Abstract
We designed AM1241, a selective CB2 cannabinoid receptor agonist, and used it to test the hypothesis that CB2 receptor activation would reverse the sensory hypersensitivity observed in neuropathic pain states. AM1241 exhibits high affinity and selectivity for CB2 receptors. It also exhibits high potency in vivo. AM1241 dose-dependently reversed tactile and thermal hypersensitivity produced by ligation of the L5 and L6 spinal nerves in rats. These effects were selectively antagonized by a CB2 but not by a CB1 receptor antagonist, suggesting that they were produced by actions of AM1241 at CB2 receptors. AM1241 was also active in blocking spinal nerve ligation-induced tactile and thermal hypersensitivity in mice lacking CB1 receptors (CB1-/- mice), confirming that AM1241 reverses sensory hypersensitivity independent of actions at CB1 receptors. These findings demonstrate a mechanism leading to the inhibition of pain, one that targets receptors localized exclusively outside the CNS. Further, they suggest the potential use of CB2 receptor-selective agonists for treatment of human neuropathic pain, a condition currently without consistently effective therapies. CB2 receptor-selective agonist medications are predicted to be without the CNS side effects that limit the effectiveness of currently available medications.
Neuropathic pain is defined as pain initiated or caused by a primary lesion or dysfunction in the nervous system (1). It affects ≈1% of the population and results from a variety of etiologies including trauma, infection, diabetes, immune deficiencies, ischemic disorders, and toxic neuropathies (1, 2). It can be excruciating, and some patients are unable to work or to perform normal daily activities. Neuropathic pain often responds poorly to medical therapy (3, 4). This may be due, in part, to adverse side effects of available medications that limit drug dosage (5). Medications currently used for the treatment of neuropathic pain act on neurotransmitter systems or ion channels and typically produce significant CNS side effects. For example, gabapentin, a drug commonly used to treat neuropathic pain because of its modest side effect profile compared with other therapeutic options, produces somnolence in 19% of patients and dizziness in 17% (Neurontin prescribing information, Parke-Davis). A therapy directed at targets not found in the CNS would avoid these problems. CB2 cannabinoid receptors are one such potential target.
CB2 receptor mRNA is not detected in brain (6, 7). In addition, the CB2 receptor-selective antagonist SR144528 did not displace the nonselective cannabinoid ligand [3H]CP55,940 from binding to rat brain (7). Finally, binding of [3H]CP55,940 to mouse brain was eliminated by disruption of the CB1 receptor gene (8) but was not affected by disruption of the CB2 receptor gene (9). These studies suggest that CB2 receptors are not found in the normal CNS, although they do not fully exclude the possibility that CB2 receptors are expressed in the CNS in small, but functionally significant, amounts. CB2 receptor-selective agonists do not produce CNS effects typically caused by nonselective cannabinoid agonists (10, 11). CB2 receptors are found primarily in peripheral tissues with immune functions (6, 12-14).
We designed AM1241, a selective CB2 receptor agonist, and used it to test the hypothesis that CB2 receptor activation will reverse the sensory hypersensitivity observed in neuropathic pain states without producing CNS side effects. These experiments were performed in light of the previous demonstration that AM1241 inhibits acute thermal nociception (10).
AM1241 belongs to a class of cannabergic ligands known as aminoalkylindoles. Early on, a number of these compounds were shown to bind to the CB1 receptor and elicit the characteristic effects produced by Δ9-tetrahydrocannabinol, the key psychoactive ingredient in Cannabis sativa. Following the discovery of the CB2 receptor (6), the second cannabinoid receptor subtype, our group has sought to develop aminoalkylindole analogs possessing high potency and selectivity for this receptor. Our efforts were rewarded with the design and synthesis of AM1241, a compound possessing high affinity and selectivity for the CB2 receptor.
Methods
Binding Assays. Binding to cannabinoid receptors was tested by using competition-equilibrium binding vs. [3H]CP55,940 as described in Lan et al. (15). AM1241 was diluted into 25 mM Tris base (pH 7.4)/5 mM MgCl2/1 mM EDTA/0.1% essentially fatty acid-free BSA and transferred to Regisil-treated 96-well plates. [3H]CP55,940 (DuPont/NEN; specific activity 100-180 Ci/mmol; 1 Ci = 37 GBq) was added to a concentration of 0.8 nM. Membranes prepared from rat brain (containing CB1 receptors) or mouse spleen (containing CB2 receptors) were added (≈50 μg of membrane protein per well), plates were incubated at 30°C for 1 h, and the contents were filtered over Packard Unifilter GF/B 96-well filters (Perkin-Elmer) by using a Packard Filtermate 196 cell harvester. Filters were washed with ice-cold 50 mM Tris base/5 mM MgCl2/0.5% BSA and dried. Bound radioactivity was quantitated and corrected for nonspecific binding, and results were normalized between 0% and 100% [3H]CP-55,940 specifically bound. IC50 was determined by nonlinear regression analysis using GraphPad prism and transformed to a Ki value (16). All data were collected in duplicate. IC50 and Ki values were determined from three independent experiments.
Animals. All procedures were approved by the University of Arizona Animal Care and Use Committee and conform to the guidelines of the International Association for the Study of Pain and the National Institutes of Health. Male Sprague-Dawley rats (Harlan Breeders, Indianapolis) were 250-350 g at the time of testing. Mice were 20-25 g at the time of testing. Animals were maintained in a climate-controlled room on a 12-h light/dark cycle and allowed food and water ad libitum.
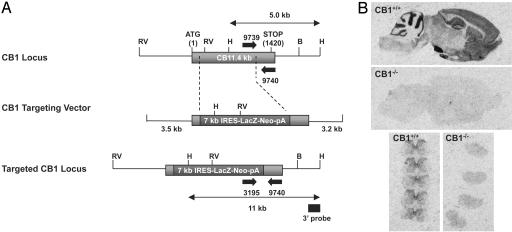
Generation of CB1-/- Mice. CB1-deficient mice were generated in concert with Deltagen (Redwood City, CA), using the 1.4-kb mouse CB1 cDNA sequence as a starting point for the generation of a CB1 targeting vector. The CB1 targeting vector includes 5.8 kb of genomic sequence and a 7-kb IRES-LacZ-Neo-pA cassette. Homologous recombination between the targeting vector and the wild-type CB1 allele results in deletion of 1,223 bp of the mouse CB1 coding sequence, encompassing bp 26-1,248, and replacement of this sequence with the IRES-LacZ-Neo-pA cassette. Linear CB1 targeting vector was electroporated into 129/SvJ-derived R1 embryonic stem cells. Homologous recombinant clones were identified by PCR and confirmed by Southern blot analysis using 5′ and 3′ probes outside of the targeting vector homology arms. Targeted clones were injected into C57BL/6 blastocysts, and chimeras were bred to 129/SvJ (The Jackson Laboratory) mice. Germ-line transmission was confirmed by Southern blot analysis using a 3′ outside-region probe to confirm the presence of the predicted 5- and 11-kb HindIII fragments diagnostic of the wild-type and mutant CB1 alleles (see Fig. 3A). All mice analyzed in this study have the genetic background 129/SvJ and were derived from heterozygous breeding. Genotyping of CB1 wild-type (CB1+/+), heterozygous (CB1+/-), and knockout (CB1-/-) mice was carried out by using a 3-primer PCR assay (see Fig. 3A), where primer pair 9739 and 9740 generates a 199-bp amplicon indicative of the CB1 wild-type allele, primer pair 3195 and 9740 generates a 314-bp amplicon indicative of the mutant allele, and the presence of both amplicons is indicative of a heterozygous complement of both alleles. Primer sequences are as follows: 9740, 5′-TATCTAGAGGCTGCGCAGTGCCTTC-3′; 9739, 5′-CCCTCTGCTTGCGATCATGGTGTATG-3′; and 3195, 5′-GGGCCAGCTCATTCCTCCCACTCAT-3′. PCR genotyping of tail DNA was carried out in a total reaction volume of 50 μl by using ExTaq polymerase (Panvera, Madison, WI) with 10 μl of 9740 (10 μM), 5 μl of 9739 (10 μM), and 5 μl of 3195 (10 μM). PCR conditions were 94°C for 5 min; 14 cycles at 98°C for 20 sec, 68°C for 45 sec; 16 cycles at 98°C for 20 sec, 68°C for 45 sec plus 15 sec per cycle; and 72°C for 10 min.
Fig. 3.
(A) Generation of CB1 receptor-deficient mice. (B) Disruption of the CB1 receptor gene eliminated binding of the nonselective cannabinoid ligand [3H]CP55,940. Shown is autoradiography of 10 nM [3H]CP55,940 binding in CB1+/+ and CB1-/- sagittal sections of mouse brain (Top and Middle) and transverse sections of lumbar spinal cord (Bottom).
Receptor Autoradiography. Autoradiography was conducted as described by Herkenham et al. (17) with minor modifications. Mouse brain or spinal cord cryosections (15 μm) were thawmounted on Superfrost Plus slides and stored at -80°C until the day of the experiment. Slides were preincubated in 50 mM Tris·HCl, pH 7.4, at 25°C for 30 min. Then 50 mM Tris·HCl (pH 7.4)/5% BSA containing 10 nM [3H]CP55,940 (specific activity, 165 Ci/mmol; DuPont NEN) was applied at 25°C to the tissue sections and placed horizontally in a humid chamber for 2 h at room temperature; 10 μM CP55,940 was used to define nonspecific binding. Slides were washed, dried, and exposed as described (17).
Drug Administration. Cannabinoid drugs were dissolved in dimethyl sulfoxide. AM630 is a CB2 receptor-selective antagonist with 70- to 165-fold selectivity for binding to the CB2 receptor in vitro (18, 19). AM251 is a 300-fold selective CB1 receptor antagonist (20, 21). Drugs were administered i.p. 15 min before behavioral testing.
Spinal Nerve Ligation (SNL). L5/L6 SNL was performed as described by Kim and Chung (22). Animals were anesthetized with halothane. An incision was made lateral to the lumbar spine. The right L5 and L6 spinal nerves were isolated and tightly ligated distal to the dorsal root ganglion. The incision was closed, and animals were allowed to recover for 10 days. Sham-operated animals were prepared in an identical fashion except that the spinal nerves were not ligated.
Measurement of Tactile Withdrawal Threshold. Tactile withdrawal threshold was determined as described by Chaplan et al. (23). Animals were acclimated for 30 min in suspended cages with wire mesh bottoms. The hindpaw was probed with calibrated von Frey filaments (Stoelting) applied perpendicularly to the plantar surface. A positive response was indicated by a sharp withdrawal of the paw. The 50% paw withdrawal threshold was determined by the nonparametric method of Dixon (24), in which the stimulus is incrementally increased until a positive response is obtained, then decreased until a negative result is observed. The protocol was repeated until three changes in behavior were determined. The maximal cut-off values used were 15 g for rats and 3.5 g for mice. The 50% paw withdrawal threshold was determined as 10Xf+kδ/10,000, where Xf = the value of the last von Frey filament used, k = Dixon value for the positive/negative pattern, and δ = the logarithmic difference between stimuli.
Measurement of Thermal Withdrawal Latency. The method of Hargreaves et al. (25) was used. Animals were acclimated within Plexiglas enclosures on a clear glass plate maintained at 30°C. A radiant heat source (high-intensity projector lamp) was focused onto the plantar surface of the paw. When the paw was withdrawn, a motion detector halted the stimulus and a timer. A maximal cut-off of 40 sec for rats and 30 sec for mice was used to prevent tissue damage.
Data Analysis. Differences in responses between groups were tested by using ANOVA followed by post hoc testing with Student's t test with Bonferroni's correction. Significance was defined as P < 0.05.
Results
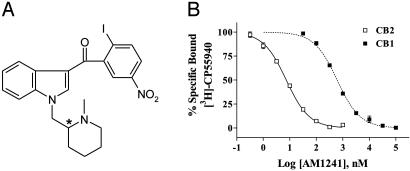
AM1241 is an aminoalkylindole analog substituted at the 1 position with a methylene group linked to an N-methylpiperidine ring at the 2 position (Fig. 1A), whereas the 3-indole substituent is a 2-iodo-5-nitrobenzoyl group. The compound has favorable physicochemical properties and can be crystallized as both the free base and its water-soluble hydrochloride salt.
Fig. 1.
AM1241 is a CB2 cannabinoid receptor-selective ligand. (A) Structure of AM1241. (B) Equilibrium-competition binding of AM1241 vs. [3H]CP55,940 using either rat brain synaptosomal membranes (CB1) or mouse spleen homogenates (CB2) illustrates the selectivity of this ligand for CB2. The curves shown are representative of single experiments that were independently replicated three times.
The affinity of AM1241 (Ki) for the CB2 receptor in a mouse spleen preparation was 3.4 ± 0.5 nM (mean ± SEM), and its affinity (Ki) for the CB1 receptor in a rat brain preparation was 280 ± 41 nM (mean ± SEM) (Fig. 1B), indicating an 82-fold selectivity for the CB2 receptor in rodent tissue.
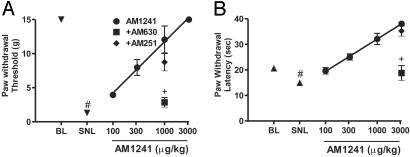
AM1241 produced a dose-dependent inhibition of SNL-induced tactile and thermal hypersensitivity in rats (Fig. 2). The effect of AM1241 was completely blocked by the CB2 receptor-selective antagonist, AM630, but was not affected by the CB1 receptor-selective antagonist, AM251.
Fig. 2.
AM1241 dose-dependently inhibits sensory hypersensitivity in rats. (A) Inhibition of tactile hypersensitivity. (B) Inhibition of thermal hypersensitivity. BL, presurgical baseline; SNL, after spinal nerve ligation. All drugs were administered i.p. AM630 and AM251 were administered at a dose of 300μg/kg. Data are expressed as mean ± SEM; n = 6 per group. #, P < 0.05 compared with presurgical baseline; +, P < 0.05 compared with AM1241 alone.
To further test whether activation of the CB2 receptor is sufficient to inhibit nerve injury-induced sensory hypersensitivity, we constructed mice deficient in the CB1 receptor (Fig. 3A) and studied the effects of AM1241 on SNL-induced tactile and thermal hypersensitivity in CB1-/- mice. Disruption of the CB1 receptor gene eliminated binding of the nonselective cannabinoid ligand [3H]CP55,940 in brain and spinal cord (Fig. 3B), indicating a total absence of cannabinoid receptors (CB1 and CB2) in the CNS. WIN55,212-2, a mixed CB1/CB2 receptor agonist, produced significant catalepsy in CB1+/+ mice but did not produce catalepsy in CB1-/- mice, demonstrating a functional lack of CB2 receptor activity in vivo (data not shown).
Presurgical tactile withdrawal thresholds were lower for CB1-/- than for CB1+/+ (wild-type) mice (Fig. 4). In contrast, presurgical thermal withdrawal latencies did not differ between CB1-/- and CB1+/+ animals (Fig. 5).
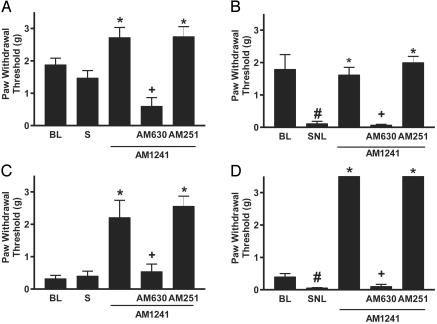
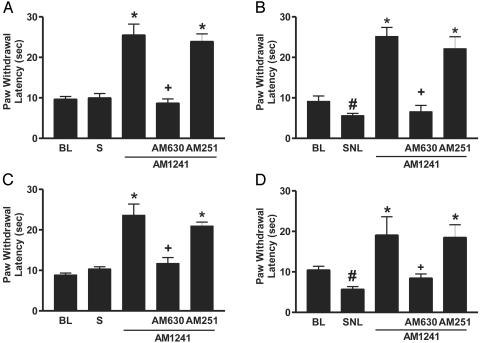
Fig. 4.
AM1241 inhibits tactile hypersensitivity in mice lacking the CB1 receptor. (A) Sham-operated wild-type mice. (B) Spinal nerve-ligated wild-type mice. (C) Sham-operated CB1-/- mice. (D) Spinal nerve-ligated CB1-/- mice. BL, presurgical baseline; S, after sham operation; SNL, after spinal nerve ligation. Doses used were as follows: AM1241, 1 mg/kg; AM 630, 1 mg/kg; AM251, 300 μg/kg. Data are expressed as mean ± SEM; n = 6 per group. #, P < 0.05 compared with presurgical baseline; *, P < 0.05 compared with postsurgical values; +, P < 0.05 compared with AM1241 alone.
Fig. 5.
AM1241 inhibits thermal hypersensitivity in mice lacking the CB1 receptor. (A) Sham-operated wild-type mice. (B) Spinal nerve-ligated wild-type mice. (C) Sham-operated CB1-/- mice. (D) Spinal nerve-ligated CB1-/- mice. BL, presurgical baseline; S, after sham operation. SNL, after spinal nerve ligation. Doses used were as follows: AM1241, 3 mg/kg; AM 630, 1 mg/kg; AM251, 300 μg/kg. Data are expressed as mean ± SEM; n = 6 per group. #, P < 0.05 compared with presurgical baseline; *, P < 0.05 compared with postsurgical values; +, P < 0.05 compared with AM1241 alone.
SNL decreased tactile withdrawal threshold in both CB1-/- and CB1+/+ mice (Fig. 4 B and D), whereas sham operation had no effect (Fig. 4 A and C); i.p. injection of AM1241 reversed SNL-induced tactile hypersensitivity. The effects of AM1241 were completely blocked by AM630 but not by AM251. In spinal nerve-ligated animals, AM1241 returned tactile withdrawal thresholds in CB1-/- mice to preligation values of CB1+/+ animals, whereas in sham-operated CB1-/- mice the compound increased tactile withdrawal thresholds until they equaled those observed in CB1+/+ animals.
Similarly, SNL decreased thermal withdrawal latency in both CB1-/- and CB1+/+ mice (Fig. 5 B and D). Conversely, sham operation had no effect (Fig. 5 A and C); i.p. injection of AM1241 reversed SNL-induced thermal hypersensitivity. AM1241 also prolonged thermal withdrawal latency beyond presurgical baseline values in both nerve-ligated and sham-operated animals. Again, the effects of AM1241 were completely inhibited by AM630 but not by AM251.
Discussion
Ligation of the L5 and L6 spinal nerves in experimental animals is used to model human neuropathic pain resulting from injury or disease of primary afferent neurons. SNL increases sensitivity to tactile and thermal stimuli (22), two features commonly observed in human neuropathic pain (26).
AM1241 reversed SNL-induced tactile and thermal hypersensitivity in rats and in mice. The effects of AM1241 were inhibited by the CB2 receptor-selective antagonist AM630 but not by the CB1 receptor-selective antagonist AM251, indicating that they are mediated by the CB2 receptor. Further, the reversal by AM1241 of SNL-induced tactile and thermal hypersensitivity in CB1-/- mice confirms that AM1241 acts through a mechanism independent of the CB1 receptor. AM1241 inhibited both thermal hypersensitivity, which is dependent upon intact capsaicin-sensitive, small, unmyelinated C-fiber afferents as well as tactile hypersensitivity, a condition that does not seem to be mediated by C-fiber afferents but may be mediated by large, myelinated Aβ fibers (27).
In addition to reversing nerve injury-induced thermal hypersensitivity, AM1241 prolonged thermal withdrawal latencies beyond preligation baseline values. This increase is consistent with the increased thermal withdrawal latencies observed in sham-operated animals and with the previously demonstrated thermal antinociceptive effects of CB2 receptor activation (10).
The greater tactile sensitivity in CB1-/- mice compared with CB1+/+ animals suggests that CB1 receptors modulate basal tactile sensitivity through the action of endogenous cannabinoid agonists and/or by intrinsic activity of the receptor. In contrast, the equivalent thermal sensitivity observed in CB1-/- and CB1+/+ mice suggests either that CB1 receptors do not participate in the modulation of basal thermal sensitivity or that compensatory changes in other regulatory systems return thermal sensitivity to normal levels in CB1-/- animals. Our finding of equivalent thermal sensitivity in CB1-/- and CB1+/+ in mice is consistent with the observation by Zimmer et al. (8) that withdrawal latencies in the tail flick assay do not differ between wild-type and CB1 knockout mice. AM1241 returned thermal withdrawal latencies in CB1-/- mice to preligation values of CB1+/+ animals, demonstrating that it is capable of reversing the tactile hypersensitivity produced by disruption of the CB1 receptor gene as well as that induced by SNL. This observation is consistent with the finding that in sham-operated CB1-/- mice, AM1241 increased tactile withdrawal thresholds until they equaled those observed in CB1+/+ animals.
The mechanism by which AM1241 acts to reverse SNL-induced somatosensory hypersensitivity is not known. The effects of this compound are unlikely to be the result of actions in the CNS because considerable evidence suggests that CB2 receptors are not present in the CNS (6-9). A site of action outside the CNS is consistent with our previous findings where, using site-specific injections of agonist and antagonists, we showed that the antinociceptive actions of AM1241 seem to be mediated at peripheral sites (10).
One possibility for a peripheral mechanism of action is direct inhibition of primary afferent neurons. However, CB2 receptor mRNA was not found in dorsal root ganglia, whereas CB1 receptor mRNA was readily detected (28), suggesting that CB2 receptors are not expressed in primary afferent neurons. This result does not, however, exclude the possibility that CB2 receptors are expressed in small, but functionally significant, amounts.
A second possibility for a peripheral mechanism is an indirect action of AM1241 to decrease the sensitivity of primary afferent neurons. Indeed, CB2 receptors are expressed primarily on mast and immune cells (6, 12-14), and CB2 receptor agonists, including AM1241, have been demonstrated to have antiinflammatory effects (11, 29). It is also known that mast and immune cells release mediators that are capable of sensitizing primary afferent neurons, including histamine, serotonin, prostaglandins, interleukin 1β, tumor necrosis factor-α, and nerve growth factor (30). Therefore, activation of peripheral CB2 receptors might decrease the sensitivity of primary afferent neurons by inhibiting the release of sensitizing substances from neighboring mast and immune cells. Congruent with the above is the observation that neurogenic inflammation seems to take place after SNL at the site of SNL and in peripheral tissues surrounding the distal terminals of primary afferent neurons. Cyclooxygenase 2-immunoreactive cells, including macrophages, are increased at the injury site in spinal nerve-ligated rats (31), and peripheral nerve injury results in polymorphonuclear leukocyte accumulation in the periphery (32, 33). Alternatively, CB2 receptor activation may reverse sensory hypersensitivity by inhibiting input into a nervous system sensitized at more central locations. Sensory hypersensitivity resulting from enhanced responses to normal levels of input from peripheral terminals of sensory neurons might be blocked by inhibition of this peripheral input. CB2 receptor activation seems to be capable of inhibiting the sensory sensitivity of peripheral terminals of primary afferent neurons in the absence of peripheral inflammation or nerve injury, as demonstrated by the ability of AM1241 to inhibit acute thermal nociception by acting at peripheral CB2 receptors (10).
Our data demonstrate that activity of CB1 cannabinoid receptors is not required for the inhibition of neuropathic pain by AM1241. They do not, however, fully exclude the involvement of other receptors. For example, a putative receptor has recently been described in brain that is modulated by the cannabinoid receptor agonist WIN55,212-2 but has different pharmacological properties from the CB1 receptor and is not inhibited by the CB1 receptor-selective antagonist AM251 (34). To date, this receptor has not been cloned, and its interactions with cannabinoid ligands have not been characterized.
In previous work, we have shown that AM1241 does not produce catalepsy, hypothermia, inhibition of spontaneous locomotor activity, or inhibition of performance on the rotarod apparatus (10). Earlier data with the CB2 receptor-selective but less potent agonist HU-308 also showed that this compound does not produce catalepsy, hypothermia, or inhibition of spontaneous locomotor activity (11).
The results presented in this communication provide evidence that CB2 receptor-selective agonists may be effective in treating neuropathic pain without CNS side effects. Such a therapeutic profile offers significant advantages over current therapies. In addition, the value of CB2 receptor-selective agonists, such as AM1241, in pain therapy is enhanced by their observed effectiveness against nociceptive (10), inflammatory (29), and neuropathic pain. Although multiple pain mechanisms may be active in the same patient (as, for example, in cancer pain), there are presently no single therapies that are consistently effective against these diverse types of pain. Our data suggest the importance of the development of CB2 receptor-selective agonists for human therapeutics.
Acknowledgments
This work was supported by National Institute on Drug Abuse Grants DA00283, DA7215, DA3801, and DA9158.
Abbreviation: SNL, spinal nerve ligation.
References
- 1.Merskey, H. & Bogduk, N. (1994) Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms (IASP Press, Seattle).
- 2.Bowsher, D. (1991) Br. Med. Bull. 47, 644-646. [DOI] [PubMed] [Google Scholar]
- 3.Kingery, W. S. (1997) Pain 73, 123-139. [DOI] [PubMed] [Google Scholar]
- 4.Jensen, T. S., Gottrup, H., Sindrup, S. H. & Bach, F. W. (2001) Eur. J. Pharmacol. 429, 1-11. [DOI] [PubMed] [Google Scholar]
- 5.Sindrup, S. H. & Jensen, T. S. (1999) Pain 83, 389-400. [DOI] [PubMed] [Google Scholar]
- 6.Munro, S., Thomas, K. L. & Abu-Shaar, M. (1993) Nature 365, 3842-3851. [DOI] [PubMed] [Google Scholar]
- 7.Griffin, G., Wray, E. J., Tao, Q., McAllister, S. D., Rorrer, W. K., Aung, M. M., Martin, B. R. & Abood, M. E. (1999) Eur. J. Pharmacol. 377, 117-125. [DOI] [PubMed] [Google Scholar]
- 8.Zimmer, A., Zimmer, A. M., Hohmann, A. G., Herkenham, M. & Bonner, T. I. (1999) Proc. Natl. Acad. Sci. USA 96, 5780-5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buckley, N. E., McCoy, K. L., Mezey, E., Bonner, T., Zimmer, A., Felder, C. C., Glass, M. & Zimmer, A. (2000) Eur. J. Pharmacol. 396, 141-149. [DOI] [PubMed] [Google Scholar]
- 10.Malan, T. P., Jr., Ibrahim, M. M., Deng, H., Liu, Q., Mata, H. P., Vanderah, T., Porreca, F. & Makriyannis, A. (2001) Pain 93, 239-245. [DOI] [PubMed] [Google Scholar]
- 11.Hanus, L., Breuer, A., Tchilibon, S., Shiloah, S., Goldenberg, D., Horowitz, M., Pertwee, R. G., Ross, R. A., Mechoulam, R. & Fride, E. (1999) Proc. Natl. Acad. Sci. USA 96, 14228-14233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galiegue, S., Mary, S., Marchand, J., Dussossoy, D., Carriere, D., Carayon, P., Bouaboula, M., Shire, D., Le Fur, G. & Casellas, P. (1995) Eur. J. Biochem. 232, 54-61. [DOI] [PubMed] [Google Scholar]
- 13.Facci, L., Dal Toso, R., Romanello, S., Buriani, A., Skaper, S. D. & Leon, A. (1995) Proc. Natl. Acad. Sci. USA 92, 3376-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schatz, A. R., Lee, M., Condie, R. B., Pulaski, J. T. & Kaminski, N. E. (1997) Toxicol. Appl. Pharmacol. 142, 278-287. [DOI] [PubMed] [Google Scholar]
- 15.Lan, R., Liu, Q., Fan, P., Lin, S., Fernando, S. R., McCallion, D., Pertwee, R. & Makriyannis, A. (1999) J. Med. Chem. 42, 769-776. [DOI] [PubMed] [Google Scholar]
- 16.Cheng, Y. C. & Prusoff, W. H. (1973) Biochem. Pharmacol. 22, 3099-3102. [DOI] [PubMed] [Google Scholar]
- 17.Herkenham, M., Lynn, A. B., Johnson, M. R., Melvin, L. S., de Costa, B. R. & Rice, K. C. (1991) J. Neurosci. 11, 563-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ross, R. A., Brockie, H. C., Stevenson, L. A., Murphy, V. L., Templeton, F., Makriyannis, A. & Pertwee, R. G. (1999) Br. J. Pharmacol. 126, 665-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hosohata, Y., Quock, R. M., Hosohata, K., Makriyannis, A., Consroe, P., Roeske, W. R. & Yamamura, H. I. (1997) Eur. J. Pharmacol. 321, R1-R3. [DOI] [PubMed] [Google Scholar]
- 20.Gatley, S. J., Gifford, A. N., Volkow, N. D., Lan, R. & Makriyannis, A. (1996) Eur. J. Pharmacol. 307, 331-338. [DOI] [PubMed] [Google Scholar]
- 21.Gatley, S. J., Lan, R., Pyatt, B., Gifford, A. N., Volkow, N. D. & Makriyannis, A. (1997) Life Sci. 61, PL191-PL197. [DOI] [PubMed] [Google Scholar]
- 22.Kim, S. H. & Chung, J. M. (1992) Pain 50, 355-363.1333581 [Google Scholar]
- 23.Chaplan, S. R., Bach, F. W., Pogrel, J. W., Chung, J. M. & Yaksh, T. L. (1994) J. Neurosci. Methods 53, 55-63. [DOI] [PubMed] [Google Scholar]
- 24.Dixon, W. J. (1980) Annu. Rev. Pharmacol. Toxicol. 20, 441-462. [DOI] [PubMed] [Google Scholar]
- 25.Hargreaves, K. M., Dubner, R., Brown, F., Flores, C. & Joris, J. (1988) Pain 32, 77-88. [DOI] [PubMed] [Google Scholar]
- 26.Rowbotham, M. C. (1995) Neurology 45, S5-S10. [Google Scholar]
- 27.Ossipov, M. H., Bian, D., Malan, T. P., Lai, J. & Porreca, F. (1999) Pain 79, 127-133. [DOI] [PubMed] [Google Scholar]
- 28.Hohmann, A. G. & Herkenham, M. (1999) Neuroscience 90, 923-931. [DOI] [PubMed] [Google Scholar]
- 29.Quartilho, A., Mata, H. P., Ibrahim, M. M., Vanderah, T. W., Porreca, F., Makriyannis, A. & Malan, T. P., Jr. (2003) Anesthesiology, in press. [DOI] [PubMed]
- 30.Dray, A. (1995) Br. J. Anaesthesiol. 75, 125-131. [Google Scholar]
- 31.Ma, W. & Eisenach, J. C. (2002) Eur. J. Neurosci. 15, 1037-1047. [DOI] [PubMed] [Google Scholar]
- 32.Daemen, M. A., Kurvers, H. A., Kitslaar, P. J., Slaaf, D. W., Bullens, P. H. & Van den Wildenberg, F. A. (1998) Neurol. Res. 20, 41-45. [DOI] [PubMed] [Google Scholar]
- 33.Daemen, M., Kurvers, H., Bullens, P., Barendse, G., Van Kleef, M. & Van den Wildenberg, F. (1998) Acta Orthop. Belg. 64, 441-447. [PubMed] [Google Scholar]
- 34.Hajos, N. & Freund, T. F. (2002) Neuropharmacology 43, 503-510. [DOI] [PubMed] [Google Scholar]