeled Chemicals, St. Louis, MO, USA, 0.1 mCi/ml) plus unlabeled DHA at a final concentration of 10 μM, or 3H-AA (Perkin-Elmer Lifescience, Boston, MA, 0.1 mCi/ml) plus unlabeled AA at a final concentration of 10 μM. Extraction, separation and identification of individual phospholipids species was performed as described above. Spots corresponding to PC, PS, PI, and PE were scraped into scintillation vials, eluted with 500 μl methanol and the radioactivity was measured by liquid scintillation spectrophotometry.
Data Analysis
Results were normalized with respect to cell DNA and expressed as means ± SEM of measurements from sextuplicate or quintuplicate dishes. Differences between means were analyzed by ANOVA, followed by Student's t test for the comparison of two means or by Dunnett's test for the comparison between multiple means and their control. Differences were accepted as significant at p<0.05.
3. Results
Fatty acids and phospholipid synthesis
Incubation of PC12 cells with the unsaturated palmitic acid or the monounsaturated oleic acid, both at 20 μM concentration, did not stimulate 14C-choline incorporation into cellular PL, while incubation with LCPUFAs AA and DHA were stimulatory (Fig 1A). In separate experiments the saturated fatty acid stearic acid (20 μM) failed to stimulate PL synthesis, while EPA, an LCPUFA, at the same concentration, was stimulatory (Fig.1B).
Fig. 1. Effect of fatty acids on phospholipid synthesis in PC12 cells.
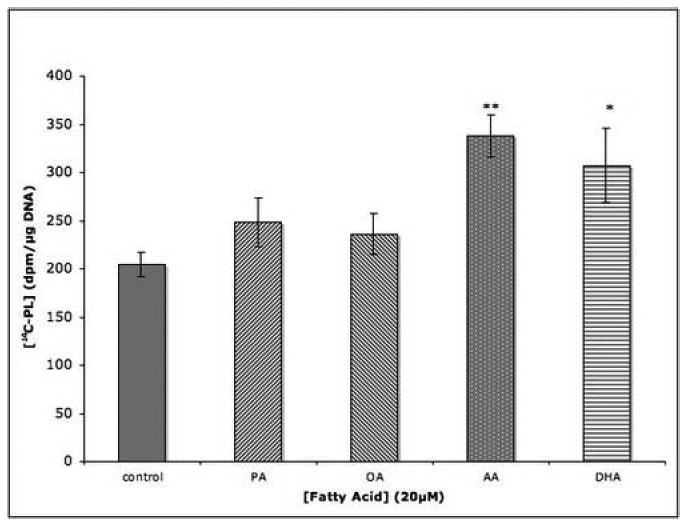
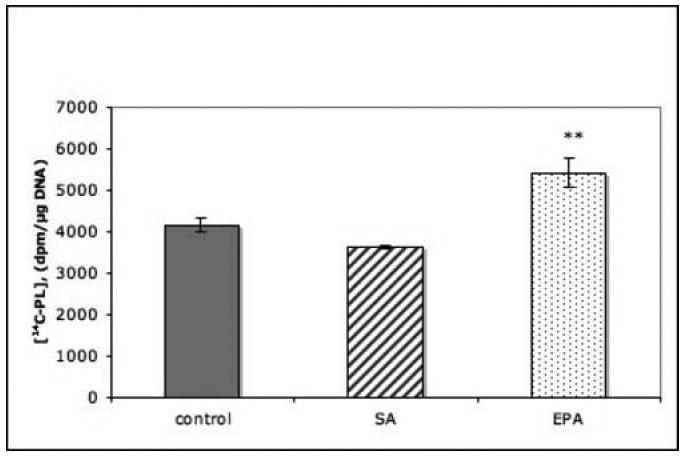
Cells were incubated for 4 hrs with fatty acids (20 μM) and, during the last 2 hrs of incubation, with 14C-choline (3 μCi/ml). Radioactivity incorporated into total cell phospholipids was measured. Data are the means ± SEM of 6 dishes and represent (A) one of 3 or (B) one of 2 independent experiments. *, P<0.05; **, P<0.01.
In order to ascertain that the observed effect of PUFAs was not due to inhibition of PL breakdown, PC12 cells were incubated in the presence of 20μM DHA and the concentration of unlabeled PC was measured. DHA supplementation was indeed found to increase cellular PC levels significantly (Fig.2). This response was concentration-related between 5 and 50 μM DHA (Fig.3). Concentrations greater than 100 μM caused numbers of cells to become detached from the dishes during incubation.
Fig. 2. DHA effect on phosphatidylcholine level in PC12 cells.
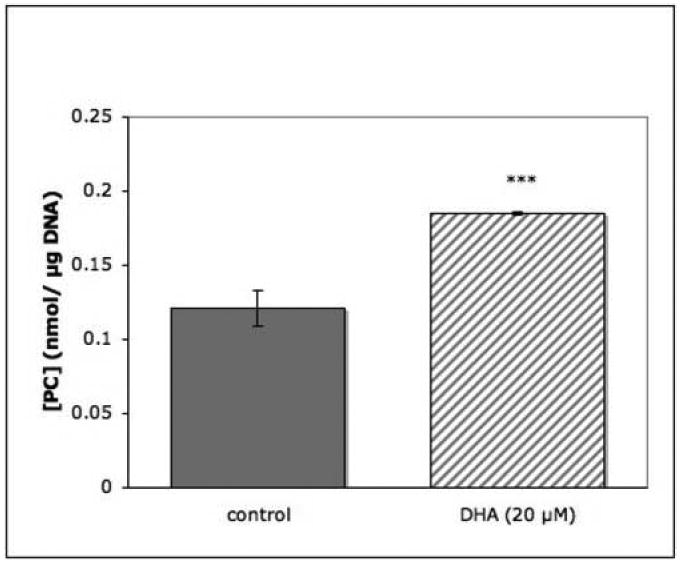
Replicate dishes were incubated for 4 hrs in the absence or presence of 20 μM DHA. Phospholipids were extracted, separated and quantitated as described in Experimental Procedures. Results are shown as the means ± SEM of 6 dishes from one of two experiments. ***, P<0.001.
Fig. 3. DHA dose and phosphatidylcholine synthesis in PC12 cells.
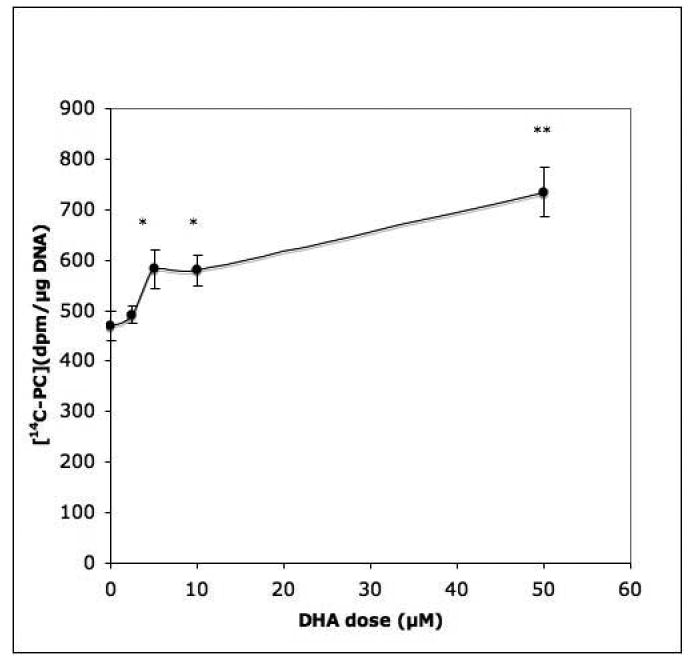
Cells were incubated for 4 hrs with the indicated doses of DHA (2.5, 5, 10, 50 μM). Incorporation of 14C-choline into total phospholipids during the last 2 hrs of incubation was measured. Data show the means ± SEM of 6 dishes, representative of 2 experiments. *, P<0.05; **, P<0.01.
Time course of DHA effect
The accumulation of PL in PC12 cells with time after the addition of 20 μM DHA to the medium is shown in Fig. 4. Because the rates of synthesis during the first hours of incubation were variable for both control and treated cells, results are expressed as ratios of labeled PL in DHA treated/control cells (dpm/μg DNA). Between 2 and 6 hours of incubation, newly synthesized PL accumulated in nearly linear fashion to a significantly greater extent in DHA-treated cells than in the corresponding controls. During these incubation periods in serum-free medium cell viability was maintained as judged by cell attachment or by Trypan Blue exclusion.
Fig. 4. Time course of DHA-stimulated phospholipid synthesis.
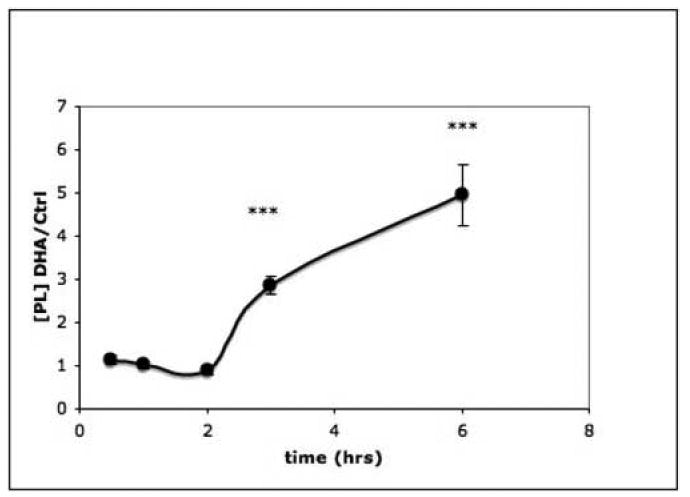
Replicate dishes of PC12 cells were incubated with or without 20 μM DHA for the time periods indicated and incorporation of 14C-choline into phospholipids during a 2 hr period was measured. For incubations less than 2 hrs, the addition of 14C-choline preceded addition of DHA. Results are from 6 dishes/group and are expressed as the ratio of DHA-treated/untreated cells. The measurement in each group is [14C-phospholipid], (dpm/μg DNA). The experiment was repeated twice. ***, P<0.001.
Incorporation of AA and DHA into phospholipids
Parallel groups of dishes were incubated with either 3H-AA or 14C-DHA. Because the specific activity of the radiolabeled molecules was low, the concentrations of unlabeled PUFAs added to the incubation medium were reduced to a final concentration of 10 μM. The concentrations of individual phospholipid esters were determined by phosphate assay from a third group of dishes and the specific activities of radiolabeled AA and DHA incorporated into each were calculated. DHA was preferentially incorporated into PS and PE, while AA incorporation predominated in PC. PI synthesis utilized both DHA and AA to a comparable extent (Fig.5).
Fig. 5. Incorporation of AA and DHA into phospholipid esters.
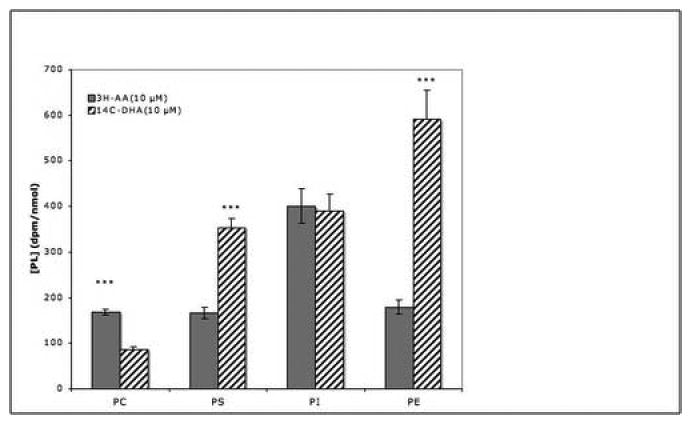
Replicate dishes of PC12 cells were incubated for 3 hrs with 0.1 μCi 3H-AA or 14C-DHA and with 10 μM of the corresponding nonradioactive fatty acids. Phospholipids were extracted, separated by TLC and the radioactivity in individual spots was measured. Quantitites of PC, PS, PI and PE were determined by phosphate analysis. Data are the means ± SEM of 5 dishes and are expressed as dpm 3H or 14C incorporated/nmol PO4. ***, P<0.001 for differences between AA and DHA-treated groups.
4. Discussion
The results presented here show that increasing the availability of such LCPUFAs as AA, EPA and DHA can increase both the synthesis (Figs. 1A and 1B) and levels (Fig. 2) of PC in undifferentiated PC12 cells. This effect is time- and concentration-dependent, and is specific for PUFAs; monounsaturated and saturated FA were ineffective. In contrast to our findings, Pelech et al. observed significant stimulation by oleic acid as well as by PUFAs of choline incorporation into phosphatidylcholine in HeLa cells [29]. This discrepancy may reflect inherent differences between human and rat cells, or perhaps differences in the concentrations of fatty acids used in the two studies (i.e. 1 mM OA cf. our 20 μM).
Previous studies have shown that other circulating PC precursors, specifically choline and cytidine or uridine, also stimulate PC synthesis in slice preparations of rat striatum [30] or in PC12 cells [24] by combining to form CDP-choline, one of the two proximate precursors of PC synthesis [31]. The final step in PC synthesis, catalyzed by CDP choline:1,2-diacylglycerol phosphotransferase [32], attaches CDP-choline to diacylglycerol (DAG). Under conditions where NGF-stimulated neurite outgrowth required new membrane formation, increased availability of DAG also was found to stimulate PC synthesis [33,34]. Because these effects of NGF would confound any stimulation of PC synthesis that might have been caused by PUFAs, our experiments used undifferentiated PC12 cells in defined medium. When gerbils received uridine in the diet and DHA by gavage, significant increases in brain phospholipids and in specific synaptic proteins were observed, while the increases caused by uridine or DHA alone were less pronounced [21].
Due to their hydrophobic nature PUFAs cross the blood/brain barrier and enter membrane-delimited compartments with relative ease, aided by intramembranous long-chain fatty acid transport proteins [35]. In order to sustain the observed intracellular accumulation of LCPUFAs in brain cells, retrograde repartitioning is prevented by the binding of PUFAs to fatty acid binding proteins, followed by their rapid conversion to fatty acid coenzyme A (CoA) derivatives [36]. Kinetic studies of FA uptake by a variety of mammalian cell preparations revealed a saturable process with Km values in the nM range [36,39–41]. This propensity to be saturated at low ligand concentrations necessarily limits intracellular concentrations of FAs. Following their uptake and translocation across the cell membrane the FAs are activated by a family of ACSs, each with particular preferences for various unsaturated, monounsaturated or polyunsaturated FAs, and with substrate Kms in the μM range [36–38,42–44]. It is therefore likely that the ACS enzymes responsible for activating LCPUFAs prior to their incorporation into PLs will be unsaturated and thus responsive to changes in substrate availability.
Differences in the patterns of saturated and unsaturated FAs found in particular phospholipid species have been described [10,23,45,46]. We also found that PC12 cells preferentially incorporated different PUFAs into particular phospholipid species. Thus, AA was incorporated into PC to a greater extent than was DHA, while DHA predominated in PS and, to a marked extent in PE; PI incorporated both FAs (Fig.5). These findings agree with those described in rat and beef brain preparations [47,48]. AA and DHA are incorporated into PI of PC12 cells at almost identical levels while in rat brain AA predominated over DHA by a factor of 4:1; in implanted rat brain tumor the ratio is 2.5:1 [47]. The neoplastic origin of PC12 cells may account for an AA/DHA ratio more closely related to that of tumor tissue than to normal brain.
It has been suggested that cholinephosphotransferase and ethanolaminephosphotransferase may have different substrate specificities with regard to the FA composition of diacylglycerol [45,46], while De Kruyff and his coworkers found no such specificity in rat liver microsomes [49]. The preferential labeling of PS by DHA most likely reflects the synthesis of PS from PE by base-exchange [50]; Holbrook documented a preference of the choline base-exchange enzyme for DHA-containing phospholipids [51]. A study of rat hepatocytes incubated in vitro with fatty acids of various chain length and degrees of unsaturation showed that the composition of diacylglycerol, as well as of the PC and PE derived from it, varied depending on the fatty acid supplied in the incubation medium [46]. We therefore propose that the observed stimulation of PC synthesis in PC12 cells by DHA and other LCPUFAs results from enhanced activation of LCPUFAs by their respective, specific acyl-CoA synthetases [38], their subsequent incorporation into diacylglycerols enriched in LCPUFAs [46], and the utilization of these diacylglycerols in phospholipid synthesis.
Several neurodegenerative diseases are known or suspected to involve the breakdown of membranes, leading to cell death. Conversely, maintenance and expansion of synaptic connections requires the formation of new membrane [8,37,52]. Thus an understanding of how the dynamic requirements for membrane synthesis can be controlled may aid our ability to sustain optimal neuronal function in health and disease. We have now demonstrated that, by increasing the availability of LCPUFAs, PC synthesis and concentration are stimulated in untransfected and undifferentiated PC12 cells, even when these cells are not undergoing NGF-stimulated neurite outgrowth.
Acknowledgments
We thank Mark Vangel for advise with the statistical analysis. This work was supported by grants from NIMH (MH-28783) and from the Center for Brain Sciences and Metabolism Charitable Trust
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lauritzen L, Hansen H, Jorgensen M, Michaelsen K. The essentiality of long chain n-3 fatty acids in relation to development and function of the brain and retina. Progr Lipid Res. 2001;40:1–94. doi: 10.1016/s0163-7827(00)00017-5. [DOI] [PubMed] [Google Scholar]
- 2.McCann JC, Ames BN. Is docosahexaenoic acid, an n-3 long-chain polyunsaturated fatty acid, required for development of normal brain function? An overview of evidence from cognitive and behavioral tests in humans and animals. Am J Clin Nutr. 2005;82:281–295. doi: 10.1093/ajcn.82.2.281. [DOI] [PubMed] [Google Scholar]
- 3.Salvati S, Attorri L, Avellino C, Di Biase A, Sanchez M. Diet, lipids and brain development. Dev Neurosci. 2001;22:481–487. doi: 10.1159/000017479. [DOI] [PubMed] [Google Scholar]
- 4.Wainwright PE. Dietary essential fatty acids and brain function: a developmental perspective on mechanisms. Proc Nutr Soc. 2002;61:61–69. doi: 10.1079/pns2001130. [DOI] [PubMed] [Google Scholar]
- 5.O'Brien JS, Sampson EL. Fatty acid and aldehyde composition of the major brain lipids in normal gray matter, white matter and myelin. J Lipid Res. 1965;4:545–551. [PubMed] [Google Scholar]
- 6.Breckenridge WC, Gombos G, Morgan IG. The lipid composition of adult rat brain synaptosomal membranes. Biochim Biophys Acta. 1972;266:695–707. doi: 10.1016/0006-3002(72)90012-1. [DOI] [PubMed] [Google Scholar]
- 7.Sun GY, Sun AY. Phospholipids and acyl groups of synaptosomal and myelin membranes of squirrel monkey (Saimiri sciureus) Biochim Biophys Acta. 1972;280:306–315. doi: 10.1016/0005-2760(72)90098-7. [DOI] [PubMed] [Google Scholar]
- 8.Martin RE, Bazan NG. Changing fatty acid content of growth cone lipids prior to synaptogenesis. J Neurochem. 1992;59:318–325. doi: 10.1111/j.1471-4159.1992.tb08906.x. [DOI] [PubMed] [Google Scholar]
- 9.Martin RE, Wickham JQ, Om AS, Sanders J, Ceballos N. Uptake and incorporation of docosahexaenoic acid (DHA) into neuronal cell body and neurite/nerve growth cone lipids: evidence of compartmental DHA metabolism in nerve growt factor-differentiated PC12 cells. Neurochem Res. 2000;25:715–723. doi: 10.1023/a:1007575406896. [DOI] [PubMed] [Google Scholar]
- 10.Anderson RE, Sperling L. Lipids of ocular tissues. VII. Positional distribution of the fatty acids in the phospholipids of bovine retina rod outer segments. Arch Biochem Biophys. 1971;144:673–677. doi: 10.1016/0003-9861(71)90374-2. [DOI] [PubMed] [Google Scholar]
- 11.Fliesler AJ, Anderson RE. Chemistry and metabolism of lipids in the vertebrate retina. Prog Lipid Res. 1983;22:79–131. doi: 10.1016/0163-7827(83)90004-8. [DOI] [PubMed] [Google Scholar]
- 12.Conquer JA, Tierney MC, Zecevic J, Bettger WJ, Fisher RH. Fatty acid analysis of blood plasma of patients with Alzheimer's disease, other types of dementia, and cognitive impairment. Lipids. 2000;35:1305–1312. doi: 10.1007/s11745-000-0646-3. [DOI] [PubMed] [Google Scholar]
- 13.Martinez M. Abnormal profiles of polyunsaturated fatty acids in the brain, liver, kidney and retina of patients with peroxisomal disorders. Brain Res. 1992;583:171–182. doi: 10.1016/s0006-8993(10)80021-6. [DOI] [PubMed] [Google Scholar]
- 14.Neuringer M, Connor WE, Lin DS, Barstad L, Luck S. Biochemical and functional effects of prenatal and postnatal omega-3 fatty acid deficiency on retina and brain in rhesus monkeys. Proc Natl Acad Sci USA. 1986;83:4021–4125. doi: 10.1073/pnas.83.11.4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soderberg M, Kristensson ECK, Dallner G. Fatty acid composition of brain phospholipids in aging and in Alzheimer's disease. Lipids. 1991;26:421–425. doi: 10.1007/BF02536067. [DOI] [PubMed] [Google Scholar]
- 16.Lukiw W, Cui J, Marcheselli V, Bodker M, Botkjaer A, Gotlinger K, Serhan C, Bazan N. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;115:2774–2783. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Wilson RS, Aggarwal N, Schneider J. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer Disease. Arch of Neurol. 2003;60:940–946. doi: 10.1001/archneur.60.7.940. [DOI] [PubMed] [Google Scholar]
- 18.Schaefer EJ, Bongard V, Beiser AS, Lamon-Fava S, Robins SJ, Au R, Tucker KL, Kyle DJ, Wilson PW, Wolf PA. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease. Arch Neurol. 2006;63:1545–1550. doi: 10.1001/archneur.63.11.1545. [DOI] [PubMed] [Google Scholar]
- 19.Kim HY, Akbar M, Lau A. Effects of docosahexaenoic acid on neuronal apoptosis. Lipids. 2003;38:453–457. doi: 10.1007/s11745-003-1083-z. [DOI] [PubMed] [Google Scholar]
- 20.Calon F, Lim GP, Yang F, Morihara T, Teter B, Ubeda O, Rostaing P, Triller A, Salem NJ, Ashe KH, Frautschy SA, Cole G. Docosahexaenoic acid protects from dendritic pathology in an Alzheimer's disease mouse model. Neuron. 2004;43:633–645. doi: 10.1016/j.neuron.2004.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wurtman RJ, Ulus IH, Cansev M, Watkins CJ, Wang L, Marzloff G. Synaptic proteins and phospholipids are increased in gerbil brain by administering uridine plus docosahexaenoic acid orally. Brain Res. 2006;1088:83–92. doi: 10.1016/j.brainres.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 22.Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat pheochromocytoma cells which responds to nerve growth factor. Proc Natl Acad Sci U S A. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knapp S, Wurtman R. Enhancement of fatty acid incorporation into phospholipids by choline plus cytidine. Brain Res. 1999;822:52–59. doi: 10.1016/s0006-8993(99)01072-0. [DOI] [PubMed] [Google Scholar]
- 24.Lopez-Coviella I, Wurtman RJ. Enhancement by cytidine of membrane phospholipid synthesis. J Neurochem. 1992;59:338–343. doi: 10.1111/j.1471-4159.1992.tb08909.x. [DOI] [PubMed] [Google Scholar]
- 25.Williams TP, M R., J The effects of membrane fatty acid modification of clonal pheochromocytoma cells on depolarization-dependent exocytosis. J Biol Chem. 1982;257:3491–3500. [PubMed] [Google Scholar]
- 26.Labarca C, Paigen K. A simple, rapid and sensitive DNA assay procedure. Anal Biochem. 1980;102:344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- 27.Folch J, Lees M, Sloane-Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226 [PubMed] [Google Scholar]
- 28.Svanborg A, Svennerholm L. Plasma total lipid, cholesterol, triglycerides, phopholipids and free fatty acids in a healthy scandinavian population. Acta Med Scand. 1961;169:43–49. [Google Scholar]
- 29.Pelech SL, Cook HW, Paddon HB, Vance DE. Membrane-bound CTP:Phosphocholine cytidylyltransferase regulates the rate of phosphatidylcholine synthesis in HeLa cells treated with unsaturated fatty acids. Biochim Biophys Acta. 1984;795:433–440. doi: 10.1016/0005-2760(84)90169-3. [DOI] [PubMed] [Google Scholar]
- 30.Savci V, Wurtman RJ. Effect of cytidine on membrane phospholipid synthesis in rat striatal slices. J Neurochem. 1995;64:378–384. doi: 10.1046/j.1471-4159.1995.64010378.x. [DOI] [PubMed] [Google Scholar]
- 31.Richardson UI, Watkins CJ, Pierre C, Ulus IH, Wurtman RJ. Stimulation of CDP-choline synthesis by uridine and cytidine in PC12 rat pheochromocytoma cells. Brain Res. 2003;971:161–167. doi: 10.1016/s0006-8993(03)02333-3. [DOI] [PubMed] [Google Scholar]
- 32.Kent C. Regulation of phosphatidylcholine biosynthesis. Progr Lipid Res. 1990;29:87–105. doi: 10.1016/0163-7827(90)90010-i. [DOI] [PubMed] [Google Scholar]
- 33.Araki W, Wurtman RJ. Control of membrane phosphatidylcholine biosynthesis by diacylglycerol levels in neuronal cells undergoing neurite outgrowth. Proc Natl Acad Sci, USA. 1997;94:11946–11950. doi: 10.1073/pnas.94.22.11946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J, Wurtman RJ. Nerve growth factor stimulates diacylglycerol de novo synthesis and phosphatidylinositol hydrolysis in pheochromocytoma cells. Brain Res. 1998;803:44–53. doi: 10.1016/s0006-8993(98)00595-2. [DOI] [PubMed] [Google Scholar]
- 35.Hajri T, Abumrad NA. Fatty acid transport across membranes: relevance to nutrition and metabolic pathology. Annu Rev Nutr. 2002;22:383–415. doi: 10.1146/annurev.nutr.22.020402.130846. [DOI] [PubMed] [Google Scholar]
- 36.Schmelter T, Trigatti BL, Gerber GE, Mangroo D. Biochemical demonstration of the involvement of fatty acyl-CoA synthetase in fatty acid translocation across the plasma membrane. J Biol Chem. 2004;279:24163–24170. doi: 10.1074/jbc.M313632200. [DOI] [PubMed] [Google Scholar]
- 37.Marszalek J, Kitidis C, Dararutana A, Lodish H. Acyl-CoA synthetase 2 overexpression enhances fatty acid internalization and neurite outgrowth. J Biol Chem. 2004;279:23882–23891. doi: 10.1074/jbc.M313460200. [DOI] [PubMed] [Google Scholar]
- 38.Marszalek J, Kitidis C, Dirusso CC, Lodish H. Long-chain acyl CoA synthetase 6 preferentially promotes DHA metabolism. J Biol Chem. 2005;280:10817–10826. doi: 10.1074/jbc.M411750200. [DOI] [PubMed] [Google Scholar]
- 39.Bonen A, Luiken JJ, Arumugam Y, Glatz JF, Tandon NN. Acute regulation of fatty acid uptake involves the cellular redistribution of fatty acid translocase. J Biol Chem. 2000;275:14501–14508. doi: 10.1074/jbc.275.19.14501. [DOI] [PubMed] [Google Scholar]
- 40.Ibrahimi A, Sfeir Z, Magharaie H, Amri EZ, Grimaldi P, Abumrad NA. Expression of the CD36 homolog (FAT) in fibroblast cells: Effects on fatty acid transport. Proc Natl Acad Sci U S A. 1996;93:2646–2651. doi: 10.1073/pnas.93.7.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stremmel W, Pohl L, Ring A, Herrman T. A new concept of cellular uptake and intracellular trafficking of long-chain fatty acids. Lipids. 2001;36:981–989. doi: 10.1007/s11745-001-0809-2. [DOI] [PubMed] [Google Scholar]
- 42.Fujino T, Kang MJ, Suzuki H, Iijima H, Yamamoto T. Molecular characterization and expression of rat Acyl-CoA synthetase 3. J Biol Chem. 1996;271:16748–16752. doi: 10.1074/jbc.271.28.16748. [DOI] [PubMed] [Google Scholar]
- 43.Li J, Wurtman RJ. Heterogeneous long chain Acyl-CoA synthetases control distribution of individual fatty acids in newly-formed glycerolipids of neuronal cells undergoing neurite outgrowth. Neurochem Res. 1999;24:739–750. doi: 10.1023/a:1020727429052. [DOI] [PubMed] [Google Scholar]
- 44.Iijima H, Fujino T, Minekura H, Suzuki H, Kang MJ, Yamamoto T. Biochemical studies of two rat acyl-CoA synthetases, ACS1 and ACS2. Eur J Biochem. 1996;242:186–190. doi: 10.1111/j.1432-1033.1996.0186r.x. [DOI] [PubMed] [Google Scholar]
- 45.Radominska-Pyrek A, Stosznajder J, Dabrowiecki Z, Chojnacki T, Horrocks LA. Effects of free fatty acids on the enzymic synthesis of diacyl and ether types of choline and ethanolamine phosphoglycerides. J Lipid Res. 1976;17:657–662. [PubMed] [Google Scholar]
- 46.Sundler R, Akesson B, Nilsson A. Effect of different fatty acids on glycerolipid synthesis in isolated rat hepatocytes. J Biol Chem. 1974;249:5102–5107. [PubMed] [Google Scholar]
- 47.Nariai T, DeGeorge JJ, Greig NH, Genka S, Rapoport SI, Purdon AD. Differences in rates of incorporation of intravenously injected radiolabeled fatty acids into phospholipids on intracereberally implanted tumor and brain in awake rats. Clin Exp Metastasis. 1994;12:213–225. doi: 10.1007/BF01753889. [DOI] [PubMed] [Google Scholar]
- 48.Yabuuchi H, O'Brien JS. Positional distributions of fatty acids in glycerophosphatides of bovine gray matter. J Lipid Res. 1968;9:65–67. [PubMed] [Google Scholar]
- 49.De Kruyff B, Van Golde LMG, Van Deenen LLM. Utilization of dicacylglycerol species by choline phosphotransferase, ethanolaminephosphotransferase and diacylglycerol acyltransferase in rat liver microsomes. Biochim Biophys Acta. 1970;210:425–435. doi: 10.1016/0005-2760(70)90038-x. [DOI] [PubMed] [Google Scholar]
- 50.Borkenhagen LF, Kennedy EP, Fielding L. Enzymatic formation and decarboxylation of phosphatidylserine. J Biol Chem. 1961;236:PC28–30. [Google Scholar]
- 51.Holbrook PG, Wurtman R. Calcium-dependent incorporation of choline into phosphatidylcholine (PC) by base-exchange in rat brain membranes occurs preferentially with phospholipid substrates containing docosahexaenoic acid (22:6(n-3)) Biochim Biophys Acta. 1990;1046:185–188. doi: 10.1016/0005-2760(90)90187-3. [DOI] [PubMed] [Google Scholar]
- 52.Darios F, Davletov B. Omega-3 and omega-6 fatty acids stimulate cell membrane expansion by acting on syntaxin 3. Nature. 2006;440:813–817. doi: 10.1038/nature04598. [DOI] [PubMed] [Google Scholar]


