Abstract
It has been reported that an insulin 2 gene knockout, when bred onto nonobese diabetic (NOD) mice, accelerates diabetes. We produced insulin 1 gene knockout congenic NOD mice. In contrast to insulin 2, diabetes and insulitis were markedly reduced in insulin 1 knockout mice, with decreased and delayed diabetes in heterozygous females and no insulitis and diabetes in most homozygous female mice. Lack of insulitis was found for insulin 1 female homozygous knockout mice at 8, 12, and 37 weeks of age. Despite a lack of insulitis, insulin 1 homozygous knockout mice spontaneously expressed insulin autoantibodies. Administration of insulin peptide B:9-23 of both insulin 1 and 2 to NOD mice induced insulin autoantibodies. Insulin 1 is not the only lymphocytic target of NOD mice. Insulin 1 homozygous knockout islets, when transplanted into recently diabetic wild-type NOD mice, became infiltrated with lymphocytes and only transiently reversed diabetes. These observations indicate that loss of either insulin gene can influence progression to diabetes of NOD mice and suggest that the preproinsulin 1 gene is crucial for the spontaneous development of NOD insulitis and diabetes.
In addition to the original Rose and Witebsky criteria (1) for autoantigens, with current molecular techniques, one can add the criteria that removal of the antigen (e.g., gene knockout) or relevant alteration of its molecular sequence should prevent or ameliorate disease. The nonobese diabetic (NOD) mouse, biobreeding rat, and Long-Evans Tokushima lean rat are important models of organ-specific autoimmunity (2, 3). For all three models, the development of diabetes depends on the expression of specific alleles of class II histocompatibility molecules [e.g., I-Ag7 of NOD (4, 5) and RT1u for the biobreeding and Long-Evans Tokushima lean rat (6, 7)]. In addition, other genes contributing to diabetes have been identified [e.g., lymphopenia Ian-4/5 gene of the biobreeding rat (3) and the Cblb gene of the Long-Evans Tokushima lean rat (7)]. Patients with type 1A diabetes (immune-mediated diabetes) also express characteristic HLA class II alleles with highest risk provided by the genotype DQ8/DQ2 and dominant protection by DQ6. In Colorado, ≈1 of 15 DQ8/DQ2 newborns develops type 1 diabetes, which represents 40% of all children developing diabetes before age 5, despite a genotype population frequency of 2.4% (8). These dramatic HLA associations suggest that presentation of specific islet peptides might be central to the development of diabetes, and there may be primary islet autoantigens.
The detection of multiple anti-islet autoantibodies in humans [e.g., to insulin, glutamic acid decarboxylase 65 (GAD65) and insulinoma-associated antigen (IA-2)] and T cell reactivity to multiple antigens in the NOD mouse (9) indicates that multiple islet antigens are targets of autoimmunity. The NOD mouse would seem to be an ideal animal model to test the primacy of given islet autoantigens. A GAD65 knockout NOD mouse develops diabetes (10), and GAD transgenic mice with widespread antigen expression designed to induce tolerance still develop diabetes (10, 11). A report of a GAD antisense transgenic indicated that, out of multiple lines, two were protected from diabetes with early backcrossing to NOD (12). NOD mice with a heat shock protein transgene had decreased development of diabetes (13). Other important antigens such as those targeted by the BDC2.5 and the NY8.3 T cell receptors of transgenic mice have not yet been studied for altered antigen expression (14, 15). However, Harrison and coworkers (16) found that widespread proinsulin expression in antigen-presenting cells prevented diabetes in NOD mice.
Mice have two insulin genes. Crosses of NOD mice with control strains have not demonstrated an insulin-dependent diabetes mellitus locus (protective or diabetogenic) linked to either the insulin 1 gene (chromosome 19) or the insulin 2 gene (chromosome 7) (17). The two genes differ in terms of preproinsulin expression with much greater preproinsulin 2 protein in the thymus but similar expression of both genes in the islets (18-21). Chentoufi and Polychronakos (21) have demonstrated that thymic expression of insulin is related directly to the number of copies of the insulin 2 gene. We and Thebault-Baumont and coworkers (22) have reported that breeding the insulin 2 gene knockout onto NOD mice accelerated the development of diabetes.
The sequence of insulin 1 differs from insulin 2 by two amino acids (insulin 1: B9 proline, B29 lysine versus insulin 2: B9 serine, B29 methionine). Insulin is produced by cleavage from preproinsulin, and there are additional amino acid differences between insulins 1 and 2 for the preproinsulin-connecting peptide and leader sequences. Polymorphisms of the insulin gene are associated with diabetes risk in humans (23, 24). The insulin gene variable nucleotide tandem repeat (VNTR) allele associated with protection from type 1 diabetes is also associated with greater insulin message within the human thymus, and given the studies of Hanahan (25) and Chentoufi and Polychronakos (21), it has been suggested that greater insulin expression in the thymus of mice may be protective.
Daniel and coworkers (26) cloned islet-reactive CD4 T cells directly from islets of NOD mice by using whole islets as initial antigens. They found that the majority of these T cells reacted with insulin, and anti-insulin clones were present as early as 4 weeks of age (the earliest age studied). Of these insulin-reactive T cell clones, >95% reacted with insulin B chain peptide, B:9-23. Many of these T cell clones can accelerate diabetes onset in young NOD mice. We have used the insulin B:9-23 peptide (insulin 2) to induce insulin autoantibodies in both NOD and BALB/c mice (autoantibodies reacting with insulin but not the peptide), with insulitis after immunization with B:9-23 peptide with PolyIC in BALB/c mice, and diabetes after immunization with B:9-23 peptide in BALB/c mice with islet B7.1 transgene expression (27). We hypothesized that insulin may be an essential islet target antigen and set out to create NOD mice with either the insulin 1 or 2 gene deleted. We find that the preproinsulin 1 gene knockout prevents the development of diabetes and insulitis.
Methods
Mice. We used speed congenic techniques to establish NOD mice with either the insulin 1 or insulin 2 gene deleted. Briefly, both the insulin 1 and insulin 2 gene knockouts were produced in 129S1/SvImJ embryonal cell lines by J. Jami (18). The knockout cell lines were microinjected into C57BL/6 blastocysts and lines developed with C57BL/6 mice. We then bred the knockouts onto NOD mice. For the insulin 1 knockout, NOD diabetogenic loci (idd 1-14) were fixed by backcross 3 and for the insulin 2 knockout by backcross 4. Both strains have been further backcrossed onto NOD. We are at the ninth backcross generation for insulin 1 knockout mice and the 10th backcross generation for insulin 2 knockout mice. Non-NOD genomic regions flanking each insulin gene knockout are now <10 centiMorgans (28). A total of 309 insulin knockout mice have been prospectively followed for the development of diabetes. They are divided into 12 groups based on sex and insulin 1 and 2 knockout genotypes (wild-type +/+, heterozygous +/-, and homozygous knockout -/-). Blood glucose is measured weekly with the FreeStyle blood glucose monitoring system (TheraSense, Alameda, CA), and the mice are considered diabetic after two consecutive blood glucose values >250 mg/dl. The mice were housed in specific pathogen-free facilities with approved University of Colorado Health Sciences Center Animal Core and Use Committee protocols.
Histology. The tissue of the pancreata, salivary glands, and graft-bearing kidneys were removed from the mice, fixed in 10% buffered formalin, and paraffin embedded. Paraffin sections were stained with hematoxylin/eosin, and in parallel sections, insulin or glucagon granules were detected with immunoperoxidase staining.
Insulin Autoantibody (IAA) Assay. IAA expression from serum of insulin knockout mice was evaluated beginning at 8 weeks of age until the development of diabetes, or until 32 weeks of age. NOD mice were injected s.c. with B:9-23 peptide (10 μg per dose in saline weekly from 4 to 25 weeks of age) from either the insulin 1 or insulin 2 sequence; IAA was also evaluated. IAA were measured with a 96-well filtration plate micro IAA assay as described (29).
Islet Transplantation and Adoptive Transfer of Splenocytes. Insulin knockout islets (insulin 1 +/- or -/-) were isolated from adult mouse pancreata by collagenase digestion (Sigma type V) and Histopaque purification (Sigma). Pellets of islets (350-400 per mouse) or a piece of thyroid gland were transplanted into the subrenal capsule of acutely diabetic NOD mice as described (30). The recipient mice were monitored for blood glucose as described. Splenocytes (2 × 107 per mouse) from one of the insulin 1 homozygous knockout islet graft recipient mice were i.v. transferred to two 8-week-old severe combined immunodeficient NOD mice.
Statistics. Survival curves were analyzed with the log rank test. Mann-Whitney U tests were used to compare the peak values of IAA expression. χ2 tests were used for insulitis score comparison. Statistical tests used prism software (GraphPad, San Diego).
Results
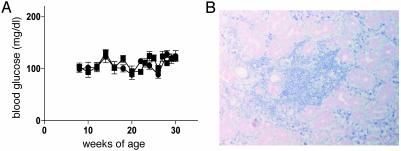
Prior studies indicate normal glucose tolerance and insulin secretion for mice having only one of two insulin genes (22). This was expected because both insulin 1 and insulin 2 are expressed within the pancreas and insulin secretion is posttranslationally regulated. Before development of diabetes, our insulin 1 knockout mice and their wild-type littermates had equivalent glucose levels (Fig. 1A). In addition, insulin 1 knockout (-/- and +/-) mice were not protected from sialitis, an additional autoimmune disorder of NOD mice (Fig. 1B).
Fig. 1.
Glucose levels and Sialitis of insulin 1 -/- knockout mice. (A) Glucose levels of insulin 1 homozygous knockout female mice (-/-, ▪, n = 7) and wild-type littermates (+/+, •, n = 7) plotted from 8 to 30 weeks of age before diabetes onset. (B) Lymphocyte infiltration in salivary glands was present in insulin 1 -/- mice despite absence of insulitis for this 45-week-old mouse.
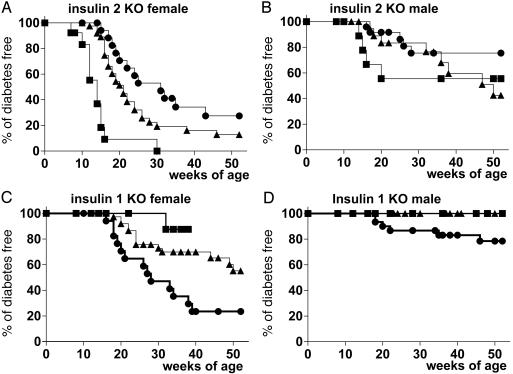
Similar to the studies of Thebault-Baumont and coworkers (22), Fig. 2 A and B illustrates the acceleration of the development of diabetes by the insulin 2 gene knockout. The order of progression to diabetes, from least to greatest, was +/+ (no knockout), +/- (heterozygous knockout), or -/- (homozygous knockout). Female insulin 2 homozygous knockout mice (-/-) have a dramatic synchronization of the development of diabetes with all but one mouse developing diabetes between 7 and 15 weeks of age. These early onset diabetic mice have severe insulitis (Fig. 3 A and D) with primarily intraislet infiltrates.
Fig. 2.
Life table analysis of insulin knockout (KO) mice. Filled squares (▪) indicate insulin homozygous KO (-/-), filled triangles (▴) indicate heterozygous KO (+/-), and filled circles (•) indicate wild-type insulin genes (+/+). (Upper) Progression to diabetes in insulin 2 KO mice is shown (A: female mice -/-, n = 13; +/-, n = 43; +/+, n = 19; B: male mice -/-, n = 13; +/-, n = 25; +/+, n = 24). (Lower) Progression to diabetes in insulin 1 KO mice is shown (C: female mice, -/-, n = 18; +/-, n = 37; +/+, n = 17; D: male mice -/-, n = 19; +/-, n = 51; +/+, n = 30). Statistical analysis was performed with prism logrank software. The three curves are dramatically different for female insulin 2 (A, P < 0.0001) and insulin 1 KO (D, P < 0.001). For male mice, insulin 1 KO mice differ significantly in progression to diabetes (C, P < 0.01).
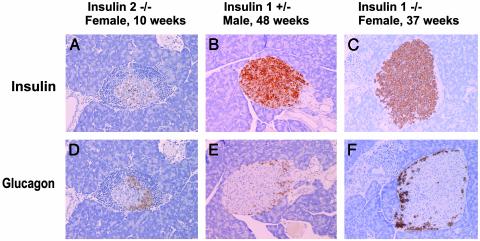
Fig. 3.
Severe insulitis in insulin 2 knockout mice and lack of insulitis in insulin 1 knockout (KO) mice. Immunohistochemical staining (anti-insulin, anti-glucagon) of pancreatic islets from insulin KO mice was performed. As early as at 10 weeks of age, beta cells are destroyed in insulin 2 KO mice with extensive insulitis (A and D). In contrast, insulin-containing beta cells remain without insulitis for older insulin 1 +/- male (age = 48 weeks) (B and E) and insulin 1 -/- female mice (age = 37 weeks) (C and F).
In contrast to the insulin 2 knockout “controls,” homozygous insulin 1 knockout mice have a remarkable lack of insulitis and diabetes. Fig. 2 C and D illustrates the lack of development of diabetes for insulin 1 knockout mice compared with their wild-type (+/+) littermates. With up to 1 year of followup, none of the male insulin 1 knockout mice (+/- or -/-) have developed diabetes. To date, 14 of 37 female insulin 1 heterozygous knockout (+/-) mice and 1 of 19 female insulin 1 homozygous knockout (-/-) mice have developed diabetes. Fig. 3 B and E illustrates the histology of a male heterozygous insulin 1 knockout mouse (+/-) with islets free of insulitis, even at 48 weeks of age. Of 40 islets examined from five different +/- male mice (ages 38-50 weeks), 27 islets had no insulitis, 12 islets had minimal periinsulitis islets, and 1 islet of a 39-week-old had what appeared to be intra-islet insulitis. In contrast, for control nondiabetic male NOD mice (ages 33, 33, and 52 weeks), 13 of 15 islets had severe intra-islet insulitis, one had periinsulitis, and one islet was atrophic with no insulitis and no insulin reactivity (χ2 test, P < 0.001). Older insulin 1 knockout female (+/-) mice have more extensive insulitis than insulin 1 knockout male (+/-) mice, concordant with their limited progression to diabetes.
The protection was even more dramatic for homozygous insulin 1 knockout mice. A homozygous insulin 1 knockout (-/-) female killed at 37 weeks had many insulin-containing islets without insulitis and minimal intraductal infiltrates (Fig. 3 C and F). Homozygous insulin 1 knockout (-/-) female mice killed at 8, 12, and 37 weeks were all free of insulitis. As reported by many investigators, essentially all NOD mice have extensive insulitis. Thus, insulin 1 knockout littermates (+/+) had severe insulitis and relatively few remaining islet beta cells as expected.
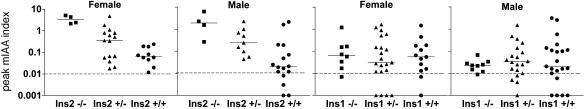
The expression of insulin autoantibodies of insulin 1 and 2 knockout mice is illustrated in Fig. 4. Both male and female insulin 2 knockout mice have higher peak levels of insulin autoantibodies than wild-type mice. Insulin 1 knockout and wild-type mice had similar peak levels of insulin autoantibodies. It is possible to induce or increase insulin autoantibodies in both NOD and BALB/c mice with the administration of the insulin 2 peptide B:9-23 sequence (autoantibodies reacting with insulin but not the immunizing peptide) (31). Therefore, we evaluated whether both the insulin 1 and 2, B:9-23 sequences would similarly induce insulin autoantibodies in NOD mice. The two peptides were equivalent in their induction of insulin autoantibodies [median before administration, 4 weeks of age: insulin 1 B:9-23 peptide, IAA index, 0.001 and insulin 2 B:9-23 peptide, IAA index, 0.003; median after administration, 15 weeks of age: insulin 1 peptide, IAA index, 1.2 (range 0.3 to 5.8, n = 9) and insulin 2 IAA index, 1.4 (range 0.3 to 5.2, n = 10)], indicating that both insulin 1 and 2 B:9-23 peptide sequences can be recognized by NOD mice.
Fig. 4.
Peak IAA of different genotypes (-/-, +/-, +/+) of insulin knockout mice. Peak IAA index between 8 and 32 weeks of age is plotted. Statistical analysis was performed with Mann-Whitney U test. Heterozygous and homozygous knockouts versus wild type are significantly different for insulin 2 female (P < 0.05) and male (P < 0.01) mice but not for insulin 1 female and male mice.
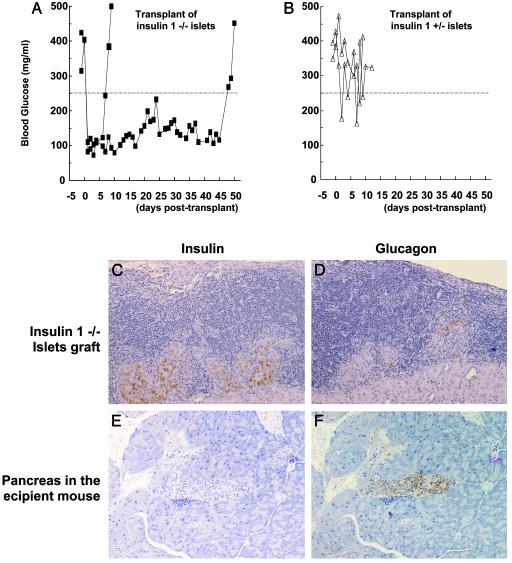
We hypothesized that the protection from diabetes of the insulin 1 homozygous knockout would be particularly relevant early in diabetogenesis and that at the time of diabetes onset (with presumed epitope spreading) even insulin 1 -/- islets would engender insulitis. Islets from insulin 1 knockout mice (heterozygous and homozygous) were transplanted under the kidney capsule of recent onset NOD mice. Heterozygous knockout islets (+/-) were rapidly destroyed (Fig. 5B), whereas homozygous insulin 1 knockout islets (-/-) transplanted into two diabetic NOD mice reversed hyperglycemia and maintained euglycemia until 50 and 7 days of posttransplant, at which time diabetes recurred (Fig. 5A). At the time of development of diabetes, the transplanted -/- islets had extensive lymphocytic infiltrates (Fig. 5 C and D), whereas an adjacent thyroid transplant was not infiltrated (thyroid not shown). Of note, despite recurrence of diabetes and infiltrates, the islets transplanted for 50 days had abundant insulin staining (Fig. 5C, insulin cells; Fig. 5D, glucagon cells). In contrast, the pancreas of the transplant recipient was devoid of insulin-staining cells (Fig. 5E) with glucagon-staining cells remaining (Fig. 5F). To rule out a lack of pathogenic lymphocytes in the recipient of the insulin 1 -/- islet transplant (the transplant reversed hyperglycemia for 50 days), splenocytes from the recipient mouse were administered to two severe combined immunodeficient NOD mice (8 weeks of age). Diabetes developed within the usual 5 weeks postsplenocyte transfer.
Fig. 5.
Restoration of normoglycemia by the transplantation of insulin 1 -/- islets but not by insulin 1 +/- islets. (A and B) Blood glucose levels of transplanted mice with insulin 1 -/- islets graft (A) or with insulin 1 +/- islets graft (B). Insulin 1 -/- islet transplant (C and D) and the native recipient pancreas (E and F) were stained for insulin (C and E) or glucagon (D and F) at the time of diabetes recurrence. The recipient mouse pancreas was devoid of islets with insulin-containing cells, with glucagon cells remaining, whereas the transplanted -/- islets had abundant insulin-containing cells.
Discussion
It is hypothesized that insulin 2 gene knockouts may accelerate diabetogenesis because of decreased expression of insulin within the thymus and therefore decreased tolerance to insulin peptides (22). Such a hypothesis would be consistent with the fascinating observation that the AIRE gene associated with polyendocrine autoimmunity controls expression of multiple “peripheral” antigens within the thymus, including insulin (32). Chentoufi and Polychronakos (21) have reported that thymic insulin expression correlates with the number of insulin 2 genes, and we and Thebault-Baumont et al. (22) find diabetes acceleration with insulin 2 knockouts. Thus, removal of one of the two insulin genes (insulin 2) does not block antiislet autoimmunity. The insulin 1 knockout, especially in the homozygous state, prevents the development of insulitis and diabetes while “normal” (for NOD mice) levels of insulin autoantibodies develop. We hypothesize that this occurs because processed peptides of preproinsulin 1 are a preferential target of anti-insulin autoimmunity, and the presence of anti-insulin autoantibodies (that usually correlate with insulitis) (33) may be related to the continuing presence of insulin 2. Differences in diabetogenesis between insulin 1 and 2 cannot simply be due to absence of response to one of the two insulin B:9-23 sequences, in that immunization of NOD mice with either peptide induced insulin autoantibodies, and both peptides can stimulate NOD T cells (34).
Preproinsulin 1 differs from preproinsulin 2 in 13 aa, 2 amino acids in the B chain, 6 in the leader sequence, and 5 in the connecting peptide of the insulin molecule. Any one of these amino acid changes may be relevant in determining the resistance of the insulin 1 knockout, particularly in determining CD8 T cell targeting and the processing of peptides from preproinsulin. At present, only one CD8 peptide target has been defined (B chain amino acids 15-23), and this portion of the molecule is invariant between the two insulins. At present, it is not known whether residual anti-islet autoimmunity of transplanted NOD insulin 1 homozygous knockout islets represents targeting of insulin 2 or of other important islet molecules [e.g., NRP (NOD-related peptide, a CD8 epitope) (36)]. To directly test this question, we are creating mice with both insulin genes knocked out, and with a preproinsulin transgene engineered to produce an insulin with alanine at the B16 position of the molecule. The B:9-23 peptide with alanine at B16 does not activate the dominant CD4 T cells described by Wegmann and coworkers (35). We hypothesize that this animal will not develop anti-insulin autoantibodies, insulitis, or diabetes, and its islets might be resistant to recurrent disease when transplanted into new onset diabetic NOD mice. Alternatively, this epitope of both insulins might not be crucial for islet transplant destruction, and/or other noninsulin molecules may be relevant in this model. In addition, production of conditional knockouts, where the timing of insulin 1 and insulin 2 expression can be controlled in several tissues, will be important to define the mechanism of protection and disease acceleration.
It has been hypothesized that beta cells may be very susceptible to immune destruction, and this explains selective loss of beta cells versus glucagon-containing alpha cells. Despite marked infiltrates of the transplanted insulin 1 homozygous knockout islets, beta cells were present with abundant insulin staining at 50 and 7 days after transplantation, at the time of hyperglycemia recurrence.
There are many targets for the natural autoimmunity of type 1 diabetes (36). The study of transgenic animals induced to express specific nonislet molecules (37, 38) also indicates that islets can be destroyed by lymphocytes targeting multiple molecules with evidence for epitope spreading. Nevertheless, dominant islet molecular targets, critical for the initiation of diabetogenesis for a given genotype (e.g., NOD mouse), are consistent with the importance of the insulin gene(s) in humans and the NOD mouse. As additional islet target molecules are characterized (e.g., NRP antigen; ref. 39), it will be important to determine whether removal of other molecules block NOD diabetogenesis similar to the insulin 1 homozygous knockout.
Acknowledgments
The animal, histology, islet, and molecular cores at the University of Colorado Health Sciences Center Diabetes Endocrine Research Center aided the present study. Research was supported by National Institutes of Health Grants DK32082, AI39213, DK55969, DK62718, AI50864, AI95380, DK32493, DK32493, DK50970, and AI46374, Diabetes Endocrine Research Center Grant P30 DK57516, the American Diabetes Association, the Juvenile Diabetes Foundation, and the Children's Diabetes Foundation (to G.S.E.), a postdoctoral fellowship from the Juvenile Diabetes Research Foundation (to H.M.), an American Diabetes Association mentor-based fellowship (to N.A.), the Academy of Finland, the Finnish Cultural Foundation, and the Foundation for Pediatric Research (to J.P.).
Abbreviations: NOD, nonobese diabetic; IAA, insulin autoantibody.
References
- 1.Rose, N. R. & Bona, C. (1993) Immunol. Today 14, 426-430. [DOI] [PubMed] [Google Scholar]
- 2.Wucherpfennig, K. W. & Eisenbarth, G. S. (2001) Nat. Immunol. 2, 1-3. [DOI] [PubMed] [Google Scholar]
- 3.MacMurray, A. J., Moralejo, D. H., Kwitek, A. E., Rutledge, E. A., Van Yserloo, B., Gohlke, P., Speros, S. J., Snyder, B., Schaefer, J., Bieg, S., et al. (2002) Genome Res. 12, 1029-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hattori, M., Buse, J. B., Jackson, R. A., Glimcher, L., Dorf, M. E., Minami, M., Makino, S., Moriwaki, K., Korff, M., Kuzuya, H., et al. (1986) Science 231, 733-735. [DOI] [PubMed] [Google Scholar]
- 5.Todd, J. A., Acha-Orbea, H., Bell, J. I., Chao, N., Fronek, Z., Jacob, C. O., McDermott, M., Sinha, A. A., Timmerman, L., Steinman, L., et al. (1988) Science 240, 1003-1009. [DOI] [PubMed] [Google Scholar]
- 6.Colle, E., Fuks, A., Poussier, P., Edouard, P. & Guttman, R. D. (1992) Diabetes 41, 1617-1623. [DOI] [PubMed] [Google Scholar]
- 7.Yokoi, N., Komeda, K., Wang, H. Y., Yano, H., Kitada, K., Saitoh, Y., Seino, Y., Yasuda, K., Serikawa, T. & Seino, S. (2002) Nat. Genet. 31, 391-394. [DOI] [PubMed] [Google Scholar]
- 8.Robles, D. T., Eisenbarth, G. S., Wang, T., Erlich, H. A., Bugawan, T. L., Babu, S. R., Barriga, K., Norris, J. M., Hoffman, M., Klingensmith, G., et al. (2002) Clin. Immunol. 102, 217-224. [DOI] [PubMed] [Google Scholar]
- 9.Wong, F. S. & Janeway, C. A. J. (1999) J. Autoimmun. 13, 290-295. [DOI] [PubMed] [Google Scholar]
- 10.Baekkeskov, S., Kanaani, J., Jaume, J. C. & Kash, S. (2000) J. Autoimmun. 15, 279-286. [DOI] [PubMed] [Google Scholar]
- 11.Geng, L., Solimena, M., Flavell, R. A., Sherwin, R. S. & Hayday, A. C. (1998) Proc. Natl. Acad. Sci. USA 95, 10055-10060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoon, J. W., Sherwin, R. S., Kwon, H. & Jun, H. S. (2000) J. Autoimmun. 15, 273-278. [DOI] [PubMed] [Google Scholar]
- 13.Birk, O. S., Douek, D. C., Elias, D., Takacs, K., Dewchand, H., Gur, S. L., Walker, M. D., van der, Z. R., Cohen, I. R. & Altmann, D. M. (1996) Proc. Natl. Acad. Sci. USA 93, 1032-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amrani, A., Verdaguer, J., Serra, P., Tafuro, S., Tan, R. & Santamaria, P. (2000) Nature 406, 739-742. [DOI] [PubMed] [Google Scholar]
- 15.Poulin, M. & Haskins, K. (2000) J. Immunol. 164, 3072-3078. [DOI] [PubMed] [Google Scholar]
- 16.French, M. B., Allison, J., Cram, D. S., Thomas, H. E., Dempsey-Collier, M., Silva, A., Georgiou, H. M., Kay, T. W., Harrison, L. C. & Lew, A. M. (1997) Diabetes 46, 34-39. [DOI] [PubMed] [Google Scholar]
- 17.Todd, J. A. & Wicker, L. S. (2001) Immunity 15, 387-395. [DOI] [PubMed] [Google Scholar]
- 18.Deltour, L., Leduque, P., Blume, N., Madsen, O., DuBois, P., Jami, J. & Bucchini, D. (1993) Proc. Natl. Acad. Sci. USA 90, 527-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heath, V. L., Moore, N. C., Parnell, S. M. & Mason, D. W. (1998) J. Autoimmun. 11, 309-318. [DOI] [PubMed] [Google Scholar]
- 20.Throsby, M., Homo-Delarche, F., Chevenne, D., Goya, R., Dardenne, M. & Pleau, J. M. (1998) Endocrinology 139, 2399-2406. [DOI] [PubMed] [Google Scholar]
- 21.Chentoufi, A. A. & Polychronakos, C. (2002) Diabetes 51, 1383-1390. [DOI] [PubMed] [Google Scholar]
- 22.Thebault-Baumont, K., Dubois-LaForgue, D., Krief, P., Briand, J. P., Halbout, P., Vallon-Geoffroy, K., Morin, J., Laloux, V., Lehuen, A., Carel, J. C., et al. (2003) J. Clin. Invest. 111, 851-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pugliese, A., Zeller, M., Fernandez, A., Zalcberg, L. J., Bartlett, R. J., Ricordi, C., Pietropaolo, M., Eisenbarth, G. S., Bennett, S. T. & Patel, D. D. (1997) Nat. Genet. 15, 293-297. [DOI] [PubMed] [Google Scholar]
- 24.Vafiadis, P., Bennett, S. T., Todd, J. A., Nadeau, J., Grabs, R., Goodyer, C. G., Wickramasinghe, S., Colle, E. & Polychronakos, C. (1997) Nat. Genet. 15, 289-292. [DOI] [PubMed] [Google Scholar]
- 25.Hanahan, D. (1998) Curr. Opin. Immunol. 10, 656-662. [DOI] [PubMed] [Google Scholar]
- 26.Wegmann, D. R., Norbury-Glaser, M. & Daniel, D. (1994) Eur. J. Immunol. 24, 1853-1857. [DOI] [PubMed] [Google Scholar]
- 27.Moriyama, H., Wen, L., Abiru, N., Liu, E., Yu, L., Miao, D., Gianani, R., Wong, F. S. & Eisenbarth, G. S. (2002) Proc. Natl. Acad. Sci. USA 99, 5539-5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paronen, J., Moriyama, H., Abiru, N., Sikora, K., Melanitou, E., Babu, S., Bao, F., Liu, E., Miao, D. & Eisenbarth, G. S. (2003) Ann. N.Y. Acad. Sci., in press. [DOI] [PubMed]
- 29.Yu, L., Robles, D. T., Abiru, N., Kaur, P., Rewers, M., Kelemen, K. & Eisenbarth, G. S. (2000) Proc. Natl. Acad. Sci. USA 97, 1701-1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicolls, M. R., Coulombe, M., Yang, H., Bolwerk, A. & Gill, R. G. (2000) J. Immunol. 164, 3627-3634. [DOI] [PubMed] [Google Scholar]
- 31.Abiru, N., Maniatis, A. K., Yu, L., Miao, D., Moriyama, H., Wegmann, D. & Eisenbarth, G. S. (2001) Diabetes 50, 1274-1281. [DOI] [PubMed] [Google Scholar]
- 32.Anderson, M. S., Venanzi, E. S., Klein, L., Chen, Z., Berzins, S., Turley, S. J., von Boehmer, H., Bronson, R., Dierich, A., Benoist, C., et al. (2002) Science 298, 1395-1401. [DOI] [PubMed] [Google Scholar]
- 33.Robles, D. T., Eisenbarth, G. S., Dailey, N. J. M., Peterson, L. B. & Wicker, L. S. (2002) Diabetes 52, 882-886. [DOI] [PubMed] [Google Scholar]
- 34.Halbout, P., Briand, J. P., Becourt, C., Muller, S. & Boitard, C. (2002) J. Immunol. 169, 2436-2443. [DOI] [PubMed] [Google Scholar]
- 35.Abiru, N., Wegmann, D., Kawasaki, E., Gottlieb, P., Simone, E. & Eisenbarth, G. S. (2000) J. Autoimmun. 14, 231-237. [DOI] [PubMed] [Google Scholar]
- 36.McDevitt, H. (2001) N. Engl. J. Med. 345, 1060-1061. [DOI] [PubMed] [Google Scholar]
- 37.Wong, F. S., Dittel, B. N. & Janeway, C. A. J. (1999) Immunol. Rev. 169, 93-104. [DOI] [PubMed] [Google Scholar]
- 38.von Herrath, M. G., Holz, A., Homann, D. & Oldstone, M. B. (1998) Semin. Immunol. 10, 87-100. [DOI] [PubMed] [Google Scholar]
- 39.Trudeau, J. D., Kelly-Smith, C., Verchere, C. B., Elliott, J. F., Dutz, J. P., Finegood, D. T., Santamaria, P. & Tan, R. (2003) J. Clin. Invest. 111, 217-223. [DOI] [PMC free article] [PubMed] [Google Scholar]