Abstract
The abundance of the cyclin-dependent kinase (CDK) inhibitor p57Kip2, an important regulator of cell cycle progression, is thought to be controlled by the ubiquitin-proteasome pathway. The Skp1/Cul1/F-box (SCF)-type E3 ubiquitin ligase complex SCFSkp2 has now been shown to be responsible for regulating the cellular level of p57Kip2 by targeting it for ubiquitylation and proteolysis. The elimination of p57Kip2 was impaired in Skp2-/- cells, resulting in abnormal accumulation of the protein. Coimmunoprecipitation analysis also revealed that Skp2 interacts with p57Kip2 in vivo. Overexpression of WT Skp2 promoted degradation of p57Kip2, whereas expression of a dominant negative mutant of Skp2 prolonged the half-life of p57Kip2. Mutation of the threonine residue (Thr-310) of human p57Kip2 that is conserved between the COOH-terminal QT domains of p57Kip2 and p27Kip1 prevented the effect of Skp2 on the stability of p57Kip2, suggesting that phosphorylation at this site is required for SCFSkp2-mediated ubiquitylation. Finally, the purified recombinant SCFSkp2 complex mediated p57Kip2 ubiquitylation in vitro in a manner dependent on the presence of the cyclin E-CDK2 complex. These observations thus demonstrate that the SCFSkp2 complex plays an important role in cell-cycle progression by determining the abundance of p57Kip2 and that of the related CDK inhibitor p27Kip1.
Ubiquitylation and subsequent proteasomal degradation of regulatory proteins control a variety of cellular processes, including cell cycle progression, gene transcription, and signal transduction. The multistage processing of target proteins begins with enzymatic tagging with a polyubiquitin chain and culminates with ubiquitin-dependent degradation by the 26S proteasome (1, 2). In the first stage of the ubiquitylation process, the COOH terminus of ubiquitin is covalently attached by a thioester bond to the cysteine residue in the active site of a ubiquitin-activating enzyme (E1). Ubiquitin is then transferred from E1 to the active-site cysteine of a ubiquitin-conjugating enzyme (E2). Finally, in a reaction catalyzed by a ubiquitin-protein ligase (E3), multiple ubiquitin monomers donated by an E2 are conjugated directly via an isopeptide bond first to the ε-amino group of lysine residues in the target protein and then to lysines in the attached ubiquityl moieties to complete synthesis of the polyubiquitin tag.
The E3 components of the ubiquitin pathway are responsible for recognizing and recruiting target proteins for polyubiquitylation. E3 enzymes have been divided into three functional classes (1, 3-6): One class includes the homologous to E6-AP COOH terminus (HECT) domain proteins, which contain an active-site cysteine residue that receives ubiquitin from an E2 and transfers it to the target protein, whereas the other two classes include RING finger and U-box domain proteins, respectively, which appear to mediate ubiquitylation, at least in part, by binding to both an E2 and the target protein and thereby bringing them into close proximity.
Two major types of E3 enzymes are thought to regulate cell cycle progression: Skp1/Cul1/F-box (SCF) complexes are implicated in G1-S progression, and the anaphase-promoting complex or cyclosome (APC/C) is required both for separation of sister chromatids at anaphase and for exit from M phase into G1 (7). SCF complexes comprise the invariable components Skp1, Cul1, and Rbx1 (also known as Roc1 or Hrt1) and a variable component, an F-box protein, that serves to recognize and recruit target proteins (8-14). F-box proteins are linked to the Cul1-Rbx1 module via the Skp1-adapter protein, which binds to an ≈40-aa degenerate sequence motif (the F box) present in F-box proteins. Skp2, which contains an F-box domain followed by leucine-rich repeats, was originally identified as a protein that interacts with the complex of cyclin A and cyclin-dependent kinase 2 (CDK2) (15). It has subsequently been implicated, however, in the ubiquitin-mediated degradation of p27Kip1 (16-18), E2F-1 (19), free cyclin E (16), ORC1 (20), CDK9 (21), and B-Myb (22). Skp2 begins to accumulate in mammalian cells during late G1 phase of the cell cycle, and its abundance is maximal during S and G2 phases (15, 17, 19, 23).
The CDK inhibitors (CKIs) p27Kip1 and p57Kip2 negatively regulate progression of the cell cycle by inhibiting the activity of cyclin-CDK complexes. Both p27Kip1 and p57Kip2 contain a conserved CDK binding-inhibitory domain and a QT domain at their NH2 and COOH termini, respectively (24, 25). Phosphorylation of the QT domain of p27Kip1 is thought to promote the ubiquitin-dependent degradation of this protein mediated by the SCFSkp2 complex (17, 18, 26). We have previously shown that targeted disruption of the Skp2 gene (Skp2) in mice results in the cellular accumulation of p27Kip1, suggesting that Skp2 plays an important role in control of the abundance of this CKI (16). Targeted disruption of the gene for Uba3, a component of the NEDD8 system that appears to be indispensable for the function of all SCF ubiquitin ligase complexes, was recently shown to result in the abnormal accumulation of p57Kip2 (27). This observation, together with the structural similarity of the two CKIs, prompted us to investigate whether SCFSkp2 complex-mediated proteolysis controls the abundance not only of p27Kip1 but also of p57Kip2.
We now show that the elimination of p57Kip2 is impaired in Skp2-/- cells, resulting in the abnormal accumulation of this protein. Furthermore, Skp2 was found to interact with p57Kip2 and promote its degradation. Finally, we demonstrate that the purified recombinant SCFSkp2 complex mediated ubiquitylation of p57Kip2 in vitro in a manner that appeared to depend on phosphorylation of the COOH-terminal QT domain of p57Kip2 by the cyclin E-CDK2 complex. Our data thus indicate that SCFSkp2 regulates the cellular abundance of p57Kip2 by targeting it for ubiquitylation and proteolysis.
Materials and Methods
Abs. Rabbit polyclonal Abs to p57Kip2 were obtained from Santa Cruz Biotechnology and Sigma. Mouse mAbs to p57Kip2, p27Kip1, HSP90, and glycogen synthase kinase-3β (GSK-3β) were from BD Biosciences (San Jose, CA). Mouse mAb to protein C (HPC4) was from Roche Molecular Biochemicals. Mouse mAbs to Skp2 and Myc were from Zymed and Roche Molecular Biochemicals, respectively.
Preparation of Mouse Embryonic Fibroblasts (MEFs). MEFs were isolated from 13.5-day-postcoitum WT, Skp2-/-, or Skp2-/- p27-/- embryos and cultured as described (28). Only nonsenescent MEFs (no more than passage 2) were used for experiments. For cell synchronization, MEFs were arrested either at G0-G1 by serum deprivation for 96 h in medium supplemented with 0.1% FBS or at S-G2 by incubation with aphidicolin (1 μg/ml) for 14 h (to block cell-cycle progression at G1-S) and subsequent culture for 3 h in aphidicolin-free medium. Cells were harvested and lysed in an ice-cold solution containing 40 mM Hepes-NaOH (pH 7.9), 300 mM NaCl, 1 mM DTT, 0.5% Triton X-100, 10% glycerol, 5 μg/ml leupeptin, 5 μg/ml antipain, 5 μg/ml pepstatin A, and 5 μg/ml aprotinin.
Production of Recombinant Proteins in Bacteria. Complementary DNAs encoding human WT p57Kip2 and mutant p57(T310A) were subcloned into pET-30a (Novagen), and cDNA encoding Cks1 was subcloned into pGEX6P-3 (Amersham Biosciences). The p57Kip2 tagged with the NH2-terminal hexahistidine (His-6), and GST-Cks1 fusion protein were expressed in Escherichia coli strain BL21(DE3)pLysS (Novagen) and purified with the use of Ni2+-agarose beads and glutathione beads, respectively, as described (23). Mouse p27Kip1 (23), Saccharomyces cerevisiae Uba1 (29), human UbcH5A (30), and a GST fusion protein of mouse ubiquitin (29) were prepared as described.
Baculovirus Expression System. A cDNA encoding human Cul1 tagged at its NH2 terminus with the hemagglutinin (HA) epitope and a cDNA for human Skp1 containing NH2-terminal hexahistidine (His-6) and T7 tags were subcloned into pBacPAK8. Complementary DNAs encoding a GST fusion protein of human CDK2 tagged at its NH2 terminus with HA, and human cyclin E containing an NH2-terminal Myc epitope were subcloned into pBacPAK9. A cDNA encoding mouse Skp2 with a NH2-terminal FLAG tag was subcloned into pFastBacHTb. Recombinant baculoviruses were generated with the BacPAK (CLONTECH) or Bac-To-BacHT (Life Technologies, Grand Island, NY) baculovirus expression systems. Baculoviruses encoding mouse Rbx1 with an NH2-terminal Myc epitope tag and human hypoxia-inducible factor-1α (HIF-1α) with NH2-terminal hexahistidine (His-6) and HPC4 tags have been described (30). The recombinant SCFSkp2 complex was purified by Ni2+-agarose chromatography as described (30) from lysates of Sf21 cells that had been coinfected with baculoviruses encoding Cul1, Skp2, Skp1, and Rbx1. The recombinant cyclin E-CDK2 complex was purified by glutathione-Sepharose affinity chromatography as described (23) from lysates of Sf21 cells that had been coinfected with baculoviruses encoding cyclin E and CDK2.
Expression of Recombinant Proteins in Mammalian Cells. Complementary DNAs encoding mouse WT Skp2 or a Skp2 mutant lacking the NH2-terminal and F-box domains (ΔNF), each tagged at their NH2 terminus with the FLAG epitope, and cDNAs for human WT p57Kip2 or the derivative p57(T310A), each containing an NH2-terminal Myc epitope, were subcloned into pcDNA3 (Invitrogen). A cDNA encoding human Skp2 tagged at its NH2 terminus with the HA epitope was subcloned into pCG-N (16). Mammalian cells were transfected with the indicated plasmids by the calcium phosphate method. After transfection (24 h), the cells were treated with cycloheximide (CHX; 50 μg/ml) or MG132 (10 μM) for various times. Cells were then harvested, lysed, and subjected to immunoprecipitation (IP) and immunoblot analysis.
IP and Immunoblot Analysis. HeLa cell lysates were incubated for 2 h at 4°C with protein A-Sepharose beads (Amersham Biosciences) and indicated Abs. The beads were washed three times with a solution containing 40 mM Hepes-NaOH (pH 7.9), 150 mM NaCl, 1 mM DTT, and 0.5% Triton X-100, after which the immunoprecipitated proteins were fractionated by SDS/PAGE, transferred to a Hybond P membrane (Amersham Biosciences), and subjected to immunoblot analysis. Immune complexes were detected with Supersignal West Pico chemiluminescent reagent (Pierce).
In Vitro Ubiquitylation Assay. To determine the ability of the purified recombinant SCFSkp2 complex to mediate the ubiquitylation of p57Kip2, p27Kip1, or HIF-1α, we incubated the complex (200 ng) for 30 min at 26°C with 50 ng of Uba1, 100 ng of UbcH5A, 3 μg of GST-ubiquitin, and 50 ng of either p57Kip2, p27Kip1, or HIF-1α in a final volume of 10 μl, containing 40 mM Hepes-NaOH (pH 7.9), 60 mM potassium acetate, 2 mM DTT, 5 mM MgCl2, 0.5 mM EDTA, 10% glycerol, and 1.5 mM ATP. Where indicated, the reaction mixture also contained 50 ng of the recombinant cyclin E-CDK2 complex or 50 ng of GST-Cks1. The reaction mixtures were then subjected to immunoblot analysis with Abs to the respective target protein.
Results
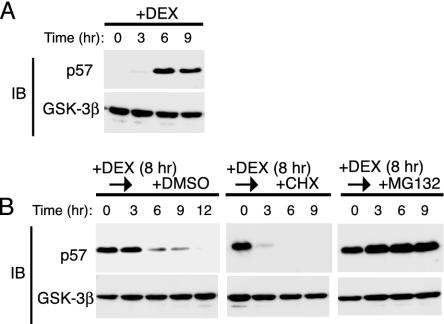
Degradation of p57Kip2 by the Proteasome. Dexamethasone (DEX) induces the expression of p57Kip2 in HeLa cells (31). We examined the expression of p57Kip2 and GSK-3β (control) by immunoblot analysis in HeLa cells that had been incubated with 100 nM DEX for various times. DEX induced a marked increase in the abundance of p57Kip2 that was maximal after 6 h (Fig. 1A) and had no effect on that of GSK-3β (Fig. 1 A). We next examined the effect of DEX on the stability of p57Kip2. HeLa cells were treated with DEX for 8 h and then cultured for various times in the absence of the steroid and the presence of either the protein synthesis inhibitor CHX (50 μg/ml), the proteasome inhibitor MG132 (10 μM), or DMSO vehicle (0.1%) (Fig. 1B). After removal of DEX, the abundance of p57Kip2 in cells cultured with DMSO decreased gradually, with the protein being undetectable at 12 h. The cells exposed to CHX after DEX removal exhibited almost a complete loss of p57Kip2 within 3 h. The gradual decrease in the amount of p57Kip2 in the control HeLa cells after removal of glucocorticoid thus appeared to reflect the net effect of a residual synthesis and a rapid degradation of the protein. The addition of MG132 to the culture medium after DEX removal completely blocked the decrease in the abundance of p57Kip2, indicating that the degradation of this protein was mediated by the proteasome.
Fig. 1.
Degradation of p57Kip2 by the proteasome pathway in HeLa cells. (A) Cells were incubated with 100 nM DEX for the indicated times, after which cell lysates were subjected to immunoblot analysis (IB) with Abs to p57Kip2 or GSK-3β. (B) Cells were treated with 100 nM DEX for 8 h and then cultured for the indicated times in DEX-free medium in the presence of 0.1% DMSO, 50 μg/ml CHX, or 10 μM MG132. Cell lysates were then subjected to immunoblot analysis with Abs to p57Kip2 or GSK-3β.
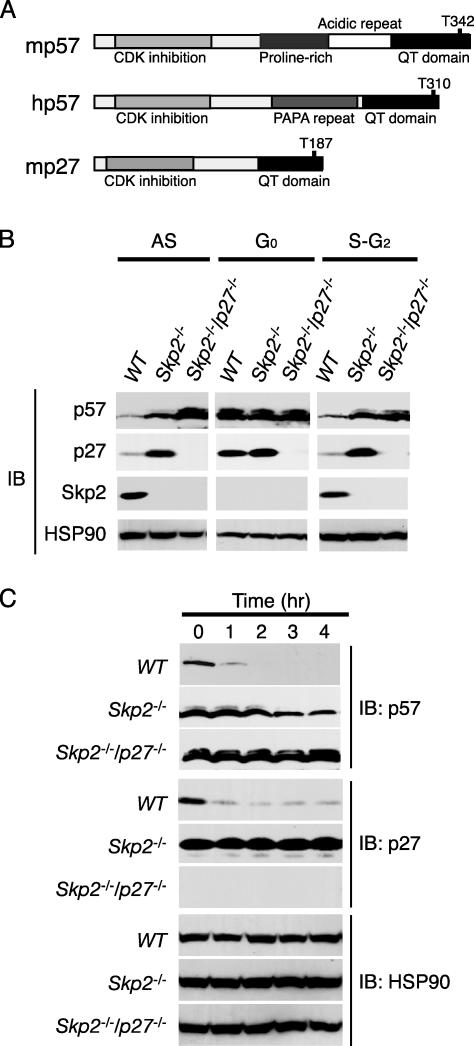
Accumulation of p57Kip2 in Skp2-/- Cells. The CKI p27Kip1 is recognized by the SCFSkp2 ubiquitin ligase in a manner dependent on phosphorylation of Thr-187, which is located in the COOH-terminal QT domain (Fig. 2A). Given that p57Kip2 exhibits sequence homology to p27Kip1 around the conserved CDK consensus phosphorylation site in the QT domain, we hypothesized that the SCFSkp2 complex is also able to mediate the ubiquitylation of p57Kip2. To investigate this hypothesis, we compared the abundance of p57Kip2, p27Kip1, and Skp2 in MEFs prepared from WT, Skp2-/-, and Skp2-/-p27-/- mice (16). Both for cells in asynchronous culture and cells synchronized at S-G2 phases, immunoblot analysis revealed that the abundance of p57Kip2 was greater in the Skp2-/- and Skp2-/-p27-/- MEFs than in the WT MEFs (Fig. 2B). p27Kip1 also accumulated in the Skp2-/- MEFs. As described (15, 17, 19, 23), the expression of Skp2 was detected at S-G2 phases but not at G0 phase. We next investigated whether p57Kip2 is eliminated in proliferating Skp2-/- and Skp2-/-p27-/- MEFs after their exposure to CHX. Whereas p57Kip2 was almost completely degraded within 1 h in WT MEFs, its abundance remained relatively high in Skp2-/- and Skp2-/-p27-/- MEFs even after incubation with CHX for 4 h (Fig. 2C). Consistent with our previous observations (16), p27Kip1 remained stable in Skp2-/- cells. These data thus suggested that Skp2 contributes to the degradation of p57Kip2 and to that of p27Kip1 and eliminated the possibility that the effects of Skp2 on p57Kip2 degradation are secondary to a cell-cycle delay induced by an accumulation of p27Kip1 in Skp2-/- cells.
Fig. 2.
Accumulation of p57Kip2 in Skp2-/- MEFs as a result of its impaired elimination. (A) Schematic representation of the domain organization of mouse p57Kip2 (mp57), human p57Kip2 (hp57), and mouse p27Kip1 (mp27). (B) Cell lysates prepared from WT, Skp2-/-, or Skp2-/-p27-/- MEFs either in asynchronous culture (AS) or synchronized at G0 phase or at S-G2 phases were subjected to immunoblot analysis with Abs to p57Kip2, p27Kip1, Skp2, or HSP90. (C) Proliferating WT, Skp2-/-, or Skp2-/-p27-/- MEFs were incubated for the indicated times in the presence of CHX (50 μg/ml), after which cell lysates were subjected to immunoblot analysis with Abs to p57Kip2, p27Kip1, or HSP90.
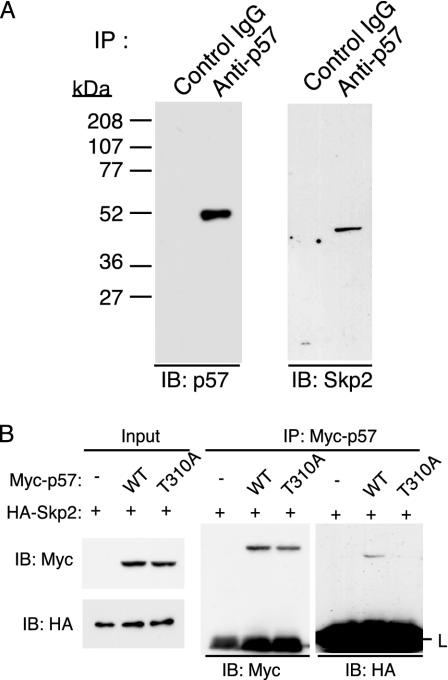
Thr-310-Dependent Interaction of p57Kip2 with Skp2. To investigate further the role of Skp2 in p57Kip2 degradation, we examined whether endogenous Skp2 associates physically with endogenous p57Kip2 in HeLa cells. Cells were incubated with DEX for 8 h to induce p57Kip2 expression and then with MG132 for an additional 6 h to facilitate the potential interaction of p57Kip2 with Skp2. Cell lysates were then subjected to IP with Abs to p57Kip2 or control IgG. Immunoblot analysis of the resulting precipitates revealed the presence of Skp2 specifically in those prepared with Abs to p57Kip2 (Fig. 3A), suggesting that Skp2 functions to bind and recruit p57Kip2 to the SCF complex.
Fig. 3.
Association between p57Kip2 and Skp2 in HeLa cells. (A) Cells were incubated with 100 nM DEX for 8 h and then with 10 μM MG132 for 6 h, after which cell lysates were subjected to IP with either rabbit Abs to p57Kip2 or control rabbit IgG. The resulting precipitates were subjected to immunoblot analysis with Abs to p57Kip2 or Skp2, as indicated. (B) After transfection (24 h) with expression vectors for the indicated proteins, cells were incubated for 6 h with MG132 (10 μM). Cell lysates were then subjected to IP with Abs to Myc. The immunoprecipitated proteins were subjected to immunoblot analysis with abs to Myc or HA.
Given that the phosphorylation of mouse p27Kip1 on Thr-187 by cyclin E-CDK2 is essential for the interaction of this CKI to Skp2, and that the sequence around this CDK consensus phosphorylation site in the QT domain of p27Kip1 is conserved in p57Kip2, we examined whether phosphorylation of the CDK consensus site of human p57Kip2 (Thr-310) is necessary for binding of this protein to Skp2. HeLa cells were transfected with the expression vector for Skp2 together with an expression plasmid for either human WT p57Kip2 or a mutant thereof in which Thr-310 is replaced with alanine (T310A). After transfection (24 h), the cells were treated with MG132 for an additional 6 h. Cell lysates were then subjected to IP with Abs to Myc. Coimmunoprecipitaion of p57Kip2 with Skp2 depended on the presence of the intact CDK consensus phosphorylation site (Fig. 3B), suggesting that phosphorylation on Thr-310 of p57Kip2 is necessary for the binding to Skp2. This result is further confirmed by pulse-chase and in vitro ubiquitylation assays (see Figs. 4C and 5B).
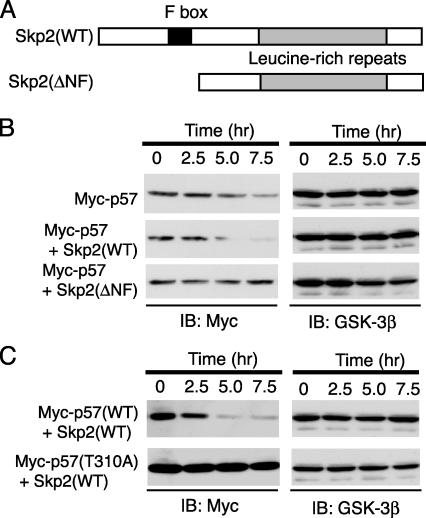
Fig. 4.
Promotion by Skp2 of the degradation of p57Kip2 in a CDK consensus phosphorylation site-dependent manner. (A) Schematic representation of the domain organization of mouse WT Skp2 and the Skp2(ΔNF) mutant. (B and C) After transfection (24 h) with expression plasmids for the indicated proteins, HEK293T cells were incubated for the indicated times with CHX (50 μg/ml). Cell lysates were then subjected to immunoblot analysis with Abs to Myc or GSK-3β.
Fig. 5.
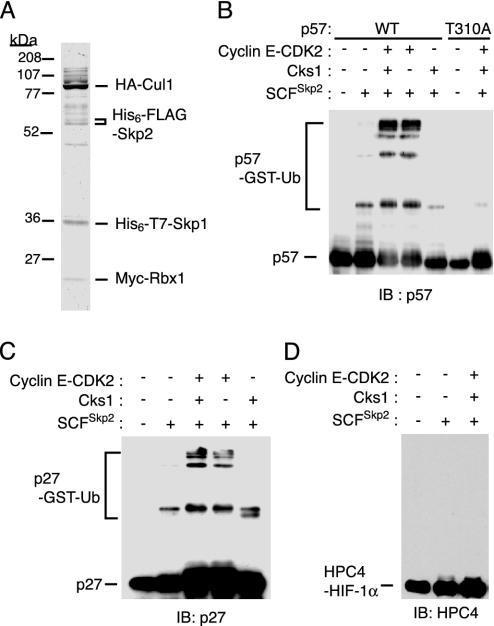
Ubiquitylation of p57Kip2 by the recombinant SCFSkp2 complex in vitro. (A) The recombinant SCFSkp2 complex purified from Sf21 insect cell lysates was analyzed by SDS/PAGE and staining with Coomassie brilliant blue. The positions of the various epitope-tagged recombinant proteins are indicated. (B-D) The recombinant SCFSkp2 complex was assayed for the ability to mediate the ubiquitylation of p57Kip2 (B), p27Kip1 (C), or HIF-1α (D) in the presence of ATP, Uba1, UbcH5A, and GST-ubiquitin (Ub), and in the absence or presence of the recombinant cyclin E-CDK2 complex or Cks1. Reaction products were subjected to immunoblot analysis with Abs to p57Kip2 (B), p27Kip1 (C), or HPC4 (D).
Promotion of p57Kip2 Degradation by Skp2. The importance of Skp2 as a determinant of p57Kip2 stability was further examined by transfecting HEK293T cells with an expression vector for human p57Kip2 in the absence or presence of a vector for either mouse WT Skp2 or a deletion mutant thereof (ΔNF) that lacks the NH2-terminal and F-box domains (Fig. 4A). After transfection (24 h), the cells were incubated with CHX for various times, lysed, and subjected to immunoblot analysis. Expression of Skp2(WT) induced a marked decrease in the stability of p57Kip2, compared to that apparent in control cells, whereas Skp2(ΔNF) prolonged the half-life of the recombinant CKI (Fig. 4B). These observations thus supported the notion that Skp2 interacts with and promotes the degradation of p57Kip2.
CDK Consensus Phosphorylation Site at Thr-310 as a Determinant of the Half-Life of p57Kip2. We next investigated the contribution of the CDK consensus phosphorylation site of human p57Kip2 (Thr-310) to the stability of this protein. HEK293T cells were transfected with the expression vector for Skp2(WT) together with an expression plasmid for either human WT p57Kip2 or the mutant p57(T310A). The cells were incubated with CHX for various times, after which cell lysates were subjected to immunoblot analysis. The stability of the p57(T310A) mutant was substantially greater than that of the WT protein (Fig. 4C), suggesting that the phosphorylation of p57Kip2 on Thr-310 triggers the degradation of this CKI.
Mediation of p57Kip2 Ubiquitylation by the Recombinant SCFSkp2 Complex in a CDK-Dependent Manner. Finally, to determine whether the SCFSkp2 complex mediates ubiquitylation of p57Kip2, we expressed the four subunits of the complex (Skp1, Cul1, Rbx1, and Skp2), each tagged with appropriate epitopes, in Sf21 cells. We then purified the recombinant complex to near homogeneity (Fig. 5A) and assayed it for its ability to catalyze the ubiquitylation of p57Kip2 in vitro in the presence of ATP, Uba1 (E1), UbcH5A (E2), and GST-ubiquitin. Polyubiquitylation of p57(WT) was evident in the additional presence of the recombinant cyclin E-CDK2 complex, whereas polyubiquitylation of p57(T310A) was not detected even in the presence of the cyclin E-CDK2 complex (Fig. 5B). We also examined whether the SCFSkp2 complex mediated the ubiquitylation of p27Kip1 and HIF-1α. As shown (16-18), the SCFSkp2 complex mediated the ubiquitylation of p27Kip1 (Fig. 5C). In contrast, HIF-1α was not ubiquitylated by the recombinant complex (Fig. 5D). The addition of recombinant Cks1 did not enhance the ubiquitylation of any of the two CKIs (see Discussion). These in vitro experiments thus demonstrated that the related CKIs p27Kip1 and p57Kip2 are both targets of the SCFSkp2 complex.
Discussion
Protein degradation by the ubiquitin-proteasome pathway plays a fundamental role in determining the abundance of important regulatory proteins. The E3 ubiquitin ligases are thought to determine the substrate specificity of this pathway, and many diverse E3 molecules are therefore thought to exist. Indeed, a large number of potential E3s has been identified from database searches based mostly on amino acid similarity to the homologous to E6-AP COOH terminus (HECT; 1), RING (3), or U-box (4-6) domain. The specific targets of E3 enzymes remain largely unknown, however. We have now provided direct biochemical and genetic evidence that Skp2 interacts with p57Kip2 and thereby induces its ubiquitin-dependent proteolysis. Both of the structurally related CKIs p57Kip2 and p27Kip1 thus accumulate in Skp2-/- cells as a result of a specific impairment in their degradation. Our observations indicate that SCFSkp2 plays a critical role in regulation of cell cycle progression by controlling the abundance of these two CKIs.
Our demonstration that SCFSkp2 regulates the stability of both p57Kip2 and p27Kip1 raises an important question: Why do such similar molecules exist? Generation of knockout mice has revealed that the loss of these proteins results in markedly different phenotypes (28, 32-36). The spatial and temporal expression patterns of these molecules during mouse development have also been characterized extensively (37). Whereas certain organs and tissues express both proteins, others express only p27Kip1 or p57Kip2. Furthermore, whereas the expression of p57Kip2 in most tissues is restricted to embryogenesis, that of p27Kip1 is maintained in many tissues into adulthood. These complex patterns of expression are consistent with the phenotypes of mice deficient in p27Kip1 or p57Kip2. Thus, the existence of two such closely related CKIs might be attributable to these differences in their expression patterns, rather than to differences in function. Given that the degradation of p27Kip1 and p57Kip2 appears to be mediated by a common mechanism, transcriptional or translational differences may largely underlie their distinct expression patterns.
It also remains unclear why, despite the similarity in the NH2-terminal and COOH-terminal regions of both human and mouse p57Kip2 and p27Kip1, the central region of p57Kip2 differs between the human and mouse molecules (38, 39). Whereas the middle portion of human p57Kip2 contains a PAPA-repeat domain, that of the mouse protein contains a proline-rich domain and an acidic-repeat domain (Fig. 2A). Such a substantial difference between human and mouse p57Kip2 orthologs is relatively unusual given the conservation through evolution apparent for most cell cycle regulators. As far as we are aware, however, no functional differences between human and mouse p57Kip2 have been demonstrated.
Compared to p27Kip1, relatively little is known about regulation of the expression of p57Kip2, largely because of the low abundance of this protein in many cell types. Recent studies (40, 41) have shown that p57Kip2 accumulates in osteoblastic cells arrested at G0 phase by serum deprivation and that it is degraded in a proteasome-dependent manner in these cells as a result of activation of the Smad-signaling pathway by transforming growth factor β1. Transcriptional regulation of Skp2 is mediated by the cell-cycle-dependent interaction of its promoter with GA-binding protein that is induced by activation of the Ras and mitogen-activated protein kinase signaling pathway (42). Although the Skp2 promoter does not appear to contain a consensus binding motif for Smad proteins, it is possible that the transforming growth factor β1 and Smad signaling pathway activates the Ras and mitogen-activated protein kinase pathway and thereby induces Skp2 expression, resulting in the degradation of p57Kip2.
E3 ubiquitin ligases are thought to recognize and ubiquitylate specific substrates in a manner dependent on the posttranslational modification of the target proteins. In S. cerevisiae, phosphorylation of Sic1 and Cln1/Cln2 triggers their ubiquitin-dependent proteolysis mediated by SCFCdc4 and SCFGrr1 complexes (9, 10, 43, 44). In mammals, in addition to phosphorylation, other modifications, including prolyl hydroxylation and N-glycosylation, are implicated in ubiquitin-mediated degradation. Thus, HIF-1α is ubiquitylated by the von Hippel-Lindau ubiquitin ligase in a prolyl hydroxylation-dependent manner (45-47), and the SCFFbx2 ubiquitin ligase binds specifically to proteins with N-linked high-mannose oligosaccharides and thereby contributes to their ubiquitylation (48). We now provide evidence that phosphorylation of p57Kip2 mediated by the cyclin E-CDK2 complex is required for degradation of this CKI by the SCFSkp2 complex. Cks1 has been shown to be necessary for the binding of Skp2 to p27Kip1 phosphorylated on Thr-187 (49, 50). Our in vitro ubiquitylation experiments, however, failed to detect an effect of Cks1 addition on the ubiquitylation of either p27Kip1 or p57Kip2, possibly because the endogenous Cks1 in insect cells copurified with the recombinant SCFSkp2 or cyclin E-CDK2 complexes. We could not show direct evidence that insect Cks1 is included in the precipitates with Skp2. Cks1 is the highly conserved protein through evolution: 48% identical and 65% similar between budding yeast and human. Given the high degree of conservation, it is likely that insect Cks1 is also present in our purified SCFSkp2 fraction and enhance the p57Kip2 and p27Kip1 ubiquitylation.
We recently showed that p27Kip1 is degraded by at least two distinct pathways (23): an SCFSkp2-dependent pathway that occurs during S-G2 phases in the nucleus and an SCFSkp2-independent pathway that occurs at the G0-G1 transition in the cytoplasm. Similarly, we have observed that p57Kip2 degradation also occurs at the G0-G1 transition even in Skp2-/- MEFs (T.K., T.H., and K.I.N., unpublished results). Further investigations are therefore necessary to clarify the regulatory mechanism of p57Kip2 degradation at the G0-G1 transition.
In conclusion, we have provided direct biochemical and genetic evidence that the degradation of p57Kip2 is mediated by the SCFSkp2 complex. Our observations thus provide a foundation for future efforts to understand the biochemical events that underlie cell cycle regulation by the SCFSkp2 complex.
Acknowledgments
We thank R. Yasukochi, K. Shimoharada, N. Nishimura, and S. Matsushita for technical assistance and C. Sugita for help in preparation of the manuscript. This work was supported in part by a grant from the Ministry of Education, Science, Sports, and Culture of Japan and a research grant from the Human Frontier Science Program.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: SCF, Skp1/Cul1/F-box; CDK, cyclin-dependent kinase; CKI, CDK inhibitor; GSK-3β, glycogen synthase kinase-3β; MEF, mouse embryonic fibroblast; HA, hemagglutinin; DEX, dexamethasone; CHX, cycloheximide; IP, immunoprecipitation; HIF-1α, hypoxiainducible factor 1α.
References
- 1.Hershko, A. & Ciechanover, A. (1998) Annu. Rev. Biochem. 67, 425-479. [DOI] [PubMed] [Google Scholar]
- 2.Hochstrasser, M. (1996) Annu. Rev. Genet. 30, 405-439. [DOI] [PubMed] [Google Scholar]
- 3.Joazeiro, C. A. & Weissman, A. M. (2000) Cell 102, 549-552. [DOI] [PubMed] [Google Scholar]
- 4.Hatakeyama, S., Yada, M., Matsumoto, M., Ishida, N. & Nakayama, K. I. (2001) J. Biol. Chem. 276, 33111-33120. [DOI] [PubMed] [Google Scholar]
- 5.Aravind, L. & Koonin, E. V. (2000) Curr. Biol. 10, R132-R134. [DOI] [PubMed] [Google Scholar]
- 6.Cyr, D. M., Hohfeld, J. & Patterson, C. (2002) Trends Biochem. Sci. 27, 368-375. [DOI] [PubMed] [Google Scholar]
- 7.Zachariae, W. & Nasmyth, K. (1999) Genes Dev. 13, 2039-2058. [DOI] [PubMed] [Google Scholar]
- 8.Bai, C., Sen, P., Hofmann, K., Ma, L., Goebl, M., Harper, J. W. & Elledge, S. J. (1996) Cell 86, 263-274. [DOI] [PubMed] [Google Scholar]
- 9.Feldman, R. M., Correll, C. C., Kaplan, K. B. & Deshaies, R. J. (1997) Cell 91, 221-230. [DOI] [PubMed] [Google Scholar]
- 10.Skowyra, D., Craig, K. L., Tyers, M., Elledge, S. J. & Harper, J. W. (1997) Cell 91, 209-219. [DOI] [PubMed] [Google Scholar]
- 11.Kamura, T., Koepp, D. M., Conrad, M. N., Skowyra, D., Moreland, R. J., Iliopoulos, O., Lane, W. S., Kaelin, W. G., Jr., Elledge, S. J., Conaway, R. C., et al. (1999) Science 284, 657-661. [DOI] [PubMed] [Google Scholar]
- 12.Ohta, T., Michel, J. J., Schottelius, A. J. & Xiong, Y. (1999) Mol. Cell 3, 535-541. [DOI] [PubMed] [Google Scholar]
- 13.Tan, P., Fuchs, S. Y., Chen, A., Wu, K., Gomez, C., Ronai, Z. & Pan, Z. Q. (1999) Mol. Cell 3, 527-533. [DOI] [PubMed] [Google Scholar]
- 14.Seol, J. H., Feldman, R. M., Zachariae, W., Shevchenko, A., Correll, C. C., Lyapina, S., Chi, Y., Galova, M., Claypool, J., Sandmeyer, S., et al. (1999) Genes Dev. 13, 1614-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang, H., Kobayashi, R., Galaktionov, K. & Beach, D. (1995) Cell 82, 915-925. [DOI] [PubMed] [Google Scholar]
- 16.Nakayama, K., Nagahama, H., Minamishima, Y. A., Matsumoto, M., Nakamichi, I., Kitagawa, K., Shirane, M., Tsunematsu, R., Tsukiyama, T., Ishida, N., et al. (2000) EMBO J. 19, 2069-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carrano, A. C., Eytan, E., Hershko, A. & Pagano, M. (1999) Nat. Cell Biol. 1, 193-199. [DOI] [PubMed] [Google Scholar]
- 18.Sutterluty, H., Chatelain, E., Marti, A., Wirbelauer, C., Senften, M., Muller, U. & Krek, W. (1999) Nat. Cell Biol. 1, 207-214. [DOI] [PubMed] [Google Scholar]
- 19.Marti, A., Wirbelauer, C., Scheffner, M. & Krek, W. (1999) Nat. Cell Biol. 1, 14-19. [DOI] [PubMed] [Google Scholar]
- 20.Mendez, J., Zou-Yang, X. H., Kim, S. Y., Hidaka, M., Tansey, W. P. & Stillman, B. (2002) Mol. Cell 9, 481-491. [DOI] [PubMed] [Google Scholar]
- 21.Kiernan, R. E., Emiliani, S., Nakayama, K., Castro, A., Labbe, J. C., Lorca, T., Nakayama, K., Nakayama, K. I. & Benkirane, M. (2001) Mol. Cell. Biol. 21, 7956-7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charrasse, S., Carena, I., Brondani, V., Klempnauer, K. H. & Ferrari, S. (2000) Oncogene 19, 2986-2995. [DOI] [PubMed] [Google Scholar]
- 23.Hara, T., Kamura, T., Nakayama, K., Oshikawa, K., Hatakeyama, S. & Nakayama, K. I. (2001) J. Biol. Chem. 276, 48937-48943. [DOI] [PubMed] [Google Scholar]
- 24.Sherr, C. J. & Roberts, J. M. (1999) Genes Dev. 13, 1501-1512. [DOI] [PubMed] [Google Scholar]
- 25.Nakayama, K. I. & Nakayama, K. (1998) BioEssays 20, 1020-1029. [DOI] [PubMed] [Google Scholar]
- 26.Tsvetkov, L. M., Yeh, K. H., Lee, S. J., Sun, H. & Zhang, H. (1999) Curr. Biol. 9, 661-664. [DOI] [PubMed] [Google Scholar]
- 27.Tateishi, K., Omata, M., Tanaka, K. & Chiba, T. (2001) J. Cell. Biol. 155, 571-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakayama, K., Ishida, N., Shirane, M., Inomata, A., Inoue, T., Shishido, N., Horii, I., Loh, D. Y. & Nakayama, K. I. (1996) Cell 85, 707-720. [DOI] [PubMed] [Google Scholar]
- 29.Kamura, T., Conrad, M. N., Yan, Q., Conaway, R. C. & Conaway, J. W. (1999) Genes Dev. 13, 2928-2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamura, T., Sato, S., Iwai, K., Czyzyk-Krzeska, M., Conaway, R. C. & Conaway, J. W. (2000) Proc. Natl. Acad. Sci. USA 97, 10430-10435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samuelsson, M. K., Pazirandeh, A., Davani, B. & Okret, S. (1999) Mol. Endocrinol. 13, 1811-1822. [DOI] [PubMed] [Google Scholar]
- 32.Kiyokawa, H., Kineman, R. D., Manova-Todorova, K. O., Soares, V. C., Hoffman, E. S., Ono, M., Khanam, D., Hayday, A. C., Frohman, L. A. & Koff, A. (1996) Cell 85, 721-732. [DOI] [PubMed] [Google Scholar]
- 33.Fero, M. L., Rivkin, M., Tasch, M., Porter, P., Carow, C. E., Firpo, E., Polyak, K., Tsai, L. H., Broudy, V., Perlmutter, R. M., et al. (1996) Cell 85, 733-744. [DOI] [PubMed] [Google Scholar]
- 34.Zhang, P., Liegeois, N. J., Wong, C., Finegold, M., Hou, H., Thompson, J. C., Silverman, A., Harper, J. W., DePinho, R. A. & Elledge, S. J. (1997) Nature 387, 151-158. [DOI] [PubMed] [Google Scholar]
- 35.Yan, Y., Frisen, J., Lee, M. H., Massague, J. & Barbacid, M. (1997) Genes Dev. 11, 973-983. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi, K., Nakayama, K. I. & Nakayama, K. (2000) J. Biochem. (Tokyo) 127, 73-83. [DOI] [PubMed] [Google Scholar]
- 37.Nagahama, H., Hatakeyama, S., Nakayama, K., Nagata, M., Tomita, K. & Nakayama, K. I. (2001) Anat. Embryol. 203, 77-87. [DOI] [PubMed] [Google Scholar]
- 38.Lee, M. H., Reynisdottir, I. & Massague, J. (1995) Genes Dev. 9, 639-649. [DOI] [PubMed] [Google Scholar]
- 39.Matsuoka, S., Edwards, M. C., Bai, C., Parker, S., Zhang, P., Baldini, A., Harper, J. W. & Elledge, S. J. (1995) Genes Dev. 9, 650-662. [DOI] [PubMed] [Google Scholar]
- 40.Nishimori, S., Tanaka, Y., Chiba, T., Fujii, M., Imamura, T., Miyazono, K., Ogasawara, T., Kawaguchi, H., Igarashi, T., Fujita, T., et al. (2001) J. Biol. Chem. 276, 10700-10705. [DOI] [PubMed] [Google Scholar]
- 41.Urano, T., Yashiroda, H., Muraoka, M., Tanaka, K., Hosoi, T., Inoue, S., Ouchi, Y. & Toyoshima, H. (1999) J. Biol. Chem. 274, 12197-12200. [DOI] [PubMed] [Google Scholar]
- 42.Imaki, H., Nakayama, K., Delehouzee, S., Handa, H., Kitagawa, M., Kamura, T. & Nakayama, K. I. (2003) Cancer Res., in press. [PubMed]
- 43.Skowyra, D., Koepp, D. M., Kamura, T., Conrad, M. N., Conaway, R. C., Conaway, J. W., Elledge, S. J. & Harper, J. W. (1999) Science 284, 662-665. [DOI] [PubMed] [Google Scholar]
- 44.Deshaies, R. J., Chau, V. & Kirschner, M. (1995) EMBO J. 14, 303-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Epstein, A. C., Gleadle, J. M., McNeill, L. A., Hewitson, K. S., O'Rourke, J., Mole, D. R., Mukherji, M., Metzen, E., Wilson, M. I., Dhanda, A., et al. (2001) Cell 107, 43-54. [DOI] [PubMed] [Google Scholar]
- 46.Ivan, M., Kondo, K., Yang, H., Kim, W., Valiando, J., Ohh, M., Salic, A., Asara, J. M., Lane, W. S. & Kaelin, W. G., Jr. (2001) Science 292, 464-468. [DOI] [PubMed] [Google Scholar]
- 47.Jaakkola, P., Mole, D. R., Tian, Y. M., Wilson, M. I., Gielbert, J., Gaskell, S. J., Kriegsheim, A., Hebestreit, H. F., Mukherji, M., Schofield, C. J., et al. (2001) Science 292, 468-472. [DOI] [PubMed] [Google Scholar]
- 48.Yoshida, Y., Chiba, T., Tokunaga, F., Kawasaki, H., Iwai, K., Suzuki, T., Ito, Y., Matsuoka, K., Yoshida, M., Tanaka, K. & Tai, T. (2002) Nature 418, 438-442. [DOI] [PubMed] [Google Scholar]
- 49.Ganoth, D., Bornstein, G., Ko, T. K., Larsen, B., Tyers, M., Pagano, M. & Hershko, A. (2001) Nat. Cell Biol. 3, 321-324. [DOI] [PubMed] [Google Scholar]
- 50.Spruck, C., Strohmaier, H., Watson, M., Smith, A. P., Ryan, A., Krek, T. W. & Reed, S. I. (2001) Mol. Cell 7, 639-650. [DOI] [PubMed] [Google Scholar]