Abstract
In many species of social insects, the cuticular hydrocarbons of adults vary with both colony identity and individual physiology (oogenesis). Such variations have been shown in some ants and social wasps to function in nestmate recognition, but as yet there is no demonstration of their use by workers to recognize egg layers. We report that in the ant Myrmecia gulosa, workers can discriminate queens and fertile workers from infertile individuals based on distinctive blends of long-chained hydrocarbons present both on the cuticle and in the postpharyngeal gland. The purified hydrocarbon fraction of cuticular extracts from queens elicited high interest in workers, unlike the nonhydrocarbon fraction. However, both fractions were necessary to trigger a response of maximal intensity. In contrast, extracts of mandibular and Dufour glands from queens or infertile workers were not treated differentially by workers. We suggest that cuticular hydrocarbons function as pheromones allowing for recognition of the queen as well as egg-laying workers.
In insect societies chemical communication plays essential roles. At the group level, individuals must recognize colony membership to avoid exploitation of resources by nonnestmates. At the individual level, the presence of a reproductive must be detected by the infertile nestmates to maximize their reproductive interests (e.g., ref. 1). In addition to their implication against desiccation (2-5), long-chained hydrocarbons (HCs) present on the cuticle seem to play a major role in both these identification mechanisms. In solitary insects, cuticular HCs (CHCs) can be sex attractants or used for species recognition (reviewed in refs. 2, 3, and 6). In social insects, CHCs are known to be colony-specific (e.g., refs. 7 and 8) and in some ants were demonstrated to be the basis of nestmate recognition (9-11). In the latter studies, the purified HC fraction of cuticular extracts from workers was applied on live ants or artificial lures, and the response of nestmates or nonnestmates was modified according to the origin of the extracts to which they were exposed. Nestmate and nonnestmate extracts elicited weak and strong aggression, respectively.
CHCs also correlate with ovarian activity in various solitary (12-14) and social (7, 8, 15-22) insects. It has been shown that CHC profiles shift in a predictable manner after either the onset or a decline in egg-laying activity. CHCs thus encode reliable information about reproductive physiology and have been suggested to function in the recognition of fertile and infertile nestmates (refs. 18-23 and V. Cuvillier-Hot, A. Lenoir, R. Crewe, C. Malosse, and C.P., unpublished data). However, bioassays are needed to demonstrate that ant workers can detect variations in CHC profiles.
Myrmecia gulosa belongs to the phylogenetically primitive ant subfamily Myrmeciinae. Queens are morphologically specialized relative to the workers. Their ovaries are bigger (44 ovarioles compared with 8-14 in workers), and thus they can lay more eggs (24). A retinue of workers constantly surrounds the queen, and in her presence, workers do not reproduce but lay unviable trophic eggs (24). Queens thus monopolize reproduction and are clearly recognized. When workers are experimentally induced to produce unfertilized, male-destined eggs in the presence of their queen, they are attacked by nestmates (25), which indicates that fertile workers of M. gulosa are recognized as already demonstrated in other ants (26-28).
The ability of workers to discriminate between individuals of contrasting reproductive status enabled us to use bioassays to investigate the underlying chemical mechanism. We first verified the correlation between CHCs and ovarian activity in M. gulosa. These HCs were extracted from the cuticle as well as the postpharyngeal gland (PPG), where CHCs accumulate during self-grooming (refs. 29-33 and R. Beard and V.D., unpublished data). The extracts from fertile queens, workers laying reproductive eggs, and infertile workers were transferred on glass slides that were presented to nestmate workers. If these extracts elicit different amounts of attention, then workers are able to recognize different blends of CHCs and use them to discriminate reproductive from nonreproductive individuals.
Methods
Ant Collection and Laboratory Rearing. Complete nests of M. gulosa were excavated in October 1999 and 2000 from a sandstone area near Waterfall, New South Wales, Australia. For practical reasons, the number of individuals brought back for laboratory rearing was reduced; together with the queen and all the eggs, 500 workers and up to 250 larvae and 100 cocoons were selected randomly. The ants were kept in plaster-of-Paris nests into which chambers had been molded. A glass plate covered each chamber to allow for observations and removal of individuals. The nests were connected to foraging arenas where food (pieces of cockroaches or entire crickets and honeywater) was deposited every 1-2 days. The temperature was maintained at 24 ± 1°C, the photoperiod was set at 10:14-h (light/darkness) cycles, and a high humidity was maintained inside the nests by regularly moistening the plaster.
Extraction of CHCs and GC. Solid-phase microextraction (34) was used to extract CHCs from live individuals (17). The ants were immobilized by blocking their postpetiole in a split cardboard, which reduced the mobility of the ant's gaster and allowed the rubbing of a solid-phase microextraction fiber (SUPELCO, Bellefonte, PA, coated with a 7-μm polydimethylsiloxane film) on the tergites. The rubbing was performed for 30 sec in a standardized manner. The fiber then was inserted in the injection port (260°C) of a Carlo Erba 8130 gas chromatograph equipped with a DB-1 nonpolar capillary column (30 m × 0.32 mm × 0.25 μm,J&W Scientific, Folsom, CA). Helium was used as a carrier gas with a column head pressure of 95 kPa. Samples were run in the splitless mode. The column temperature was maintained at 60°C for 4 min and then raised to 250°C at a rate of 20°C·min-1 and from 250°C to 300°C at 2.5°C·min-1. Flame ionization detector temperature was set at 310°C.
We extracted CHCs of 12 mated queens from 12 colonies as well as CHCs of 12 virgin queens from 2 colonies. The queen-right workers measured (n = 12) originated from five colonies, and the workers laying reproductive eggs (n = 12) originated from five orphaned groups of workers. Because worker reproduction only occurs in orphaned situations (24), we also extracted 10 trophic egg-laying workers from two orphaned colonies to separate the effects of queen loss and of reproductive activity on the CHCs of workers.
The solid-phase microextraction/GC analysis yielded 92 peaks. The number of variables to be used in multivariate statistical analysis was reduced (see ref. 18). We selected the peaks that represented >0.3% relative peak area and that were present in all the individuals. The relative areas of the 17 selected peaks were restandardized to 100% and transformed following Aitchison's formula (35),
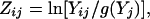 |
where Zij is the standardized peak area i for individual j, Yij is the peak area i for individual j, and g(Yj) is the geometric mean of all peaks for individual j. The homogeneity of variance of these variables was tested with Levene's test, and Bonferroni's correction was applied. Only 13 variables had homogeneous variances and were considered. The transformed areas were used as variables in a principal component analysis. The four principal components extracted then were used as variables in a discriminant analysis. This procedure does not allow the identification of the discriminating compounds. A stepwise analysis was not performed, because the proportion of describing variables vs. sample size was too large to fulfill the statistical requirements for this procedure.
In M. gulosa a large proportion of both large and small workers produce trophic eggs in the presence of their queen; these fragile yolk sacs without a rigid chorion are mostly given to larvae (24). To determine whether the cuticular profiles varied with the rate of trophic egg laying, two categories of workers were extracted: workers without oocytes or with only submature trophic oocytes (n = 9) and workers with mature trophic oocytes in most ovarioles (n = 9). Their cuticular profiles were compared with those of the orphaned reproductive workers used in the previous analysis, but 18 compounds were selected and three principal components were used in a discriminant analysis.
Bioassays of Glandular Extracts. Individuals were freeze-killed at -70°C for 10 min, and PPGs, Dufour glands, and mandibular glands were dissected out after thawing. The glands were excised in Ringer's solution with pentane-cleaned forceps and stored in glass vials. The vials were frozen at -70°C for several hours, and after 1 h of thawing, 100 μl of hexane was added. The vials then were placed in an ultrasonic bath for 5 min to release the chemicals they contained as completely as possible. The long-chain HCs were extracted from either the cuticle or the PPGs. The PPGs store CHCs in several ant species (29-33), and large quantities of compounds can be collected from the reservoirs (31, 36). Poison glands of queens were always degenerated and empty (n = 12; V.D., unpublished data) and thus were excluded from this study.
We compared the interest triggered by queen vs. infertile worker extracts of each gland. In M. gulosa, most workers produce unviable trophic eggs in the presence of their queen (24). For extraction, we selected workers that had just laid a trophic egg.
Gland extracts from orphaned reproductive workers were also tested against those of infertile individuals. These workers originated from groups of 100-200 individuals without the queen. These groups were created by randomly choosing workers from all chambers of the nests to avoid biasing the sample toward particular age or task classes. M. gulosa egg layers are difficult to identify by direct observations. Some workers present near the egg pile had a protruding sting sheath and stood high on their legs, suggesting they were about to oviposit. To confirm this, they were taken out of the nest and individually isolated overnight in a plastic box with moist cotton. On the next day, the presence of eggs in the box was checked. The workers that did not lay eggs were not used in the experiment. The ovarian status of all the workers was verified later by dissection.
For the bioassays, we used one of the inhabited chambers (90 × 110 mm) of the rearing nests to provide a biologically relevant context. After blocking access to this chamber with a cotton ball, it was emptied from its workers. Ten large and 10 small workers were selected randomly and left in the chamber for 10 min to calm down. One-tenth of the gland extracts of either queen/infertile worker or trophic/reproductive egg layers was then applied on two 18 × 18-mm glass cover slides cleaned with pentane. After solvent evaporation, the cover slides were introduced in the middle of the chamber without disturbing the ants. The slides were placed 4 cm apart from each other. The extracts were tested only with nestmates of the extracted individuals. The extracts were presented to 6-13 different groups of 20 queenright nestmate workers in each of three colonies. In the queen vs. infertile worker bioassay, the same extracts were used in six repetitions (because only one queen is available per nest), whereas in the reproductive vs. infertile worker bioassay, a different extract was used for each repetition. The bioassays were done in the 48 h after the extraction of queens and workers. Controls consisted of two cover slides on which 5 μl of hexane was applied. They were presented to workers as described above. Gland extracts and controls were presented to each group of workers in sequences of randomized order.
The interest of workers was quantified as the cumulated number of antennal inspections received by the extracts or controls during 5 min. An inspection started as an individual's antennae touched the extract and was deemed to finish once the antennae left the vertical projection area of the cover slides. The position of the two extracts tested was alternated for each repetition by using a blind protocol. The cumulated numbers of antennal inspections were compared with a Wilcoxon test for paired samples for each colony.
Bioassays of Polar vs. Nonpolar Cuticular Compounds. HCs are the major lipid constituents of insect epicuticle, but non-HC polar lipids are also present (cf. refs. 2 and 4). Non-HCs are found only in traces in M. gulosa and do not differ among individuals of different morphological caste or reproductive status (R. Beard and V.D., unpublished data). However, we tested the biological activity of polar and nonpolar constituents separately. The procedure of Lahav et al. (9) to fractionate lipid extracts into HC and non-HC compounds was adapted for M. gulosa. Total lipid extracts (37) were obtained from the cuticle of single queens (after freeze-killing but before dissection, see above) and pools of five nestmate workers. Pools of several workers were used because the queen produces more CHCs than individual workers (25). The extracts were evaporated and rediluted in 200 μl of hexane. These solutions were loaded onto a silica gel column (Chromabond 500 mg, Macherey & Nagel) and eluted with 10 ml of hexane to obtain the HC fraction and then with 4 ml of hexane/chloroform (1:1), 5 ml of chloroform, and 5 ml of methanol to obtain the more polar non-HC components. The fractions obtained were left to evaporate under a fume hood. The HC fractions were rediluted in 200 μl of hexane, and all the others were pooled in 200 μl of chloroform/methanol (1:2). The quality and yield of the fractioning process were verified by GC analysis.
One hundredth of the queen extracts and a volume of worker extracts containing the equivalent quantity of HCs were used in the bioassays. Extracts in hexane (5 μl) and hexane controls (5 μl) were applied on glass cover slides, and the interest of nestmate workers was quantified and compared as described above.
Results
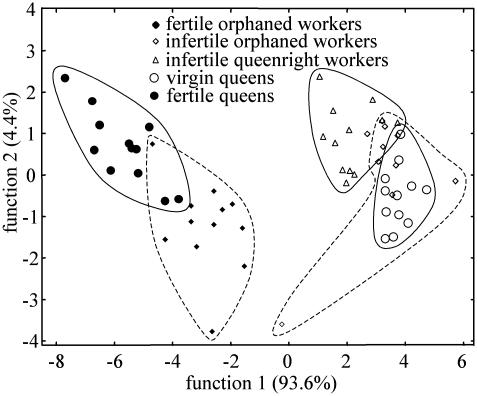
HC Profiles and Reproductive Status. The CHCs of M. gulosa are a complex mixture of alkanes, alkenes, and methyl-branched alkanes ranging from C23 to C39 (R. Beard and V.D., unpublished data). Two compounds (9-pentacosene and 3-methyl-pentacosane; Fig. 1) were present in high proportions (range 12-43%) in reproductive individuals (both queens and workers) but were absent or in trace quantities in nonreproductive individuals. In addition to these conspicuous differences, a discriminant analysis with 13 other compounds (peaks numbered in Fig. 1) revealed that each category of individuals has typical blends of these HCs [Wilk's λ = 0.03, F(16,153) = 21.7, P < 0.01; Fig. 2]. All individuals able to lay reproductive eggs had similar CHC profiles regardless of morphological caste. Although all categories were statistically separated, virgin queens and infertile workers (both queenright and orphaned) clustered together, whereas fertile queens and workers laying reproductive eggs formed a second group (Fig. 2). These reproductive and nonreproductive poles were separated by the first discriminant function explaining 93.6% of the variance. Within these poles, misclassification occurred, but the model permitted the correct classification of 87.9% of the individuals.
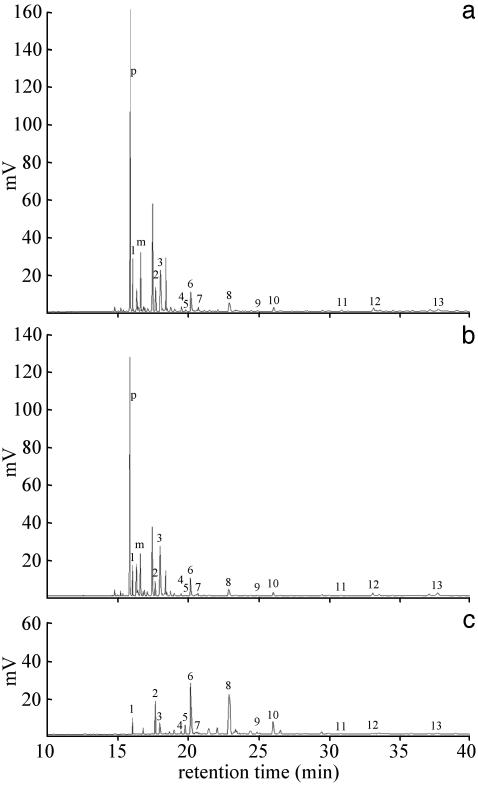
Fig. 1.
Chromatograms of CHCs of M. gulosa obtained by solid-phase microextraction. (a) Fertile queen. (b) Worker laying reproductive eggs. (c) Infertile worker. Two compounds [9-pentacosene (p) and 3-methylpentacosane (m)] clearly differentiate reproductives from nonreproductives. Used in the statistical analysis are pentacosane (peak 1), heptacosane (peak 2), 9,11,13,15-methylheptacosane (peak 3), 10,11,12,13,14-methyloctacosane (peak 4), nonacosene (peak 5), nonacosane (peak 6), 9,11,13,15-methylnonacosane (peak 7), 3-methylnonacosane (peak 8), hentriacontane (peak 9), 9,11,13,15-methylhentriacontane (peak 10), 9,11,13,15-methylheptatriacontane (peak 11), and unknown (peaks 12 and 13).
Fig. 2.
Discriminant analysis of five categories of individuals based on the relative proportions of 13 cuticular compounds. Reproductive (filled symbols) and nonreproductive (open symbols) individuals are separated completely. The groups are encircled arbitrarily. Solid lines indicate individuals present in queenright colonies and dotted lines designate orphaned workers.
In M. gulosa, many of the infertile workers exhibit a limited degree of ovarian activity that allows them to lay trophic eggs. A discriminant analysis with 18 compounds [Wilk's λ = 0.09, F(6,50) = 19.92, P < 0.01] showed that individuals with high and low ovarian development could not be separated (P = 0.36) but that their cuticular profiles were very distinct from those of workers laying reproductive eggs (P < 0.01 in both cases).
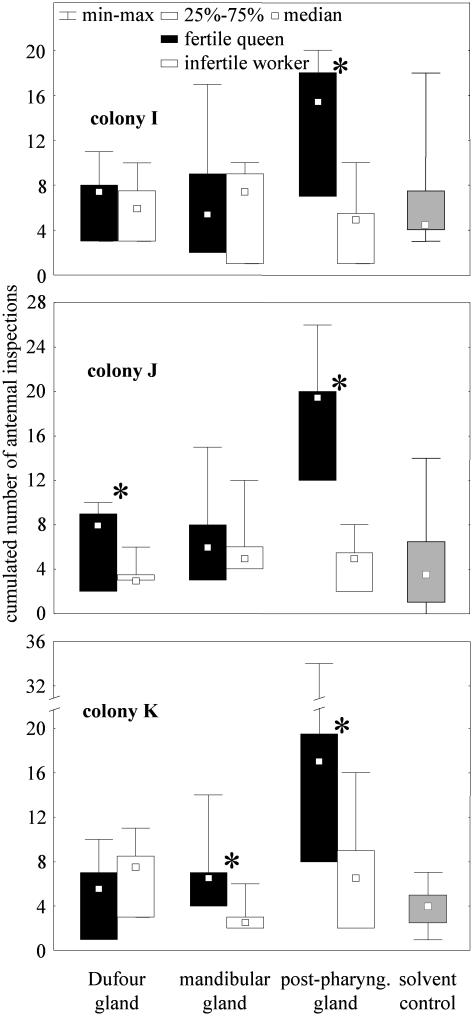
Gland Extracts and Queen Recognition. In all three colonies studied (I-K), the extracts of the queens' PPGs triggered significantly more interest than those of the infertile workers (TI = 0.0 and pI = 0.03; TJ = 0.0 and pJ = 0.03; TK = 1.0 and pK = 0.046) (Fig. 3). In contrast, the Dufour and mandibular gland extracts of queens were each preferred in only one colony (Wilcoxon test, n = 6 in all cases, Dufour gland: TI = 6.0 and pI = 0.35; TJ = 1.0 and pJ = 0.046; TK = 5.0 and pK = 0.25; mandibular gland: TI = 10.0 and pI = 0.92; TJ = 8.5 and pJ = 0.68; TK = 1.0 and pK = 0.046) (Fig. 3).
Fig. 3.
Bioassay of glandular extracts from queens and infertile workers. Twenty workers were exposed to 1/10th of mandibular, Dufour, and PPGs extracts in each of three colonies. The cumulated number of antennal inspections elicited in 5 min was compared with the Wilcoxon test (* indicates significant differences, P < 0.05). Five microliters of hexane applied on two cover slides constituted the solvent control. Because the attention elicited was not significantly different for the two control slides (Wilcoxon test), results were pooled for clarity. The number of replications was six for each category.
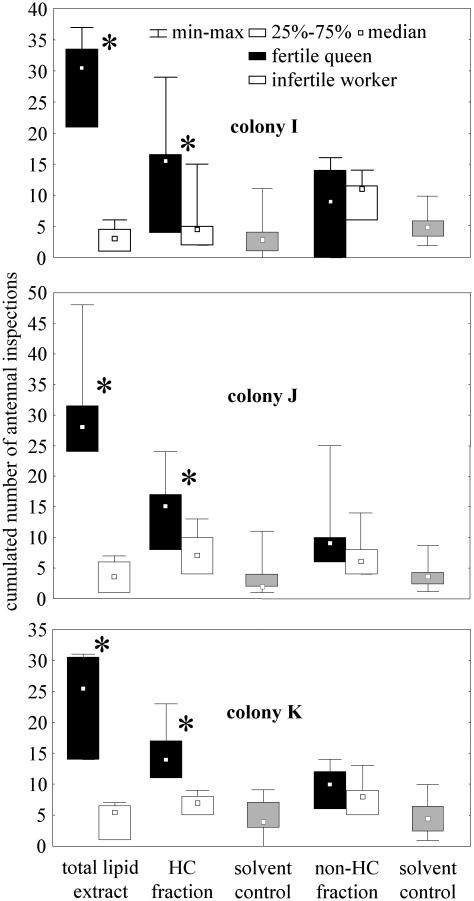
HC Fraction of Cuticle Extracts and Queen Recognition. Workers showed more interest in the total lipid extracts of queens' cuticles than in those of the infertile workers (Wilcoxon test, n = 6 in all cases: TI = 0.0 and pI = 0.03; TJ = 0.0 and pJ = 0.03; TK = 0.0 and pK = 0.03) (Fig. 4). The same was true for the purified HC fraction from these extracts (Wilcoxon test, n = 6 in all cases: TI = 0.0 and pI = 0.03; TJ = 0.0 and pJ = 0.04; TK = 0.0 and pK = 0.03). No preference was shown when the non-HC fractions of the queen and infertile worker extracts were compared (Wilcoxon test, n = 6 in all cases: TI = 7.0 and pI = 0.46; TJ = 3.0 and pJ = 0.47; TK = 3.0 and pK = 0.23). The intensity of antennation directed at the queen extracts was higher for the total lipid extract compared with the HC fraction (Mann-Whitney test, ntotal lipids = 6 in all cases and nHC = 6 in all cases: UI = 5.0 and pI = 0.04; UJ = 5.0 and pJ < 0.01; UK = 2.5 and pK = 0.01) (Fig. 4).
Fig. 4.
Bioassay of cuticular extracts from queens and infertile workers. Twenty workers were exposed to 1/100th of cuticular extracts of nestmate queens and to the matching quantity of nestmate worker extracts. Total lipid extracts as well as their HC and non-HC fractions were tested. We monitored the preference of the workers for each extract in terms of cumulated number of antennal inspection in 5 min (* indicates significant differences, Wilcoxon test, P < 0.05). Five microliters of hexane applied on two cover slides constituted the solvent control. Because the attention elicited was not significantly different for the two control slides (Wilcoxon test), results were pooled for clarity. The number of replications was six for each category.
Gland Extracts and Recognition of Reproductive Workers. The comparison of gland extracts from infertile workers and reproductive workers gives similar results to those for queen recognition. Only the PPG extracts allowed discrimination between the two categories. Reproductive worker PPG extracts elicited significantly more interest in nestmates than PPG extracts from infertile workers (Wilcoxon test, n = 9-13, PPG: TI = 0.0 and pI < 0.01; TJ = 4.5 and pJ = 0.01; TK = 5.5 and pK = 0.04; Dufour gland: TI = 12.0 and pI = 0.74; TJ = 28.5 and pJ = 0.69; TK = 7.5 and pK = 0.14; mandibular gland: TI = 22.0 and pI = 0.95; TJ = 22.0 and pJ = 0.58; TK = 12.5 and pK = 0.23).
Discussion
CHCs Correlate with Reproductive Status. Variations in the CHCs of workers and queens in M. gulosa reflect individual reproductive status. Distinct CHC profiles were always associated with reproductive activity in both morphological castes. Two compounds (both with 25 carbon atoms) were characteristic of reproductives. The workers that shifted to the production of reproductive eggs also produced these molecules together with proportions of other CHCs typical of the fertile queens (Fig. 2). Although the reproductive workers of M. gulosa cannot mate, the resemblance of their cuticular profiles to those of the queens is similar to that found for the gamergates and fertile queens in the ponerine ant Harpegnathos saltator (18). These intracolonial variations are independent of age, because the age structure of orphaned and queenright groups was randomized in our experiments. Orphaned infertile workers kept a profile similar to that of queenright workers, showing that changes in CHCs are not caused by our manipulations. Furthermore, the profiles of fertile and virgin (infertile) queens did not overlap. Thus, as in other social Hymenoptera (see the introduction), CHCs of M. gulosa encode reliable information about fertility.
Although fertility has a striking effect on the CHCs, workers laying trophic eggs had different cuticular profiles than those of reproductives. Our study of the variations of CHCs in an ant producing trophic eggs gives insight into which physiological traits affect the synthesis of CHCs.
Recognition of Reproductive Status. In colonies of M. gulosa, queens and reproductive workers are recognized by their nest-mates in which they elicit high interest; a retinue is formed around queens, whereas workers that start reproducing are often surrounded by nestmates and immobilized (25). We bring evidence that these behaviors can be based on the detection of specific CHC profiles. Indeed, cuticular compounds from the PPG of queens and reproductive workers elicited more interest in nestmates than those of infertile workers. In M. gulosa, as in other ants studied, the PPG content closely reflects the CHC profile of an individual, and PPG contents of queens and infertile and reproductive workers vary (R. Beard and V.D., unpublished data). Furthermore, the HC fraction of the queen cuticular lipid extracts played the major role in eliciting interest. The non-HC fraction on its own lacked such effect, but its presence together with the HC fraction increased the intensity of the nestmate workers' response. Indeed, the total lipid extract (HC + non-HC fractions) elicited more antennal inspections than the HC fraction alone. Non-HC compounds represent only a small proportion of the cuticle constituents and are similar in all individuals irrespective of morphological caste or reproductive status (R. Beard and V.D., unpublished data). They may therefore only have made the glass slides smell more ant-like and thus more attractive in our experiment. Although the activity of both fractions was not tested separately for the PPG extracts of reproductive workers and infertile workers, the HC fraction presumably also plays the major role in their discrimination.
As hypothesized in various social Hymenoptera (see the introduction), workers of M. gulosa detect changes in CHC profiles and use this information to discriminate reproductive from nonreproductive nestmates. According to the signaling hypothesis, fertile queens produce honest signals to which workers respond adaptively by refraining from reproduction (38). We suggest that the CHCs represent such a signal by allowing the workers to detect the presence of the queen. Further experiments are needed to show that CHCs also have primer effects in workers. However, the fact that changes in workers' CHCs correlate with the triggering of attacks as well as with the onset of reproductive activity in this species (25) supports the idea that CHCs are important in the regulation of reproduction. None of the other gland products tested in this study (mandibular and Dufour glands) unambiguously allowed for the recognition of queens or reproductive workers.
CHCs as Queen Pheromone. Until recently, the origin of ant queen pheromones was only sought in exocrine glands, which often release their products on the cuticle. The excision of gland reservoirs allows for collection of these products in large quantities for bioassays. However, only in fire ants and the honey bees have queen pheromones been identified from glands (e.g., refs. 39-42). In contrast, numerous studies demonstrated the existence of pheromones on the cuticle of queens but failed to locate their glandular source (e.g., refs. 43-47). This can be explained in the light of our findings. CHCs are produced by the oenocytes (48, 49), which are glandular cells spread in the body cavity; the secretions are released in their hemolymph and transported to the cuticle (2, 48-54). The absence of a structured reservoir storing CHCs has hindered the investigation of this pheromonal source. Only two studies envisaged the origin of queen pheromones to be epidermal gland cells (45, 46). The possibility that CHCs could function as queen or gamergate (mated workers) recognition pheromones was suggested only recently (18-23).
CHC profiles correlate with ovarian activity in a growing number of species investigated (see the introduction). Their use as queen-recognition pheromones thus could be widespread in the social Hymenoptera. Perception of the poorly volatile long-chained CHCs necessitates direct contacts between emitters and receivers. Indeed in M. gulosa, direct contact is needed for the workers to perceive the presence of the queen (25). With increasing colony size, however, the probability of a worker encountering the queen diminishes. In species with larger colonies, queen pheromones can be transmitted efficiently in an indirect manner via other workers or the substrate, as occurs in the honey bee (55, 56). Alternatively, more volatile secretions might be needed in addition to the queen's CHCs to attract workers to her immediate vicinity.
Intracolonial Variation in CHC Profiles and Colonial Odor. The ability of ant workers to use variations in CHC profiles to identify reproductives is in apparent contradiction to the Gestalt model for nestmate recognition. Indeed, it is claimed that all nestmates share a colonial odor as a result of mixing their CHC profiles (29, 30, 36, 57-59). Our results and others clearly show that this is not the case because reproductives have a specific profile. Furthermore, the molecules characterizing reproductives are the same in all colonies. In most of the ants investigated, CHC profiles are a complex mixture of dozens of compounds. The presence or absence of some compounds or the variations in their relative proportion allow for enough combinations to encode both kinds of information. It has already been demonstrated that nestmate queens or their extracts were preferred over nonnestmate queens in several ant species (43, 59-63). Similarly, in M. gulosa, extracts of nestmate queens were preferred over those of foreign ones (25). It is thus likely that CHCs are used as both reproductive and colony labels. Determining the specific cuticular compounds involved in the recognition of either nestmates or reproductives is the next step for future studies.
Acknowledgments
We thank Richard Beard, Abraham Hefetz, and Graeme Jones for chemical analyses and helpful discussions, and Michael Schwarz, Katja Hogendoorn, Remko Leijs, Steve Shattuck, Archie McArthur, and Russell for hospitality and assistance during fieldwork. Thomas Seeley and Stefano Turillazzi made helpful comments on the manuscript. This work was funded by the Deutsche Forschungsgemeinschaft SFB 554 (C3) and the Graduiertenkolleg “Grundlagen des Arthropodenverhaltens.”
Abbreviations: HC, hydrocarbon; CHC, cuticular HC; PPG, postpharyngeal gland.
References
- 1.Bourke, A. F. G. (1988) Q. Rev. Biol. 63, 291-311. [Google Scholar]
- 2.Lockey, K. H. (1988) Comp. Biochem. Physiol. 89, 595-654. [Google Scholar]
- 3.Howard, R. W. (1993) in Insect Lipids: Biochemistry and Biology, eds. Stanley-Samuelson, D. W. & Nelson, D. R. (Univ. of Nebraska Press, Lincoln), pp. 179-226.
- 4.Gibbs, A. G. (1998) Am. Zool. 38, 471-482. [Google Scholar]
- 5.Gibbs, A. G. (2002) J. Insect Physiol. 48, 391-400. [DOI] [PubMed] [Google Scholar]
- 6.Singer, T. L. (1998) Am. Zool. 38, 394-405. [Google Scholar]
- 7.Butts, D. P., Camann, M. A. & Espelie, K. E. (1995) Insectes Soc. 42, 45-55. [Google Scholar]
- 8.Dahbi, A. & Lenoir, A. (1998) Insectes Soc. 45, 301-313. [Google Scholar]
- 9.Lahav, S., Soroker, V. & Hefetz, A. (1999) Naturwissenschaften 86, 246-249. [Google Scholar]
- 10.Thomas, M. L., Parry, L. J., Allan, R. A. & Elgar, M. E. (1999) Naturwissenschaften 86, 87-92. [Google Scholar]
- 11.Wagner, D., Tissot, M., Cuevas, W. & Gordon, D. M. (1999) J. Chem. Ecol. 26, 2245-2257. [Google Scholar]
- 12.Dillwith, J. W., Adams, T. S. & Blomquist, G. (1983) J. Insect Physiol. 29, 377-386. [Google Scholar]
- 13.Trabalon, M., Campan, M., Porcheron, P., Clément, J. L., Baehr, J. C., Morinière, M. & Joulie, C. (1990) Gen. Comp. Endocrinol. 80, 216-222. [DOI] [PubMed] [Google Scholar]
- 14.Schal, C., Gu, X., Burns, E. L. & Blomquist, G. J. (1994) Arch. Insect Biochem. Physiol. 25, 375-391. [DOI] [PubMed] [Google Scholar]
- 15.Bonavita-Cougourdan, A., Theraulaz, G., Bagnères, A. G., Roux, M., Pratte, M., Provost, E. & Clément, J. L. (1991) Comp. Biochem. Physiol. 100B 4, 667-680. [Google Scholar]
- 16.Ayasse, M., Marlovits, T., Tengo, J., Taghizadeh, T. & Franke, W. (1995) Apidologie (Celle) 26, 163-180. [Google Scholar]
- 17.Monnin, T., Malosse, C. & Peeters, C. (1998) J. Chem. Ecol. 24, 473-490. [Google Scholar]
- 18.Liebig, J., Peeters, C., Oldham, N. J., Markstädter, C. & Hölldobler, B. (2000) Proc. Natl. Acad. Sci. USA 97, 4124-4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuvillier-Hot, V., Cobb, M., Malosse, C. & Peeters, C. (2001) J. Insect Physiol. 47, 485-493. [DOI] [PubMed] [Google Scholar]
- 20.Sledge, M., Boscaro, F. & Turillazzi, S. (2001) Behav. Ecol. Sociobiol. 49, 401-409. [Google Scholar]
- 21.Hannonen, M., Sledge, M. F., Turillazzi, S. & Sundström, L. (2002) Anim. Behav. 64, 477-485. [Google Scholar]
- 22.Heinze, J., Stengl, B. & Sledge, M. F. (2002) Behav. Ecol. Sociobiol. 52, 59-65. [Google Scholar]
- 23.Peeters, C., Monnin, T. & Malosse, C. (1999) Proc. R. Soc. London 266, 1323-1327. [Google Scholar]
- 24.Dietemann, V., Hölldobler, B. & Peeters, C. (2002) Insectes Soc. 49, 289-298. [Google Scholar]
- 25.Dietemann, V. (2002) Ph.D. thesis (Würzburg University, Würzburg, Germany).
- 26.Gobin, B., Billen, J. & Peeters, C. (1999) Anim. Behav. 58, 1117-1122. [DOI] [PubMed] [Google Scholar]
- 27.Kikuta, N. & Tsuji, K. (1999) Behav. Ecol. Sociobiol. 46, 180-189. [Google Scholar]
- 28.Liebig, J., Peeters, C. & Hölldobler, B. (1999) Proc. R. Soc. London Ser. B 266, 1865-1870. [Google Scholar]
- 29.Soroker, V., Vienne, C. & Hefetz, A. (1994) Naturwissenschaften 81, 510-513. [Google Scholar]
- 30.Soroker, V., Fresneau, D. & Hefetz, A. (1998) J. Chem. Ecol. 24, 1077-1090. [Google Scholar]
- 31.Bagnères, A. G. & Morgan, E. D. (1991) Experentia 47, 106-111. [Google Scholar]
- 32.Do Nascimento, R. R., Billen, J. & Morgan, E. D. (1993) Comp. Biochem. Physiol. 104, 505-508. [Google Scholar]
- 33.Soroker, V. & Hefetz, A. (2000) J. Insect Physiol. 46, 1097-1102. [DOI] [PubMed] [Google Scholar]
- 34.Arthur, C. L. & Pawliszyn, J. (1990) Anal. Chem. 62, 2145-2148. [Google Scholar]
- 35.Aitchison, J. (1986) The Statistical Analysis of Compositional Data: Monographs in Statistics and Applied Probability (Chapman & Hall, London).
- 36.Soroker, V., Vienne, C. & Hefetz, A. (1995) J. Chem. Ecol. 21, 365-378. [DOI] [PubMed] [Google Scholar]
- 37.Bligh, E. G. & Dyer, W. J. (1959) Can. J. Biochem. Physiol. 37, 911-917. [DOI] [PubMed] [Google Scholar]
- 38.Keller, L. & Nonacs, P. (1993) Anim. Behav. 45, 787-794. [Google Scholar]
- 39.Barbier, J. & Lederer, E. (1960) C. R. Acad. Sci. 251, 1131-1135. [Google Scholar]
- 40.Callow, R. K. & Johnston, N. C. (1960) Bee World 41, 152-153. [Google Scholar]
- 41.Vander Meer, R. K., Glancey, B. M., Lofgren, C. S., Glover, A., Tumlinson, J. H. & Rocca, J. (1980) Ann. Entomol. Soc. Am. 73, 609-612. [Google Scholar]
- 42.Vargo, E. L. (1997) Naturwissenschaften 84, 507-510. [Google Scholar]
- 43.Brian, M. V. (1973) Anim. Behav. 21, 691-698. [DOI] [PubMed] [Google Scholar]
- 44.Passera, L. (1980) Insectes Soc. 27, 212-225. [Google Scholar]
- 45.Coglitore, C. & Cammaerts, M. C. (1981) Insectes Soc. 28, 353-370. [Google Scholar]
- 46.Cariou-Etienne, A. & Passera, L. (1992) Behav. Processes 27, 179-186. [DOI] [PubMed] [Google Scholar]
- 47.Bloch, G. & Hefetz, A. (1999) J. Chem. Ecol. 25, 881-896. [Google Scholar]
- 48.Diehl, P. A. (1975) J. Insect Physiol. 21, 1237-1246. [Google Scholar]
- 49.Fan, Y., Zureck, L., Dykstra, M. & Schal, C. (2003) Naturwissenschaften 90, 121-126. [DOI] [PubMed] [Google Scholar]
- 50.Wigglesworth, V. B. (1970) Tissue Cell 2, 155-179. [DOI] [PubMed] [Google Scholar]
- 51.Ismail, M. T. & Zachary, D. (1984) J. Chem. Ecol. 10, 1385-1398. [DOI] [PubMed] [Google Scholar]
- 52.Gu, X., Quilici, D., Juarez, P., Blomquist, G. J. & Schal, C. (1995) J. Insect Physiol. 41, 257-267. [Google Scholar]
- 53.Colla-Ruvolo, M. C. & Cruz-Landim, C. (1993) Mem. Inst. Oswaldo Cruz 88, 387-395. [Google Scholar]
- 54.Jensen, P. V. & Børgesen, L. W. (2000) Arthropod Struct. Dev. 29, 171-184. [DOI] [PubMed] [Google Scholar]
- 55.Seeley, T. D. (1979) Behav. Ecol. Sociobiol. 5, 391-415. [Google Scholar]
- 56.Naumann, K., Winston, M. L., Slessor, K., Prestwich, G. D. & Webster, F. X. (1991) Behav. Ecol. Sociobiol. 29, 321-332. [Google Scholar]
- 57.Meskali, M., Bonavita-Cougourdan, A., Provost, P., Bagnères, A. G., Dusticier, G. & Clément, J. L. (1995) J. Chem. Ecol. 21, 1127-1148. [DOI] [PubMed] [Google Scholar]
- 58.Vienne, C., Soroker, V. & Hefetz, A. (1995) Insectes Soc. 42, 267-277. [Google Scholar]
- 59.Lahav, S., Soroker, V., Vander Meer, R. K. & Hefetz, A. (1998) Behav. Ecol. Sociobiol. 43, 203-212. [Google Scholar]
- 60.Watkins, J. F. & Cole, T. W. (1966) Tex. J. Sci. 18, 264-265. [Google Scholar]
- 61.Jouvenaz, D. P., Banks, W. A. & Lofgren, C. S. (1974) Ann. Entomol. Soc. Am. 67, 442-445. [Google Scholar]
- 62.Keller, L. & Passera, L. (1989) Anim. Behav. 37, 733-740. [Google Scholar]
- 63.Berton, F., Lenoir, A., Nowbahari, E. & Barreau, S. (1991) Insectes Soc. 38, 293-305. [Google Scholar]