Abstract
The optical transitions of a chiral, three-substituted polythiophene with an amino acid function can be tuned by interactions with synthetic peptides. The addition of a positively charged peptide with a random-coil formation will force the polymer to adopt a nonplanar conformation, and the intensity of the emitted light is increased and blue-shifted. After the addition of a negatively charged peptide with a random-coil conformation, the backbone of the polymer adopts a planar conformation and an aggregation of the polymer chains occurs, seen as a red shift and a decrease of the intensity of the emitted light. By adding the positively charged peptide designed to form a four-helix bundle with the negatively charged peptide, the polymer aggregates are disrupted and the intensity of the emitted light is increased because of separation of the polymer chains. This technique could be used as a platform for making novel sensors and biomolecular switches.
Conjugated polymers (CPs) that are capable of continuously and selectively detecting biomolecular interactions have large potential for being used as biosensors and in molecular electronics. In particular, CPs such as polythiophene and polypyrrole can be used to couple analyte-receptor interactions as well as nonspecific interactions into observable responses (1-8). CP-based sensors are very sensitive to very minor perturbations due to amplification by a collective system response and offer a key advantage compared with small molecule elements (9, 10). By making conjugated polyelectrolytes, the CPs can be used as detecting elements for biological molecules in an aqueous environment, as recently used to detect biomolecules by their impact on the conditions for photoinduced charge or excitation transfer (11-13). Conjugated polyelectrolytes offer possibilities for very sensitive luminescence measurements, and these materials may become ubiquitous for biosensors in the future if luminescence is used to track biospecific interactions.
The physical and chemical properties of polythiophenes can be modified by the introduction of suitable side chains in the 3 position. Polythiophene derivatives that display biotin (1, 14) and different carbohydrates (3) have been synthesized and shown to undergo colorimetric transitions in response to binding of streptavidin and different types of bacteria and viruses, respectively. The chromic transitions, from deep violet (absorption maximum ≈550 nm) to bright yellow (absorption maximum near 400 nm), are related to a planar-to-nonplanar (from highly conjugated to less conjugated) conformational transition of the backbone. The highly conjugated form is believed to be associated with intermolecular and intramolecular (through chain folding) aggregates. Molecular forces influencing the conformational change of the polymer backbone are hydrogen bonding and nonpolar and electrostatic interactions, leading to aggregation, gelation, or crystallization (15, 16).
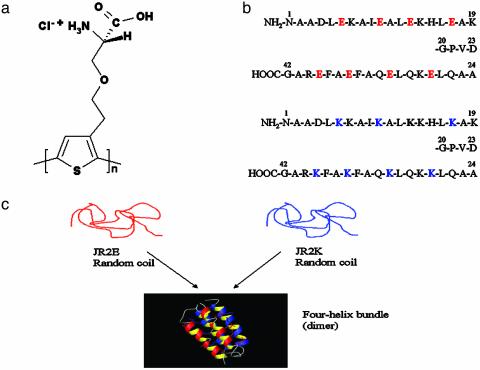
Previous studies (17-19) of the first polythiophene carrying an amino acid side chain, poly{3-[(S)-5-amino-5-carboxyl-3-oxapentyl]-2,5-thiophenylene hydrochloride} (POWT) (Fig. 1), have shown interesting optical phenomena due to different interactions within the polymer chain and between adjacent polymer chains. These interactions cause different conformational transitions of the polymer backbone and aggregation of the polymer chains. The functional groups of the zwitterionic side chain, charged anionic or cationic at different pH values, make this polythiophene derivative suitable for forming polyelectrolyte complexes with negatively or positively charged oligomers and polymers. In addition, the zwitterionic groups are able to create versatile hydrogen-bonding patterns with different molecules, thus offering a new route for CP-biopolymer interactions.
Fig. 1.
(a) Chemical structure of the repeating unit of POWT. (b) Amino acid sequences for JR2E (Upper) and JR2K (Lower). (c) Schematic drawing of the conformational changes of the synthetic peptides JR2E and JR2K. Neither JR2E nor JR2K form homodimers because of electrostatic repulsion; they only form the heterodimer.
Conformational alterations of biomolecules are very important in biological systems, forming part of the chain of interactions leading to signal transduction. Although redox processes in biomacromolecules have been wired through classical electronic conductors (metals and semiconductors) (20), polymer bioelectronics may include the possibility to extend beyond the collection of charge from redox processes in biological systems. The conformational flexibility of polymers, also found in CPs, allows direct connection between the geometry of chains and the resulting electronic structure and processes. This does not necessarily require charge transfer but requires that the CP chain geometry will be governed by biomolecules. It would be of great interest to use dominant biomolecules, forcing the geometry of CPs. If conformational changes of biomolecules could lead to different conformations of the polymer backbone, an alteration of the absorption and emission properties from the polymer would be observed. This could therefore be used as a platform for making novel sensors and biomolecular switches. In this article we report conformational transitions of a water-soluble, zwitterionic, electroactive, and photoactive polythiophene derivative induced by noncovalent coupling of synthetic peptides designed (21, 22) to adopt alternative conformations.
Experimental Procedures
Polymer and Peptide Synthesis. The synthesis of POWT was reported elsewhere (17). The peptides JR2E and JR2K (Fig. 1) were synthesized with a PerSeptive Biosystems (Framingham, MA) Pioneer automated peptide synthesizer by using a standard fluorenylmethoxycarbonyl (Fmoc) chemistry protocol and Fmoc-Gly-polyethylene glycol-polystyrene resin. After synthesis, the peptide resins were washed with methylene chloride and dried under vacuum for 2 h. The peptides were cleaved from the polymer and deprotected with 19 ml of trifluoroacetic acid, 0.5 ml of triisopropylsilane, and 0.5 ml of H2O per gram of polymer for 3 h at room temperature, precipitated by cold diethyl ether, and lyophilized. The peptides were purified by reversed-phase HPLC on a semipreparative C-8 Hichrome column. JR2Es were eluted isocratically with 34% 2-propanole in 0.1% trifluoroacetic acid at a flow rate of 10 ml/min, and JR2Ks were eluted isocratically with 29% 2-propanole in 0.1% trifluoroacetic acid at a flow rate of 10 ml/min. The purity was checked by analytical HPLC, and the peptides were identified by matrix-assisted laser desorption ionization/time-of-flight MS.
Optical Measurements. A stock solution containing 3.7 mg·ml-1 POWT in deionized water was prepared. Ten microliters of the polymer solution was mixed with 10 or 20 μl of the negative peptide or the positive peptide solution (2.2 mg·ml-1), respectively, and diluted with deionized water to a final volume of 300 μl. After 15 min of incubation, the samples were diluted with a stock buffer solution (sodium phosphate, pH 7.4) and 10 μl of deionized water or 10 μl of the positive/negative peptide solution (2.2 mg·ml-1) to a final volume of 2,000 μl containing 20 mM sodium phosphate. The samples were incubated for 10 min in room temperature, and the emission spectra were recorded with an ISA Jobin Yvon (Longjumeau, France) Spex FluoroMax-2 apparatus. All the spectra were recorded with excitation at 400 nm. The CD spectra were recorded with an ISA Jobin Yvon CD6 (5-mm quartz cell). A Perkin-Elmer Lambda 9 UV/VIS/NIR spectrophotometer was used for the absorption measurements.
NMR Measurements. NMR spectra were recorded by using a Varian Inova 600-MHz spectrometer. 1H NMR spectra were recorded in 2H2O solution at 298 K by using peptide concentrations of ≈1 mM and a polymer concentration of ≈2.4 mg·ml-1. The pH (7.4) was adjusted with NaOD and DCl and measured directly in the NMR tube.
Results and Discussion
Absorption and Emission Measurements. The emission spectrum of POWT (170 nmol on a monomer basis) after 10 min of incubation in a buffer solution (20 mM sodium phosphate, pH 7.4) is shown in Fig. 2, and the absorption maximum for the same solution is presented in Table 1. A previous study (19) of POWT has shown that alkaline pH will lead to planarization of the polymer backbone and aggregation of the polymer chains. An absorption maximum of 438 nm is related to a certain degree of planarization of the polymer backbone. The emission peak at 540 nm is associated with an intrachain event and has been seen previously for solutions where the polymer adopts a nonplanar helical conformation (18, 19). The peak at 600 nm and the shoulder at longer wavelengths are due to planarization of the polymer backbone. The intensity of the emitted light at longer wavelength is also decreased because of aggregation of the polymer chains (19).
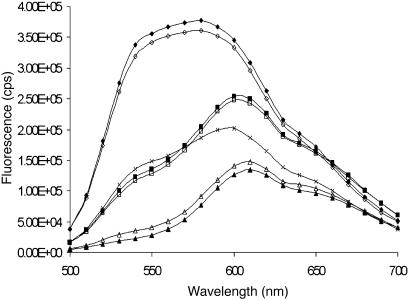
Fig. 2.
Emission spectra of 100 nmol of POWT (on a monomer basis) (×) with 1.0 equivalent (on a monomer basis) of JR2K (⋄), 1.0 equivalent of JR2E (▵), 0.5 equivalents of JR2E plus an addition of 0.5 equivalents of JR2K (□), 0.5 equivalents of JR2K plus an addition of 0.5 equivalents of JR2E (▪), 2.0 equivalents of JR2K (♦), and 2.0 equivalents of JR2E (▴) after 10 min of incubation in a 20 mM sodium phosphate buffer, pH 7.4. All the emission spectra were recorded with excitation at 400 nm.
Table 1. Absorption maximum and the ratio of the intensity of the emitted light at 540:610 nm and 540:670 nm for POWT and POWT-peptide complexes.
| Absorption maximum, nm | Ratio of the intensity of the emitted light at 540/610 nm | Ratio of the intensity of the emitted light at 540/670 nm | |
|---|---|---|---|
| POWT | 438 | 0.72 | 1.63 |
| POWT + JR2E | 451 | 0.24 | 0.44 |
| POWT + JR2K | 419 | 1.08 | 2.88 |
| POWT + JR2E + JR2K | 440 | 0.49 | 0.97 |
After the addition of 1.0 or 2.0 equivalents (on a monomer basis) of a negatively charged peptide (JR2E) (Fig. 1), the intensity of the emitted light is decreased further, and the emission is slightly red-shifted. Interestingly, the peak at 540 nm decreases dramatically, and a peak at 610 nm with less intensity occurs because of planarization of the polymer backbone and interchain events (Fig. 2). These interchain events have been observed earlier in solid solutions of POWT (18) and will reduce the fluorescence quantum yield from the single-chain level of 26% (observed in dilute methanol solutions) to 4% in the solid polymer because of nonradiative deexcitation. This new channel for deexcitation is created because of contact between polymer chains. Hence, the interaction between JR2E and POWT will force the polymer chains to aggregate. We suggest that the negatively charged glutamate (E) groups of the peptide most likely will interact electrostatically with the positive amino groups of the polymer side chains. The internal interactions between the amino and carboxyl groups are disrupted, leading to a planarization of the polymer backbone. Other amino acids and the peptide backbone are subsequently able to form hydrogen bonds with the amino and carboxyl groups of a nearby polymer chain, and aggregation of the polymer chains occurs. The absorption maximum of the POWT/JR2E solution (Table 1) is also red-shifted compared with the pure polymer solution, indicating that the polymer backbone adopts a more rod-shaped conformation due to the interactions with JR2E.
The addition of 1.0 or 2.0 equivalents (on a monomer basis) of a positively charged peptide (JR2K) (Fig. 1) will induce a more twisted conformation of the polymer backbone, seen as a shift of the emitted light to shorter wavelengths (Fig. 2) and a shift of the absorption maximum to a shorter wavelength (Table 1). The intensity of the emitted light is also increased because of separation of the polymer chains. The positively charged lysine (K) residues of the peptide most likely will interact with the negatively charged carboxyl groups of the polymer side chains and thereby force the polymer to adopt a nonplanar conformation. A similar nonplanar conformation has been seen earlier for the polymer in deionized water and in buffer aqueous solutions with pH equal to pI for serine (19).
The addition of equal amounts (1.0 equivalent on a monomer basis) of JR2K to the JR2E-POWT (1:1 dilution on a monomer basis) complex will disrupt the aggregates, seen as a shift of the emission peak back to shorter wavelengths and an increased intensity of the emitted light, both at 540 and 610 nm (Fig. 2). The absorption maximum (Table 1) is also shifted to a shorter wavelength, thus the polymer backbone adopts a more twisted conformation after formation of the new complex. Because the synthetic peptides have been designed to form a four-helix bundle (21, 22), the negatively charged glutamate groups of JR2E will interact with the positively charged lysine groups of JR2K rather than the amino groups of the polymer side chains. Consequently, the interactions responsible for the aggregation of the POWT chains are disrupted. The zwitterionic polymer side chains are now able to form hydrophobic interactions, electrostatic interactions, or hydrogen bonding with the four-helix bundle or adjacent polymer chains. The difference in the emission spectra for the pure POWT solution and the POWT/peptides mixture indicates that the polymer is in fact interacting with the four-helix bundle and a new complex between the polymer and the peptides is being formed. Interestingly, the addition of JR2E to the POWT-JR2K complex will give a similar spectrum (Fig. 2).
Taking the ratio of the intensity of the emitted light at 540:610 nm (Table 1) clearly demonstrates that the geometry of the polymer chains and the optical processes can be tuned differently depending on the peptide being used. This might offer a new route for creating materials for photonic devices. The peptides can be used to increase the quantum efficiency by separation of the polymer chains, and alteration of the amino acid sequence of the peptide offers a possibility to tune the wavelength of the light being emitted. The addition of a polypeptide most likely will decrease the charge transport in the bulk in light emitting diodes. However, it may be acceptable for the design of stimulated emission in photonic devices.
An earlier study (18) of POWT has shown that the emission maximum for the intrachain and interchain events in thin polymer films was 565 and 670 nm, respectively. The ratio of the intensity of emitted light at 540 nm (the intrachain event with a helical backbone) and 670 nm (the interchain event with a planar backbone) is influenced even more by the different peptides (Table 1), indicating that the net charge and conformation of the peptide will alter the intra- and interchain processes in the polymer. This phenomenon has also been used for the detection of single nucleotide polymorphism in oligonucleotides (23) and offers a new route for the detection of biospecific interactions.
CD Measurement. As reported previously (17, 19), the polymer exhibits a split-type induced CD (ICD) in the π-π* transition region. The ICD is probably a result of main-chain chirality such as a predominantly one-handed helical structure induced by the zwitterionic group of the polymer side chain (19, 24, 25). The shape and sign of the ICD pattern are characteristic of a right-handed helical form of polythiophene (26, 27).
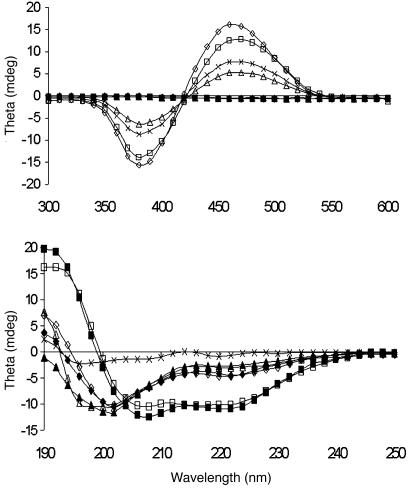
The addition of 2.0 equivalents (on a monomer basis) of JR2E will force the polymer backbone to adopt a more planar conformation, seen as a decreased intensity of the CD signal compared with the pure polymer solution (Fig. 3). The CD spectrum in the far-UV region (Fig. 3) shows that JR2E has a slightly altered conformation if the polymer is present in the solution. The CD signal becomes more positive around 190-195 nm; a new strong negative peak at 197 nm and new weaker negative peak at 221 nm occurs. Although the spectrum is slightly different, probably because of an interaction between the peptide and the polymer, no evidence for an ordered structure such as helix or β-sheets can be found.
Fig. 3.
CD spectra of 170 nmol of POWT (on a monomer basis) (×) with 2.0 equivalents of JR2E (▵), 2.0 equivalents of JR2K (⋄), and 1.0 equivalent of JR2E plus an addition of 1.0 equivalent of JR2K (□) after 10 min of incubation in a 20 mM sodium phosphate buffer, pH 7.4. CD spectra of the peptide solutions, without the polymer and incubated under the same conditions, are marked with filled symbols.
After the addition of 2.0 equivalents (on a monomer basis) of JR2K, the ICD signal from the polymer is increased and slightly shifted to shorter wavelength (Fig. 3). Hence, the polymer backbone adopts a nonplanar conformation with a high helicity due to interactions between the polymer side chains and JR2K. This observation agrees with the results from the absorption measurements (Table 1) and has been seen previously for a neutrally charged POWT (19). The peptide has a positive CD signal around 190-195 nm, a strong negative peak at 202 nm, and a weaker negative peak at 223 nm, indicative of a random coil or a β-like structure. The conformation of JR2K is similar whether the polymer is present or not, because the spectrum in the far-UV region is altered just slightly. The spectrum for JR2K is slightly different than the one obtained for JR2E, indicating that the peptides adopt alternative conformations.
As mentioned above, the JR2E and JR2K sequences were designed to form a heterodimeric four-helix bundle motif. The CD spectra of JR2E, JR2K, and an equimolar mixture of JR2E and JR2K are shown in Fig. 3. The strong positive CD signal around 190-195 nm and the strong negative peaks at 208 and 222 nm in the spectrum of the equimolar mixture are indicative of a helical structure and thus of heterodimer formation. In contrast, the weak CD signals of the single-peptide solutions demonstrate little helicity and little dimer formation, in agreement with the design.
The spectrum in the far-UV region for the JR2E-JR2K-POWT complex (Fig. 3) is altered just slightly and has the same features indicative of a helical conformation. The ratio of the mean residue ellipticities at 222 and 208 nm is considered to be a measure of helical shape, and the change in ratio observed for the heterodimer suggests that there is a transition from α-helix to 310-helix after binding the polymer. Interestingly, the ICD signal of the polymer is increasing compared with the polymer in pure buffer solution, suggesting that the polymer adopts a more helical conformation. These observations disagree with the results obtained from the absorption measurements (Table 1). A red shift of the absorption maximum (4 nm) is normally related to a nonplanar-to-planar (from less conjugated to more conjugated) conformational transition of the backbone. An explanation for this observation could be the longer measurement time needed for the CD experiments. The polymer has not reached equilibrium after 10 min of incubation in the buffered solution, and the backbone will be more planar and the polymer chains will aggregate more with time. For instance, after 2 h of incubation the emission peak at 540 nm has disappeared almost completely and the peak at longer wavelengths is dominating, and after several hours the polymer starts to precipitate (data not shown). Hence, the weaker CD signal for the polymer in pure buffer solution can be a result of increased aggregation and planarization of the polymer chains with time. In the polymer/peptide solutions the backbone conformations reach equilibrium much faster, and no shift of the emission peaks can be seen with increasing incubation time. No precipitation can be seen in the POWT/JR2K or the POWT/JR2E/JR2K solutions after several days of incubation, but the POWT-JR2E complex will precipitate out of the solution after a couple of hours. These observations are in good agreement with the results from the optical studies.
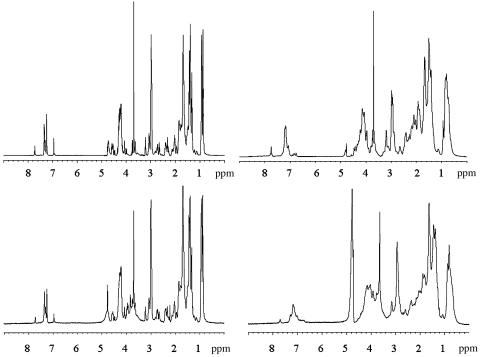
NMR Measurements. NMR spectroscopic experiments might shed some light on the nature of the structural effects of polypeptide polymer interactions. 1H NMR spectra are excellent qualitative probes of molecular interactions, because line widths and chemical shifts are sensitive to molecular environmental changes and to changes in rotational correlation time (28). The spectrum of JR2K (Fig. 4) is typical of a poorly ordered polypeptide with poor shift dispersion and narrow resonances due to sampling of a large number of conformations at a rate that is fast on the NMR time scale. The mixture of JR2K and JR2E gives rise to severe line broadening, because a four-helix bundle is formed that is more ordered and sampled at an intermediate rate on the NMR time scale. The shift dispersion is also increased, because the molecular environment is better defined in the more ordered structure. Interactions between JR2K and POWT are unequivocally established by the observation of line broadening and chemical-shift changes, and the severe broadening of the spectrum of the heterodimer in the presence of POWT establishes clearly that the bundle and POWT interact in a qualitative sense. High-resolution structures cannot be obtained because of the dynamic nature of the complex. Unfortunately, no 1H NMR data could be obtained for the POWT-JR2E complex. At the high concentrations required for the experiment, the POWT-JR2E complex precipitates almost immediately. Interestingly, the remaining solution did not show any NMR signals from the peptide or the polymer, clearly showing that the interactions between POWT and JR2E will force the polymer chains to aggregate.
Fig. 4.
1H NMR spectra (600 MHz) of JR2K (Upper Left), JR2E/JR2K (Upper Right), JR2K/POWT (Lower Left), and JR2E/JR2K/POWT (Lower Right) in 2H2O at pH 7.4 and 25°C.
Conclusions
We have shown that the backbone conformation, the separation and aggregation of different polymer chains of a conjugated polythiophene with a free amino acid side chain, can be switched by conformational changes in synthetic peptides. The addition of a positively charged peptide with a random-coil formation will force the polymer to adopt a nonplanar conformation, and the intensity of the emitted light is increased and blue-shifted. After the addition of a negatively charged peptide, with a random coil conformation, the polymer backbone adopts a planar conformation and the polymer chains aggregates. By adding the positively charged peptide that forms a four-helix bundle with the negatively charged peptide, the polymer chains are separated and the polymer backbone adopts a more helical conformation. The molecular complexes being formed clearly affect the conformation of the polymer backbone and the interactions between adjacent polymer chains. This has thus far been detected by optical measurement, but electrical detection of these interactions most likely will be possible. Because conformational alterations and net charge alterations (e.g., phosphorylation) of biomolecules are used as communication tools in biological systems, the discovery of a system that is able to mimic and record such processes is of great importance. We suggest that the method described in this article can be used for a wide range of different biosensors and for the design of novel bioelectronic devices. Assembly of electronic devices with the help of synthetic peptides is one of the tantalizing possibilities; using semiconducting polymer wires to connect to metal ions in synthetic biocatalysts may be another.
Acknowledgments
We thank Mats R. Andersson and coworkers (Chalmers University, Göteborg, Sweden) for the synthesis of POWT. This work was funded to a degree by the Swedish Research Council.
Abbreviations: CP, conjugated polymer; POWT, poly{3-[(S)-5-amino-5-carboxyl-3-oxapentyl]-2,5-thiophenylene hydrochloride}; ICD, induced CD.
References
- 1.Korri-Youssoufi, H., Garnier, F., Srivastava, P., Godillot, P. & Yassar, A. (1997) J. Am. Chem. Soc. 119, 7388-7389. [Google Scholar]
- 2.Faïd, K. & Leclerc, M. (1998) J. Am. Chem. Soc. 120, 5274-5278. [Google Scholar]
- 3.Baek, M., Stevens, R. C. & Charych, D. H. (2000) Bioconjugate Chem. 11, 777-788. [DOI] [PubMed] [Google Scholar]
- 4.Kumpumbu-Kalemba, L. & Leclerc, M. (2000) Chem. Commun., 1847-1848.
- 5.Gaylord, B. S., Heeger, A. J. & Bazan, G. C. (2002) Proc. Natl. Acad. Sci. USA 99, 10954-10957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaylord, B. S., Heeger, A. J. & Bazan, G. C. (2003) J. Am. Chem. Soc. 125, 896-900. [DOI] [PubMed] [Google Scholar]
- 7.Ho, H.-A., Boissinot, M., Bergeron, M. G., Corbeil, G., Dore, K., Boudreau, D. & Leclerc, M. (2002) Angew. Chem. Int. Ed. Engl. 41, 1548-1551. [DOI] [PubMed] [Google Scholar]
- 8.Liu, B., Gaylord, B. S., Wang, S. & Bazan, G. C. (2003) J. Am. Chem. Soc. 125, 6705-6714. [DOI] [PubMed] [Google Scholar]
- 9.Zhou, Q. & Swager, T. M. (1995) J. Am. Chem. Soc. 117, 7017-7018. [Google Scholar]
- 10.Chen, L., McBranch, D. W., Wang, H.-L., Helgeson, R., Wudl, F. & Whitten, D. G. (1999) Proc. Natl. Acad. Sci. USA 96, 12287-12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heeger, P. S. & Heeger, A. J. (1999) Proc. Natl. Acad. Sci. USA 96, 12219-12221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang, J., Wang, D., Miller, E. K., Moses, D., Bazan, G. C. & Heeger, A. J. (2000) Macromolecules 33, 5153-5158. [Google Scholar]
- 13.Wang, D., Gong, X., Heeger, P. S., Rininsland, F., Bazan, G. C. & Heeger, A. J. (2002) Proc. Natl. Acad. Sci. USA 99, 49-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pande, R., Kamtekar, S., Ayyagari, M. S., Kamath, M., Marx, K. A., Kuma, J., Tripcthy, S. K. & Kaplan, D. L. (1996) Bioconjugate Chem. 7, 159-164. [DOI] [PubMed] [Google Scholar]
- 15.McCullough, R. D., Ewbank, P. C. & Loewe, R. S. (1997) J. Am. Chem. Soc. 119, 633-634. [Google Scholar]
- 16.Kim, B., Chen, L., Gong, J. & Osada, Y. (1999) Macromolecules 32, 3964-3969. [Google Scholar]
- 17.Andersson, M., Ekeblad, P. O., Hjertberg, T., Wennerström, O. & Inganäs, O. (1991) Polym. Commun. 32, 546-548. [Google Scholar]
- 18.Berggren, M., Bergman, P., Fagerström, J., Inganäs, O., Andersson, M., Weman, H., Granström, M., Stafström, S., Wennerström, O. & Hjertberg, T. (1999) Chem. Phys. Lett. 304, 84-90. [Google Scholar]
- 19.Nilsson, K. P. R., Andersson, M. R. & Inganäs, O. (2002) J. Phys. Condens. Matter 14, 10011-10020. [Google Scholar]
- 20.Willner, I. & Katz, E. (2000) Angew. Chem. Int. Ed. Engl. 39, 1180-1218. [DOI] [PubMed] [Google Scholar]
- 21.Olofsson, S., Johansson, G. & Baltzer, L. (1995) J. Chem. Soc. Perkin Trans. 2, 2047-2056.
- 22.Baltzer, L., Nilsson, H. & Nilsson, J. (2001) Chem. Rev. (Washington, D.C.) 101, 3153-3163. [DOI] [PubMed] [Google Scholar]
- 23.Nilsson, K. P. R. & Inganäs, O. (2003) Nat. Mater. 2, 419-424. [DOI] [PubMed] [Google Scholar]
- 24.Yashima, E., Matsushima, T. & Okamoto, Y. (1997) J. Am. Chem. Soc. 119, 6345-6359. [Google Scholar]
- 25.Yashima, E., Goto, H. & Okamoto, Y. (1999) Macromolecules 32, 7942-7945. [Google Scholar]
- 26.Langeveld-Voss, B. M. W., Janssen, R. A. J., Christiaans, M. P. T., Meskers, S. C. J., Dekkers, H. P. & Meijer, E. W. (1996) J. Am. Chem. Soc. 118, 4908-4909. [Google Scholar]
- 27.Langeveld-Voss, B. M. W., Christiaans, M. P. T., Janssen, R. A. J. & Meijer, E. W. (1998) Macromolecules 31, 6702-6704. [Google Scholar]
- 28.Olofsson, S. & Baltzer, L. (1996) Folding Des. 1, 347-356. [DOI] [PubMed] [Google Scholar]