Abstract
The impact of progestins on estrogen-inducible mechanisms of neuroprotection was investigated. Previously, we showed that estrogen and progesterone are neuroprotective against excitotoxicity, whereas the synthetic progestin medroxyprogesterone acetate (MPA; Provera) is not. Here, we demonstrate that 17β-estradiol (E2) and progesterone (P4) treatment of hippocampal neurons attenuated the excitotoxic glutamate-induced rise in intracellular calcium concentration. Although MPA had no effect alone, MPA completely antagonized E2-induced attenuation of intracellular calcium concentration. Activation of extracellular receptor kinase (ERK) is required for estrogen-induced neuroprotection and calcium regulation. Paradoxically, E2, P4, and MPA all elicited similar rapid and transient activation of ERK, presenting a contradiction between the dependence on ERK for gonadal hormone-induced neuroprotection and the lack of neuroprotection induced by MPA. Subcellular analysis of ERK demonstrated that the phospho-ERK signal is transduced to the nucleus only by E2 and P4, not by MPA. These results indicate that the profile of nuclear translocation of ERK is consistent with the neuroprotective profile. Further, the E2-induced nuclear translocation of ERK was blocked by coadministration of MPA. Results of this study reveal that nuclear ERK induction by ovarian steroids is predictive of the neuroprotective effects of estrogen and progestin treatments, revealing a hitherto unrecognized divergence of progestin signaling through the src/MAPK pathway. These results have much broader implications encompassing the impact of progestins on estrogen-mediated effects in multiple tissues. The recent results from the Women's Health Initiative trial, which used MPA as the progestinal agent, indicate that differences between progestin formulations are crucial to health outcomes in women.
Neurologic benefits of estrogen replacement therapy in humans include reversal of estrogen deficiency-induced memory dysfunction and reduced risk of Alzheimer's disease (AD) (1-5). Recently, the Cache County Study confirmed a reduced risk of AD in elderly women on hormone replacement therapy (HRT) (6). Because progestins are added to HRT to prevent hyperplasia of the endometrium (7) and resulting uterine cancer (8), possible impacts of progestins need to be determined. Numerous studies have found contradictory effects of estrogen/progestin use and breast cancer (9), coronary heart disease (10), and cognition (11, 12). Although some of these studies used the same hormone formulation [conjugated estrogens (Premarin) with medroxyprogesterone acetate (MPA; Provera)], many did not determine or subdivide the type of progestin used (9-14), raising the possibility that the apparent discrepancies in outcomes are due to the differences in cellular responses induced by different progestins. Such concerns have been underscored by the termination of the combined regimen arm of the Women's Health Initiative trial (11, 12, 15).
In earlier work, we found that 17β-estradiol (E2) and progesterone (P4), but not MPA, exerted neuroprotection against glutamate neurotoxicity (16). Not only was MPA an ineffective neuroprotectant, it attenuated E2-induced neuroprotection when coadministered (16). Estrogen-induced neuroprotection against excitotoxic glutamate is correlated with an attenuated rise in intracellular calcium concentration ([Ca2+]i) (17, 18). Thus, we sought to determine the impact of P4 and MPA on excitotoxic glutamate-induced [Ca2+]i rise. Here we show that E2 and P4 attenuated the glutamate-induced rise in [Ca2+]i. Although MPA had no effect on the glutamate-induced Ca2+ signal, it blocked E2-induced attenuation.
Although the full underlying cascade of mechanisms remains unidentified, the mitogen-activated protein kinase (MAPK) cascade is required for estrogen-mediated neuroprotection (19) and estrogen-induced attenuation of the [Ca2+]i rise (17). E2, P4, and MPA all activate p44/p42MAPK [extracellular receptor kinase (ERK)] (16), yet only E2 and P4 are neuroprotective (16). To resolve the paradox between dependence on MAPK for gonadal hormone-induced neuroprotection and lack of neuro-protection induced by some progestins that activate MAPK, we analyzed the temporal and subcellular profile of ERK activation by E2, P4, and MPA. We show that E2 and P4 rapidly and transiently activated nuclear ERK in hippocampal neurons. In contrast, ERK activated by MPA remained cytosolic with no nuclear signal. Further, MPA blocked the E2-induced nuclear ERK activation. The dramatic differences in signaling elicited by P4 and MPA indicate that all progestins are not alike in their induction of cellular responses and, hence, health outcomes.
Materials and Methods
Chemicals. Culture materials were from GIBCO/BRL. Chemicals were from Sigma, unless otherwise noted. Steroids were dissolved in ethanol and diluted in culture medium with final ethanol concentration <0.001%. Fura 2-AM (the acetoxymethyl ester) was from Molecular Probes.
Neuronal Culture. Hippocampal neurons from embryonic day 18 (E18) rat fetuses were cultured as described and generated cultures 98% neuronal in phenotype (17). In brief, embryonic rat hippocampi were dissociated by passage through fire-polishconstricted Pasteur pipettes. Neurons plated on poly-D-lysine-coated coverslips (22-mm-diameter round), four-well chamber slides (Falcon), or polyethylenimine-coated six-well plates were grown in Neurobasal medium (GIBCO/BRL) supplemented with 5 units/ml penicillin, 5 mg/ml streptomycin, and B27 supplement at 37°C in humidified 5% CO2/95% air atmosphere for 10-12 days before experimentation.
Measurement of Cytoplasmic Ca2+ by Using Fura 2-AM. Hippocampal neurons were treated with E2 (10 ng/ml), P4 (10 ng/ml), and/or MPA (10 ng/ml) or vehicle control for 48 h before loading in the dark with fura 2-AM (2 μM) in Hanks' balanced salt solution (45 min; 37°C). [Ca2+]i was determined by comparing the A340/A380 ratio to a standard curve as described (17). Data are presented as representative traces averaged from at least 10 cells per coverslip. Responses to steroids were quantified as the difference between the average [Ca2+]i for 1 min during glutamate exposure (30-90 sec after glutamate exposure) and the average [Ca2+]i for 1 min before exposure. Changes in [Ca2+]i are presented as mean ± SEM from four independent experiments with at least 30 cells per experiment. Equal dye loading was determined as described (18).
Western Blotting. Immunoblottings of whole-cell lysates and sub-cellular fractions were performed with Anti-Active MAPK antibody (1:750 in PBS-T/1% horse serum; Cell Signaling Technologies, Beverly, MA; PBS-T is PBS containing Tween) or total ERK2 antibody (C-14) (1:5,000 in PBS-T/1% horse serum; Santa Cruz Biotechnology) as described (16). Cytosolic and nuclear proteins were prepared by differential centrifugation. Cell pellets resuspended in 5 vol of cytoplasmic extract (CE) buffer [10 mM Hepes (pH 7.5)/60 mM KCl/1 mM EDTA/1mM DTT/1 mM sodium orthovanadate/1 mM PMSF/0.075% (vol/vol) Igepal CA-630, Sigma] were homogenized by repeated passage through a 23-gauge needle. Homogenate was centrifuged at 500 × g for 5 min at 4°C to pellet out nuclei. Supernatants were centrifuged at 10,000 × g for 10 min at 4°C. The resulting supernatants were used as cytoplasmic extracts. Nuclear pellets were washed with 100 μl of CE buffer and resuspended in 1 vol of nuclear extract (NE) buffer [20 mM Tris·HCl/420 mM NaCl/60 mM KCl/1 mM EDTA/1 mM sodium orthovanadate/1 mM PMSF/25% (vol/vol) glycerol; pH 8.0]. Salt concentration was adjusted to 400 mM by addition of 5 M NaCl, followed by addition of 1 vol of NE buffer. Nuclear extracts were incubated for 10 min at 4°C and centrifuged at 10,000 × g for 10 min at 4°C. The resulting supernatants were used as nuclear extracts. Specificity of subcellular fractionations was determined by probing parallel Western blots with antihistone (nuclear) and anti-neuron-specific enolase (cytoplasm).
Immunocytochemistry. Hippocampal neurons exposed to E2 (10 ng/ml), P4 (10 ng/ml), or MPA (10 ng/ml) for 30 min were paraformaldehyde (4%)-fixed and Triton X-100 (0.001%)permeabilized before incubation with anti-Active MAPK antibody (1:300; Cell Signaling Technologies) followed by incubation in fluorescein-conjugated goat anti-rabbit secondary antibody (1:250, Vector Laboratories) for 45 min at RT. Cells were mounted in Vectashield mounting medium with 4′, 6-diamidino-2-phenylindole (DAPI) (Vector Laboratories). Relative immunoreactive intensity was calculated by using INCYTIM1 software (Intracellular Imaging, Cincinnati). The area of DAPI staining was mapped to the FITC images to define the nucleus as the region of interest or as a mask to define the cytoplasm as the region of interest. The relative fluorescence intensities of 20 randomly selected cells were normalized to the average fluorescence intensity of control cells and presented as mean ± SEM. The cytoplasm and nucleus were analyzed independently of each other.
Statistics. Statistically significant differences between groups were determined by an ANOVA followed by a Newman-Keuls post hoc analysis.
Results
E2 and P4 Attenuate the Glutamate-Induced Rise in Intracellular Calcium. Estrogen-induced neuroprotection against excitotoxic glutamate is correlated with an attenuated rise in [Ca2+]i (17, 18). Thus, we sought to determine the impact of P4 and MPA on excitotoxic glutamate-induced [Ca2+]i rise. Accordingly, our first aim was to compare the rise in [Ca2+]i, as measured by Fura2, in E2-, P4-, and MPA-treated neurons.
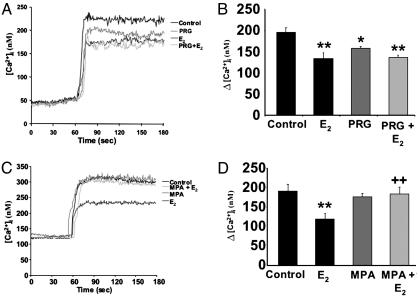
Glutamate induced a rapid increase in [Ca2+]i (Fig. 1) with a mean change in [Ca2+]i obtained on stimulation with 200 μM glutamate alone of 196.8 ± 10.5 nM (n = 4; Fig. 1B). Hippocampal neurons pretreated with E2 exhibited a marked (≈30%) and significant decrease in [Ca2+]i generated by 200 μM glutamate (Fig. 1 A) with a mean change in [Ca2+]i of 135.1 ± 10.2 nM (P < 0.01; n = 4 experiments; Fig. 1B). Likewise, P4-treated neurons exhibited a marked and significant decrease in [Ca2+]i generated by 200 μM glutamate (Fig. 1 A) with a mean change in [Ca2+]i of 158.7 ± 3.6 nM (P < 0.05; n = 4 experiments; Fig. 1B). The attenuation of the glutamate-induced rise in [Ca2+]i was non-significantly less with P4 treatment than with E2 treatment (Fig. 1B). Hippocampal neurons pretreated with the combination of E2 (10 ng/ml) and P4 (10 ng/ml) showed attenuation of the glutamate-induced rise in [Ca2+]i (Fig. 1 A), with a mean change in [Ca2+]i of 137.1 ± 5.6 nM (P < 0.01 as compared with control; n = 4; Fig. 1B), an effect more like that of E2 than P4.
Fig. 1.
E2 and P4, but not MPA, attenuate the excitotoxic glutamate-induced rise in [Ca2+]i. Hippocampal neurons pretreated with E2 (10 ng/ml), P4 (PRG), or both exhibited a significantly lower response to glutamate (200 μM) than control neurons. MPA had no effect on glutamate-induced Ca2+ signaling, but blocked the estrogen-mediated attenuation. (A) Representative tracings of the average [Ca2+]i from 10 neurons over time in response to glutamate in the presence or absence of P4 and/or E2.(B) Quantitative changes in [Ca2+]i in response to glutamate in the presence or absence of P4 and/or E2.(C) Representative tracings of the average [Ca2+]i from 10 neurons over time in response to glutamate in the presence or absence of MPA and/or E2. (D) Quantitative changes in [Ca2+]i in response to glutamate in the presence or absence of MPA and/or E2. *, P < 0.05 vs. control neurons; **, P < 0.01 vs. control neurons; ++, P < 0.01 vs. E2-treated neurons; n = 4 independent experiments with ≥10 neurons per experiment.
MPA Blocks the E2-Induced Attenuation of the Glutamate-Induced Rise in Intracellular Calcium. Hippocampal neurons were exposed to MPA (10 ng/ml) or vehicle control in the presence and absence of E2 (10 ng/ml) for 48 h before monitoring [Ca2+]i in response to glutamate (200 μM). Glutamate induced a rapid increase in [Ca2+]i (Fig. 1C), with a mean change in [Ca2+]i of 191.1 ± 17.8 nM (Fig. 1C). Neurons pretreated with E2 again exhibited a significant attenuation of glutamate-stimulated increase in [Ca2+]i (Fig. 1C), with a mean change in [Ca2+]i of 119.2 ± 14.5 nM (P < 0.01; n = 4; Fig. 1D). Neurons pretreated with MPA exhibited no change in glutamate-stimulated rise in [Ca2+]i (n = 4; Fig. 1C), with a mean change in [Ca2+]i of 176.9 ± 8.9 (n = 4; Fig. 1D). Coadministration of MPA with E2 significantly antagonized the E2-induced attenuation of the glutamate-stimulated rise in [Ca2+]i (Fig. 1C), with a mean change in [Ca2+]i of 183.5 ± 18.1 nM (P < 0.01 as compared with E2-treated neurons; n = 4; Fig. 1D).
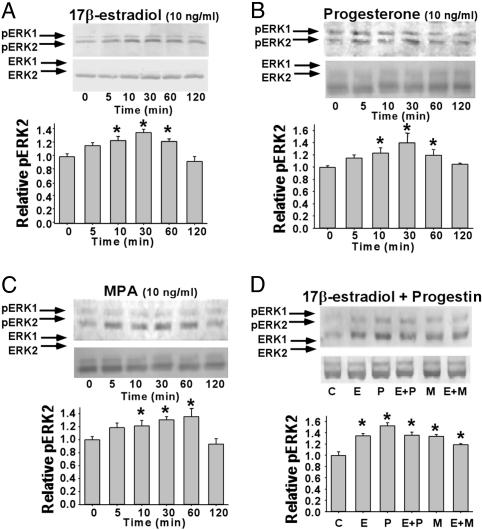
MAPK Activation in Response to E2, P4, and MPA in Primary Hippocampal Neurons. Blockade of MAPK activation prevents estrogen-mediated attenuation of excitotoxic glutamate-induced rise in [Ca2+]i (16) and the associated neuroprotection (19). These findings suggest that the ovarian steroid-induced neuroprotection and the attenuation of excitotoxin-induced [Ca2+]i signaling rise are dependent on active MAPK. Yet MPA, which neither exerts neuroprotection nor alters Ca2+ signaling, activates MAPK equally as well as E2 and P4 (16). To resolve the paradox between the dependence on MAPK for gonadal hormone-induced neuroprotection and the lack of neuroprotection induced by MPA, we chose to analyze first the temporal nature of ERK activation by E2, P4, and MPA, because the duration of MAPK activation can result in different outcomes (20). Using an antibody recognizing the phosphorylated form of p44/p42 MAPK (ERK1/ERK2) as an indicator of activation (21), Western blot analyses indicated that activation of ERK in primary hippocampal neurons occurs rapidly and transiently after treatment with E2, P4, and MPA. The kinetics of ERK activation by E2, P4, and MPA were similar, with increased immunoreactivity apparent 5 min after treatment and maximal immunoreactive intensity apparent at 30 min, with a return to basal levels by 120 min (Figs. 2A and 1 B and C). Treatment with E2 (10 ng/ml) resulted in a maximal phospho-ERK2 (pERK2) immunoreactivity increase of ≈60% at 30 min (Fig. 2 A), which is consistent with previous reports (19, 22). Treatment with P4 (10 ng/ml) or MPA (10 ng/ml) maximally increased pERK2 immunoreactivity by ≈123% (Fig. 2B) and ≈60% (Fig. 2C), respectively, at 30 min. Coadministration of P4 or MPA with E2 for 30 min increased pERK2 immunoreactivity by ≈100% (Fig. 2D) and ≈75% (Fig. 2D), respectively, compared with vehicle-treated neurons.
Fig. 2.
Rapid activation of ERK-2 in primary hippocampal neurons treated with E2, P4, or MPA. Western blots show levels of pERK2 and total ERK2 in whole-cell lysates from primary hippocampal neurons treated with E2 (A), P4 (B), MPA (C), or combined E2 and progestin (D). C, vehicle; E, E2; P, P4; M, MPA. Bar graphs represent mean ± SEM; *, P < 0.05 as compared with control; n = 4.
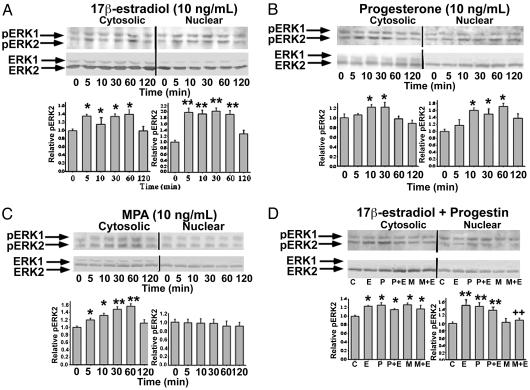
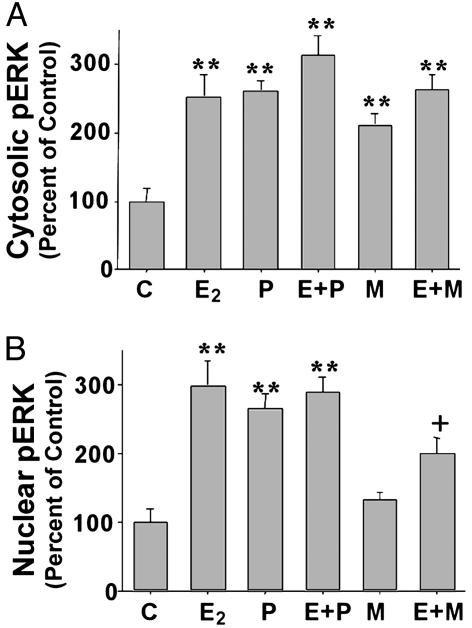
Increased Nuclear pERK in Primary Hippocampal Neurons in Response to E2 and P4, but Not MPA. Nuclear signaling by many cellular stimuli depends on activation of the MAPK cascade and nuclear localization of active MAPK, where these enzymes can act on their target substrates (23, 24). Such nuclear signaling depends on translocation of MAPK from the cytoplasm to the nucleus (24, 25). To determine whether this critical step was a point of divergence between the progestins, Western blot analysis was performed on cytosolic and nuclear fractions from primary hippocampal neurons treated with E2, P4, and MPA (Fig. 3). Results demonstrated that pERK2 immunoreactivity was present at very low levels in both cytosolic and nuclear fractions from control neurons (Fig. 3). In neurons treated with E2 or P4, a rapid and transient increase in pERK2 in both cytosolic and nuclear fractions occurred within 5 min (Fig. 3 A and B). The kinetics of ERK activation in the cytosolic fraction in response to E2 and P4 were similar, with increased immunoreactivity observed at 5 min and maximal staining occurring at 30 min, and immunoreactivity returning to basal levels by 120 min (Figs. 3 A and B). Increased immunoreactivity for pERK2 in the nuclear fraction in response to E2 was observed at 5 min, and maximal staining occurred at 60 min, with immunoreactivity returning to basal levels by 120 min (Fig. 3A). Increased immunoreactivity for pERK2 in the nuclear fraction in response to P4 was observed at 10 min and maximal staining occurred at 60 min, with a slight decrease in staining intensity at 120 min (Fig. 3B). In contrast to the response to E2 and P4, MPA treatment significantly increased pERK2 immunoreactivity in only the cytosolic fraction (Fig. 3C). Increased immunoreactivity was observed at 5 min and maximal staining occurred at 60 min with a return to basal levels by 120 min (Fig. 3C). No detectable increase in pERK2 immunoreactivity occurred in the nuclear fraction in response to MPA treatment at any of the times examined (Fig. 3C).
Fig. 3.
Rapid activation of nuclear ERK-2 in hippocampal neurons treated with E2 and P4, but not with MPA. Western blots show levels of pERK2 and total ERK2 in cytoplasmic and nuclear fractions from primary hippocampal neurons treated with E2 (A), P4 (B), MPA (C), or combined E2 and progestin (D). Bar graphs represent mean ± SEM; *, P < 0.05 as compared with control; **, P < 0.01 as compared with control; ++, P < 0.01 as compared with E2 alone; n = 4.
Coadministration of P4 or MPA with E2 resulted in a significant increase in pERK2 immunoreactivity in the cytosolic fraction (Fig. 3D). Coadministration of P4 with E2 resulted in a significant increase in pERK2 immunoreactivity in the nuclear fraction that was similar to that seen for either steroid alone (Fig. 3D). Coadministration of MPA with E2 completely blocked the increased pERK2 immunoreactivity in the nuclear fraction seen with E2 alone (Fig. 3D).
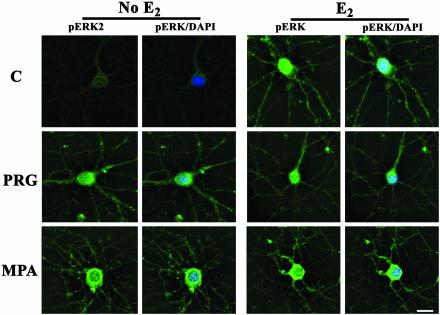
Intracellular Distribution of pERK After E2, P4, or MPA Treatment of Hippocampal Neurons. To verify the differential pattern of pERK localization observed with Western blot analysis, immunostaining of primary hippocampal neurons was performed to visualize the subcellular distribution of pERK. Untreated control neurons showed weak immunoreactivity for the active form of ERK, which was restricted to the cytoplasm (Fig. 4A). pERK immunofluorescence increased after 30-min treatment with E2, P4, or MPA, with staining intensity varying from cell to cell (Fig. 4). In estrogen-responsive neurons, immunoreactive pERK was distributed throughout the cell, appearing in cytoplasm, neuronal processes, and nucleus (Fig. 4B) with a significant increase in pERK immunoreactivity occurring in the nucleus (Fig. 4B). Treatment with P4 also resulted in increased pERK in the nuclear compartment of the neuron (Figs. 4C and 5B). Although MPA treatment resulted in increased staining intensity (Figs. 4E and 5A), pERK immunoreactivity was located cytoplasmically in MPA-treated cells with nuclei devoid of pERK immunoreactivity (Figs. 4E and 5B).
Fig. 4.
Nuclear localization of pERK in hippocampal neurons induced by E2 or P4, but not MPA. pERK (green; FITC) immunofluorescence and DAPI nuclear counterstain (blue) in primary hippocampal neurons treated with vehicle (C, Top Left), E2 (Right), P4 (PRG, Middle), and MPA (Bottom). (Bar, 10 μm.)
Fig. 5.
Subcellular compartmentalization of the fluorescent intensity of the pERK signal is altered by E2, P4, and MPA. Bar graphs represent relative fluorescence intensities for pERK localized in cytoplasm (A) and nucleus (B)of primary hippocampal neurons treated with vehicle (C), E2 (E), P4 (P), and MPA (M). Data are presented as mean ± SEM; **, P < 0.01 as compared with control; +, P < 0.0 as compared with E2 alone; n = 20 neurons.
Coadministration of P4 with E2 increased the intensity of pERK immunoreactivity in the cytoplasm and nucleus as compared with baseline levels (Figs. 4D and 5). Coadministration of MPA with E2 for 30 min increased pERK immunoreactivity, but it restricted the localization of the increased immunoreactive signal to the cytoplasm, which is a pattern of pERK similar to that seen with MPA alone (Figs. 4F and 5).
Discussion
We demonstrate that different progestins can induce divergent neural responses directly and regulate E2-mediated regulation of calcium signaling and nuclear activation of ERK. Relevance of these effects for neural survival is predicated on a mechanistic pathway leading to E2-inducible neuroprotection. Neuroprotection induced by full estrogen agonists against glutamate-induced excitotoxicity is mediated by shunting of Ca2+i into mitochondria, leading to attenuation of [Ca2+]i (17, 18, 26). In turn, estrogen-induced attenuation of [Ca2+]i rise depends on MAPK/ERK, because blockade of MAPK activation results in a loss of estrogen-induced neuroprotection (16) and in a loss of estrogen-induced attenuation of excitotoxic glutamate-induced rise in [Ca2+]i (19). These data indicate that activation of MAPK is required for the attenuation of excitotoxic glutamate-induced rise in [Ca2+]i that leads to an increase in neuronal survival. Here we show that, like E2, P4 attenuates the excitotoxic glutamate-induced rise in [Ca2+]i. In contrast, not only was MPA ineffective at altering glutamate-mediated Ca2+ signaling alone, MPA blocked E2-induced attenuation. Paradoxically, however, MPA, like E2 and P4, activated MAPK (16), yet was ineffective at neuroprotection and attenuation of excitotoxic glutamate-mediated Ca2+ signaling. These data indicate that ERK activation per se is not predictive of neuroprotection, presenting a paradox of the MAPK requirement for steroid-induced neuroprotection.
We sought to resolve the paradox between the dependence of MAPK activation for gonadal hormone-induced neuroprotection and the lack of neuroprotection induced by MPA that also activated ERK. Results of Western blot analysis and immunocytochemistry demonstrated that all three steroids elicited similar rapid and transient activation of ERK. Thus, the divergence between P4 and MPA must occur downstream of MAPK activation. Building on the findings of Singh and colleagues (22, 27) showing E2-induced nuclear translocation of ERK, we determined whether E2, P4, and MPA induced nuclear translocation of ERK. Surprisingly, the pERK signal was transduced to the nucleus only by E2 and P4, not by MPA. Further, E2-induced nuclear translocation of pERK was blocked by coadministration of MPA. These data indicate that translocation of ERK to the nucleus is a pivotal and necessary requirement for gonadal hormone protection of neurons against excitotoxic insults associated with neurodegenerative disease. A probable downstream consequence of failing to translocate ERK to the nucleus is to prevent E2 activation of CREB, which is MAPK-dependent (28), and to thwart E2-induced increase in the antiapoptotic protein Bcl-2. This postulate is consistent with previous findings that E2 and P4 induced Bcl-2 expression and MPA blocked E2-induced Bcl-2 expression (16). The divergence between the induction of ERK nuclear translocation offers a plausible predictive mechanism by which MPA fails to protect neurons against toxic insults and antagonizes E2-induced neuroprotection and predicts the neuroprotective efficacy of HRT.
Mechanisms underlying the divergent ERK translocation remain to be determined but may include different steps in initiating ERK signaling, differential activation of regulators of ERK nuclear translocation, or activation of distinct pools of MAPK. Several events cooperatively determine the amount of nuclear ERK, such as cytoplasmic anchoring, phosphorylation, and subsequent dimerization, active transport of ERK across the nuclear membrane, and retention in the nucleus (29, 30). Interference at any step by MPA could prevent nuclear translocation of ERK. E2-induced nuclear translocation of pERK can be blocked by protein synthesis inhibitors (27), implicating an active process, which could be antagonized by MPA. Alternatively, spatial organization of kinases and substrates can determine the transmission and target site of action, providing a localization strategy whereby distinct populations of MAPK can restrict activation of downstream targets (23, 31, 32). Activation of a nontranslocated pool of MAPK could lead to inactivation of proteins responsible for cell survival. Predictive relationships between nuclear pERK and neuroprotective effectiveness of sex steroids suggests a requirement for transcriptional activation (33-35).
The use of HRT as a protective agent against age-related cognitive decline and AD has been supported by the recent Cache County Study (6) and numerous epidemiological retrospective and prospective analyses (for review see ref. 36), which demonstrate significant reduction in incidence and risk of AD with HRT use. In striking contrast, analysis from the Women's Health Initiative Memory Study clinical trial reported that estrogen plus progestin therapy did not improve cognitive function in older women and resulted in a doubling of the diagnosis of AD (12). The formulation of HRT used in the Women's Health Initiative Memory Study was a combination of MPA and conjugated estrogens [Prempro]. The results of our studies (16, 37) indicate that inclusion of MPA would negate the potential beneficial effects of estrogens on cognitive function and prevention of AD and would thus provide a potential explanation for the adverse outcomes of the Women's Health Initiative Memory Study clinical investigation.
Results obtained in neurons reported here and previously (16, 37) are potentially relevant to other tissues. Multiple studies using different hormone formulations have reported contradictory effects of estrogen/progestin use in breast cancer risk (9) and coronary heart disease (10). In light of our findings, discrepancies in outcomes could be, in part, attributable to differences in the cellular responses induced by different progestins. For example, MPA, but not P4, mitigated E2 protection against coronary artery vasospasm in rhesus monkeys (38). In addition, the Postmenopausal Estrogen/Progestins Intervention trial, which compared different HRT regimens, found that P4 induced a more favorable lipid profile when administered with estrogen than did MPA (14). Collectively, these data demonstrate that all progestins are not alike in induction of cellular responses and, hence, health outcomes. Further, our data indicate that the progestin within a HRT formulation will be pivotal for achieving therapeutic benefit to prevent neurodegenerative disease and to sustain cognitive function throughout the menopausal years.
Acknowledgments
This study was supported by grants from the National Institute on Aging (PO1 AG1475: Project 2), the Kenneth T. and Eileen L. Norris Foundation, the L. K. Whittier Foundation, and the Stanley Family Trust (to R.D.B.) and from the Alzheimer's Association (NIRG-01-2626) and the John Douglas French Alzheimer's Foundation (to J.N.).
Abbreviations: AD, Alzheimer's disease; [Ca2+]i, intracellular calcium concentration; E2, 17β-estradiol; P4, progesterone; HRT, hormone replacement therapy; MPA, medroxyprogesterone acetate; MAPK, mitogen-activated protein kinase; ERK, extracellular receptor kinase; pERK, phospho-ERK; DAPI, 4′, 6-diamidino-2-phenylindole.
References
- 1.Brinton, R. D. (2001) Learn. Mem. 8, 121-133. [DOI] [PubMed] [Google Scholar]
- 2.McEwen, B. S. (1999) J. Clin. Endocrinol. Metab. 84, 1790-1797. [DOI] [PubMed] [Google Scholar]
- 3.Green, P. S. & Simpkins, J. W. (2000) Int. J. Dev. Neurosci. 18, 347-358. [DOI] [PubMed] [Google Scholar]
- 4.Wise, P. M., Dubal, D. B., Wilson, M. E., Rau, S. W., Bottner, M. & Rosewell, K. L. (2001) Brain Res. Brain Res. Rev. 37, 313-319. [DOI] [PubMed] [Google Scholar]
- 5.Hurn, P. D. & Brass, L. M. (2003) Stroke (Dallas) 34, 338-341. [DOI] [PubMed] [Google Scholar]
- 6.Zandi, P. P., Carlson, M. C., Plassman, B. L., Welsh-Bohmer, K. A., Mayer, L. S., Steffens, D. C. & Breitner, J. C. (2002) J. Am. Med. Assoc. 288, 2123-2129. [DOI] [PubMed] [Google Scholar]
- 7.Hirvonen, E. (1996) Maturitas 23, Suppl., S13-S18. [DOI] [PubMed] [Google Scholar]
- 8.Gambrell, R. D., Jr. (1986) Maturitas 8, 159-168. [DOI] [PubMed] [Google Scholar]
- 9.Marchbanks, P. A., McDonald, J. A., Wilson, H. G., Folger, S. G., Mandel, M. G., Daling, J. R., Bernstein, L., Malone, K. E., Ursin, G., Strom, B. L., et al. (2002) N. Engl. J. Med. 346, 2025-2032. [DOI] [PubMed] [Google Scholar]
- 10.Falkeborn, M., Persson, I., Adami, H. O., Bergstrom, R., Eaker, E., Lithell, H., Mohsen, R. & Naessen, T. (1992) Br. J. Obstet. Gynaecol. 99, 821-828. [DOI] [PubMed] [Google Scholar]
- 11.Shumaker, S. A., Legault, C., Thal, L., Wallace, R. B., Ockene, J. K., Hendrix, S. L., Jones, B. N., III, Assaf, A. R., Jackson, R. D., Morley Kotchen, J., et al. (2003) J. Am. Med. Assoc. 289, 2651-2662. [DOI] [PubMed] [Google Scholar]
- 12.Rapp, S. R., Espeland, M. A., Shumaker, S. A., Henderson, V. W., Brunner, R. L., Manson, J. E., Gass, M. L., Stefanick, M. L., Lane, D. S., Hays, J., et al. (2003) J. Am. Med. Assoc. 289, 2663-2672. [DOI] [PubMed] [Google Scholar]
- 13.Hulley, S., Grady, D., Bush, T., Furberg, C., Herrington, D., Riggs, B. & Vittinghoff, E. (1998) J. Am. Med. Assoc. 280, 605-613. [DOI] [PubMed] [Google Scholar]
- 14.Anonymous (1995) J. Am. Med. Assoc. 273, 199-208. [Google Scholar]
- 15.Rossouw, J. E., Anderson, G. L., Prentice, R. L., LaCroix, A. Z., Kooperberg, C., Stefanick, M. L., Jackson, R. D., Beresford, S. A., Howard, B. V., Johnson, K. C., et al. (2002) J. Am. Med. Assoc. 288, 321-333. [DOI] [PubMed] [Google Scholar]
- 16.Nilsen, J. & Brinton, R. D. (2002) Endocrinology 143, 205-212. [DOI] [PubMed] [Google Scholar]
- 17.Nilsen, J., Chen, S. & Brinton, R. D. (2002) Brain Res. 903, 216-234. [DOI] [PubMed] [Google Scholar]
- 18.Nilsen, J. & Brinton, R. D. (2003) Proc. Natl. Acad. Sci. USA 100, 2842-2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singer, C. A., Figueroa-Masot, X. A., Batchelor, R. H. & Dorsa, D. M. (1999) J. Neurosci. 19, 2455-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Traverse, S., Gomez, N., Paterson, H., Marshall, C. & Cohen, P. (1992) Biochem. J. 288, 351-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robbins, D. J., Zhen, E., Owaki, H., Vanderbilt, C. A., Ebert, D., Geppert, T. D. & Cobb, M. H. (1993) J. Biol. Chem. 268, 5097-5106. [PubMed] [Google Scholar]
- 22.Singh, M., Setalo, G., Jr., Guan, X., Warren, M. & Toran-Allerand, C. D. (1999) J. Neurosci. 19, 1179-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen, R. H., Sarnecki, C. & Blenis, J. (1992) Mol. Cell. Biol. 12, 915-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lenormand, P., Brondello, J. M., Brunet, A. & Pouyssegur, J. (1998) J. Cell Biol. 142, 625-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaaro, H., Rubinfeld, H., Hanoch, T. & Seger, R. (1997) Proc. Natl. Acad. Sci. USA 94, 3742-3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi, D. W. (1987) J. Neurosci. 7, 369-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Setalo, G., Jr., Singh, M., Guan, X. & Toran-Allerand, C. D. (2002) J. Neurobiol. 50, 1-12. [DOI] [PubMed] [Google Scholar]
- 28.Wade, C. B. & Dorsa, D. M. (2003) Endocrinology 144, 832-838. [DOI] [PubMed] [Google Scholar]
- 29.Adachi, M., Fukuda, M. & Nishida, E. (1999) EMBO J. 18, 5347-5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fukuda, M., Gotoh, Y. & Nishida, E. (1997) EMBO J. 16, 1901-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reszka, A. A., Bulinski, J. C., Krebs, E. G. & Fischer, E. H. (1997) Mol. Biol. Cell 8, 1219-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furuchi, T. & Anderson, R. G. (1998) J. Biol. Chem. 273, 21099-21104. [DOI] [PubMed] [Google Scholar]
- 33.Cowley, S., Paterson, H., Kemp, P. & Marshall, C. J. (1994) Cell 77, 841-852. [DOI] [PubMed] [Google Scholar]
- 34.Szeberenyi, J. (1996) Neurobiology 4, 1-11. [PubMed] [Google Scholar]
- 35.Szeberenyi, J. & Erhardt, P. (1994) Biochim. Biophys. Acta 1222, 187-202. [DOI] [PubMed] [Google Scholar]
- 36.Henderson, V. W. (2000) Novartis Found. Symp. 230, 254-265. [DOI] [PubMed] [Google Scholar]
- 37.Nilsen, J. & Brinton, R. D. (2002) NeuroReport 13, 825-830. [DOI] [PubMed] [Google Scholar]
- 38.Miyagawa, K., Rosch, J., Stanczyk, F. & Hermsmeyer, K. (1997) Nat. Med. 3, 324-327. [DOI] [PubMed] [Google Scholar]