Abstract
Between January 4 and 10, 1998, a severe ice storm impacted large areas of northern New York, New England, and eastern Canada. This storm struck the Hubbard Brook Experimental Forest in New Hampshire on January 7-8, 1998, and caused extensive forest crown damage (>30%) in a narrow elevation band (600-740 m) across the south-facing experimental watersheds. Stream water has been collected and chemically analyzed since 1963 in six experimental watersheds at Hubbard Brook Experimental Forest; thus, we were able to examine the effect of this severe natural disturbance on watershed nutrient export and changes in instream nitrate (NO3-) processing. The ice storm caused large increases in watershed export of NO3- for 2 years after the disturbance, but our examination of in-stream processing suggests that NO3- losses would have been much more dramatic had there not been an increase in in-stream, nitrogen-processing efficiency after the ice storm. The canopy damage that resulted from the ice storm led to increased light availability and large inputs of woody debris to the stream. We suspect that increases in algal production and storage and processing of terrestrial litter account for the increase in inorganic nitrogen processing in these streams. Our results indicate that, without in-stream processing, export of NO3- from the damaged watersheds would have been 80-140% higher than was observed. These results point to an intriguing negative feedback mechanism whereby the same disturbance that causes watershed NO3- loss may simultaneously lead to increased in-stream retention and transformation.
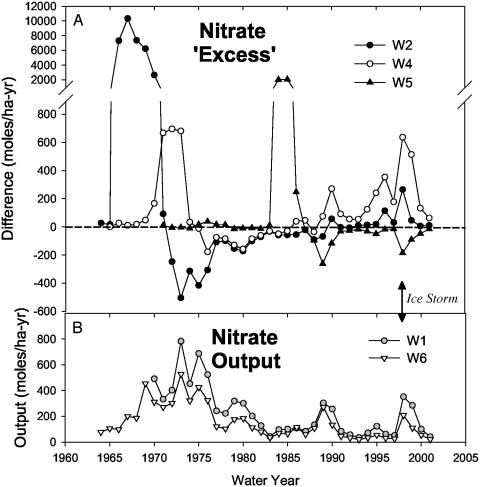
One of the major findings of the long-term Hubbard Brook Ecosystem Study is that disturbance of northern hardwood forests results in increased watershed losses of nitrogen (primarily as NO3-) (1-4). Nitrate export to stream water increases after deforestation because of reduced plant uptake, and increased rates of N mineralization and nitrification in upland soils (1, 4). Dramatic increases in streamwater NO3- fluxes followed whole watershed deforestation experiments in 1965-1968, 1974-1976, and 1983 (Fig. 1A). Despite differences in the severity of the deforestation treatment, all watersheds showed a similar pattern of nitrogen loss (3, 5). After a winter cutting or logging disturbance, NO3- concentrations in streams increased during the following growing season and peaked during the second growing season in a consistent pattern (Fig. 1A). The magnitude of the streamwater response varied with the type and intensity of disturbance.
Fig. 1.
(A) Nitrate concentrations in stream water from the three experimentally deforested watersheds (W2, W4, and W5). These data are normalized to the [NO3-] in-stream export from W6, the reference watershed, to more clearly show the effects of the cutting events without including the variation in export common to all six watersheds. (B) Patterns of NO3- export over the same time period for W1 and W6, with a clear increase in streamwater NO3- concentrations in the 2 years after the ice storm.
The ice storm that struck New England in January 1998 was the most severe of the century (6) and as such provided a natural corollary to previous deforestation experiments. After the ice storm, there was extensive tree canopy damage (>50% loss) in a narrow elevation band (600-740 m in elevation) across the south-facing watersheds (7). The impact was estimated at 30% canopy loss for two well studied watersheds (watersheds 1 and 6) at Hubbard Brook Experimental Forest (HBEF) (7, 8).
This natural disturbance provided us with the unique opportunity to confirm several major paradigms of our long-term studies: (i) that forest disturbance leads to reduced watershed retention of nitrogen and increased export of NO3- in stream water (1, 2, 9-11); (ii) that solute pulses added to streams are rapidly attenuated (12-16); and (iii) that this attenuation is often the result of in-stream retention and processing, which are important regulators of nutrient export from watersheds (14, 15, 17-19).
Methods
Site Description. This research was done in two south-facing watersheds within the HBEF. Both watersheds 1 (W1) and 6 (W6) are small (11.8 and 13.2 hectares, respectively) and have not had any active land use change since forest harvesting ceased around 1920. The HBEF is located in north central New Hampshire (43°56′N, 71°45′W), and has a cool continental climate. Most of the basin is forested by American beech (Fagus grandifolia), sugar maple (Acer saccharum), and yellow birch (Betula allegheniensis). Both streams drain steep forest watersheds and have a characteristic stairstep sequence of waterfalls and pools with a substrate of cobbles and boulders.
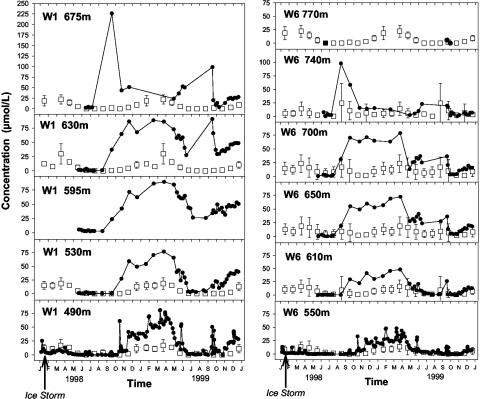
Data Collection. Stream samples were collected in W1 and W6 at monthly intervals. During summer 1999, immediately after the ice storm, stream samples were collected at approximately biweekly intervals. Streamwater samples were collected in acid-washed 0.5-liter high-density polyethylene bottles. Stream grab samples were collected along longitudinal elevation gradients: 675, 630, 595, 530, and 490 (weir) meters on W1, and 770, 740, 700, 650, 610, and 550 (weir) meters on W6. Longitudinal stream sampling began in 1990 in W1 and 1982 in W6. For the purposes of this analysis, we compared 2 years of post-ice storm data to 5 years (1993-1997) of predisturbance data. Stream discharge in both watersheds is monitored continuously over V-notch weirs at the outflow (20).
Hydrologic Flux Measurements. Annual volume-weighted stream NO3- concentrations were calculated for the sampling point just below the ice-storm damage zone (700 m elevation site for W6 and 630 m elevation site for W1) by using the period-weighted method (for a description, see ref. 20). To calculate annual hydrologic flux of NO3-, we multiplied the volume-weighted concentration by the total annual water yield from each site. Previous work at HBEF has demonstrated a strong relationship between watershed area and stream discharge in these watersheds, thus we calculated discharge and nutrient flux (discharge times nutrient concentration) at the damage-zone sampling site by multiplying discharge at the weir by the proportional watershed area of the subwatershed above the damage-zone sampling site.
In-Stream Uptake Measurements. To calculate in-stream uptake, we calculated the change in nutrient flux between the damage zone and weir sampling sites. To account for inputs of NO3- in subsurface flow, we multiplied total inflow (the difference between water yield from the damage zone sampling site and the weir) by a conservative estimate of subsurface NO3- (0.005 μg of N per m3) derived from a valleywide survey of groundwater seep concentrations (D.C.B., unpublished data).
Results and Discussion
Annual streamwater NO3- losses from the experimental watersheds after the ice storm were less than those seen for the whole-watershed deforestation experiments, no doubt because forest canopy damage in this case was confined to a discrete elevation band across the watershed. Thus, on a watershed scale, the weighted average crown loss was less than ≈30% of the total (7). The timing and pattern of NO3- loss, however, was similar to previous deforestation events (Fig. 1). After the ice storm, NO3- concentrations remained within the average for the preceding decade throughout the spring and summer months (Fig. 2). By September, increases in streamwater NO3- were observed (Fig. 2), and throughout the following year, streamwater losses of NO3- from the uncut W1 and W6 were significantly higher than they had been during the previous decade (Fig. 1). This natural “experiment” confirmed the results of three previous whole-watershed deforestation experiments. That is, large-scale tree damage and mortality within the watershed results in significant streamwater losses of NO3- from the system, with maximum increases in watershed N export lagging a growing season behind the actual disturbance.
Fig. 2.
Nitrate concentrations in stream water along the longitudinal sampling transects in W1 and W6. Samples taken during the 7 years before the ice storm are denoted with an open square, with mean ± SD for 1990-1997. Samples taken at each site after the ice storm are denoted with a filled circle.
Having established that the ice storm generated large increases in losses of NO3- from the terrestrial ecosystem to the stream, we explored the fate of that NO3- in stream water. We found that (i) NO3- pulses to streams declined rapidly with distance downstream from the damaged subwatersheds; (ii) that based on mass flux comparisons between the upper, damaged subwatersheds and the lower, whole-watershed stream monitoring sites, these differences could not be explained by dilution alone; and (iii) after the ice storm, in-stream uptake of NO3- exceeded export in both watersheds.
During 1998 and 1999, despite large inputs of NO3- into the ice-storm-damaged sections of W1 and W6 streams, longitudinal sampling of stream water revealed that NO3- concentrations in stream water at the base of the watersheds (at ≈500 m in elevation) were lower than streamwater NO3- concentrations from the damaged upper portion of the watersheds (>600 m in elevation) (Fig. 2). Inorganic NO3- concentrations in streams of the HBEF typically decline with declining elevation (21), and these patterns in stream water are correlated with elevational changes in soil-solution nitrogen concentrations (22, 23). Conversely, stream discharge always increases with downstream distance; thus, it is necessary to compare NO3- fluxes rather than concentration to separate between the mechanisms of dilution and in-stream uptake.
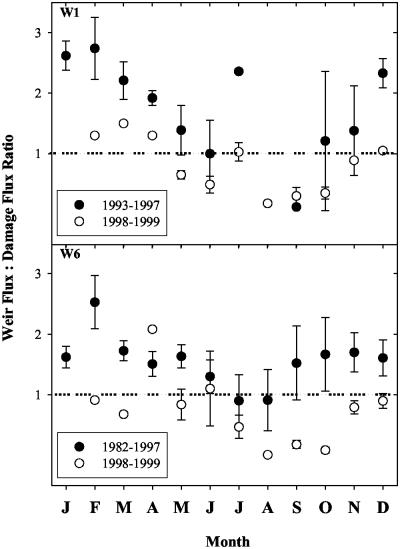
Nitrate mass fluxes declined between the damage zone and the weir, indicating that dilution alone could not explain the change in NO3- flux between damage zone and weir samples. Therefore, in-stream uptake was altering the patterns of inorganic nitrogen loss. For the 2 years after the ice storm, annual losses of NO3- over gauging weirs at the base of the watershed (although higher than they had been for the previous decade) were smaller than losses from the damage zones in both W1 and W6. This dramatic reduction of watershed N exports after the ice storm differed in magnitude from NO3- flux comparisons made throughout the preceding 5 years (Table 1). Before the ice storm, NO3- exports at the weir typically exceeded or equaled exports from the high-elevation subwatersheds in all months of the year (Table 1, Fig. 3). After the ice storm, the ratio of weir to damage-zone NO3- fluxes was consistently lower than it had been before the ice storm, and during the summer and early fall months, watershed export of NO3- was only a small fraction (<50%) of the NO3- flux from the damaged subwatersheds (Fig. 3).
Table 1. Losses and in-stream uptake of NO3-N before and after ice storm damage to W1 and W6 of the HBEF.
| Output from weir, mol·yr−1* | Output from damaged zone, mol·yr−1 | Subsurface inputs, mol·yr−1† | Instream uptake mol·yr−1‡ | Ratio uptake/export | Uptake rate, mg·m −2·d−1§ | |
|---|---|---|---|---|---|---|
| W1 | ||||||
| Before ice storm | 1,284 | 825 | 225 | No net uptake | NA | NA |
| After ice storm | 4,573 | 10,886 | 241 | 6,553 | 1.43 | 221 |
| W6 | ||||||
| Before ice storm | 743 | 531 | 362 | 150 | 0.20 | 5 |
| After ice storm | 3,001 | 5,088 | 406 | 2,493 | 0.83 | 85 |
NA, not applicable. Before ice storm measurements are from 1993-1997, and after ice storm measurements are from 1998-1999.
Output is calculated by multiplying the annual volume weighted NO3− concentration (from all synoptic survey dates) by the annual water yield from each site. Annual water yield from the damage zone is determined by multiplying water yield over the weir by the ratio of subwatershed/watershed area.
Subsurface inputs are estimated by multiplying the difference in annual water yield between the weir and the damage zone by a conservative estimate of subsurface NO3-N of 0.005 μmol/m3 (the lowest recorded values from a 2002 survey of HBEF seeps; D.C.B., unpublished data).
Annual instream uptake is calculated as the difference between weir output and NO3− inputs from the upstream damage zone and groundwater inflows.
Uptake rates are the annual instream uptake scaled to the area of the stream reach in each watershed between the damage zone and the weir and converted to per-day estimates.
Fig. 3.
Monthly mean ratios of weir/damage zone NO3- fluxes. When the ratio exceeds 1, there is a net gain in streamwater NO3- between the damage zone and the weir. When the ratio falls below 1, only the mechanism of in-stream uptake can explain the reduction in NO3- export.
The mean annual in-stream uptake rates calculated from these flux comparisons were greater than annual watershed NO3- export (Table 1). A large fraction of NO3- inputs to stream water in the extensive, crown-damage zone was not exported from W1 or W6 as NO3-, but was instead retained or transformed (and lost as gaseous N, dissolved organic N, or particulate N) within the stream reach. Uptake rates for NO3- based on the flux comparisons (24) are well within the range of values estimated from multiple short-term NO3- experimental releases for other south-facing watershed streams at the HBEF (0.3 to 716 mg of N per m2 per d; ref. 25). Because we used a low estimate of NO3- in subsurface flow (Table 1), our estimates are probably conservative estimates of actual in-stream uptake. Without in-stream uptake mechanisms, our flux comparisons indicate that the ice storm would have resulted in 80-140% greater annual exports of NO3- from W1 and W6 in the 2 years after the ice storm.
What mechanisms may account for this dramatic change in in-stream nitrogen processing? At the same time that the ice-damaged forest became less able to retain NO3-, algal populations in the stream bloomed as a function of more light reaching the stream channel, and the density of woody debris within the stream channel increased from fallen tree crowns (E.S.B. and D.C.B., personal observation). These changes were especially evident within the high damage zone (600-740 m elevation), and apparent to a lesser extent at lower elevations where the canopy damage was less severe. Seasonal variation in light penetration and organic matter input can strongly influence patterns of nutrient uptake in streams (12, 26, 27). Thus, large-scale canopy damage should be expected to lead to even more dramatic changes in stream nutrient cycling, because of prolonged impacts on light penetration and organic matter availability. Commercial deforestation can lead to higher rates of in-stream uptake of inorganic nutrients due to increased algal biomass (28), and woody debris is associated with greater in-stream retention of organic material and more “hotspots” for microbial activity (29-31). Recent work in the Adirondack Mountains of New York found increased woody debris inputs and debris dam density in first- through third-order streams as a result of the same 1998 ice storm (32), supporting our observational data.
Several recent studies have suggested that low-order streams are sites of maximum N transformations and retention (33, 34). The small streams draining the HBEF have low average water velocities, shallow water depths, and consequently greater water/sediment contact time, which in turn promotes the uptake of inorganic N in low-order streams (35, 36). Our study compares the effects of in-stream processing of NO3- before and after a large natural disturbance. These results suggest that biogeochemical cycles in stream ecosystems, though sensitive to the biogeochemical processes of their drainage basin, may respond differently to watershed disturbance. Therefore, mechanisms of in-stream NO3- removal should be considered when using streams to integrate processes occurring within terrestrial environments (i.e., ecosystem input-output budgets; ref. 37). In addition, the importance of in-stream processing for ameliorating terrestrial inputs found in this study suggests that protection and rehabilitation of healthy stream ecosystems is critical to preventing high inorganic nitrogen loading of rivers, estuaries, and oceans.
Changes in the patterns of NO3- export from watersheds associated with the 1998 ice storm confirmed the findings from many previous studies at HBEF, providing support for three of the major paradigms on which the Hubbard Brook Ecosystem Study has been built. After vegetation damage, NO3- inputs to draining streams increased dramatically over the following 2 years. These NO3- inputs to streams were rapidly attenuated as the water moved downstream, with annual watershed NO3- exports significantly lower than NO3- export from the upstream, damaged subwatersheds. Simple mass balances allow us to conclude that large amounts of NO3- were removed from stream water by in-stream processing, and that without in-stream uptake, watershed exports of NO3- would have been twice as high as actually measured. After the ice storm, the streams became more active processors of NO3- at the same time that inorganic N export from their watersheds increased. We have not yet determined whether the removal of NO3- from the water column was the result of retention (through denitrification and/or biotic assimilation) or simply the result of transformation and subsequent export of diverse organic forms of N. Nevertheless, our results illustrate an interesting and important interaction between streams and their drainage areas after major disturbance, with the two components of the watershed (upland and stream) reacting in opposite ways with respect to nutrient retention and following different recovery trajectories. We show that the stream can act as a buffer to changes in biogeochemical flux of inorganic nitrogen from the watershed caused by disturbance to terrestrial vegetation. Although the pattern is clear, more research is necessary to provide a mechanistic understanding of how in-stream processing may dampen or alter the forms of terrestrial export signals.
Acknowledgments
Reviews by Patrick Mulholland and Bruce Peterson significantly improved this manuscript. We thank Brian Dresser, Jason Demers, Christine Johnson, and Adam Welman for field assistance. Bob Hall and Ben Houlton provided helpful comments. E.S.B. thanks Duke University and the University of Maryland for support during the writing of this paper. This research was supported by The Andrew W. Mellon Foundation and the National Science Foundation, and is a contribution to the Hubbard Brook Ecosystem Study and the Program of the Institute of Ecosystem Studies. The Hubbard Brook Experimental Forest is operated and maintained by the Northeastern Forest Research Station, U.S. Department of Agriculture (Newtown Square, PA).
Abbreviations: HBEF, Hubbard Brook Experimental Forest; Wn, watershed n.
See commentary on page 10137.
References
- 1.Likens, G. E., Bormann, F. H. & Johnson, N. M. (1969) Science 163, 1205-1206. [DOI] [PubMed] [Google Scholar]
- 2.Likens, G. E., Bormann, F. H., Johnson, N. M., Fisher, D. W. & Pierce, R. S. (1970) Ecol. Monogr. 40, 23-47. [Google Scholar]
- 3.Aber, J. D., Ollinger, S. V., Driscoll, C. T., Likens, G. E., Holmes, R. T., Freuder, R. J. & Goodale, C. L. (2002) Ecosystems 5, 648-658. [Google Scholar]
- 4.Bormann, F. H. & Likens, G. E. (1979) Pattern and Process in a Forested Ecosystem (Springer, New York).
- 5.Martin, C. W., Pierce, R. S., Likens, G. E. & Bormann, F. H. (1986) Clearcutting Affects Stream Chemistry in the White Mountains of New Hampshire (U.S. Department of Agriculture, Broomall, PA), Publication no. NE579.
- 6.DeGaetano, A. T. (2000) Bull. Am. Meteorol. Soc. 81, 237-254. [Google Scholar]
- 7.Houlton, B. Z., Driscoll, C. T., Fahey, T. G., Groffman, P. M., Likens, G. E., Bernhardt, E. S. & Buso, D. (2003) Ecosystems, in press.
- 8.Rhoads, A. G., Hamburg, S. P., Fahey, T. J., Siccama, T. G., Hane, E. N., Battles, J., Cogbill, C., Randall, J. & Wilson, G. (2002) Can. J. Forest Res. 32, 1763-1775. [Google Scholar]
- 9.Bormann, F. H., Likens, G. E., Fisher, D. W. & Pierce, R. S. (1968) Science 159, 882-884. [DOI] [PubMed] [Google Scholar]
- 10.Bormann, F. H., Likens, G. E., Siccama, T. G., Pierce, R. S. & Eaton, J. S. (1974) Ecol. Monogr. 44, 255-277. [Google Scholar]
- 11.Vitousek, P. M., Gosz, J. R., Grier, C. C., Melillo, J. M., Reiners, W. A. & Todd, R. L. (1979) Science 204, 469-474. [DOI] [PubMed] [Google Scholar]
- 12.Meyer, J. L. (1979) Limnol. Oceanogr. 24, 365-375. [Google Scholar]
- 13.Hall, R. J., Driscoll, C. T. & Likens, G. E. (1987) Freshwater Biol. 18, 17-43. [Google Scholar]
- 14.Hall, R. O., Macneale, K. H., Bernhardt, E. S., Field, M. & Likens, G. E. (2001) Freshwater Biol. 46, 291-302. [Google Scholar]
- 15.Richey, J. S., McDowell, W. H. & Likens, G. E. (1985) Hydrobiologia 124, 129-139. [Google Scholar]
- 16.McDowell, W. H. (1985) Biogeochemistry 1, 329-352. [Google Scholar]
- 17.Fisher, S. G. & Likens, G. E. (1973) Ecol. Monogr. 43, 421-439. [Google Scholar]
- 18.Meyer, J. L. & Likens, G. E. (1979) Ecology 60, 1255-1269. [Google Scholar]
- 19.Bernhardt, E. S. (2001) Ph.D. thesis (Cornell University, Ithaca, NY), p. 185.
- 20.Likens, G. E. & Bormann, F. H. (1995) Biogeochemistry of a Forested Ecosystem (Springer, New York).
- 21.Johnson, N. M., Driscoll, C. T., Eaton, J. S., Likens, G. E. & McDowell, W. H. (1981) Geochim. Cosmochim. Acta 45, 1421-1437. [Google Scholar]
- 22.Lawrence, G. B., Fuller, R. D. & Driscoll, C. T. (1987) J. Environ. Qual. 16, 383-390. [Google Scholar]
- 23.Bohlen, P. J., Groffman, P. M., Driscoll, C. T., Fahey, T. J. & Siccama, T. G. (2001) Ecology 82, 965-978. [Google Scholar]
- 24.Newbold, J. D., Oneill, R. V., Elwood, J. W. & Vanwinkle, W. (1982) Am. Nat. 120, 628-652. [Google Scholar]
- 25.Bernhardt, E. S., Hall, R. O. & Likens, G. E. (2002) Ecosystems 5, 419-430. [Google Scholar]
- 26.Mulholland, P. J., Newbold, J. D., Elwood, J. W. & Webster, J. R. (1985) Ecology 66, 1012-1023. [Google Scholar]
- 27.Mulholland, P. J. & Hill, W. R. (1997) Water Resourc. Res. 33, 1297-1306. [Google Scholar]
- 28.Sabater, F., Butturini, A., Marti, E., Munoz, I., Romani, A., Wray, J. & Sabater, S. (2000) J. North Am. Benthol. Soc. 19, 609-620. [Google Scholar]
- 29.Bilby, R. E. (1981) Ecology 62, 1234-1243. [Google Scholar]
- 30.Hedin, L. O. (1990) Oikos 57, 94-105. [Google Scholar]
- 31.Steinhart, G., Likens, G. & Groffman, P. (2001) Verh. Int. Ver. Theor. Angew. Limnol. 27, 1331-1336. [Google Scholar]
- 32.Kraft, C. E., Schneider, R. L. & Warren, D. R. (2002) Can. J. Fish. Aquat. Sci. 59, 1677-1684. [Google Scholar]
- 33.Alexander, R. B., Smith, R. A. & Schwarz, G. E. (2000) Nature 403, 758-761. [DOI] [PubMed] [Google Scholar]
- 34.Seitzinger, S. P., Styles, R. V., Boyer, E. W., Alexander, R. B., Billen, G., Howarth, R. W., Mayer, B. & Van Breemen, N. (2002) Biogeochemistry 57, 199-237. [Google Scholar]
- 35.Peterson, B. J., Wollheim, W. M., Mulholland, P. J., Webster, J. R., Meyer, J. L., Tank, J. L., Marti, E., Bowden, W. B., Valett, H. M., Hershey, A. E., et al. (2001) Science 292, 86-90. [DOI] [PubMed] [Google Scholar]
- 36.Wollheim, W. M., Peterson, B. J., Deegan, L. A., Hobbie, J. E., Hooker, B., Bowden, W. B., Edwardson, K. J., Arscott, D. B. & Hershey, A. E. (2001) Limnol. Oceanogr. 46, 1-13. [Google Scholar]
- 37.Bormann, F. H. & Likens, G. E. (1967) Science 155, 424-429. [DOI] [PubMed] [Google Scholar]