Abstract
We report in vitro characterization of eleven SIVsmm strains of six lineages co-circulating in naturally-infected sooty mangabeys (SMs) from US Primate Centers and showed no major differences in the in vitro replication pattern between different SIVsmm lineages. Primary SIVsmm isolates utilized CCR5 and Bonzo co-receptors in vitro. SIVsmm growth in human T cell lines was isolate-, not lineage specific, with poor replication on Molt4-Clone8, CEMss and PM1 cells and better replication on MT2, SupT1 and CEMx174 cells. All primary SIVsmm isolates replicated on SM and human PBMCs. In vitro replication in macaques varied widely, with moderate to high replication in pig-tailed macaque PBMCs, enhanced by CD8+ T cell depletion, and highly variable replication on rhesus macaque (Rh) PBMCs. Primary SIVsmm isolates replicated in Rh monocyte-derived dendritic cells (MDDCs) and monocyte-derived macrophages (MDMs). In vivo, SIVsmm isolates replicated at high levels in all SIVsmm-infected Rh. The poor in vitro replication of primary SIVsmm isolates in Rh cells did not correlate with in vivo replication, emphasizing the value of in vivo studies.
Introduction
SIVsmm naturally infects sooty mangabeys (SMs Cercocebus atys) (Apetrei et al., 2005b; Chen et al., 1996; Hirsch et al., 1989b; Marx et al., 1991; Peeters et al., 1994; Santiago et al., 2005) and is genetically closely related to HIV-2 (Chen et al., 1997a; Damond et al., 2004; Gao et al., 1994; Gao et al., 1992; Yamaguchi, Devare, and Brennan, 2000) and SIVmac (Chakrabarti et al., 1987). Phylogenetic analyses and geographic coincidence support the idea that multiple cross-species transmission events from SMs to humans gave rise to HIV-2 (Chen et al., 1997a; Damond et al., 2004; Gao et al., 1994; Gao et al., 1992; Yamaguchi, Devare, and Brennan, 2000). Accidental or experimental cross-species transmission of SIVsmm to macaque species is the origin of SIVmac/mne and SIVstm (Apetrei et al., 2006; Khan et al., 1991; Lowenstine et al., 1992; Mansfield et al., 1995; Murphey-Corb et al., 1986). In the new hosts, cross-species transmitted viruses show pathogenic potential. Thus, over 80% of SIVmac251-infected rhesus macaques (Rh) develop profound immune suppression within 2 years of infection (Smith et al., 1999; Westmoreland, Halpern, and Lackner, 1998). SIV disease progression is even more rapid in pig-tailed macaques (PTMs) (Hirsch and Johnson, 1994). Humans infected with HIV-2 also progress to AIDS, although the incubation period is longer than that of HIV-1 (Simon et al., 1993). Therefore, it is a widely held belief that SIVsmm is highly pathogenic upon cross-species transmission. However, one should note that the eight cross-species transmission events documented in humans had unbalanced epidemiologic consequences, with only two epidemiologically successful cross-species transmissions generating the epidemic groups HIV-2 A and HIV-2 B (Damond et al., 2001; Gao et al., 1994). Groups C-H of HIV-2 (Chen et al., 1997a; Damond et al., 2004; Gao et al., 1994; Yamaguchi, Devare, and Brennan, 2000) are weakly pathogenic, non-epidemic strains that replicate poorly in infected humans and are found only within the range of SMs or in persons who emigrated from Western Africa (Chen et al., 1997a; Gao et al., 1994). Therefore, it appears that cross-species transmission is not the only requirement for HIV emergence and additional effects, such as viral adaptation through serial passages, may have contributed to successful adaptation of HIV-2 into its new host (Apetrei and Marx, 2004; Drucker, Alcabes, and Marx, 2001). This was clearly demonstrated in the case of SIVmac/SIVsmm strains, for which the increase in pathogenicity was associated with serial passage (Apetrei et al., 2006; Hirsch et al., 1997; Mansfield et al., 1995).
In SMs, chronic SIVsmm infection is generally not associated with disease despite high viral loads (Rey-Cuille et al., 1998; Silvestri, 2005; Silvestri et al., 2005; Silvestri et al., 2003). Different mechanisms have been associated with this absence of pathogenicity, and a recent consensus currently states that the lack of SIV disease progression in SMs is due to an effective control of immune activation and proliferation (Chakrabarti et al., 2000; Dunham et al., 2006; Silvestri et al., 2005; Silvestri et al., 2003). SIVsmm was recently shown to induce AIDS both in the host species (SM) (Ling et al., 2004) and in another African non-human primate host, the black mangabey, upon cross-species transmission (Apetrei et al., 2004).
During our previous studies, we did extensive genetic characterization of SIVsmm diversity in SM colonies in the United States and showed the co-circulation of 9 genetic clusters. Two of these lineages were the ancestral lineages of the macaque reference strains SIVmac and SIVstm (Apetrei et al., 2005a; Ling et al., 2003). These clusters originated from the transmission of SIVsmm founder viruses from SMs naturally infected before importation (Apetrei et al., 2005a). Most of these SIVsmm lineages went undetected until our initial studies in 2003 (Ling et al., 2003). Three lineages (including the two that generated SIVmac/mne and SIVstm) are currently extinct in American colonies. However, the co-circulation of highly divergent viruses in SMs offer opportunities for comparative pathogenesis and vaccine studies since these SIVsmm lineages show the same degree of divergence as HIV-1 subtypes (Apetrei et al., 2005a).
Although SIVsmm was the first virus isolated from natural African NHP hosts of SIVs (Fultz et al., 1986; Murphey-Corb et al., 1986), and is the root source of the extensively used strains characterized in the macaque model for AIDS (Apetrei et al., 2005a; Hirsch et al., 1997; Letvin, 1990), very few available reports address the in vitro replicative capacities of primary SIVsmm isolates. Most studies carried out thus far utilize strains heavily adapted for use in the laboratory or in macaques. It was reported that SIVsmm strains primarily use chemokine co-receptor CCR5, although some strains can also use CXCR4 (Chen et al., 1997b; Owen et al., 2000; Zhang et al., 2000). SIVsmm was shown to efficiently infect in vitro human peripheral blood mononuclear cells (PBMCs) (Chen et al., 1995; Hirsch et al., 1989a) and monocyte-derived macrophages (MDMs) (Grimm et al., 2003). This is in agreement with molecular epidemiology data showing that this virus crossed the species barrier into humans eight times during the last century (Damond et al., 2004; Hahn et al., 2000). In vitro replication of SIVsmm in human T cell lines is variable and the efficacy of growth increase for SIVsmm strains that have been serially passaged in macaques (Fultz et al., 1986; Fultz et al., 1989).
In this study we have performed a thorough in vitro characterization of currently available SIVsmm primary isolates belonging to 6 different phylogenetic lineages (Apetrei et al., 2005a). We show here that there are no discernable differences in the in vitro replication capacities between different SIVsmm lineages. We also show that Rh PBMCs vary greatly in their ability to support in vitro replication of primary SIVsmm isolates. Finally, we demonstrated that this in vitro variability of viral replication is not paralleled by in vivo replication patterns.
Results
Stock preparation
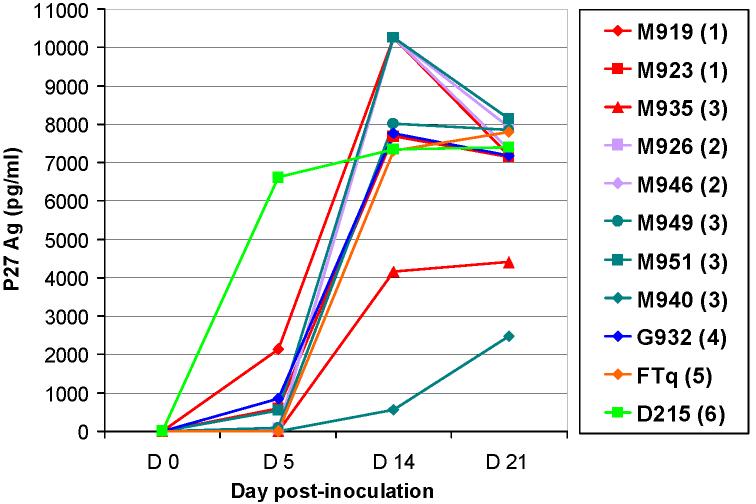
To obtain viral stocks for further in vitro studies, primary SIVsmm isolates belonging to different lineages were first propagated in vitro on SM PBMCs. Results are showed in Figure 1. All but two SIVsmm strains grew rapidly and at high titers in SM PBMCs. Significant P27 Ag production was observed starting from week 1. Strain SIVsmmM940 (Lineage 3) only replicated at low levels on SM PBMCs. SIVsmmM935 (Lineage 1) replicated at moderate levels on SM PBMCs (Figure 1). All the isolates showed cytopathic effects on SM cells. There was no discernable difference in the in vitro replication profiles on SM PBMCs between strains belonging to different lineages. Supernatants from these positive SM PBMC cultures were used as viral stocks for further experiments.
Figure 1.
Primary SIVsmm isolates replication in sooty mangabey PBMCs. SIVsmm strain belonging to different lineages are color-coded (red-Lineage 1; purple-Lineage 2; turquoise-Lineage 3; blue-Lineage 4; orange-Lineage 5; light green-Lineage 6).
Use of CCR5 and STRL33 is a common feature of SIVsm
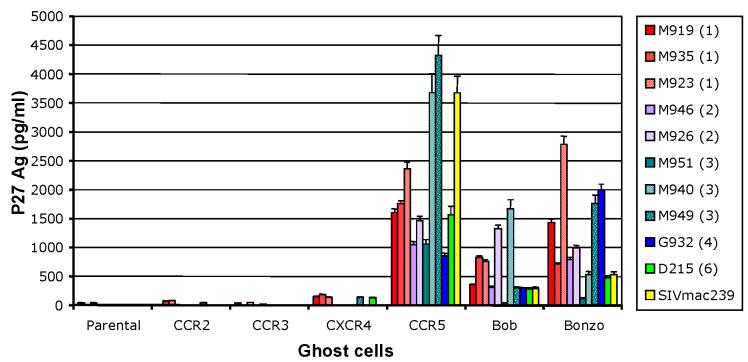
The previously characterized SIVsmm strains utilized CCR5 as their major co-receptor (Chen et al., 1998a; Zhang et al., 2000), and, to a lesser extent, CXCR4 (Owen et al., 2000). Therefore, to investigate the differences in viral replication between primary SIVsmm isolates, co-receptor usage was determined using the GHOST cell assay, focusing on the major coreceptors: CCR5, CXCR4, CCR2B, CCR3, STRL33 (Bonzo), and GPR15 (Bob) (Figure 2). Replication capacity in GHOST cell lines, as assessed by supernatant P27 Ag activity, showed no qualitative differences in co-receptor usage between SIVsmm strains belonging to different lineages. Our results showed that most of the primary SIVsmm isolates tested here used CCR5 and STRL33; primary SIVsmm isolates also used to a lesser extent GPR15, but did not utilize CCR3, CCR2b, or CXCR4, thus confirming previous reports on co-receptor usage of SIVsmm (Chen et al., 1998a; Zhang et al., 2000). Some strains showed reactivity above background level on CXCR4 cells, that was isolate-related, rather than lineage-related (Figure 2). This experiment showed that the differences in pathogenicity between primary SIVsmm isolates and reference strains in Rh are apparently not due to differences in co-receptor use.
Figure 2.
Co-receptor usage of primary SIVsmm isolates belonging to 6 different lineages, as measured at day 6 post-inoculation. SIVsmm strain belonging to different lineages are color-coded (red-Lineage 1; purple-Lineage 2; turquoise-Lineage 3; blue-Lineage 4; orange-Lineage 5; light green-Lineage 6; yellow-SIVmac239).
Primary SIVsmm isolate growth profile on Hu-derived cell lines
All the primary SIVsmm isolates were tested for their ability to replicate on different Hu cell lines and the results are shown in Table 1. All of the tested strains replicated slowly on CEMx174 cells. Detectable amounts of viral antigen were seen only after 3 weeks post-inoculation. In most cases, cultures became positive very late after the inoculation (>w6 for 5 strains). In the T cell lines, there were differences in viral growth between the different strains. Strains SIVsmmM919, SIVsmmM935 (lineage 1), SIVsmmM946 (lineage 2), SIVsmmM951 (lineage 3) and SIVsmmD215 (lineage 6) grew on SupT1. On MT2 cells, three isolates showed no sign of replication [SIVsmmM949 and SIVsmmM951 (lineage 3), and SIVsmmD215 (lineage 6)], while other isolates [SIVsmmM919, SIVsmmM935, SIVsmmM923 (lineage 1), SIVsmmG932 (lineage 4) and SIVsmmM946 (lineage 2)] showed efficient, although delayed (w4 to w6), replication. Only three strains [SIVsmmM935 (lineage 1), SIVsmmM926 (lineage2) and SIVsmmD215 (lineage 6)] replicated at low titers on PM1 cells. None of the viruses replicated on Molt4-Clone8 or CEMss cells. Altogether, these results show that primary SIVsmm isolates have a variable tropism on human T cell lines. This experiment showed that although strain-related differences were observed in this experiment, there are no major differences in the in vitro replication pattern on human T cell lines for SIVsmm lineages.
Table 1.
In vitro growth of primary SIVsm isolates belonging to different lineages on human cell lines
| MT2 | SupT1 | CEMx174 | CEMss | PM1 | Molt4 Clone8 | ||
|---|---|---|---|---|---|---|---|
| Lineage 1 | M919 | +++2(w53) | +++(w3) | ++(w8) | - | - | - |
| M935 | +++(w5) | +++(w6) | +++(w8) | - | +(w4) | - | |
| M923 | ++(w6) | - | +(w4) | - | - | - | |
| Lineage 2 | M926 | NT | NT | +++(w6) | NT | ±(w4) | - |
| M946 | +++(w4) | +++(w6) | +++(w3) | - | - | - | |
| Lineage 3 | M949 | - | - | +++(w5) | - | - | - |
| M951 | - | ±(w6) | +++(w6) | - | - | - | |
| Lineage 4 | M932 | +++(w6) | - | +++(w5) | - | - | - |
| Lineage 6 | D215 | - | +++(w7) | +++(w6) | - | +(w5) | - |
Uninfected SM PBMC co-cultivated with PBMC from SIVsm naturally-infected SM;
defined based on P27 antigen concentration at the peak of viral replication (noted in the parenthesis); ±30-100 pg;+100-500pg;++501-2000pg;+++>2000pg per well;
w =week post cell inoculation
Primary SIVsmm isolate growth profiles in Hu PBMCs
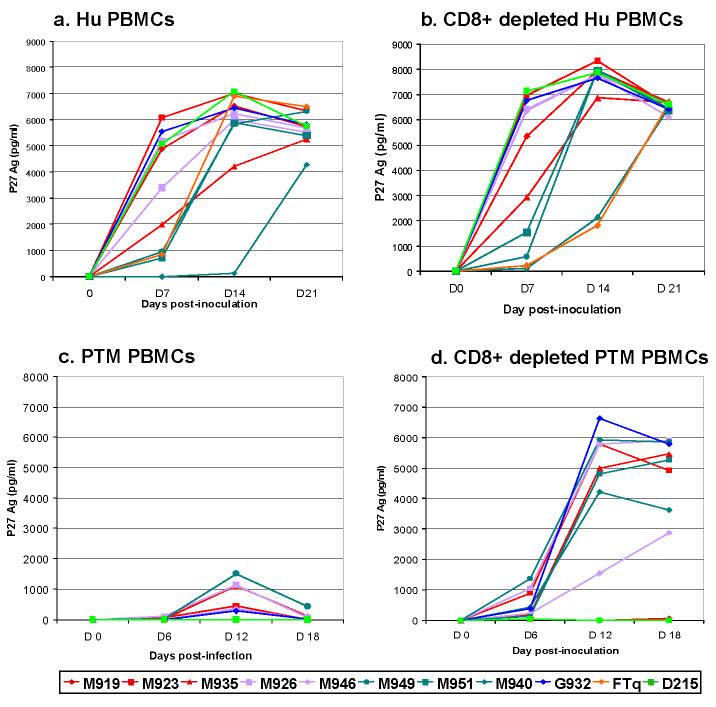
The ability of primary SIVsmm isolates to replicate on human cells was further tested on both Hu PBMC, and CD8+ depleted Hu PBMCs. All the viruses replicated efficiently in Hu PBMC as well as in CD8+ depleted Hu PBMCs. All but one (SIVsmmM940) cultures showed detectable P27 Ag after day 7 post-inoculation. P27 levels at the peak of antigen production ranged from 7000 to 8000 pg. Lineage 3 strains grew slower on both Hu PBMCs and CD8+-depleted Hu PBMCs (Figure 3a and 3b). Strain SIVsmmFTq (lineage 5) grew slower than the other strains on CD8+ depleted Hu PBMCs. However, this difference was not observed on undepleted Hu PBMCs, in which SIVsmmFTq replicated similarly to other primary SIVsmm isolates. Again, these results showed no significant difference in primary SIVsmm isolates ability to infect Hu PBMCs in vitro and confirmed previous reports indicating that SIVsm, the source virus of HIV-2 (Chen et al., 1997a; Gao et al., 1994; Gao et al., 1992; Marx et al., 1991), replicate efficiently on Hu PBMCs.
Figure 3.
Primary SIVsmm isolate replication in Human (Hu) PBMCs (a), CD8+ depleted Hu PBMCs (b), Pig-tailed macaque (PTM) PBMCs (c), CD8+ depleted PTM PBMCs (d). SIVsmm strain belonging to different lineages are color-coded (redLineage 1; purple-Lineage 2; turquoise-Lineage 3; blue-Lineage 4; orange-Lineage 5; light green-Lineage 6).
Primary SIVsmm isolate growth profiles in PTM PBMCs
PTM PBMCs, and CD8+depleted PTM PBMCs were used to replicate primary SIVsmm strains. Total PTM PBMCs supported only limited replication of primary SIVsmm isolates. Only six out of ten strains were isolated on PTM PBMCs, and only at low P27 Ag levels, ranging from 100 to 1500 pg (Figure 3c). Strains SIVsmmM919 (lineage 1), SIVsmmM951, SIVsmmM940 (both lineage 3) and SIVsmmD215 (lineage 6) did not replicate on the PTM PBMCs. Conversely, CD8+depleted PTM PBMCs from the same animal supported SIVsmm replication for all but two (M919 and D215) primary SIVsmm isolates (Figure 3d). Moreover, CD8+depleted PTM PBMCs had significantly higher levels of SIVsmm replication compared to non-CD8+ depleted PTM PBMCs, ranging from 3000 to over 7000 pg/ml. However, the highest viral replication in CD8+depleted PTM PBMC occurred at later time points than in SM-PBMCs (Figure 1) or Hu-PBMCs (Figure 3a and 3b).
Variable growth of primary SIVsmm isolates in Rh PBMCs
The first attempts to replicate primary SIVsmm isolates in Rh PBMCs were unsuccessful. None of the tested strains replicated on PBMCs from 3 different Rh (data not shown). This result was unexpected, since all Rh inoculated with macaque-passaged SIVsmm strains replicate the virus and eventually progress to AIDS (Hirsch and Johnson, 1994). Therefore, a detailed analysis of the in vitro replication profiles of primary SIVsmm isolates on a large number of animals was performed.
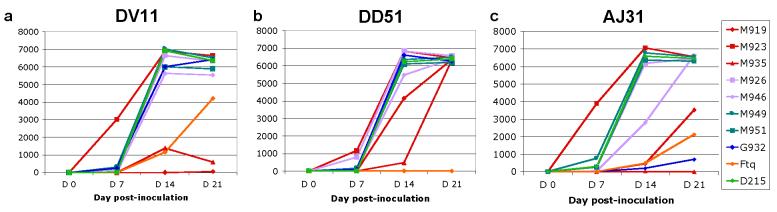
In the first step, Rh PBMCs from three different Rh (DV11, DD51 and AJ31) were CD8+ depleted and inoculated with ten primary SIVsmm isolates belonging to six different lineages. Results are shown in Figure 4a, b, c. In vitro replication profiles varied between the different animals. CD8+ depleted PBMCs from DV11 supported the replication of all but one (SIVsmmM919-lineage 1) strain. Two strains (SIVsmmFTqlineage 5 and SIVsmmM935-lineage 1) replicated at lower titers compared to the remaining strains (Figure 4a). All but one (SIVsmmFTq) strain replicated at high levels on CD8+ depleted PBMCs from DD51 (Figure 4b). Finally, all but one (SIVsmmM935) strain replicated on CD8+ depleted PBMCs from AJ31 (Figure 4c). Strains SIVsmmG932 (lineage 4), SIVsmmFTq (lineage 5) and SIVsmmM919 (lineage 1) replicated at lower levels compared to the remaining strains (Figure 4c). These results suggested a certain variability of the in vitro replication of primary SIVsmm isolates on Rh PBMCs.
Figure 4.
Primary SIVsmm replication in CD8+ depleted PBMCs from 3 rhesus macaques (Rh): RhDV11 (a); RhDV51 (b); RhAJ31 (c). SIVsmm strain belonging to different lineages are color-coded (red-Lineage 1; purple-Lineage 2; turquoise-Lineage 3; blue-Lineage 4; orange-Lineage 5; light green-Lineage 6).
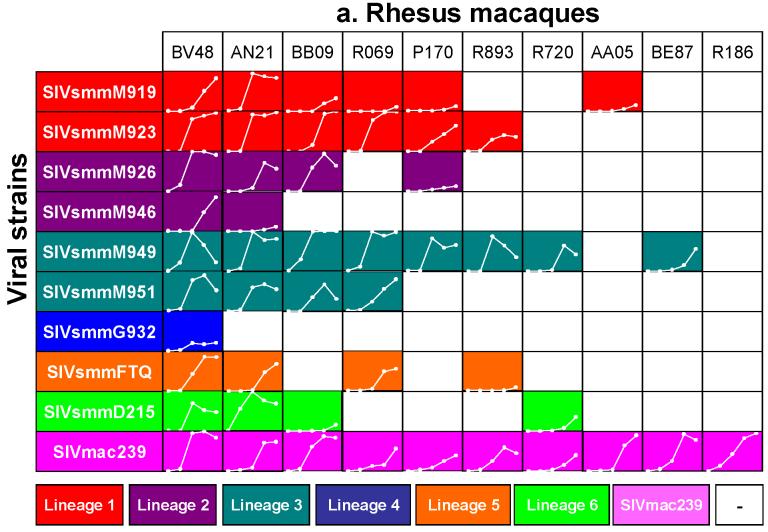
Since such variability was not observed in previous experiments on human, SM or PTM PBMCs, we extended this experiment to include a significant number of Rh. Total PBMCs from ten different Rh were used in a chess-board testing using nine primary SIVsmm isolates and SIVmac239 as a control. Results are shown in Figure 5a. Primary SIVsmm isolates showed a variable replication pattern, dependent on the animal used for in vitro cultures, that ranged from complete or almost complete replication support (BV48 and AN21) to complete restriction (R186). Quantitative replication patterns of primary SIVsmm isolates on Rh PBMCs also varied widely between different Rh (Figure 5a). This comparative in vitro study also showed that lineage 1 and lineage 3 strains are able to replicate better in vitro on Rh PBMCs compared to the other lineages (Figure 5a). Differently from primary SIVsmm isolates, serially passaged SIVmac239 replicated in all Rh (Figure 5a).
Figure 5.
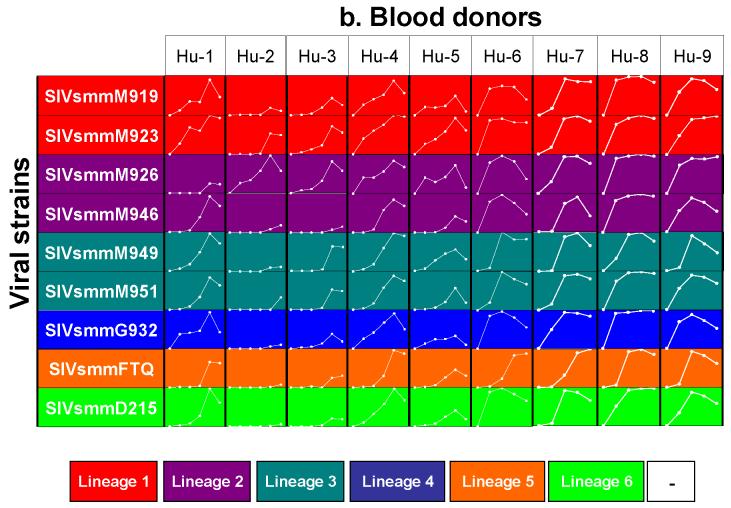
Variabile susceptibility of Rh PBMCs to primary SIVsmm isolates belonging to different lineages (a), as compared to consistent susceptibility of Hu-PBMCs. SIVsmm strain belonging to different lineages are color-coded (red-Lineage 1; purple-Lineage 2; turquoise-Lineage 3; blue-Lineage 4; orange-Lineage 5; light green-Lineage 6). White lines respresent the dynamics of in vivo replication at different time points post-inoculation (days 0, 7, 10, 14, 21 or 28 p.i.). Vertical scale ranges from 0 to 8,000 pg of P27 Ag. White squares signify no in vitro growth.
Finally, in order to confirm that this variable viral replication on Rh PBMCs is characteristic for Rh, we have detailed the in vitro replication of SIVsmm on Hu PBMCs. Total PBMCs from nine different Hu blood donors were used in a chess-board testing using nine primary SIVsmm isolates. Results are shown in Figure 5b. As it can be observed from this figure, although the viral replication patterns varied between the different blood donors, all the primary SIVsmm isolates were consistently replicated in vitro using Hu PBMCs from blood donors (Figure 5b).
Primary SIVsmm isolates grow at low levels in Rh MDDCs
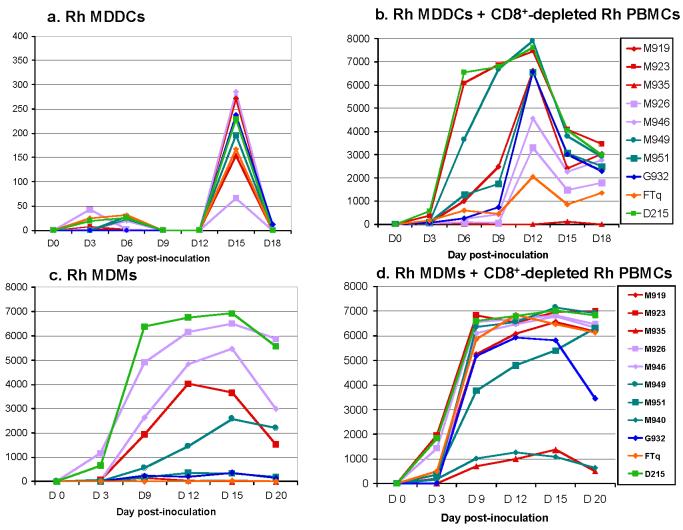
Dendritic cells are antigen-presenting cells that are susceptible to HIV-1 infection (Donaghy et al., 2003). Since MDDC were reported to enhance CD4+ T cell infection (McDonald et al., 2003) we investigated the ability of primary SIVsmm isolates to grow on MDDCs from Rh in order to understand the differences observed between in vitro and in vivo replication patterns. As observed from Figure 6a, MDDC supported the in vitro replication of primary SIVsmm isolates. The replication levels were, however, very low (100-400 pg P27 Ag), in agreement with previous reports on HIV-1 growth in MDDCs (Donaghy et al., 2003). Only strain SIVsmmM926 showed a replication pattern that was indistinguishable from baseline levels (Figure 6a). To confirm SIVsmm replication on the MDDCs, infected MDDCs were co-cultivated with autologous CD8+ depleted PBMCs. As it can be observed from Figure 6b, all cultures became positive, with the exception of SIVsmmM935, which only showed a low blip at day 15 post-innoculation. This experiment demonstrated the ability of primary SIVsmm isolates to replicate in Rh MDDCs. Moreover, our co-culture experiment demonstrated that prior exposure of DCs to SIVsmm can increase the efficacy of SIVsmm isolation in vitro.
Figure 6.
Primary SIVsmm isolate replication on Rh MDDC cultures (a), CD8+ depleted Rh PBMCs following co-cultivation with SIV-infected MDDC (b); Rh monocyte-derived macrophages (MDMs) (c) and on MDMs+ CD8+ depleted Rh PBMCs (d). SIVsmm strain belonging to different lineages are color-coded (red-Lineage 1; purple-Lineage 2; turquoise-Lineage 3; blue-Lineage 4; orange-Lineage 5; light green-Lineage 6).
Primary SIVsmm isolates show variable growth in Rh MDMs
Macrophages may play a pivotal role in SIV infection and some SIV strains are macrophage tropic (Grimm et al., 2003). Macrophage tropism is almost exclusively linked to use of the chemokine coreceptor CCR5 (Moore et al., 2004). However, CCR5 tropism is sometimes not sufficient for replication in macarophages (e.g. SIVmac239) (Moore et al., 2004). Since our SIVsmm strains belonging to different lineages also use the CCR5 co-receptor, we investigated a potential role of MDMs in Rh infection with primary SIVsmm isolates. Over a 3-week period, ten primary SIVsmm isolates were grown in Rh MDMs (Figure 6c). Seven SIVsmm strains replicated productively in MDMs, whereas measurable antigen production was not observed for the remaining three [SIVsmmM919 and SIVsmmM935 (lineage 1) and SIVsmmFTQ (lineage 5)]. The extent of replication in Rh MDMs was variable between different isolates and lineages. Thus, lineage 6 (SIVsmmD215) and lineage 2 (SIVsmmM926 and SIVsmmM946) replicated at the highest levels in Rh MDMs (Figure 6c). Lineage 3 (SIVsmmM949, SIVsmm M951 and SIVsmmM940) and 4 (SIVsmmG932) strains replicated at lower levels. Surprisingly, although lineage 1 is the most pathogenic in vivo in Rh (McClure et al., 1989) (Apetrei, unpublished data), two out the three lineage 1 strains did not replicate on Rh MDMs, and the remaining strain only showed moderate levels of growth. When SIVsmm-infected macrophages were co-cultured with CD8+ depleted PBMCs, all ten virus strains showed significant levels of replication (Figure 6d). The three isolates which did not show detectable levels of replication in MDM alone, replicated fairly well when co-cultured with autologous CD8+ depleted cells (Figure 6d). This experiment confirmed the ability of primary SIVsmm isolates to replicate in Rh MDMs in vitro (Hirsch et al., 1997).
No correlation between the in vitro growth pattern in Rh and the in vivo viral replication
Our results showing a limited in vitro replication of primary SIVsmm on Rh PBMCs were surprising because SIVsmm is a virus which is pathogenic in Rh upon in vivo iinnoculation in all exposed Rh (Hirsch and Johnson, 1994). Therefore, in order to understand if variability in in vitro replication of primary SIVsmm isolates on Rh PBMCs has any impact on viral replication in vivo, we compared viral replication in Rh in vivo and in vitro on a significant group of animals. In this experiment used PBMCs sampled prior to SIVsmm infection to grew in vitro the SIVsmm strains which were employed for in vivo infection and we compared the in vivo and in vitro replication. Results are presented in Figure 7. The different SIVsmm strains replicated efficiently in vivo in all exposed animals, generating high levels of P27 antigenemia (Figure 7a), with the dynamics of plasma viral loads paralleling that of P27 antigenemia and being in the same range with those observed during pathogenic infections with reference SIVmac/SIVsmm strains (Figure 7b). However, the in vitro replication was again variable. Only 38% of cultures of SIVsmm strains on Rh PBMCs from the same animals which were used for experimental infections were positive. Lineage 1 (SIVsmmM923) and lineage 3 (SIVsmmM951) strains did not replicate in the PBMCs from the Rh that they infected successfully in vivo. Half of the cultures were positive for monkeys infected with the lineage 2 (SIVsmmM946) and lineage 6 (SIVsmmD215) strains. Only one out of three cultures were positive for SIVsmmFTq. Rh AT30 which was infected with SIVsmmG932 (lineage 4) replicated the virus at high titers. Another difference in the in vitro growth is that, in the positive cultures, strains SIVsmmM946, SIVsmmG932 and SIVsmmFTq showed acceptable growth on total PBMCs, whereas the lineage 6 strain showed significantly better in vitro growth on CD8+ depleted Rh PBMCs (data not shown). Altogether, our data show that primary SIVsmm isolate in vitro replication profiles on Rh PBMCs has no predictive value for the dynamics of in vivo replication in Rh.
Figure 7.
Comparison between in vitro and in vivo SIVsmm replication in Rh. Sixteen Rh were infected with 6 different primary SIVsmm isolates belonging to different lineages (red-Lineage 1; purple-Lineage 2; turquoise-Lineage 3; blue-Lineage 4; orange-Lineage 5; light green-Lineage 6). The in vitro replication was evaluating by quantifying the production of P27 Ag in supernatants over 3 weeks of infection (a); the in vivo replication was quantifying by measuring the P27 antigenemia during primary infection (days 0-28 post-inoculation) (b) or by quantifying plasma SIVsmm loads over the same period (c).
Discussion
We have performed an extensive in vitro characterization of primary SIVsmm isolates belonging to 6 different lineages. We show that (1) these viruses uses CCR5 as a main co-receptor for virus entry; (2) primary SIVsmm isolates efficiently replicate in Hu PBMCs; (3) primary SIVsmm isolates replicate at low levels in PTM PBMCs and the replication efficacy significantly improves on CD8+ depleted PTM PBMCs; (4) primary SIVsmm strains show variable in vitro replication on Rh PBMCs but not in Hu PBMCs; and (5) no correlation can be established between the in vitro and in vivo replication patterns in Rh.
All primary SIVsmm isolates replicated at high titers in Hu PBMCs and showed variable replication on Hu cell lines. This result confirms previous reports (Chen et al., 1995; Fultz et al., 1986; Marx et al., 1991) and is highly significant because SIVsmm was cross-species transmitted to humans in several instances during the last century and is the root cause of HIV-2 (Chen et al., 1997a; Damond et al., 2004; Gao et al., 1994; Gao et al., 1992; Marx, 2005; Yamaguchi, Devare, and Brennan, 2000). Our results showed that all primary SIVsmm isolates have a consistent ability to efficiently replicate in vitro on human PBMCs, suggesting an ongoing risk for human exposed to SIVsmm in the endemic area. Note, however, that only two of the eight documented cross-species SIVsmm transmission to humans resulted in epidemics (Damond et al., 2001), whereas the remaining cases seem to be epidemiological dead-ends, probably as a result of the immune control by the human hosts (Gao et al., 1994) or action of specific yet undetermined host restriction factors (Huthoff and Malim, 2005; Ylinen et al., 2005). In this context, our results suggest that the inability of SIVsmm strains to establish themselves as human pathogens is not due to cellular host restriction factors, since all the primary SIVsmm isolates tested here and in previous studies (Chen et al., 1995; Chen et al., 1996) replicated efficiently on PBMCs originating from different human blood donors, but to other defense barriers, such as effective immune responses. This may be an important result, because it has been suggested that human exposure to a plethora of SIVs in Central and West Africa may result in the emergence of HIVs (Hahn et al., 2000; Peeters et al., 2002). Our results show that in vitro replication of SIVs on Hu PBMCs may not have predictive value for the ability of a cross-species transmitted pathogen to establish itself as a new pathogen in the new host. In this context, the risk of emergence of new pathogens following exposure to SIV-infected monkeys and apes in Africa may need to be reconsidered, especially since numerous SIVs do not even have the ability to replicate on human PBMCs (Beer et al., 1999; Emau et al., 1991; Osterhaus et al., 1999) (Apetrei, unpublished observations).
Primary SIVsmm isolates also replicated at moderate levels in PTM PBMCs. The efficacy of replication dramatically improved on CD8+ depleted PTM PBMCs. The ability to replicate on PTM PBMCs in vitro is not surprising because previous studies reported a higher susceptibility to SIVsmm infections in PTMs than Rh. The best known example is the strain SIVsmmPBj and its derivatives, which is extremely virulent in different species of macaques and even in SMs (Fultz, 1994; Fultz et al., 1989). This strain was derived from a virus isolated from PTMs infected with SIVsmm9 (McClure et al., 1989). Also, unlike Rh, which progress to AIDS only when infected with SIVsmm and show a certain resistance to other cross-species transmitted SIVs (Osterhaus et al., 1999; Smith et al., 1998; Takehisa et al., 2001), PTMs are more susceptible to cross-species transmitted SIV infections. To date, PTMs were shown to develop AIDS when infected with SIVagm (Goldstein et al., 2005; Hirsch et al., 1995) (Pandrea et al., 2006), SIVsun (Beer et al., 2005) and SIVlhoest (Beer et al., 2005; Hirsch et al., 1999). In contrast, Rh infected with SIVagm or with SIVrcm, SIVmnd-2 and SIVtal replicated these viruses transiently and then cleared the infections (Osterhaus et al., 1999; Pandrea et al., 2006; Smith et al., 1998; Takehisa et al., 2001).
The most notable result of this study is the variable susceptibility of Rh-PBMCs to primary SIVsmm isolates. Numerous studies reported that SIVsmm is pathogenic in Rh upon direct inoculation from naturally-infected SMs (McClure et al., 1989; Murphey-Corb et al., 1986; Novembre et al., 1998). In Rh, SIVsmm induces persistent infection, with variable clinical expression. Serially passaged pathogenic SIVmac or SIVsmm543-3 strains are able to induce severe infection in which most of the monkeys progress to AIDS within 1 year post-inoculation (Hirsch et al., 1997; Westmoreland et al., 1999). Less adapted strains, or strains derived directly from SMs generally induce a less pathogenic infection, characterized by a better control of viral loads and longer and variable incubation period (Hirsch and Johnson, 1994) (Apetrei, unpublished data). Whatever the outcome of these infections in Rh, most of the Rh exposed to SIVsmm replicate the virus at high levels during the primary infection, in the absence of any immune control. Conversely, our in vitro results showed a limited ability of primary SIVsmm isolates to replicate in Rh PBMCs. This limited replication is not virus related, because there is significant variability in the replicative capacity of the same viral strain on PBMCs from different Rh. When CD8+ cells are depleted from the PBMCs, the efficacy of the in vitro replication of primary SIVsmm isolates on Rh cells does not significantly improve in resistant macaques (data not shown). Moreover, we demonstrated the ability of primary SIVsmm isolates to infect two types of antigen-presenting cells, MDDCs and MDMs. Both MDDC and MDM data show that viral replication in vivo may imply involvement of some other immune cell populations and complex cellular interactions, which may explain the differences observed between replication patterns of primary SIVsmm isolates in vitro and in vivo. Note that this variable in vitro replication of primary SIVsmm isolates is specific for Rh PBMCs, in vitro replication of SIVsmm strains being significantly more consistent in Hu PBMCs.
Finally, to investigate the predictive value of in vitro replication capacity for in vivo replication of these primary SIVsmm isolates, we compared peak levels of virus production in vitro and in vivo in the same animals. Previous studies reported that the intrinsic susceptibility of Rh PBMCs to SIV infection in vitro is predictive of viremia after SIV challenge (Lifson et al., 1997). However, in our study, even though many of the SIVsmm strains failed to replicate efficiently in vitro in a donor macaque's PBMC, the majority resulted in robust replication in vivo upon inoculation of this same Rh (Figure 7). Therefore, we concluded that the in vitro growth of SIVsmm strains on Rh PBMCs is not predictive for virus replication in vivo, showing that the in vitro results should always be interpreted in the light of the in vivo data.
What is the significance of Rh-PBMC's variable susceptibility to SIVsmm replication? We first hypothesized that the lack of viral replication in vitro on Rh PBMCs is due to a high number of CD8+ T cells in PBMC cultures inhibiting SIVsmm growth (Goldstein et al., 2000). However, Rh CD8+ depleted PBMCs cells still showed variable replication patterns between Rh. To investigate if the lack of replication was due to a total species-specific restriction of SIVsmm in some Rh (Huthoff and Malim, 2005; Ylinen et al., 2005), we co-cultivated infected MDDCs or MDMs with CD8+ Rh PBMCs. By using antigen-presenting cells to infect CD8+ depleted Rh PBMCs, we generated better results than when growing SIVsmm by conventional methods. The fact that in vitro susceptibility of Rh PBMCs varies widely may finally be explained by differences in host susceptibility.
In summary, the present study provides a thorough characterization of the in vitro growth of primary SIVsmm isolates belonging to different lineages. By showing wide variability in the ability of Rh PBMCs to support in vitro replication of SIVsmm strains, our study suggests that in vitro replication has no predictive value for in vivo pathogenesis studies using these strains. Our results thus confirm the importance of in vivo studies for understanding the pathogenesis of AIDS.
Materials and Methods
Strain selection
Eleven SIVsmm isolates were used in this study. Ten were isolated from chronic naturally infected SMs housed at the Tulane National Primate Research Center (TNPRC): M919, M923, M935 (lineage1); M926, M946 (lineage 2); M940, M949, M951 (lineage3); G932 (lineage 4); and D215 (lineage 6). Additionally, strain FTq (lineage 5) was isolated from a naturally infected SM housed at the Yerkes National Primate Research Center (YNPRC). All the SMs from which SIVsmm strains were isolated were free of simian T-cell lymphotropic virus (STLV).
All protocols and procedures for the animal studies were reviewed and approved by the Tulane University Institutional Animal Care and Use Committee (IACUC), and all protocols and procedures for the human studies were approved by the Tulane University Institutional Review Board (IRB).
PBMC isolation
PBMCs from both naturally SIVsmm-infected SMs and from SIV-free SMs (n=5), Rh (n=13), pig-tailed macaques (PTMs) (n=3) and humans (Hu) (n=10) were isolated by density gradient centrifugation over lymphocyte separation media (MP Biomedical, Irvine, CA). Briefly, blood was layered over density gradient media in a ratio of 2:1 and centrifuged at 18°C at 400 × g for 25 min. The monolayer containing PBMCs was resuspended with RPMI1640 media supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 1% penicillin-streptomycin (1 mg/ml, Invitrogen, CA) for subsequent studies. Hu PBMCs were obtained from Tulane University blood bank.
SIVsmm stock preparation
PBMCs (5×106) from infected and uninfected SMs were stimulated with 10 μg/ml phytohemagglutinin (PHA) (Invitrogen, Carlsbad, CA) for 2 days, followed by overnight incubation in interleukin-2 (IL-2) media, RPMI1640 medium supplemented with 10% heat-inactivated FBS and IL-2 (20 U/ml). Subsequently, PBMCs from SIVsmm-uninfected and SIVsmm-infected SMs were co-cultured in IL-2 media for 5 weeks, and replication was monitored weekly by quantifying the SIV P27 Gag antigen by ELISA (Zeptometrix Corp., Buffalo, NY) in supernatants. Virus stocks prepared from these infected cells were kept at −80°C for use in subsequent infectivity studies. These experiments were performed in triplicate, on PBMCs from five different SMs.
In vitro SIVsmm replication on human T-cell lines
Hu CD4+ T cell lines (MT2, SupT1, CEMss, CEMx174, PM1, and Molt4clone8) were maintained in RPMI1640, supplemented with 10% heat-inactivated FBS, and 1% penicillin-streptomycin (1mg/ml). For infection studies, 106 cells were infected with supernatants produced on SM PBMCs, corresponding to 4000 pg of P27 Ag in 12-well plates (Costar, Corning, N.Y.). Cells were incubated for 4 hours and then washed extensively to remove cell-free virus. Virus production in culture supernatants was monitored weekly by SIV P27 Ag capture assay (Zeptometrix). These experiments were performed in triplicate, on PBMCs from one human subject.
Coreceptor assays on GHOST cells
The coreceptor usage of primary SIVsmm isolates was determined on GHOST cells as previously described (Chen et al., 1998a; Zhang et al., 2000). The GHOST cells expressed CD4 alone or CD4 in combination with the following coreceptors: CCR2b, CCR5, CXCR4, BOB/GPR15, Bonzo/STRL33. Cells were cultured in complete Dulbecco's minimal essential medium containing G418 (5 μg/ml), hygromycin (1 μg/ml), and puromycin (1 μg/ml). GHOST cells expressing only CD4 were used as controls; they were cultured in the same medium except that puromycin was omitted. Expression of the coreceptors on these cells was verified at the day of infection. 2 × 105 cells were exposed to at least three distinct infectious titers of each virus. Productive viral replication was monitored by P27 measurement in the culture supernatants on days 0, 2, 4, 6, and 9. Supernatants were clarified from cells by short-term centrifugation. Several positive controls were included: HIV-1 strain B117, which uses CXCR4, CCR5, Bonzo and Bob (Bjorndal et al., 1997); SIVmac239, which uses CCR5 and Bonzo, and SIVrcm (Georges-Courbot et al., 1998) as a CCR5 CXCR4 negative control because it has been previously reported to specifically use CCR2 as its main co-receptor (Chen et al., 1998b). Uninfected cells were used as negative control. Co-receptor studies were performed in triplicate.
Infection of PBMC from different host species
PBMCs were isolated from nine blood donors, three PTMs and thirteen Rh blood samples, as described earlier. Freshly isolated PBMCs were stimulated with 10 μg PHA per ml of medium for 2 days followed by overnight incubation in IL-2 media. Activated PBMCs and resting PBMCs (5 × 106) were infected with SIV stocks containing 4,000 pg of P27 at 37°C for 4 h; cells were then washed extensively to remove any cell-free virus. Cells were maintained in IL-2 media for 7 weeks. Virus production in culture supernatants was monitored weekly by SIV P27 antigen capture assay.
Isolation and infection of CD8+-depleted cells
Hu-PBMCs, PTM-PBMCs and Rh-PBMCs were depleted of CD8+ cells employing a positive selection of the CD8+ T cells (CD8 microbead kit, Miltenyi Biotech, Auburn, CA). The CD8+ depleted cells were then stimulated with 10 μg/ml PHA for 2 days, followed by overnight incubation in IL-2 media. 5×105 CD8+-depleted cells were incubated with 400 pg of SIVsmm isolates for 4 hrs, followed by two washes to remove cell free virus. For 4 weeks, one half of the supernatant was collected every third day and replaced with fresh IL-2 containing media. Virus production in culture supernatants was monitored by SIV P27 antigen capture assay. These experiments were performed in triplicate.
Generation of monocyte-derived dendritic cells (MDDCs)
Rh MDDCs were generated using CD14+ monocytes, positively selected by MACS Ab-conjugated magnetic bead selection according to the manufacturer's protocol (Miltenyi Biotech). Briefly, isolated CD14+ monocytes were placed into six-well tissue culture plates at 106cells in 2 ml of complete (cRPMI) medium. Cells were cultured for 6 days in the presence of 50 ng/ml of granulocyte/macrophage-colony stimulating factor (GM-CSF) (Peprotech, Rockey Hill, NJ) and 20 ng/ml of IL-4 (Peprotech) per ml. Cytokines were replenished every other day by adding 2 ml of fresh medium and cytokines. After 6 days, the floating cells and weakly adherent cells were harvested by gently pipetting and used for subsequent studies. The phenotype of the cultured cells was monitored by flow cytometry. The following combinations of mouse anti-human monoclonal Abs were used: Fluorescein isothiocyanate (FITC)-labeled anti-CD14 (M5E2, BD Pharmingen, San Jose, CA), FITC-labeled anti-CD83 (HB15e; BD Pharmingen), Phycoerythrin (PE)-labeled anti-CD86 (FUN-1; BD Pharmingen); PE-labeled anti-DC-SIGN (CD209) (120507, R&D Systems, Minneapolis, MN), Peridinin chlorophyll A protein (PerCP)-labeled anti-HLA-DR (L243; BD Pharmingen), and allophycocyanin (APC)-labeled anti-CD11b (M1/70.15.11.5; Miltenyi Biotech). The cells were stained with these reagents for 30 min at 4°C, washed with PBS, and fixed with 2.0% formaldehyde in PBS. Samples were collected on a FACSCalibur flow cytometer (Becton Dickinson), and acquired data were analyzed using FloJo software (Tree Star). The culture cells were characterized by the CD14+, CD11b+, CD83+/−, HLA-DR+++, CD86++, and DC-SIGN++ phenotype corresponding to immature DC.
In vitro infection of MDDC-T cell cultures
For the infection of MDDCs, 5×105 cells were incubated with SIVsmm stocks containing 400 pg of P27 Ag in a total volume of 500 μl at 37°C for 4 hrs in eppendorf tubes. After the incubation steps, cells were extensively washed to remove cell free virus and were cultured for 3 weeks with or without autologous CD8+ depleted Rh PBMCs in the presence of GM-CSF (50 ng/ml) and IL-4 (20 ng/ml). Every 3 days, one half of the supernatant was removed and replaced with fresh medium. Supernatants were analyzed for SIV P27 Gag antigen by ELISA. These experiments were performed in triplicate.
Generation of monocyte-derived macrophages (MDM)
Rh MDM were generated using CD14+ monocytes, positively selected by MACS Ab-conjugated magnetic bead selection according to the manufacturer's protocol (Miltenyi Biotech). Recovered CD14+ monocytes were placed into six-well tissue culture plates at 106 cells in 2 ml of cRPMI medium. Cells were cultured for 6 days in the presence of 50 ng/ml of macrophage-colony stimulating factor (M-CSF; Peprotech) and 20 ng/ml of IL-4. Cytokines were replenished every other day by adding 2 ml of fresh medium and cytokines. At 7 days, adherent macrophages were collected with PBS containing 5 mM EDTA. The phenotype of the culture cells was checked by measuring CD14, CD86, HLA-DR, and CD11b expression by flow cytometry. The cultured cells expressed CD14++, CD86++, HLA-DR+++, and CD11++.
In vitro infection of MDM-T cell cultures
For the infection of MDMs, the cells (5×105) were infected with SIVsmm stocks corresponding 400 pg of P27 Ag at 37°C for 4 hrs. After this incubation step, cells were extensively washed to remove cell free virus and were cultured for 3 weeks with or without autologous CD8+ depleted Rh PBMCs in the presence of GM-CSF (50 ng/ml) and IL-4 (20 ng/ml). Every 3 days, one half of the supernatant was removed and replaced with fresh medium. Supernatants were analyzed for SIV P27 Gag antigen by ELISA. These experiments were performed in triplicate.
Comparison of in vivo and in vitro replication of primary SIVsmm isolates in Rh
To investigate the significance of in vitro growth patterns of primary SIVsmm isolates in Rh, we compared viral replication in vivo and in vitro on a significant group of Rh. Sixteen animals experimentally infected with different primary SIVsmm isolates were included. They received SIVsmm M923-lineage 1 (Rh CA59 and AP31), SIVsmm946-lineage 2 (Rh AT80 and GO99), SIVsmmM951-lineage 3 (Rh DB28 and BD51), SIVsmmG932-lineage 4 (Rh AT30), SIVsmmFTq (Rh DV11, DD58 and DG24) and SIVsmmD215 (Rh AK41, BE51, BV85, DD93, DG34 and DH44). The strains used to infect these animals were grown in vitro on PBMCs collected from each monkey prior to SIVsmm infection. The comparison between in vitro and in vivo replication was done by measuring the P27 Ag in supernatants and by quantifying the dynamics of the P27 antigenemia during the primary SIVsmm infection of Rh (days 0-28 post-inoculation). Also, in order top better relate the dynamics of P27 antigenemia to SIVsmm replication in vivo, we compared the P27 Ag dynamics in vivo to that of plasma viral load during the primary SIVsmm infection of Rh. Plasma viral loads were determined by bDNA (Bayer Laboratory, Emeryville, CA).
Acknowledgements
This work was supported by funds from grants R01, AI065325 and P20 RR020159 (CA), RO1 AI064066 (IP) from the National Institute of Allergy and Infectious Diseases and from development grants G20 RR016930, RR018397, RR019628, RR013466, RR05169, C06 RR12112 and P51 RR000164 (TNPRC) from the National Center for Research Resources. We thank Melissa Pattison, Crystal Quave, Thaidra Gaufin, Megan Mefford, Meredith Hunter and Tessa Williams for technical assistance. We also thank the veterinary and animal care staff of TNPRC for their service and expertise.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Apetrei C, Gormus B, Pandrea I, Metzger M, ten Haaft P, Martin LN, Bohm R, Alvarez X, Koopman G, Murphey-Corb M, Veazey RS, Lackner AA, Baskin G, Heeney J, Marx PA. Direct inoculation of simian immunodeficiency virus from sooty mangabeys in black mangabeys (Lophocebus aterrimus): first evidence of AIDS in a heterologous African species and different pathologic outcomes of experimental infection. J Virol. 2004;78(21):11506–18. doi: 10.1128/JVI.78.21.11506-11518.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apetrei C, Kaur A, Lerche NW, Metzger M, Pandrea I, Hardcastle J, Fakelstein S, Bohm R, Kohler J, Traina-Dorge V, Williams T, Staprans S, Plauche G, Veazey RS, McClure H, Lackner AA, Gormus B, Robertson DL, Marx PA. Molecular epidemiology of SIVsm in US Primate Centers unravels the origin of SIVmac and SIVstm. J Virol. 2005a;79:8991–9005. doi: 10.1128/JVI.79.14.8991-9005.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apetrei C, Lerche NW, Pandrea I, Gormus B, Metzger M, Silvestri G, Kaur A, Bohm R, Robertson DL, Hardcastle J, Lackner AA, Marx PA. Kuru experiments triggered the emergence of pathogenic SIVmac. AIDS. 2006;21:317–321. doi: 10.1097/01.aids.0000206498.71041.0e. [DOI] [PubMed] [Google Scholar]
- Apetrei C, Marx PA. Simian retroviral infections in human beings. Lancet. 2004;364(9429):137–8. doi: 10.1016/S0140-6736(04)16620-8. [DOI] [PubMed] [Google Scholar]
- Apetrei C, Metzger MJ, Robinson D, Ling B, Telfer PT, Reed P, Robertson DL, Marx PA. Detection and partial characterization of new simian immunodeficiency virus (SIVsm) strains from bush meat samples from rural Sierra Leone. J Virol. 2005b;79(4):2631–2636. doi: 10.1128/JVI.79.4.2631-2636.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer BE, Bailes E, Goeken R, Dapolito G, Coulibaly C, Norley SG, Kurth R, Gautier JP, Gautier-Hion A, Vallet D, Sharp PM, Hirsch VM. Simian immunodeficiency virus (SIV) from sun-tailed monkeys (Cercopithecus solatus): evidence for host-dependent evolution of SIV within the C. lhoesti superspecies. J Virol. 1999;73(9):7734–44. doi: 10.1128/jvi.73.9.7734-7744.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer BE, Brown CR, Whitted S, Goldstein S, Goeken R, Plishka R, Buckler-White A, Hirsch VM. Immunodeficiency in the absence of high viral load in pig-tailed macaques infected with simian immunodeficiency virus SIVsun and SIVlhoest. J Virol. 2005;79(22):14044–14056. doi: 10.1128/JVI.79.22.14044-14056.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorndal A, Deng H, Jansson M, Fiore JR, Colognesi C, Karlsson A, Albert J, Scarlatti G, Littman DR, Fenyo EM. Coreceptor usage of primary human immunodeficiency virus type 1 isolates varies according to biological phenotype. J Virol. 1997;71(10):7478–87. doi: 10.1128/jvi.71.10.7478-7487.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti L, Guyader M, Alizon M, Hirsch I, Sonigo P. Sequence of simian immunodeficiency virus from macaque and its relationship to other human and simian retroviruses. Nature. 1987;328(6130):543–7. doi: 10.1038/328543a0. [DOI] [PubMed] [Google Scholar]
- Chakrabarti LA, Lewin SR, Zhang L, Gettie A, Luckay A, Martin LN, Skulsky E, Ho DD, Cheng-Mayer C, Marx PA. Age-dependent changes in T cell homeostasis and SIV load in sooty mangabeys. J Med Primatol. 2000;29(34):158–65. doi: 10.1034/j.1600-0684.2000.290309.x. [DOI] [PubMed] [Google Scholar]
- Chen Z, Gettie A, Ho DD, Marx PA. Primary SIVsm isolates use the CCR5 coreceptor from sooty mangabeys naturally infected in west Africa: a comparison of coreceptor usage of primary SIVsm, HIV-2, and SIVmac. Virology. 1998a;246(1):113–24. doi: 10.1006/viro.1998.9174. [DOI] [PubMed] [Google Scholar]
- Chen Z, Kwon D, Jin Z, Monard S, Telfer P, Jones MS, Lu CY, Aguilar RF, Ho DD, Marx PA. Natural infection of a homozygous delta24 CCR5 red-capped mangabey with an R2b-tropic simian immunodeficiency virus. J Exp Med. 1998b;188(11):2057–65. doi: 10.1084/jem.188.11.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Luckay A, Sodora DL, Telfer P, Reed P, Gettie A, Kanu JM, Sadek RF, Yee J, Ho DD, Zhang L, Marx PA. Human immunodeficiency virus type 2 (HIV-2) seroprevalence and characterization of a distinct HIV-2 genetic subtype from the natural range of simian immunodeficiency virus-infected sooty mangabeys. J Virol. 1997a;71(5):3953–60. doi: 10.1128/jvi.71.5.3953-3960.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Telfer P, Reed P, Zhang L, Getti A, Ho DD, Marx PA. Isolation and characterization of the first simian immunodeficiency virus from a feral sooty mangabey (Cercocebus atys) in West Africa. J Med Primatol. 1995;24(3):108–15. doi: 10.1111/j.1600-0684.1995.tb00155.x. [DOI] [PubMed] [Google Scholar]
- Chen Z, Telfier P, Gettie A, Reed P, Zhang L, Ho DD, Marx PA. Genetic characterization of new West African simian immunodeficiency virus SIVsm: geographic clustering of household-derived SIV strains with human immunodeficiency virus type 2 subtypes and genetically diverse viruses from a single feral sooty mangabey troop. J Virol. 1996;70(6):3617–27. doi: 10.1128/jvi.70.6.3617-3627.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Zhou P, Ho DD, Landau NR, Marx PA. Genetically divergent strains of simian immunodeficiency virus use CCR5 as a coreceptor for entry. J Virol. 1997b;71(4):2705–14. doi: 10.1128/jvi.71.4.2705-2714.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damond F, Apetrei C, Robertson DL, Souquiere S, Lepretre A, Matheron S, Plantier JC, Brun-Vezinet F, Simon F. Variability of human immunodeficiency virus type 2 (HIV-2) infecting patients living in France. Virology. 2001;280(1):19–30. doi: 10.1006/viro.2000.0685. [DOI] [PubMed] [Google Scholar]
- Damond F, Worobey M, Campa P, Farfara I, Colin G, Matheron S, Brun-Vézinet F, Robertson DL, Simon F. Identification of a highly divergent HIV-2 and proposal for a change in HIV-2 classification. AIDS Res Hum Retroviruses. 2004;20:666–672. doi: 10.1089/0889222041217392. [DOI] [PubMed] [Google Scholar]
- Donaghy H, Gazzard B, Gotch F, Patterson S. Dysfunction and infection of freshly isolated blood myeloid and plasmacytoid dendritic cells in patients infected with HIV-1. Blood. 2003;101(11):4505–11. doi: 10.1182/blood-2002-10-3189. [DOI] [PubMed] [Google Scholar]
- Drucker E, Alcabes PG, Marx PA. The injection century: massive unsterile injections and the emergence of human pathogens. Lancet. 2001;358(9297):1989–92. doi: 10.1016/S0140-6736(01)06967-7. [DOI] [PubMed] [Google Scholar]
- Dunham R, Pagliardini P, Gordon S, Sumpter B, Engram J, Moanna A, Lawson B, McClure HM, Xian-Xu H, Ibegbu C, Katz N, Pandrea I, Apetrei C, Sodora DL, Feinberg MB, Staprans SI, Silvestri G. The AIDS-resistance of naturally SIV-infected sooty mangabeys is independent of cellular immunity to the virus. Blood. 2006;108:209–217. doi: 10.1182/blood-2005-12-4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emau P, McClure HM, Isahakia M, Else JG, Fultz PN. Isolation from African Sykes' monkeys (Cercopithecus mitis) of a lentivirus related to human and simian immunodeficiency viruses. J Virol. 1991;65(4):2135–40. doi: 10.1128/jvi.65.4.2135-2140.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fultz PN. SIVsmmPBj14: an atypical lentivirus. Curr Top Microbiol Immunol. 1994;188:65–76. doi: 10.1007/978-3-642-78536-8_4. [DOI] [PubMed] [Google Scholar]
- Fultz PN, McClure HM, Anderson DC, Swenson RB, Anand R, Srinivasan A. Isolation of a T-lymphotropic retrovirus from naturally infected sooty mangabey monkeys (Cercocebus atys) Proc Natl Acad Sci U S A. 1986;83(14):5286–90. doi: 10.1073/pnas.83.14.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fultz PN, McClure HM, Anderson DC, Switzer WM. Identification and biologic characterization of an acutely lethal variant of simian immunodeficiency virus from sooty mangabeys (SIV/SMM) AIDS Res Hum Retroviruses. 1989;5(4):397–409. doi: 10.1089/aid.1989.5.397. [DOI] [PubMed] [Google Scholar]
- Gao F, Yue L, Robertson DL, Hill SC, Hui H, Biggar RJ, Neequaye AE, Whelan TM, Ho DD, Shaw GM, et al. Genetic diversity of human immunodeficiency virus type 2: evidence for distinct sequence subtypes with differences in virus biology. J Virol. 1994;68(11):7433–47. doi: 10.1128/jvi.68.11.7433-7447.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Yue L, White AT, Pappas PG, Barchue J, Hanson AP, Greene BM, Sharp PM, Shaw GM, Hahn BH. Human infection by genetically diverse SIVSM-related HIV-2 in west Africa. Nature. 1992;358(6386):495–9. doi: 10.1038/358495a0. [DOI] [PubMed] [Google Scholar]
- Georges-Courbot MC, Lu CY, Makuwa M, Telfer P, Onanga R, Dubreuil G, Chen Z, Smith SM, Georges A, Gao F, Hahn BH, Marx PA. Natural infection of a household pet red-capped mangabey (Cercocebus torquatus torquatus) with a new simian immunodeficiency virus. J Virol. 1998;72(1):600–8. doi: 10.1128/jvi.72.1.600-608.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S, Brown CR, Dehghani H, Lifson JD, Hirsch VM. Intrinsic susceptibility of rhesus macaque peripheral CD4+ T cells to simian immunodeficiency virus in vitro is predictive of in vivo viral replication. J Virol. 2000;74(20):9388–95. doi: 10.1128/jvi.74.20.9388-9395.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S, Ourmanov I, Brown CR, Plishka R, Buckler-White A, Byrum R, Hirsch VM. Plateau levels of viremia correlate with the degree of CD4+-T-cell loss in simian immunodeficiency virus SIVagm-infected pigtailed macaques: variable pathogenicity of natural SIVagm isolates. J Virol. 2005;79(8):5153–62. doi: 10.1128/JVI.79.8.5153-5162.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm TA, Beer BE, Hirsch VM, Clouse KA. Simian immunodeficiency viruses from multiple lineages infect human macrophages: implications for cross-species transmission. J Acquir Immune Defic Syndr. 2003;32(4):362–9. doi: 10.1097/00126334-200304010-00003. [DOI] [PubMed] [Google Scholar]
- Hahn BH, Shaw GM, De Cock KM, Sharp PM. AIDS as a zoonosis: scientific and public health implications. Science. 2000;287(5453):607–14. doi: 10.1126/science.287.5453.607. [DOI] [PubMed] [Google Scholar]
- Hirsch V, Adger-Johnson D, Campbell B, Goldstein S, Brown C, Elkins WR, Montefiori DC. A molecularly cloned, pathogenic, neutralization-resistant simian immunodeficiency virus, SIVsmE543-3. J Virol. 1997;71(2):1608–20. doi: 10.1128/jvi.71.2.1608-1620.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch VM, Campbell BJ, Bailes E, Goeken R, Brown C, Elkins WR, Axthelm M, Murphey-Corb M, Sharp PM. Characterization of a novel simian immunodeficiency virus (SIV) from L'Hoest monkeys (Cercopithecus l'hoesti): implications for the origins of SIVmnd and other primate lentiviruses. J Virol. 1999;73(2):1036–45. doi: 10.1128/jvi.73.2.1036-1045.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch VM, Dapolito G, Johnson PR, Elkins WR, London WT, Montali RJ, Goldstein S, Brown C. Induction of AIDS by simian immunodeficiency virus from an African green monkey: species-specific variation in pathogenicity correlates with the extent of in vivo replication. J Virol. 1995;69(2):955–67. doi: 10.1128/jvi.69.2.955-967.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch VM, Edmondson P, Murphey-Corb M, Arbeille B, Johnson PR, Mullins JI. SIV adaptation to human cells. Nature. 1989a;341(6243):573–4. doi: 10.1038/341573a0. [DOI] [PubMed] [Google Scholar]
- Hirsch VM, Johnson PR. Pathogenic diversity of simian immunodeficiency viruses. Virus Res. 1994;32(2):183–203. doi: 10.1016/0168-1702(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Hirsch VM, Olmsted RA, Murphey-Corb M, Purcell RH, Johnson PR. An African primate lentivirus (SIVsm) closely related to HIV-2. Nature. 1989b;339(6223):389–92. doi: 10.1038/339389a0. [DOI] [PubMed] [Google Scholar]
- Huthoff H, Malim MH. Cytidine deamination and resistance to retroviral infection: towards a structural understanding of the APOBEC proteins. Virology. 2005;334(2):147–53. doi: 10.1016/j.virol.2005.01.038. [DOI] [PubMed] [Google Scholar]
- Khan AS, Galvin TA, Lowenstine LJ, Jennings MB, Gardner MB, Buckler CE. A highly divergent simian immunodeficiency virus (SIVstm) recovered from stored stump-tailed macaque tissues. J Virol. 1991;65(12):7061–5. doi: 10.1128/jvi.65.12.7061-7065.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letvin NL. Animal models for AIDS. Immunol Today. 1990;11(9):322–6. doi: 10.1016/0167-5699(90)90127-u. [DOI] [PubMed] [Google Scholar]
- Lifson JD, Nowak MA, Goldstein S, Rossio JL, Kinter A, Vasquez G, Wiltrout TA, Brown C, Schneider D, Wahl L, Lloyd AL, Williams J, Elkins WR, Fauci AS, Hirsch VM. The extent of early viral replication is a critical determinant of the natural history of simian immunodeficiency virus infection. J Virol. 1997;71(12):9508–14. doi: 10.1128/jvi.71.12.9508-9514.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling B, Apetrei C, Pandrea I, Veazey RS, Lackner AA, Gormus B, Marx PA. Classic AIDS in a sooty mangabey after an 18-year natural infection. J Virol. 2004;78(16):8902–8. doi: 10.1128/JVI.78.16.8902-8908.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling B, Santiago ML, Meleth S, Gormus B, McClure HM, Apetrei C, Hahn BH, Marx PA. Noninvasive detection of new simian immunodeficiency virus lineages in captive sooty mangabeys: ability to amplify virion RNA from fecal samples correlates with viral load in plasma. J Virol. 2003;77(3):2214–26. doi: 10.1128/JVI.77.3.2214-2226.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstine LJ, Lerche NW, Yee JL, Uyeda A, Jennings MB, Munn RJ, McClure HM, Anderson DC, Fultz PN, Gardner MB. Evidence for a lentiviral etiology in an epizootic of immune deficiency and lymphoma in stump-tailed macaques (Macaca arctoides) J Med Primatol. 1992;21(1):1–14. [PubMed] [Google Scholar]
- Mansfield KG, Lerche NW, Gardner MB, Lackner AA. Origins of simian immunodeficiency virus infection in macaques at the New England Regional Primate Research Center. J Med Primatol. 1995;24(3):116–22. doi: 10.1111/j.1600-0684.1995.tb00156.x. [DOI] [PubMed] [Google Scholar]
- Marx PA. Unsolved questions over the origin of HIV and AIDS. ASM News. 2005;71(1):15–20. [Google Scholar]
- Marx PA, Li Y, Lerche NW, Sutjipto S, Gettie A, Yee JA, Brotman BH, Prince AM, Hanson A, Webster RG, et al. Isolation of a simian immunodeficiency virus related to human immunodeficiency virus type 2 from a west African pet sooty mangabey. J Virol. 1991;65(8):4480–5. doi: 10.1128/jvi.65.8.4480-4485.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure HM, Anderson DC, Fultz PN, Ansari AA, Lockwood E, Brodie A. Spectrum of disease in macaque monkeys chronically infected with SIV/SMM. Vet Immunol Immunopathol. 1989;21(1):13–24. doi: 10.1016/0165-2427(89)90126-8. [DOI] [PubMed] [Google Scholar]
- McDonald D, Wu L, Bohks SM, KewalRamani VN, Unutmaz D, Hope TJ. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science. 2003;300(5623):1295–7. doi: 10.1126/science.1084238. [DOI] [PubMed] [Google Scholar]
- Moore JP, Kitchen SG, Pugach P, Zack JA. The CCR5 and CXCR4 coreceptors--central to understanding the transmission and pathogenesis of human immunodeficiency virus type 1 infection. AIDS Res Hum Retroviruses. 2004;20(1):111–26. doi: 10.1089/088922204322749567. [DOI] [PubMed] [Google Scholar]
- Murphey-Corb M, Martin LN, Rangan SR, Baskin GB, Gormus BJ, Wolf RH, Andes WA, West M, Montelaro RC. Isolation of an HTLV-III-related retrovirus from macaques with simian AIDS and its possible origin in asymptomatic mangabeys. Nature. 1986;321(6068):435–7. doi: 10.1038/321435a0. [DOI] [PubMed] [Google Scholar]
- Novembre FJ, De Rosayro J, O'Neil SP, Anderson DC, Klumpp SA, McClure HM. Isolation and characterization of a neuropathogenic simian immunodeficiency virus derived from a sooty mangabey. J Virol. 1998;72(11):8841–51. doi: 10.1128/jvi.72.11.8841-8851.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterhaus AD, Pedersen N, van Amerongen G, Frankenhuis MT, Marthas M, Reay E, Rose TM, Pamungkas J, Bosch ML. Isolation and partial characterization of a lentivirus from talapoin monkeys (Myopithecus talapoin) Virology. 1999;260(1):116–24. doi: 10.1006/viro.1999.9794. [DOI] [PubMed] [Google Scholar]
- Owen SM, Masciotra S, Novembre F, Yee J, Switzer WM, Ostyula M, Lal RB. Simian immunodeficiency viruses of diverse origin can use CXCR4 as a coreceptor for entry into human cells. J Virol. 2000;74(12):5702–8. doi: 10.1128/jvi.74.12.5702-5708.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandrea I, Gautam R, Barbercheck J, Butler IF, Rasmussen T, Marx PA, Silvestri G, Lackner AA, Veazey RS, Apetrei C. Acute loss of intestinal CD4+ T cells is not predictive of SIV virulence. 2006 doi: 10.4049/jimmunol.179.5.3035. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters M, Courgnaud V, Abela B, Auzel P, Pourrut X, Bibollet-Ruche F, Loul S, Liegeois F, Butel C, Koulagna D, Mpoudi-Ngole E, Shaw GM, Hahn BH, Delaporte E. Risk to human health from a plethora of simian immunodeficiency viruses in primate bushmeat. Emerg Infect Dis. 2002;8(5):451–7. doi: 10.3201/eid0805.01-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters M, Janssens W, Fransen K, Brandful J, Heyndrickx L, Koffi K, Delaporte E, Piot P, Gershy-Damet GM, van der Groen G. Isolation of simian immunodeficiency viruses from two sooty mangabeys in Cote d'Ivoire: virological and genetic characterization and relationship to other HIV type 2 and SIVsm/mac strains. AIDS Res Hum Retroviruses. 1994;10(10):1289–94. doi: 10.1089/aid.1994.10.1289. [DOI] [PubMed] [Google Scholar]
- Rey-Cuille MA, Berthier JL, Bomsel-Demontoy MC, Chaduc Y, Montagnier L, Hovanessian AG, Chakrabarti LA. Simian immunodeficiency virus replicates to high levels in sooty mangabeys without inducing disease. J Virol. 1998;72(5):3872–86. doi: 10.1128/jvi.72.5.3872-3886.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago ML, Range F, Keele BF, Li Y, Bailes E, Bibollet-Ruche F, Fruteau C, Noe R, Peeters M, Brookfield JF, Shaw GM, Sharp PM, Hahn BH. Simian immunodeficiency virus infection in free-ranging sooty mangabeys (Cercocebus atys atys) from the Tai Forest, Cote d'Ivoire: implications for the origin of epidemic human immunodeficiency virus type 2. J Virol. 2005;79(19):12515–27. doi: 10.1128/JVI.79.19.12515-12527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestri G. Naturally SIV-infected sooty mangabeys: are we closer to understanding why they do not develop AIDS? J Med Primatol. 2005;34(56):243–52. doi: 10.1111/j.1600-0684.2005.00122.x. [DOI] [PubMed] [Google Scholar]
- Silvestri G, Fedanov A, Germon S, Kozyr N, Kaiser WJ, Garber DA, McClure H, Feinberg MB, Staprans SI. Divergent host responses during primary simian immunodeficiency virus SIVsm infection of natural sooty mangabey and nonnatural rhesus macaque hosts. J Virol. 2005;79(7):4043–54. doi: 10.1128/JVI.79.7.4043-4054.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestri G, Sodora DL, Koup RA, Paiardini M, O'Neil SP, McClure HM, Staprans SI, Feinberg MB. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity. 2003;18(3):441–52. doi: 10.1016/s1074-7613(03)00060-8. [DOI] [PubMed] [Google Scholar]
- Simon F, Matheron S, Tamalet C, Loussert-Ajaka I, Bartczak S, Pepin JM, Dhiver C, Gamba E, Elbim C, Gastaut JA, et al. Cellular and plasma viral load in patients infected with HIV-2. Aids. 1993;7(11):1411–7. doi: 10.1097/00002030-199311000-00002. [DOI] [PubMed] [Google Scholar]
- Smith SM, Holland B, Russo C, Dailey PJ, Marx PA, Connor RI. Retrospective analysis of viral load and SIV antibody responses in rhesus macaques infected with pathogenic SIV: predictive value for disease progression. AIDS Res Hum Retroviruses. 1999;15(18):1691–701. doi: 10.1089/088922299309739. [DOI] [PubMed] [Google Scholar]
- Smith SM, Makuwa M, Lee F, Gettie A, Russo C, Marx PA. SIVrcm infection of macaques. J Med Primatol. 1998;27(23):94–8. doi: 10.1111/j.1600-0684.1998.tb00232.x. [DOI] [PubMed] [Google Scholar]
- Takehisa J, Harada Y, Ndembi N, Mboudjeka I, Taniguchi Y, Ngansop C, Kuate S, Zekeng L, Ibuki K, Shimada T, Bikandou B, Yamaguchi-Kabata Y, Miura T, Ikeda M, Ichimura H, Kaptue L, Hayami M. Natural infection of wild-born mandrills (Mandrillus sphinx) with two different types of simian immunodeficiency virus. AIDS Res Hum Retroviruses. 2001;17(12):1143–54. doi: 10.1089/088922201316912754. [DOI] [PubMed] [Google Scholar]
- Westmoreland SV, Halpern E, Lackner AA. Simian immunodeficiency virus encephalitis in rhesus macaques is associated with rapid disease progression. J Neurovirol. 1998;4(3):260–8. doi: 10.3109/13550289809114527. [DOI] [PubMed] [Google Scholar]
- Westmoreland SV, Williams KC, Simon MA, Bahn ME, Rullkoetter AE, Elliott MW, deBakker CD, Knight HL, Lackner AA. Neuropathogenesis of simian immunodeficiency virus in neonatal rhesus macaques. Am J Pathol. 1999;155(4):1217–28. doi: 10.1016/S0002-9440(10)65224-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi J, Devare SG, Brennan CA. Identification of a new HIV-2 subtype based on phylogenetic analysis of full-length genomic sequence. AIDS Res Hum Retroviruses. 2000;16(9):925–30. doi: 10.1089/08892220050042864. [DOI] [PubMed] [Google Scholar]
- Ylinen LM, Keckesova Z, Wilson SJ, Ranasinghe S, Towers GJ. Differential restriction of human immunodeficiency virus type 2 and simian immunodeficiency virus SIVmac by TRIM5alpha alleles. J Virol. 2005;79(18):11580–7. doi: 10.1128/JVI.79.18.11580-11587.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lou B, Lal RB, Gettie A, Marx PA, Moore JP. Use of inhibitors to evaluate coreceptor usage by simian and simian/human immunodeficiency viruses and human immunodeficiency virus type 2 in primary cells. J Virol. 2000;74(15):6893–910. doi: 10.1128/jvi.74.15.6893-6910.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]