Abstract
A molecular basis for memory failure in Alzheimer's disease (AD) has been recently hypothesized, in which a significant role is attributed to small, soluble oligomers of amyloid β-peptide (Aβ). Aβ oligomeric ligands (also known as ADDLs) are known to be potent inhibitors of hippocampal long-term potentiation, which is a paradigm for synaptic plasticity, and have been linked to synapse loss and reversible memory failure in transgenic mouse AD models. If such oligomers were to build up in human brain, their neurological impact could provide the missing link that accounts for the poor correlation between AD dementia and amyloid plaques. This article, using antibodies raised against synthetic Aβ oligomers, verifies the predicted accumulation of soluble oligomers in AD frontal cortex. Oligomers in AD reach levels up to 70-fold over control brains. Brain-derived and synthetic oligomers show structural equivalence with respect to mass, isoelectric point, and recognition by conformation-sensitive antibodies. Both oligomers, moreover, exhibit the same striking patterns of attachment to cultured hippocampal neurons, binding on dendrite surfaces in small clusters with ligand-like specificity. Binding assays using solubilized membranes show oligomers to be high-affinity ligands for a small number of nonabundant proteins. Current results confirm the prediction that soluble oligomeric Aβ ligands are intrinsic to AD pathology, and validate their use in new approaches to therapeutic AD drugs and vaccines.
Alzheimer's disease (AD) is a progressive dementia for which the earliest manifestation is memory failure. There is no cure for AD, and its molecular basis is not yet established. Considerable evidence, however, indicates the disease is triggered by neurotoxic assemblies of the 42-aa amyloid β-peptide (Aβ) (1-3).
Aβ1-42 is an amphipathic molecule that derives from specific proteolytic processing of its transmembrane precursor protein (amyloid precursor protein, APP) (4). Because mutations in APP cause a subset of familial AD (5), and also cause increased accumulation of Aβ1-42 (6), an extensive effort over the past 15 years has sought to establish pathogenic mechanisms that link Aβ with AD. Aβ1-42 exhibits a remarkable capacity for self-association (7), which gives rise to the large, insoluble amyloid fibrils found in AD neuritic plaques (8, 9). Similar fibrils assemble in vitro from synthetic peptide (10). Self-association is functionally significant, because seminal studies a decade ago (11, 12) determined that solutions containing large fibrillar Aβ killed cultured neurons, whereas solutions of monomer were innocuous. The amyloid cascade hypothesis, formulated in 1992 (13), took these insoluble amyloid fibrils as the primary molecular pathogens of AD. Although stimulating extensive research, the proposed role of amyloid fibrils has not been accepted. A significant failing has been the poor correlation between neurological deficits and amyloid plaque burden (14), a discrepancy recapitulated in (human) hAPP transgenic mice AD models (15, 16).
Recently, the amyloid cascade hypothesis was modified to include additional pathogenic Aβ assemblies, which are quite different in structure from amyloid fibrils (1, 16). The toxins comprise soluble Aβ oligomers. Unlike the large and conspicuous fibril deposits, oligomers would be undetected in typical pathology assays, and thus would constitute, in essence, missing links in the pathogenic cascade (17). The neurologically disruptive nature of Aβ oligomers has been established in various models. Experimentally generated oligomers applied to brain slices or injected in vivo cause failure of hippocampal long-term potentiation (LTP) (18-20), which is a form of synaptic information storage well-known as a paradigm for memory mechanisms. Soluble oligomers also have been implicated in the physical degeneration of synapses (15) and in age-onset memory failure in hAPP transgenic mice (21-23). In two studies (22), memory failure in hAPP mice was actually reversed by Aβ-antibodies, a remarkable recovery that occurred without reduction in amyloid plaque level. In one case, recovery was observed in plaque-filled mice within 24 h of a single Aβ-antibody injection. Reversal of memory failure by antibodies in mouse models confirmed predictions developed earlier from studies of oligomers and LTP (16, 18), and is consistent with the emerging concept that AD is an oligomer-induced synaptic failure (24).
Neurological damage by oligomeric Aβ in experimental models has underscored the need to ascertain the abundance and properties of oligomers in human brain. By using oligomer-sensitive immunoassays, this article verifies that human brain contains readily soluble Aβ oligomers whose levels are greatly elevated in AD. Oligomers from AD brain show properties equivalent to those of synthetic oligomers, including a striking capacity to attach to neurons at small clusters of surface binding sites.
Materials and Methods
Aβ1-42 was from American Peptide (Sunnyvale, CA), California Peptide Research (Napa, CA), or Recombinant Peptide (Athens, GA). Ham's F-12 medium phenol red-free was from Bio-Source International (Camarillo, CA). Hibernate was from Life Technologies (Rockville, MD). Neurobasal, horse serum, and B27 supplements were from Invitrogen. All other cell culture reagents were from Mediatech (Herndon, VA). Unless otherwise indicated, chemicals and reagents were from Sigma-Aldrich. The cell proliferation (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide; MTT) kit was from Roche Boehringer Mannheim (Indianapolis). The Coomassie Plus and bicinchoninic acid (BCA) protein assays, and the Super-Signal West femto chemiluminescence kit were from Pierce. SDS/4-20% PAGE Tris-glycine gels, 2D strips, and buffers were from Bio-Rad. Hybond enhanced chemiluminescence (ECL) nitrocellulose and horseradish peroxidase (HRP)-conjugated secondary were from Amersham Pharmacia Biosciences. Oligomer-selective antibodies (M93 and M94) were produced and characterized earlier (25). Alexa Fluor 488-conjugated secondary antibody was from Molecular Probes. Timed pregnant Sprague-Dawley rats were obtained from Charles River Breeding Laboratories. Samples of frontal cortex and cerebellum from AD- and age-matched control brains were obtained from the Northwestern Alzheimer's Disease Center Neuropathology Core, and were stored at -80°C until used. Synthetic Aβ-derived diffusible ligands (ADDLs) were prepared according to published protocols (25, 26).
Cell Culture. Hippocampal cells were prepared and maintained according to Brewer et al. (27), by using (0.002%) poly-l-lysine-coated coverslips plated at a density of 1.8 × 104 cells per cm2 in Neurobasal, with B27 supplements and l-glutamine (2.5 μM). Cortical and cerebellar cells were cultured as described (28), with cerebellar cultures given higher KCl (25 mM). Cells were exposed to 5 μM cytosine arabinonucleoside (araC) for 24 h, followed by 2.5 μM of araC for 24 h. For assays of metabolic activity, cells were plated onto poly-l-lysine-coated 24-well plates at a density of 0.4 × 106 cells per well. When ADDLs were added, medium was changed to F12 medium with 50 or 100 nM synthetic ADDLs (plus 25 mM KCl for cerebellar cultures), and metabolic activity (MTT reduction) was measured after 48 h by using the cell proliferation kit according to manufacturer's instructions.
Immunocytochemistry. Cultures were rinsed once with culture media and fixed with 3.7% formaldehyde. The coverslips were washed, permeabilized with 0.1% Triton X-100 in 10% normal goat serum and PBS (NGS:PBS) for 90 min at room temperature, immunolabeled with polyclonal M94 antibody (1:500) overnight at 4°C, followed by an incubation with Alexa Fluor 488 anti-rabbit (2 μg/ml) for ∼3 h at room temperature. The cells were rinsed, mounted with ProLong reagent, and visualized by using metamorph imaging software (Universal Imaging, Media, PA).
Membrane Preparation. All manipulations of human and adult rat brain tissues were performed at 4°C. Cerebellum, cortex, and hippocampus were homogenized in 20 vol of buffer A (PBS, pH 7.4/0.32 M sucrose/50 mM Hepes/25 mM MgCl2/0.5 mM DTT/200 μg/ml PMSF/2 μg/ml pepstatin A/4 μg/ml leupeptin/30 μg/ml benzamidine hydrochloride), and were centrifuged at 1,000 × g for 10 min. The pellet was rehomogenized in 10 vol of buffer A and centrifuged again. The combined supernatants were centrifuged at 100,000 × g for 1 h, and the pellet was used for total membrane fraction.
Tissue Extracts. Frontal cortex from AD or control brain (0.2 g) was homogenized in 20 volumes of F12 containing protease inhibitors (as above), and was centrifuged at 100,000 × g for 1 h. The pellet was rehomogenized in 10 volumes of F12 plus protease inhibitors and was recentrifuged. The protein concentration of the combined supernatants was determined. An aliquot of protein (1.8 mg) was then concentrated to a volume of 60 μl or less, by using a Centricon-10 concentrator.
2D Electrophoresis. Proteins of soluble cortical tissue extracts (1.8 mg) were separated according to published procedures (29), by using Bio-Lytes carrier ampholytes, pH 3-10. Synthetic ADDLs, 1 nmol in 10 μl of F12, was treated exactly as cortex, and was stained with silver as described (25).
Immunoassays. Ligand blots were based on published procedures (30). Membrane preparations were extracted with detergent (31) for 15 min on ice, then solubilized proteins (75 μg, unless otherwise indicated) were separated by SDS/PAGE for 3-4 h at 120 v, and were transferred to nitrocellulose. Blots were incubated with Tris-buffered saline (TBST) containing 5% nonfat dry milk overnight, washed three times with cold F12 medium, and incubated with 10 nM ADDLs for 3 h at 4-8°C. After washing away unbound material with TBST, bound ADDLs were labeled with M93 (1:1,000), and were visualized with enhanced chemiluminescence. Immunoblots and dot blots were completed as described (25, 30).
Results
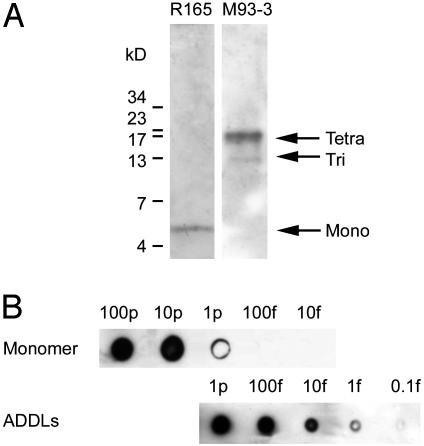
Immunoassay for Soluble Aβ Assemblies. To develop a sensitive assay for low levels of oligomers, we first obtained an antibody (R165) known to bind Aβ in Western blots at femtomolar levels (32). This antibody, although highly sensitive, proved selective for monomers. We therefore vaccinated rabbits with full-length Aβ1-42 oligomers to generate sensitive antibodies specific for assembled forms of Aβ (M71, M93, and M94). Specificities of R165 and the oligomer-generated M93 antibodies for different forms of Aβ are illustrated (Fig. 1A). The predominant oligomer detected in this preparation was tetramer, the most abundant oligomer formed in cold solutions. Depending on conditions, stable oligomers up to 24-mer have been observed, with trimer, tetramer, and 12-mer routinely observed (ref. 26; see also Fig. 3). Specificity of M93 for assembled forms of Aβ was indistinguishable from other oligomer-generated rabbit antibodies (M71 and M94). Dot immunoblot assays with these conformation-sensitive antibodies detected oligomers at <0.1 fmol (total Aβ; Fig. 1B) with great specificity. Signal in monomer preparations was at least three orders of magnitude less sensitive, and was likely due to trace amounts of oligomer. Dot immunoblots for oligomers were linear for at least a 100-fold range (data not shown), making the assay useful for determining relative levels in brain extracts.
Fig. 1.
Selective, sensitive dot-blot assay for assembled forms of soluble Aβ1-42. (A) Immunoblot of ADDL preparations (100 fmol) shows that M93 antibody selectively identifies oligomers (Right), whereas R165 antibody identifies only monomer (Left). (B) A dot blot immunoassay using M93 detects ADDLs with a sensitivity of 1 fmol, but detects monomers only at levels 1,000-fold higher.
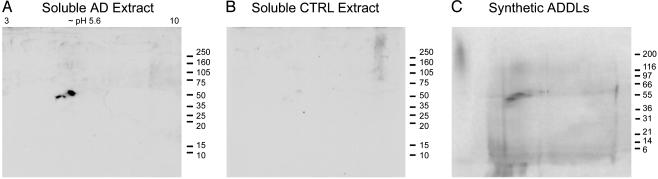
Fig. 3.
Assembled forms of soluble Aβ in AD brain show identity with synthetic Aβ oligomers. 2D immunoblots of soluble protein from AD cortex (A) or control cortex (B), and silver stain of synthetic ADDLs (C). AD extract and synthetic ADDLs show a prominent 53-kDa protein at pI 5.6, corresponding to a putative 12-mer of Aβ1-42.
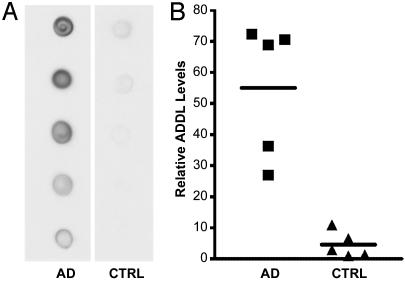
Increased Soluble Aβ Oligomers in AD. Dot blot assays were used to test for assembled forms of Aβ in soluble extracts of human frontal cortex. Five AD samples were compared with age-matched controls. To best preserve in vivo conditions, cortical tissue was homogenized without detergent in nerve cell culture medium. Soluble fractions were clear supernatants from 100,000 × g 60-min spins. Immunoreactivity was robust in AD brain extracts, but was near background for controls (Fig. 2). Essentially identical results were obtained in three separate trials. Population averages for AD brain were 12-fold higher than for control brain (P < 0.001). Some control subjects showed detectable oligomers, but at levels lower than for any AD subject. Differences between individual AD and control samples were as much as 70-fold.
Fig. 2.
AD-affected brain is associated with large increases in assembled forms of soluble Aβ.(A) Dot blot immunoassay for assemblies of Aβ in soluble extracts of five AD-affected brains and five age-matched control brains (1 μg of total extracted brain protein per dot). (B) Results from densitometric imaging of these same samples. The line indicates the mean of each set.
Composition of AD-Derived Oligomers. To verify that immunoreactivity from AD brain (Fig. 2) was due only to oligomers, and to obtain further information concerning molecular composition, soluble extracts were concentrated, resolved by 2D electrophoresis, and were analyzed by immunoblot. Extracts of AD brain contained a prominent oligomer at ∼56 kDa and pI 5.6 (Fig. 3A), whereas control brain showed no immunoreactive material (Fig. 3B). Absence of fibrils in soluble AD brain fractions was consistent with the use of mild, detergent-free extraction conditions and high-speed ultracentrifugation. High-speed pellets, which contained the amyloid fraction, had copious immunoreactive material unable to enter SDS/PAGE gels (see Fig. 6). Adding strong detergent (1% SDS) to the extraction protocol gave fibril-free supernatants with additional oligomeric species (4-mers and 24-mers, besides the 12-mers), but it cannot be certain these were of natural origin. Confirmation that immunoreactive molecules in freely soluble extracts were Aβ oligomers was established by comparison with synthetic Aβ preparations (Fig. 3C). As noted elsewhere (26), physiological temperatures favor accumulation of 12-mer in synthetic preparations, which was most abundant here. The prominent immunoreactive molecule in soluble AD brain extracts thus was identified as an Aβ 12-mer (Mr = 53 ± 4 kDa for three subjects).
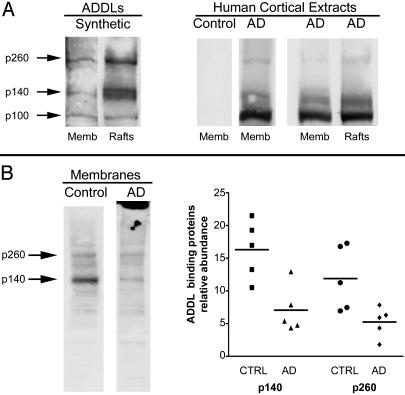
Fig. 6.
Comparative ligand overlay assays using rat and human membrane proteins. (A Left) Synthetic ligands and rat membranes. Shown is an overlay assay with synthetic Aβ oligomers and rat brain total membranes or fractions enriched in rafts. Additional binding was evident at p100, a band not always detected; compare with Fig. 5A. Binding at p140 and p260 was enriched in rafts. (A Right) Human ligands and rat membranes. Shown is an overlay assay with soluble extracts from human brain (AD or controls) applied to rat brain membranes (total membranes or rafts). AD-soluble extracts showed prominent binding at p100, but binding at p140 and p260 binding was evident in total membranes and in rafts. No signal was found in extracts from control brains. (B Left) synthetic ligands and human membranes. Shown is an overlay assay using total membranes pelleted from human brain and 10 nM synthetic ADDLs. Comparison of control and AD subjects indicated fewer binding sites in AD (note fibrillar amyloid in AD membrane fraction; top of gel). (B Right) Densitometric quantitation of p140 and p260 substantiated fewer binding sites in AD brain.
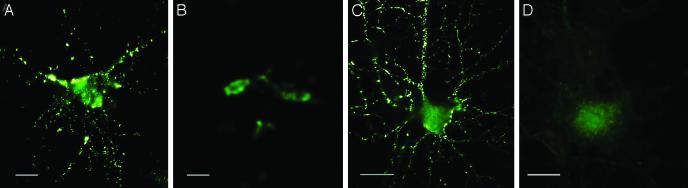
Ligand-Like Attachment of Oligomers to Neurons at Clustered Binding Sites. In further tests of AD-derived oligomers, we investigated their attachment to cultured neurons. Aβ oligomers potentially could attach nonspecifically by insertion into lipid bilayers (33, 34), or, alternatively, by acting as specific ligands for particular surface binding sites (18). Brain extracts were incubated with cultured rat hippocampal neurons for 5 min, washed, and immunolabeled without permeabilization. Prominent binding was evident for AD extracts, but not for controls (Fig. 4 A vs. B). Binding was highly patterned, occurring at small clusters of binding sites. Identical patterns occurred for synthetic oligomers (Fig. 4C) and were seen at doses as low as 20 nM (total Aβ). Punctate binding was absent whether oligomers were preincubated with primary antibodies (Fig. 4D), ruling out the possibility that binding was nonspecific, because of the presence of unusually adhesive molecules. Binding puncta were most abundant in neurites (shown here in stacked z-sections obtained by confocal imaging), and resembled signaling specializations such as clustered rafts or synaptic terminals. Binding was robust in hippocampal and cortical cultures, but not in cerebellar cultures (data not shown), and even within hippocampal cultures, many neurons exhibited no binding. Oligomers thus were specific cell-surface ligands, which was consistent with previous predictions (16, 18) based on oligomer size and diffusibility. Identical patterns seen for neuronal attachment of synthetic and AD-derived oligomers suggested a conformational equivalence.
Fig. 4.
ADDLs from AD brain or prepared in vitro show identical punctate binding to neuronal cell-surface proteins. Cultured hippocampal neurons were incubated with soluble extracts of human brain or synthetic ADDLs. Binding was visualized by immunofluorescence microscopy by using M93 antibody. Soluble AD-brain proteins (A), soluble control-brain proteins (B), synthetic ADDLs (C), and synthetic ADDLs (D) pretreated (1 h) with oligomer-selective antibody M71 are shown. Small puncta, typically <1 μm, and largely distributed along neurites, are evident for AD extracts and synthetic ADDLs, but not for control extracts or antibody-pretreated ADDLs. (Bar, 10 μm.)
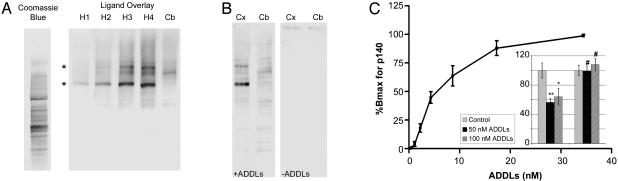
Flow cytometry previously established (18) that binding of synthetic oligomers to intact cells was trypsin-sensitive. To test whether oligomers might act as ligands for particular proteins, we separated membrane proteins by SDS/PAGE, and carried out ligand overlay assays. Similar to Western blots, except with an intermediate ligand binding step, overlay assays can uncover specific protein-protein interactions (30, 35). Assay conditions for high-affinity binding were developed by using synthetic oligomers and rat brain membrane proteins. At low doses (10 nM total Aβ), binding was most prominent at two nonabundant bands of higher molecular weight (p140 and p260). Signal depended on the level of membrane protein per well (Fig. 5A) and oligomer dosage (half-maximal binding ∼10 nM; Fig. 5C), and was trypsin-sensitive (data not shown). Binding proteins were neither abundant (Fig. 5A; compare with Coomassie blue protein stain), nor were they unusually sticky, because controls for antibody binding in the absence of oligomers were negative (e.g., Fig. 5B Center). Binding sites were prominent in hippocampal and cortical membranes, but not cerebellar membranes (Fig. 5 A and B). Regional expression paralleled sensitivity to oligomer toxicity, which was evident in cortical cultures, but not cerebellar cultures at low doses of oligomers (Fig. 5C Inset).
Fig. 5.
ADDLs are high-affinity ligands for particular solubilized proteins from rat brain regions sensitive to ADDL toxicity. (A) Coomassie blue staining or overlay assays using SDS/PAGE-separated membrane proteins from rat hippocampus (H1-4; 12.5, 25, 50, and 75 μg) or cerebellum (Cb; 75 μg). Ligands were synthetic ADDLs (10 nM). Immunodetection used Aβ assembly-dependent rabbit polyclonal antibodies. Selective binding at p140 and p260 kDa bands (*) was readily evident in hippocampus, but not in cerebellum. Binding proteins were not abundant, which was shown by comparison with total hippocampal proteins stained by Coomassie blue. (B) Overlay assays as in A, using 75-μg membrane proteins from rat cortex (Cx) or cerebellum (Cb). Binding at p140 and p260 bands was evident in cortex, but not in cerebellum. Without oligomers (-ADDLs), no signal from the horseradish peroxidase-conjugated secondary antibody was evident. (C) Densitometry of synthetic binding to rat cortical p140, using increasing doses of synthetic ADDLs. Half-maximal binding was at ∼10 nM. (Inset) Impact of ADDLs on MTT reduction in rat cortical (Left) and cerebellar (Right) cultures shows that ADDLs were selectively toxic to cortical cells, with maximal response by 50 nM. Control MTT reduction was taken as 100%.
Synthetic oligomers in some experiments bound to a third band (p100), although binding was less favorable than to p140 and p260 (Fig. 6A Left). Unlike p140 and p260 binding, the p100 sites were evident in cerebellum, as well as in cortex and hippocampus. Another difference was that p140 and p260, but not p100, binding sites were enriched in raft preparations. Rafts have been linked to signal transduction (36), aspects of Aβ metabolism (37, 38), and synapse organization (39). Overlay patterns were different for AD-derived oligomers, which bound the same three bands as synthetic oligomers, but bound most robustly to p100 (Fig. 6A Right). Whether overlay binding of AD-derived oligomers was affected by additional proteins in the extracts is not yet known. Binding, however, was completely disease dependent, as control extracts showed no signal.
Overlay assays for binding of synthetic oligomers to human membrane proteins showed conservation of the cortical sites at p140 and p260 (Fig. 6B Left), which, as illustrated, were more abundant in control brain than AD brain. Decreased relative abundance of p260 and p140 binding sites in five AD samples compared with five normal elderly samples (Fig. 6B Right; P < 0.05 for p260, and P < 0.01 for p140 using Student's t test) was consistent with possible occurrence of oligomer binding proteins on cells vulnerable to degeneration in AD.
Discussion
This study has found that AD-affected brains exhibit a striking increase in soluble oligomeric Aβ, with AD-derived oligomers being indistinguishable from synthetic molecules in terms of structure and selective attachment to nerve cell surfaces. These findings provide major support for the hypothesized involvement of oligomers in AD, and substantiate the potential of oligomers as novel targets for AD drug discovery. Accumulation of Aβ oligomers in AD provides precedent, moreover, that subfibrillar assemblies now identified for multiple amyloidogenic proteins (40) could be pathogenically significant.
Levels of soluble Aβ oligomers showed no overlap between AD samples and age-matched controls, with maximum differences of >70-fold. Some controls showed mildly elevated oligomers. Although it has been proposed that oligomers play a role in the earliest stages of AD, and even in pre-AD dysfunction (26), this will be difficult to test without clinically useful assays. Buildup of oligomers in AD pathology is consistent with results from transgenic mouse AD models, in which oligomers increase 100-fold in a regionally specific manner (41). Memory failure in transgenic mice serves as a model for early AD, and its remarkable reversal by Aβ-directed antibodies has been attributed to immunoneutralization of soluble Aβ assemblies (23). Reversal in mouse models suggests the possibility that transient changes in oligomer levels in human brain could cause fluctuations in memory performance.
Comparison of synthetic and AD-derived oligomers indicated structural equivalence. Previous studies (26) have noted that sizes of synthetic oligomers are influenced by multiple factors, and range from trimer to 24-mer. For AD brain extracts and for synthetic preparations made under bioassay conditions, 2D electrophoresis showed prominent 12-mers at pI 5.6. Although the antibody used can detect the presence of large amyloid fibrils (e.g., Fig. 6), the 2D blots established that the soluble fractions from AD brain were free of fibrillar and protofibrillar Aβ species. Protofibrils, which can be neurologically active (42), have been reported to occur in cerebrospinal fluid (43). Strong detergent (SDS) released additional oligomers from AD brain, indicating some oligomers were tightly bound to other molecules. Because human oligomers were not purified, they could not be compared with synthetic oligomers for globular appearance by atomic force microscopy, nor could they be tested for neurotoxicity. One difference noted between synthetic and AD-derived oligomers was the greater binding of AD-derived molecules to the p100 band in overlay assays. The basis for the difference is unknown, but conceivably may derive from the multiple components present in AD extracts. Overall, the immunochemical assays indicated structural equivalence, which was shown by overlapping mass, pI values, recognition by conformation-sensitive antibodies, and ability to act as specific ligands for particular binding sites. Particularly striking was the equivalent pattern of attachment of synthetic and AD-derived oligomers to neuronal cell surfaces.
Although it has been proposed that toxic Aβ acts by inserting directly into membrane lipids (33, 34), the current data show oligomers are specific ligands for discretely clustered cell-surface molecules. Overlay assays also showed oligomers act as high-affinity ligands for a small number of solubilized membrane proteins. Binding to cells and solubilized proteins was blocked by antibodies, ruling out simple adsorption at sticky sites. Binding to p140 and p260 exhibited properties suggestive of physiological relevance (high affinity, appropriate regional expression, raft association, species conservation, and decreases in AD), but whether they mediate binding observed by microscopy requires further study.
The size and patterned distribution of cell-surface attachment strongly suggests physiological relevance. Supporting this possibility, recent detailed studies¶ with synthetic oligomers applied to differentiated hippocampal neuron cultures have shown colocalization of binding sites with the synaptic marker PSD-95. Targeting of synapses by oligomers would support the hypothesis that AD is in large part a synaptic pathology (24), and would be consistent with their rapid, selective inhibition of LTP (18, 19). Molecular changes after attachment are poorly understood, although signaling cascades essential for synaptic plasticity appear to be involved. cAMP-response element-binding protein (CREB) signaling is inhibited by nondegenerative doses of Aβ under conditions that produce oligomers (44). Hypothetically, inhibition of CREB signaling could derive from displacement of Fyn (45), which normally is tethered by PSD-95 to signaling scaffolds (46), and has been linked to oligomer-induced neuronal death (18), experimental memory deficits (47), and AD pathology (48). Trafficking of receptors also may be affected. Oligomers block the reversal of long-term depression (LTD) (20), as well as the formation of LTP. These phenomena are associated with glutamate receptor insertion into postsynaptic membranes (49), a process linked to Src-family kinases (50). Disrupted signaling responsible for changes in LTP and LTD eventually may lead to synapse destabilization (20). It has been proposed that Aβ oligomers are responsible for the plaque-independent decreases in synapse levels observed in hAPP mice (15).
Because oligomers are neurologically disruptive in experimental models and show significant increases in AD brain, they provide an alternative to plaques as a target for drug discovery. Monoclonal antibodies may provide effective therapeutics, given the plaque-independent reversal of memory failure demonstrated in AD mouse models. One prerequisite will be to develop antibodies that target soluble, but not insoluble, Aβ assemblies. Clinical vaccine trials recently were suspended because of CNS inflammation (51), which was likely induced by binding of antibodies to amyloid plaque deposits. Whether Aβ oligomers manifest unique epitopes absent from fibrils is unknown. Precedent, however, comes from prions, which oligomerize via a pathway distinct from their fibrillogenesis (52). If antibodies can be developed that uniquely target oligomers, it would raise the exciting possibility that human memory function in AD could be preserved safely, and in some circumstances, perhaps even restored.
Acknowledgments
We thank Karen Ashe, David Teplow, Catherine Wooley, Nelson Spruston, and Albert Farbman for their comments on the manuscript; Eileen Bigio of Northwestern University Alzheimer's Center Pathology Core for analysis of human tissue; Joy Ramos for assistance with immunolabeling; William Russin of the Biological Imaging Facility of Northwestern University for his help with confocal microscopy; and Brett Chromy for his expert discussions of ADDL structure. This work was supported by National Institutes of Health Grants R01-AG18877 and P01-AG15501; the Boothroyd, Feiger, and French Foundations; the Institute for the Study of Aging; and a benefactor of Northwestern University.
Abbreviations: AD, Alzheimer's disease; Aβ, amyloid β-peptide; ADDLs, Aβ-derived diffusible ligands; APP, amyloid precursor protein; LTP, long-term potentiation.
Footnotes
Lacor, P. N., Viola, K. L., Lambert, M. P., Finch, C. E., Krafft, G. A. & Klein, W. L. (2002) Soc. Neurosci. Abstr. 28, 751.7.
References
- 1.Hardy, J. & Selkoe, D. J. (2002) Science 297, 353-356. [DOI] [PubMed] [Google Scholar]
- 2.Kirkitadze, M. D., Bitan, G. & Teplow, D. B. (2002) J. Neurosci. Res. 69, 567-577. [DOI] [PubMed] [Google Scholar]
- 3.Klein, W. L. (2000) in Molecular Mechanisms of Neurodegenerative Diseases, ed. Chesselet, M.-F. (Humana, Totowa, NJ), pp. 1-49.
- 4.Kang, J., Lemaire, H. G., Unterbeck, A., Salbaum, J. M., Masters, C. L., Grzeschik, K. H., Multhaup, G., Beyreuther, K. & Muller-Hill, B. (1987) Nature 325, 733-736. [DOI] [PubMed] [Google Scholar]
- 5.Goate, A., Chartier-Harlin, M. C., Mullan, M., Brown, J., Crawford, F., Fidani, L., Giuffra, L., Haynes, A., Irving, N. & James, L. (1991) Nature 349, 704-706. [DOI] [PubMed] [Google Scholar]
- 6.Ertekin-Taner, N., Graff-Radford, N., Younkin, L. H., Eckman, C., Baker, M., Adamson, J., Ronald, J., Blangero, J., Hutton, M. & Younkin, S. G. (2000) Science 290, 2303-2304. [DOI] [PubMed] [Google Scholar]
- 7.Harper, J. D. & Lansbury, P. T., Jr. (1997) Annu. Rev. Biochem. 66, 385-407. [DOI] [PubMed] [Google Scholar]
- 8.Glenner, G. G. & Wong, C. W. (1984) Biochem. Biophys. Res. Commun. 122, 1131-1135. [DOI] [PubMed] [Google Scholar]
- 9.Masters, C. L., Simms, G., Weinman, N. A., Multhaup, G., McDonald, B. L. & Beyreuther, K. (1985) Proc. Natl. Acad. Sci. USA 82, 4245-4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jarrett, J. T., Berger, E. P. & Lansbury, P. T., Jr. (1993) Biochemistry 32, 4693-4697. [DOI] [PubMed] [Google Scholar]
- 11.Lorenzo, A. & Yankner, B. A. (1994) Proc. Natl. Acad. Sci. USA 91, 12243-12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pike, C. J., Burdick, D., Walencewicz, A. J., Glabe, C. G. & Cotman, C. W. (1993) J. Neurosci. 13, 1676-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardy, J. A. & Higgins, G. A. (1992) Science 256, 184-185. [DOI] [PubMed] [Google Scholar]
- 14.Terry, R. D. (1999) in Alzheimer Disease, eds. Terry, R. D., Katzman, R., Bick, K. L. & Sisodia, S. S. (Lippincott, Williams & Wilkins, Philadelphia), pp. 187-206.
- 15.Mucke, L., Masliah, E., Yu, G. Q., Mallory, M., Rockenstein, E. M., Tatsuno, G., Hu, K., Kholodenko, D., Johnson-Wood, K. & McConlogue, L. (2000) J. Neurosci. 20, 4050-4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein, W. L., Krafft, G. A. & Finch, C. E. (2001) Trends Neurosci. 24, 219-224. [DOI] [PubMed] [Google Scholar]
- 17.Klein, W. L. (2002) Neurobiol. Aging 23, 231-235. [DOI] [PubMed] [Google Scholar]
- 18.Lambert, M. P., Barlow, A. K., Chromy, B. A., Edwards, C., Freed, R., Liosatos, M., Morgan, T. E., Rozovsky, I., Trommer, B., Viola, K. L., et al. (1998) Proc. Natl. Acad. Sci. USA 95, 6448-6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walsh, D. M., Klyubin, I., Fadeeva, J. V., Cullen, W. K., Anwyl, R., Wolfe, M. S., Rowan, M. J. & Selkoe, D. J. (2002) Nature 416, 535-539. [DOI] [PubMed] [Google Scholar]
- 20.Wang, H. W., Pasternak, J. F., Kuo, H., Ristic, H., Lambert, M. P., Chromy, B., Viola, K. L., Klein, W. L., Stine, W. B., Krafft, G. A., et al. (2002) Brain Res. 924, 133-140. [DOI] [PubMed] [Google Scholar]
- 21.Morgan, D., Diamond, D. M., Gottschall, P. E., Ugen, K. E., Dickey, C., Hardy, J., Duff, K., Jantzen, P., DiCarlo, G., Wilcock, D., et al. (2000) Nature 408, 982-985. [DOI] [PubMed] [Google Scholar]
- 22.Dodart, J. C., Bales, K. R., Gannon, K. S., Greene, S. J., DeMattos, R. B., Mathis, C., DeLong, C. A., Wu, S., Wu, X., Holtzman, D. M., et al. (2002) Nat. Neurosci. 5, 452-457. [DOI] [PubMed] [Google Scholar]
- 23.Kotilinek, L. A., Bacskai, B., Westerman, M., Kawarabayashi, T., Younkin, L., Hyman, B. T., Younkin, S. & Ashe, K. H. (2002) J. Neurosci. 22, 6331-6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selkoe, D. J. (2002) Science 298, 789-791. [DOI] [PubMed] [Google Scholar]
- 25.Lambert, M. P., Viola, K. L., Chromy, B. A., Chang, L., Morgan, T. E., Yu, J., Venton, D. L., Krafft, G. A., Finch, C. E. & Klein, W. L. (2001) J. Neurochem. 79, 595-605. [DOI] [PubMed] [Google Scholar]
- 26.Klein, W. L. (2002) Neurochem. Int. 41, 345-352. [DOI] [PubMed] [Google Scholar]
- 27.Brewer, G. J., Torricelli, J. R., Evege, E. K. & Price, P. J. (1993) J. Neurosci. Res. 35, 567-576. [DOI] [PubMed] [Google Scholar]
- 28.Samdani, A. F., Newcamp, C., Resink, A., Facchinetti, F., Hoffman, B. E., Dawson, V. L. & Dawson, T. M. (1997) J. Neurosci. 17, 4633-4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friso, G. & Wikstrom, L. (1999) Electrophoresis 20, 917-927. [DOI] [PubMed] [Google Scholar]
- 30.Denda, S., Reichardt, L. F. & Muller, U. (1998) Mol. Biol. Cell 9, 1425-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang, C., Lambert, M. P., Bunch, C., Barber, K., Wade, W. S., Krafft, G. A. & Klein, W. L. (1994) J. Biol. Chem. 269, 25247-25250. [PubMed] [Google Scholar]
- 32.Potempska, A., Mack, K., Mehta, P., Kim, K. S. & Miller, D. L. (1999) Amyloid 6, 14-21. [DOI] [PubMed] [Google Scholar]
- 33.Lin, H., Bhatia, R. & Lal, R. (2001) FASEB J. 15, 2433-2444. [DOI] [PubMed] [Google Scholar]
- 34.Kourie, J. I., Culverson, A. L., Farrelly, P. V., Henry, C. L. & Laohachai, K. N. (2002) Cell Biochem. Biophys. 36, 191-207. [DOI] [PubMed] [Google Scholar]
- 35.Bowe, M. A., Mendis, D. B. & Fallon, J. R. (2000) J. Cell Biol. 148, 801-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simmons, K. & Toomre, D. (2000) Nat. Rev. Mol. Cell Biol. 1, 31-39. [DOI] [PubMed] [Google Scholar]
- 37.Ehehalt, R., Keller, P., Haass, C., Thiele, C. & Simons, K. (2003) J. Cell Biol. 160, 113-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kakio, A., Nishimoto, S., Yanagisawa, K., Kozutsumi, Y. & Matsuzaki, K. (2002) Biochemistry 41, 7385-7390. [DOI] [PubMed] [Google Scholar]
- 39.Bruses, J. L., Chauvet, N. & Rutishauser, U. (2001) J. Neurosci. 21, 504-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bucciantini, M., Giannoni, E., Chiti, F., Baroni, F., Formigli, L., Zurdo, J., Taddei, N., Ramponi, G., Dobson, C. M. & Stefani, M. (2002) Nature 416, 507-511. [DOI] [PubMed] [Google Scholar]
- 41.Chang, L., Gong, Y., Bakhos, L., Yu, J., Venton, D. L. & Klein, W. L. (2003) J. Mol. Neurosci., in press. [DOI] [PubMed]
- 42.Hartley, D. M., Walsh, D. M., Ye, C. P., Diehl, T., Vasquez, S., Vassilev, P. M., Teplow, D. B. & Selkoe, D. J. (1999) J. Neurosci. 19, 8876-8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pitschke, M., Prior, R., Haupt, M. & Riesner, D. (1998) Nat. Med. 4, 832-834. [DOI] [PubMed] [Google Scholar]
- 44.Tong, L., Thornton, P. L., Balazs, R. & Cotman, C. W. (2001) J. Biol. Chem. 276, 17301-17306. [DOI] [PubMed] [Google Scholar]
- 45.Takasu, M. A., Dalva, M. B., Zigmond, R. E. & Greenberg, M. E. (2002) Science 295, 491-495. [DOI] [PubMed] [Google Scholar]
- 46.Ying, S. W., Futter, M., Rosenblum, K., Webber, M. J., Hunt, S. P., Bliss, T. V. & Bramham, C. R. (2002) J. Neurosci. 22, 1532-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grant, S. G. & Silva, A. J. (1994) Trends Neurosci. 17, 71-75. [DOI] [PubMed] [Google Scholar]
- 48.Shirazi, S. K. & Wood, J. G. (1993) NeuroReport 4, 435-437. [DOI] [PubMed] [Google Scholar]
- 49.Sheng, M. (2001) Proc. Natl. Acad. Sci. USA 98, 7058-7061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grosshans, D. R., Clayton, D. A., Coultrap, S. J. & Browning, M. D. (2002) Nat. Neurosci. 5, 27-33. [DOI] [PubMed] [Google Scholar]
- 51.Birmingham, K. & Frantz, S. (2002) Nat. Med. 8, 199-200. [DOI] [PubMed] [Google Scholar]
- 52.Baskakov, I. V., Legname, G., Baldwin, M. A., Prusiner, S. B. & Cohen, F. E. (2002) J. Biol. Chem. 277, 21140-21148. [DOI] [PubMed] [Google Scholar]