Abstract
Previous experiments using intermolecular thiol cross-linking to determine surface-exposed positions in the transmembrane helices of the lactose permease suggest that only positions accessible from the aqueous phase are susceptible to cross-linking. This approach is now extended to most of the remaining positions in the molecule. Of an additional 143 single-Cys mutants studied, homodimer formation is observed with both a 5-Å- and a 21-Å-long crosslinking agent containing bis-methane thiosulfonate reactive groups in 33 mutants and exclusively with the 21-Å-long reagent in 43 mutants. Furthermore, intermolecular cross-linking has little or no effect on transport activity, thereby providing further support for the argument that lactose permease is functionally, as well as structurally, a monomer in the membrane. In addition, evidence is presented indicating that reentrance loops are unlikely in this polytopic membrane transport protein.
Keywords: bioenergetics, transport, transport proteins, oligomeric state
The lactose permease (LacY) of Escherichia coli is typical of membrane transport proteins that transduce free energy stored in electrochemical ion gradients into solute concentration gradients (1-3). Thus, LacY catalyzes the coupled stoichiometric translocation of galactosides and H+ (galactoside/H+ symport) by using the free energy released from the downhill translocation of H+ to drive galactoside accumulation and vice versa. LacY has been solubilized from the membrane and purified in a completely functional state (reviewed in ref. 4) and is a monomer both functionally and structurally in the membrane (see refs. 5-8).
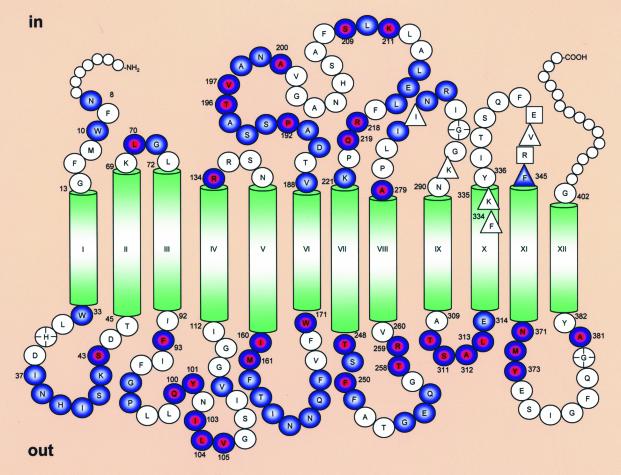
LacY has 12 transmembrane helices with the N and C termini on the cytoplasmic face of the membrane (Fig. 1) (2, 9, 10). In a functional LacY mutant devoid of native Cys residues, each residue has been replaced with Cys or other amino acids (reviewed in ref. 11). Systematic study of single-Cys and other site-directed mutants has led to the identification of functionally essential residues (11), as well as a working model for lactose/H+ symport (3). Analysis of the mutant library with a battery of site-directed biophysical and biochemical techniques has also led to the formulation of a tertiary structure model for this polytopic membrane protein (12).
Fig. 1.
Secondary structure model of LacY showing intermolecular thiol cross-linking of single-Cys mutants in periplasmic and cytoplasmic loops. Residues enclosed in red and blue circles exhibit homodimer formation with both cross-linking agents used (MTS-3-MTS and MTS-17-O5-MTS); those in filled blue circles exhibit homodimer formation with MTS-17-O5-MTS only. Residues in clear circles do not exhibit significant cross-linking. Circles marked with a cross indicate mutants with extremely low expression that were not tested. Smaller circles at the N and C termini represent residues not tested. Residues enclosed in squares or triangles in cytoplasmic loops VIII/IX and X/XI are primary and secondary epitope determinants, respectively, for mAb 4B11 (16).
In addition to other methods, intramolecular thiol crosslinking in situ has been particularly useful for estimating helix packing, tilts, and ligand-induced conformational changes in LacY (3, 12). More recently (8), intermolecular cross-linking in situ was used in an effort to identify surfaces exposed to the bilayer in the transmembrane helices of LacY. However, of 250 single-Cys replacement mutants in each transmembrane helix, only nine mutants in five helices exhibit intermolecular crosslinking in the presence of 1,1-propanediyl bis-methanethiosulfonate (MTS-3-MTS), a 5-Å-long homobifunctional thiol crosslinking agent. All of the mutants are located at or near helix-loop interfaces, and all are positioned on the periphery of the 12-helix bundle facing away from the middle of LacY. The surprisingly small number of mutants that exhibit intermolecular crosslinking may be because of several factors. (i) LacY may have an irregular shape. (ii) In the low dielectric of the bilayer, the thiol form of Cys is greatly favored over the much more reactive thiolate anion (13). (iii) The amphipathic bis-MTS reagent may have limited accessibility to Cys residues buried in the bilayer. (iv) One functional group in the cross-linking agent may react with Cys replacements facing the interior of the molecular so that the other reactive group is not in sufficiently close proximity to react with the homologous Cys residue in another molecule. (v) The position that defines the ends of the transmembrane helices is an approximation based on single amino acid deletion analysis (14, 15) that is probably accurate to about one turn of a helix. Thus, the ends of some of the transmembrane helices may have been underestimated.
In an effort to further characterize LacY by using this experimental approach, we have now examined mutants with a single-Cys residue at most of the remaining positions in the molecule. The results demonstrate that 53% of 143 single-Cys replacement mutants in presumably extramembaneous regions undergo intermolecular cross-linking, supporting the notion that Cys residues in transmembrane helices in the bilayer have markedly different properties from those exposed to the aqueous milieu. Furthermore, intermolecular cross-linking of Cys residues located at various positions in LacY has little effect on transport activity, thereby providing additional support for the contention that LacY functions as a monomer. Finally, with the exception of the cytoplasmic loop between helices X and XI (loop X/XI), which forms part of the epitope for mAb 4B11 (16), more than one Cys replacement mutant in each loop exhibits intramolecular cross-linking. Therefore, it is unlikely that any of the loops in LacY reenter the bilayer.
Experimental Procedures
LacY Mutants. Construction of all single-Cys mutants in plasmid pT7-5 has been described (17-26). Two mutants (R134C and A381C) contain a 100-residue biotin acceptor domain (BAD) in the middle of cytoplasmic loop (loop VI/VII), whereas mutant P192C contains a BAD at the C terminus.
Expression of LacY and Membrane Preparation. E. coli T184 [lacI+O+Z-Y- (A) rpsL met- thr- recA hsdM hsdR/F′ lacIq O+ZD118 (lacZ-Y+)] (27) was transformed with plasmid pT7-5 encoding the cassette lacY gene with given single-Cys mutants. Cells were grown, induced with isopropyl-1-thio-β-d-galactopyranoside, and membranes were prepared as described (8).
Cross-Linking. Most cross-linking experiments were carried out on aliquots of membrane suspensions prepared by sonification as described (8), unless the effect of cross-linking on transport activity was to be studied.
Cross-Linking and Transport. Right-side-out membrane vesicles were prepared from 2-liter cultures of E. coli T184 expressing a given single-Cys mutant by lysozyme-EDTA treatment and osmotic lysis (28, 29). The vesicles were resuspended to a protein concentration of 10 mg/ml in 100 mM potassium phosphate (KPi; pH 7.2)/10 mM MgSO4, frozen in liquid nitrogen, and stored at -80°C until use. Before use, the vesicles were diluted to 4 mg of protein per ml in 100 mM KPi, pH 7.2/10 mM MgSO4 (OD600 = 4) and divided into 0.4-ml aliquots. After addition of MTS-3-MTS (5 Å) or 3,6,9,12,15-pentaoxaheptadecane-1,17-diyl bis-methanethiosulfonate (MTS-17-O5-MTS; 21 Å) in DMSO to a final concentration of 50 μM, the samples were incubated for 1 h at room temperature. The same volume of DMSO was added to the control sample (final concentration 0.25%). The cross-linking reaction was terminated by addition of methylmethane thiosulfonate to a final concentration of 5 mM. After harvesting by centrifugation for 2 min at 10,000× g, the vesicles were washed twice with 100 mM KPi (pH 7.2)/10 mM MgSO4 and resuspended in 0.4 ml of the same buffer. An aliquot (40 μl) of each sample was subjected to SDS/12% PAGE and Western blot analysis with anti-C-terminal antibody.
For transport assays, aliquots (50 μl) of each sample were equilibrated at room temperature, and [l-14C]lactose was added to a final concentration of 0.4 mM. Lactose uptake was measured over a 5-min time course in the presence of ascorbate and phenazine methosulfate under oxygen (30). Reactions were quenched at a given time by addition of 3 ml of 100 mM KPi (pH 5.5)/100 mM LiCl followed by immediate filtration (31).
Results
Cross-Linking. One hundred and forty-three LacY mutants containing a single-Cys replacement in each putative extramembraneous domain (loop) were tested for intermolecular cross-linking in situ with the thiol-specific cross-linkers MTS-3-MTS (5 Å) or MTS-17-O5-MTS (21 Å). Almost the entire extramembraneous region was scanned, including positions 8-13 (N terminus), 33-45, 69-72, 92-112, 134-137, 160-171, 188-221, 248-260, 279-290, 309-314, 336-345, 371-382, and position 402 (Fig. 1). Positions 403-417 were not tested because LacY truncated at position 401 is expressed normally and is completely functional (32-34). Similarly, positions 1-7 at the N terminus were not tested because these residues can also be deleted without altering expression or activity (35). In addition, three single-Cys mutants that are expressed poorly, H35C (25), G287C, and G380C (36), were not tested.
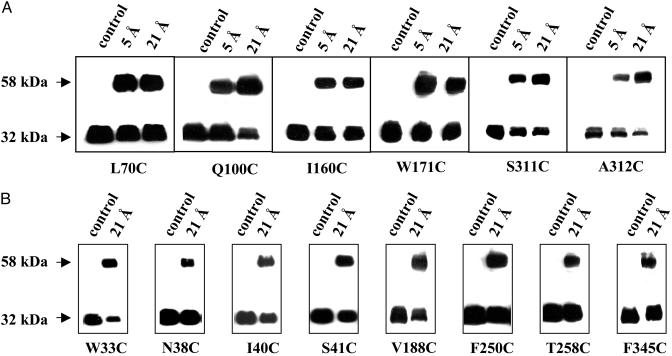
The efficiency of cross-linking varies significantly with different mutants. Examples of single-Cys mutants that exhibit relatively high cross-linking efficiency are shown in Fig. 2. Despite a true molecular weight of ≈46.5 kDa, intact LacY migrates during electrophoresis with a relative Mr of ≈32 kDa, and the homodimer migrates at ≈58 kDa (8). Mutants with a single-Cys at position 134, 192, or 381 (data not shown) contain a BAD and exhibit Mrs of ≈40 and 80 kDa, respectively, before and after cross-linking (5). Some of the single-Cys replacement mutants cross-link with both the 5- and the 21-Å homobifunctional reagents (Fig. 2 A and Tables 1 and 2), whereas others cross-link with only the 21-Å reagent (Fig. 2B and Tables 1 and 2).
Fig. 2.
Representative cross-linking studies with given single-Cys mutants. Membranes prepared from E. coli T184 containing given single-Cys mutants were treated with 50 μM MTS-3-MTS (5 Å) or MTS-17-O5-MTS (21 Å) at room temperature for 1 h, and reactions were terminated with 5 mM methyl methanethiosulfonate. Samples were then subjected to SDS/12% PAGE, transferred onto Immunobilon-poly(vinylidene difluoride) membranes, and immunoblotted with site-directed polyclonal antibodies against the C terminus of LacY (43). Control samples were treated with 2.5% DMSO, the solvent in which the cross-linking agents were dissolved. LacY monomer migrates with an Mr of ≈32 kDa, and the cross-linked dimer migrates with an Mr of ≈58 kDa. (A) Single-Cys mutants that exhibit significant cross-linking with both MTS-3-MTS (5 Å) and MTS-17-O5-MTS (21 Å). (B) Single-Cys mutants that exhibit significant cross-linking with MTS-17-O5-MTS (21 Å) only.
Table 1. Intermolecular thiol cross-linking of single-Cys mutants in periplasmic loops.
| Mutant | 5 Å | 21 Å | Mutant | 5 Å | 21 Å | Mutant | 5 Å | 21 Å |
|---|---|---|---|---|---|---|---|---|
| Loop I/II | V105C | +++ | +++ | F251C | − | ++ | ||
| W33C | − | +++ | V109C | − | + | G254C | − | ++ |
| I37C | − | + | Loop V/VI | E255C | − | + | ||
| N38C | − | +++ | I160C | +++ | +++ | Q256C | − | + |
| H39C | − | + | M161C | + | + | T258C | + | +++ |
| I40C | − | +++ | F162C | − | ++ | R259C | +++ | +++ |
| S41C | − | +++ | T163C | − | + | Loop IX/X | ||
| K42C | − | + | I164C | − | + | T310C | +++ | +++ |
| S43C | + | ++ | N165C | − | + | S311C | +++ | +++ |
| Loop III/IV | N166C | − | + | A312C | ++ | +++ | ||
| F93C | ++ | ++ | Q167C | − | + | L313C | ++ | +++ |
| G96C | − | + | F168C | − | + | E314C | − | + |
| P97C | − | ++ | W171C | +++ | +++ | Loop XI/XII | ||
| Q100C | +++ | +++ | Loop VII/VIII | N371C | + | +++ | ||
| Y101C | ++ | ++ | T248C | + | + | M372C | + | + |
| I103C | ++ | +++ | S249C | − | + | Y373C | + | +++ |
| L104C | ++ | ++ | F250C | + | +++ | A381C | + | + |
The data shown were obtained as described in Experimental Procedures. The number of +s represents efficiency of cross-linking: +, 0-20%; ++, 20-40%; +++, >40%.
Table 2. Intermolecular thiol cross-linking of single-Cys mutants in cytoplasmic loops.
| Mutant | 5 Å | 21 Å | Mutant | 5 Å | 21 Å | Mutant | 5 Å | 21 Å |
|---|---|---|---|---|---|---|---|---|
| N terminus | S193C | − | + | E215C | − | + | ||
| N8C | − | + | S194C | − | + | L216C | − | + |
| W10C | − | + | A195C | − | + | R218C | + | +++ |
| Loop II/III | T196C | + | + | Q219C | + | +++ | ||
| L70C | +++ | +++ | V197C | + | + | K221C | − | + |
| G71C | − | + | A198C | − | + | Loop VIII/X | ||
| Loop IV/V | N199C | − | + | A279C | + | + | ||
| R134C | + | ++ | A200C | + | + | I282C | − | + |
| Loop VI/VII | S209C | + | + | N284C | − | + | ||
| V188C | − | +++ | L210C | − | + | R285C | − | + |
| D190C | − | + | K211C | + | + | Loop X/XI | ||
| A191C | − | + | L214C | − | + | F345C | − | +++ |
| P192C | + | +++ |
The data shown were obtained as described in Experimental Procedures. The number of +s represents efficiency of cross-linking: +, 0-20%; ++, 20-40%; +++, >40%.
The total number of single-Cys mutants that exhibit significant homodimer formation with one or both cross-linking agents is 76 (53%). Thirty-three mutants exhibit homodimer formation in the presence of both cross-linkers, and 43 mutants cross-link in the presence of the 21-Å cross-linking agent only (Fig. 1 and Tables 1 and 2). Consistent with previous findings (16), single-Cys mutants at positions 101 (loop III/IV) and 313 (loop IX/X) demonstrate spontaneous cross-linking. Cys replacement mutants at positions 104 (loop III/IV), 192 (loop VI/VII), 211 (loop VI/VII), and 216 (loop VI/VII) also cross-link spontaneously at room temperature (data not shown).
Single-Cys replacements located in periplasmic loops are more susceptible to cross-linking than those located in cytoplasmic loops (Fig. 1). Thus, 45 Cys-replacement mutants of 75 (60%; Table 1) and 31 of 70 (44%; Table 2) in periplasmic and cytoplasmic loops, respectively, exhibit significant cross-linking. Finally, Cys replacements in periplasmic loops are more susceptible to cross-linking with the 5-Å reagent, and the efficiency of homodimer formation is higher relative to Cys replacements in cytoplasmic loops (Fig. 1 and compare Tables 1 and 2).
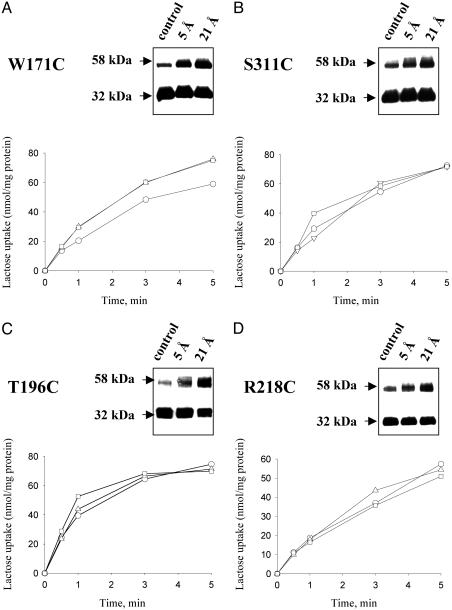
Effect of Cross-Linking on Transport Activity. Right-side-out membrane vesicles obtained from cells expressing single-Cys mutants W171C, S311C, T196C, or R218C (Fig. 3 A-D, respectively) exhibit significant cross-linking with both reagents. However, no significant effect on lactose transport is observed after crosslinking with either reagent.
Fig. 3.
Effect of cross-linking on transport activity of given single-Cys mutants in right-side-out (RSO) vesicles. Cross-linking of single-Cys mutants W171C or S311C, which are located in periplasmic loops, are shown in A and B, respectively; cross-linking of single-Cys mutants T196C or R218C, which are located in cytoplasmic loops, are shown in C and D, respectively. (Insets) Results of cross-linking experiments carried out with RSO membrane vesicles. The transport studies were carried out as described in Experimental Procedures on the same preparations. ○, Control (vesicles treated with 0.25% DMSO); ▵, vesicles treated with MTS-3-MTS; □, vesicles treated with MTS-17-O5-MTS.
Discussion
Intermolecular thiol cross-linking has been used previously (8) in an attempt to determine exposed positions on the surface of transmembrane helices in LacY, and the number of single-Cys mutants found to exhibit intermolecular cross-linking is remarkably small. Thus, homodimer formation was observed with only nine single-Cys mutants of 250 mutants tested in 5 of 12 transmembrane helices. All these positions are located at or near helix-loop interfaces, and all are positioned on the periphery of the 12-helix bundle facing away from the middle of the molecule. Furthermore, with each mutant, the native residue replaced by Cys is either hydrophobic or contains an aromatic ring. Seven of the nine mutants are located toward the cytoplasmic surface, whereas only two are located toward the periplasmic surface. The previous work also suggests several possible explanations for the low yield of mutants that cross-link, which are enumerated above. However, many of the constraints on Cys replacements in transmembrane helices of LacY are not applicable to Cys residues in loops that are presumably more accessible to the aqueous milieu. To pursue the problem further, the same approach has now been applied to single-Cys replacement mutants at essentially each position of the extramembraneous domains in the molecule.
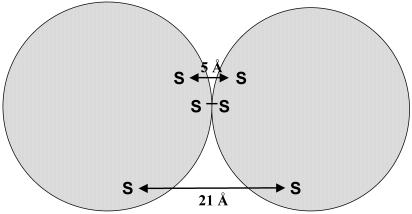
The results of this study demonstrate clearly that more than one-half of the mutants with single-Cys replacements in the putative extramembraneous portions of LacY cross-link either with both homobifunctional MTS cross-linking reagents that are ≈5 and 21 Å in length or with only the 21-Å reagent. These data may reflect the dependence of homodimer formation on location of cysteine residue in the loop and the distance between two interacting residues. Fig. 4 schematically represents the general location of residues that cross-link spontaneously, in the presence of MTS-3-MTS (5 Å) or MTS-17-O5-MTS (21 Å).
Fig. 4.
Schematic representation demonstrating the general location of residues that cross-link spontaneously, in the presence of MTS-3-MTS (5 Å)or MTS-17-O5-MTS (21 Å).
In marked contrast to results obtained for the transmembrane helices, where only ≈4% of the 250 mutants tested exhibit significant cross-linking, 53% of the mutants in the hydrophilic domains exhibit significant intermolecular cross-linking.
Although the number of Cys-replacement mutants in hydrophilic domains that form homodimers is much greater than in the transmembrane helices, cross-linking efficiency differs from mutant to mutant and with the two different cross-linking agents. One possible explanation for this observation is that cross-linking efficiency reflects differences in the conformational flexibility of the loops. It is also possible that Cys residues at certain positions may be sterically favored to undergo cross-linking with a reagent of a particular length. In this respect, MTS-17-O5-MTS is more flexible than MTS-3-MTS and can adopt a range of conformations due to rotation of the bonds that form the linker arm (37). There are several C—O bonds in the spacer of MTS-17-O5-MTS that might adopt gauche conformations and bring the two reactive ends closer together. Thus, MTS-17-O5-MTS is more likely to yield positive results than MTS-3-MTS, because the distance between the reactive MTS moieties can vary considerably more than the shorter more constrained reagent. Although the Cys replacements tested here are all presumably exposed to a high dielectric environment and can therefore dissociate to form the reactive thiolate anion, cross-linking efficiency may also reflect favorable disposition of certain Cys residues on the exposed surface of the protein. It is noteworthy that many of the loops in LacY are structured (7, 16, 38-41), as opposed to being in a random coil conformation, and are predicted by various computer algorithms to contain helical segments. Therefore, all of the positions may not be equally accessible to cross-linking agents. In this regard, it is interesting that Cys residues located in the periplasmic loops of LacY are more susceptible to cross-linking with the short cross-linking agent than those in the cytoplasmic loops (Fig. 1).
With the exception of position 345 at the cytoplasmic end of helix X, none of the other Cys replacements in loop X/XI exhibit significant intermolecular cross-linking, nor do Cys replacements at positions 285-290 at the cytoplasmic N terminus of helix IX. Interestingly, it has been shown (16) that loops VIII/IX and X/XI comprise a discontinuous epitope for mAb 4B11 (42). Furthermore, the primary epitope determinants are Glu-342 and Arg-344 in loop X/XI, whereas Ile-283 and Lys-289 in loop VIII/IX, Phe-334 and Lys-335 at the cytoplasmic end of helix X, and Val-343 and Phe-345 in loop X/XI appear to be in close proximity to the primary epitope determinants (Fig. 1). These findings provide a strong indication that not only must loops VIII/IX and X/XI be in close proximity, but also this region of the protein is structured. Therefore, it seems reasonable to conclude that sparsity of Cys replacements in loop X/XI that form intermolecular crosslinks is due to steric constraints imposed by structural properties of this loop. In any case, because loops VIII/IX and X/XI are accessible to mAb 4B11, these regions must be exposed on the cytoplasmic surface of the membrane. In view of the observation that at least two Cys-replacement mutants in each extramembraneous domain exhibit intermolecular cross-linking, with the exception of loop X/XI, which forms a major part of the 4B11 epitope, it is unlikely that any of the loops in LacY reenter the bilayer.
Finally, evidence has been presented that, despite significant cross-linking of Cys residues placed at various positions in LacY (Fig. 3), little or no inhibition of lactose transport activity is observed. These findings provide further support for a body of evidence (see ref. 5) indicating that LacY is completely functional as a monomer.
Note Added in Proof: The conclusions presented here are consistent with the x-ray structure of LacY published recently (44).
Acknowledgments
We thank Melissa Sondej and Irina Smirnova for valuable discussions, useful advice, and help with experimental procedures. This work was supported by National Institutes of Health Grant DK51131:08 (to H.R.K.).
Abbreviations: LacY, lactose permease; MTS-3-MTS, 1,3 propanediyl bis-methanethiosulfonate; MTS-17-O5-MTS, 3,6,9,12,15-pentaoxaheptadecane-1,17-diyl bis-methanethiosulfonate; BAD, biotin acceptor domain.
References
- 1.Kaback, H. R. (1996) in Handbook of Biological Physics: Transport Processes in Eukaryotic and Prokaryotic Organisms, eds. Konings, W. N., Kaback, H. R. & Lolkema, J. S. (Elsevier, Amsterdam), Vol. II, pp. 203-227. [Google Scholar]
- 2.Kaback, H. R. & Wu, J. (1997) Q. Rev. Biophys. 30, 333-364. [DOI] [PubMed] [Google Scholar]
- 3.Kaback, H. R., Sahin-Títh, M. & Weinglass, A. B. (2001) Nat. Rev. Mol. Cell. Biol. 2, 610-622. [DOI] [PubMed] [Google Scholar]
- 4.Viitanen, P., Garcia, M. L. & Kaback, H. R. (1984) Proc. Natl. Acad. Sci. USA 81, 1629-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sahin-Títh, M., Lawrence, M. C. & Kaback, H. R. (1994) Proc. Natl. Acad. Sci. USA 91, 5421-5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costello, M. J., Escaig, J., Matsushita, K., Viitanen, P. V., Menick, D. R. & Kaback, H. R. (1987) J. Biol. Chem. 262, 17072-17082. [PubMed] [Google Scholar]
- 7.Sun, J. & Kaback, H. R. (1997) Biochemistry 36, 11959-11965. [DOI] [PubMed] [Google Scholar]
- 8.Guan, L., Murphy, F. D. & Kaback, H. R. (2002) Proc. Natl. Acad. Sci. USA 99, 3475-3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foster, D. L., Boublik, M. & Kaback, H. R. (1983) J. Biol. Chem. 258, 31-34. [PubMed] [Google Scholar]
- 10.Calamia, J. & Manoil, C. (1990) Proc. Natl. Acad. Sci. USA 87, 4937-4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frillingos, S., Sahin-Títh, M., Wu, J. & Kaback, H. R. (1998) FASEB J. 12, 1281-1299. [DOI] [PubMed] [Google Scholar]
- 12.Sorgen, P. L., Hu, Y., Guan, L., Kaback, H. R. & Girvin, M. E. (2002) Proc. Natl. Acad. Sci. USA 99, 14037-14040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts, D. D., Lewis, S. D., Ballou, D. P., Olson, S. T. & Shafer, J. A. (1986) Biochemistry 25, 5595-5601. [DOI] [PubMed] [Google Scholar]
- 14.Wolin, C. & Kaback, H. R. (1999) Biochemistry 38, 8590-8597. [DOI] [PubMed] [Google Scholar]
- 15.Wolin, C. D. & Kaback, H. R. (2001) Biochemistry 40, 1996-2003. [DOI] [PubMed] [Google Scholar]
- 16.Sun, J., Li, J., Carrasco, N. & Kaback, H. R. (1997) Biochemistry 36, 274-280. [DOI] [PubMed] [Google Scholar]
- 17.Sahin-Títh, M. & Kaback, H. R. (1993) Protein Sci. 2, 1024-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunten, R. L., Sahin-Títh, M. & Kaback, H. R. (1993) Biochemistry 32, 12644-12650. [DOI] [PubMed] [Google Scholar]
- 19.Sahin-Títh, M., Persson, B., Schwieger, J., Cohan, M. & Kaback, H. R. (1994) Protein Sci. 3, 240-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sahin-Títh, M., Frillingos, S., Bibi, E., Gonzalez, A. & Kaback, H. R. (1994) Protein Sci. 3, 2302-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frillingos, S., Sahin-Títh, M., Persson, B. & Kaback, H. R. (1994) Biochemistry 33, 8074-8081. [DOI] [PubMed] [Google Scholar]
- 22.Weitzman, C. & Kaback, H. R. (1995) Biochemistry 4, 2310-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He, M. M., Sun, J. & Kaback, H. R. (1996) Biochemistry 35, 12909-12914. [DOI] [PubMed] [Google Scholar]
- 24.Frillingos, S. & Kaback, H. R. (1996) Biochemistry 35, 5333-5338. [DOI] [PubMed] [Google Scholar]
- 25.Frillingos, S., Sun, J., Gonzalez, A. & Kaback, H. R. (1997) Biochemistry 36, 269-273. [DOI] [PubMed] [Google Scholar]
- 26.Frillingos, S., Gonzalez, A. & Kaback, H. R. (1997) Biochemistry 36, 14284-14290. [DOI] [PubMed] [Google Scholar]
- 27.Teather, R. M., Bramhall, J., Riede, I., Wright, J. K., Furst, M., Aichele, G., Wilhelm, U. & Overath, P. (1980) Eur. J. Biochem. 108, 223-231. [DOI] [PubMed] [Google Scholar]
- 28.Kaback, H. R. (1971) Methods Enzymol. 22, 99-120. [Google Scholar]
- 29.Short, S. A., Kaback, H. R. & Kohn, L. D. (1975) J. Biol. Chem. 250, 4291-4296. [PubMed] [Google Scholar]
- 30.Konings, W. N., Barnes, E. M., Jr., & Kaback, H. R. (1971) J. Biol. Chem. 246, 5857-5861. [PubMed] [Google Scholar]
- 31.Kaback, H. R. (1974) Methods Enzymol. 31, 698-709. [DOI] [PubMed] [Google Scholar]
- 32.Roepe, P. D., Zbar, R. I., Sarkar, H. K. & Kaback, H. R. (1989) Proc. Natl. Acad. Sci. USA 86, 3992-3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKenna, E., Hardy, D., Pastore, J. C. & Kaback, H. R. (1991) Proc. Natl. Acad. Sci. USA 88, 2969-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKenna, E., Hardy, D. & Kaback, H. R. (1992) J. Biol. Chem. 267, 6471-6474. [PubMed] [Google Scholar]
- 35.Bibi, E., Stearns, S. M. & Kaback, H. R. (1992) Proc. Natl. Acad. Sci. USA 89, 3180-3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jung, K., Jung, H., Colacurcio, P. & Kaback, H. R. (1995) Biochemistry 34, 1030-1039. [DOI] [PubMed] [Google Scholar]
- 37.Green, N. S., Reisler, E. & Houk, K. N. (2001) Protein Sci. 10, 1293-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun, J., Wu, J., Carrasco, N. & Kaback, H. R. (1996) Biochemistry 35, 990-998. [DOI] [PubMed] [Google Scholar]
- 39.Sun, J., Frillingos, S. & Kaback, H. R. (1997) Protein Sci. 6, 1503-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Voss, J., Hubbell, W. L., Hernandez, J. & Kaback, H. R. (1997) Biochemistry 36, 15055-15061. [DOI] [PubMed] [Google Scholar]
- 41.Kwaw, I., Sun, J. & Kaback, H. R. (2000) Biochemistry 39, 3134-3140. [DOI] [PubMed] [Google Scholar]
- 42.Carrasco, N., Tahara, S. M., Patel, L., Goldkorn, T. & Kaback, H. R. (1982) Proc. Natl. Acad. Sci. USA 79, 6894-6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carrasco, N., Herzlinger, D., Mitchell, R., DeChiara, S., Danho, W., Gabriel, T. F. & Kaback, H. R. (1984) Proc. Natl. Acad. Sci. USA 81, 4672-4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abramson, J., Smirnova, I., Kasho, V., Verner, G., Kaback, H. R. & Iwata, S. (2003) Science 301, 610-615. [DOI] [PubMed] [Google Scholar]