Abstract
Oral opioids are the treatment of choice for chronic cancer pain. Morphine is the strong opioid of choice for the treatment of moderate to severe cancer pain according to guidelines from the World Health Organization (WHO). This recommendation by the WHO was derived from availability, familiarity to clinicians, established effectiveness, simplicity of administration, and relative inexpensive cost. It was not based on proven therapeutic superiority over other options. Patients who experience inadequate pain relief or intolerable side effects with one opioid may often be successfully treated with another agent or with the same agent administered by a different route. Opioid rotation, or switching to an alternative opioid, helps some patients achieve better pain control with fewer associated adverse effects. Oxycodone is a μ-opioid receptor specific ligand, with clear agonist properties. It is an active potent opioid, which is in part a κ-receptor agonist. Like morphine and other pure agonists, there is no known ceiling to the analgesic effects of oxycodone. The active metabolites of oxycodone (eg, oxymorphone) could be important in oxycodone-mediated analgesia. The main pharmacokinetic difference between oxycodone and morphine is in oral bioavailability. The bioavailability of oxycodone is >60% and the bioavailability of morphine is 20%. Controlled-release oxycodone is absorbed in a bi-exponential fashion. There is a rapid phase with a mean half-life of 37 min, accounting for 38% of the dose, and a slow phase with a half-life of 6.2 h, which accounts for the residual 62%. Oxycodone elimination is impaired by renal failure because there are both an increased volume of distribution and reduced clearance. A lot of studies prove that the efficacy of controlled-release oxycodone in cancer-pain control is at least the same as morphine, immediate-release oxycodone and hydromorphone. Its toxicity profile seems better than that of morphine. There are actually several illustrations of a lower incidence of side-effects in the central nervous system. It is therefore possible to conclude that oxycodone represents a valid alternative to morphine in the management of moderate to severe cancer pain, also as first-line treatment.
Keywords: oxycodone, opioids, cancer pain, analgesic, morphine
Introduction
Oral opioids are the treatment of choice for chronic cancer pain (Levy 1996). Morphine is the major opioid of choice for the treatment of moderate to severe cancer pain according to guidelines from the World Health Organization (WHO). This recommendation was based upon physicians’ familiarity with the molecule, established effectiveness, simplicity of administration, and the general availability of drug and its relatively inexpensive cost. It was not based on proven therapeutic superiority over other options (Riley et al 2006).
Recently, the Research Network of the European Association for Palliative Care (EAPC) performed a survey of 3030 cancer patients from 143 palliative-care centers in 21 European countries. Patients were treated with analgesics corresponding to the WHO pain ladder steps I (n=855), II (n=509), and III (n=1589). The investigators assessed 32% of the patients as having moderate or severe pain. In general there were small differences in pain intensities among different countries. Morphine was the most frequently used opioid for moderate to severe pain (oral normal release morphine: 21%; oral sustained-release morphine: 19%; intravenous [IV] or subcutaneous [SC] morphine: 10%). Other opioids for moderate to severe pain were transdermal fentanyl (14%), oxycodone (4%), methadone (2%), diamorphine (2%), and hydromorphone (1%) (Klepstad et al 2005).
Patients who experience inadequate pain relief or intolerable side effects with one opioid may often be successfully treated with another agent or with the same agent administered by a different route (Ripamonti and Dickerson 2001). Opioid rotation, or switching to an alternative opioid, helps some patients achieve better pain control with fewer associated adverse effects (Mercadante 1999). The pharmacological mechanism underlying this phenomenon involves the diverse and combined effects of agonist binding to the three opioid receptors (μ, κ, δ), incomplete cross-tolerance, the diverse genetic background of patients including allelic variations in the opioid receptors themselves, as well as differences in drug clearance mechanisms (Knapp et al 1989; Gaveriaux-Ruff and Kieffer 1999).
A prospective study was performed in the Department of Palliative Care of the Royal Marsden National Hospital Service Trust in London. In this trial 74% (138/186) of the patients treated with morphine had a good response. Twenty-five percent (47/186) did not respond to morphine. These non-responders were switched to alternative opioids. Furthermore, of the 186 patients, 37 achieved a successful outcome when switched to oxycodone and an additional 4 were well controlled when switched to more than one alternative opioid. Overall, successful pain control with minimal side effects was achieved in 96% of patients. (Riley et al 2006)
Pharmacokinetic and pharmacodynamic
Oxycodone was derived from thebaine in 1916 (Gaveriaux-Ruff and Kieffer 1999). It was introduced into clinical practice in Germany in 1917 (Falk 1917). Oxycodone has a liposolubility similar to morphine, and both are significantly less lipid soluble than fentanyl (Poyhia et al 1993; Poyhia and Seppala 1994).
Oxycodone is a μ-opioid receptor specific ligand, with clear agonist properties. The Ki (nM) of oxycodone for the μ-opioid receptor is 18±4 compared with 958±499 for the δ-opioid receptor and 677±326 for the κ-opioid receptor. The μ-opioid receptor binding affinity of oxycodone is, however, less than that of morphine or methadone. Oxymorphone, the active metabolite of oxycodone, has a significantly higher μ-opioid receptor binding affinity (Kalso 2005). The active metabolites of oxycodone (eg, oxymorphone) could be important in oxycodone-mediated analgesia. Studies using Dark Aguti rats that are deficient in the enzyme CYP2D1, which is required to O-demethylate oxycodone in rat, and various opioid receptor antagonists have suggested that the antinociceptive effects of oxycodone could be κ-opioid receptor-mediated (Ross and Smith).
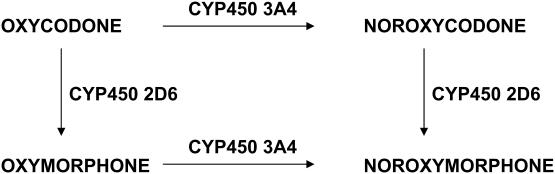
The metabolism of oxycodone in humans is still poorly characterized. The main known metabolic pathways of oxycodone are through O-demethylation to oxymorphone and via N-demethylation to noroxycodone. Noroxycodone concentrations in plasma and urine have been significantly higher after oral than after intramuscular administration, suggesting a prominent role of N-demethylation in the first-pass metabolism of oxycodone. The conversion of oxycodone to oxymorphone, as well as the conversion of noroxycodone to noroxymorphone are catalyzed by the liver enzyme cytochrome P4502D6 (CYP2D6) (Figure 1) (Kress 2005)
Figure 1.
Oxycodone metabolic pathways (Kress 2005).
This enzyme has two phenotypes in the white population: 5%–10% are poor metabolizers with diminished CYP2D6 activity. Most of oxycodone and noroxycodone is excreted in the urine as the free (unconjugated) form, whereas oxymorphone is mainly excreted in the conjugated form (Kalso 2005). Gender, but not age, influences oxycodone elimination: women eliminate oxycodone 25% more slowly than men (Kaiko et al 1996).
The main pharmacokinetic difference between oxycodone and morphine is in the oral bioavailability. The bioavailability of oxycodone is >60% and the bioavailability of morphine is 20% (Hoskin et al 1989).
Unlike normal release (NR) oxycodone, controlled-release (CR) oxycodone is absorbed in a bi-exponential fashion. There is a rapid phase with a mean half-life of 37min, accounting for 38% of the dose, and a slow phase with a half-life of 6.2 h, which accounts for the residual 62% (Mandema et al 1996).
Oxycodone elimination is impaired by renal failure because there is both an increased volume of distribution and a reduced clearance. Delayed clearance results in lower concentrations of oxycodone and noroxycodone and reduced elimination of free unconjugated oxymorphone (Kirvela et al 1996).
The mean elimination half-life of oxycodone in end-stage liver disease is 13.9 h (range 4.6–24.4 h). After liver transplant it returns to 3.4 h (range 2.6–5.1 h) (Tallgren et al 1997). Therefore, care must be exercised when oxycodone is used in cirrhosis or end-stage liver disease, and it is necessary either to reduce the dose or extend dosage intervals (Davis et al 2003).
Efficacy and safety in cancer pain
Oxycodone is mainly used as controlled-release tablets for chronic pain. The immediate-release solution and tablets are used for acute pain or for breakthrough pain. Parenteral oxycodone is a good alternative when opioids cannot be administered orally (Kalso 2005). Controlled-release oxycodone is marketed as a twice-daily oral opioid for the control of moderate-to-severe pain and has become one of the most frequently prescribed opioids in the US (Davis et al 2003).
The first controlled studies of oxycodone in cancer pain were performed by Beaver and colleagues (1978a, 1978b). These studies indicated that oxycodone could be a useful oral analgesic as it had a higher oral bioavailability than morphine. The first repeated-dose, cross-over studies comparing IV patient-controlled analgesia (PCA) and oral solutions of oxycodone and morphine were performed by Kalso and Vainio (1990) and Kalso et al (1990). These studies suggested that the oral bioavailability as calculated from the daily consumption of each drug was 0.70 for oxycodone and 0.31 for morphine. The daily oral dose of oxycodone solution was suggested to be about 67% of the morphine solution (2005). Controlled-release formulations of both oxycodone and morphine have made a major difference in the ease and simplicity of providing stable opioid analgesia in cancer pain. Both CR oxycodone and CR morphine provide, at proper doses, pain relief for 12 h. The onset of analgesia is faster with CR oxycodone.
Four studies demonstrated that CR oxycodone every 12 h is as effective as NR oxycodone in moderate to severe cancer pain (Kaplan et al 1998; Parris et al 1998; Salzman et al 1999; Stamburgh et al 2001). Parris et al (1998) compared the effectiveness and safety of CR oxycodone tablets with immediate-release (IR) oxycodone in patients with chronic cancer pain. With this aim, a multicenter, randomized, double-blind, parallel-group study was performed in 111 patients with cancer pain. Patients received CR oxycodone tablets every 12 h or IR oxycodone four times daily for 5 days. There was no significant difference between treatment groups with regard to the incidence of adverse events. This study demonstrates that cancer pain patients can be equally well treated with CR oxycodone administered every 12 h or IR oxycodone four times daily at the same total daily dose. CR oxycodone offers the benefits of twice daily dosing.
Kaplan et al (1998) randomized cancer patients who required therapy for moderate to severe pain to CR oxycodone every 12 h (n=81) or IR oxycodone four times daily (n=83) for 5 days. Pain intensity remained slight during the study, with mean oxycodone doses of 114 mg/d (range, 20 mg/d to 400 mg/d) for CR and 127 mg/d (range, 40 mg/d to 640 mg/d) for IR. Acceptability of therapy was fair to good with both treatments. Fewer adverse events were reported with CR (109) than with IR (186) oxycodone (p=0.006).
Salzman et al (1999) randomized 48 patients with cancer pain to open-label titration with either CR or IR oxycodone for a period of up to 21 days. Results of this study showed no difference between CR and IR oxycodone with respect to both the percentage of patients achieving stable pain control, the time to achieve stable pain control, and the degree of pain control achieved.
Stambaugh et al (2001) randomized thirty patients with cancer pain to receive CR oxycodone or IR oxycodone for 3 to 7 days followed by crossover at the same daily doses. Following repeat dosing under double-blind conditions, oral CR oxycodone administered every 12 h provided analgesia comparable to IR oxycodone given four times daily. Adverse events were similar for both medications.
Three studies have compared the CR formulations of both oxycodone and morphine in cancer patients. In a randomized, double-blind, cross-over trial, CR oxycodone and morphine were administered to 45 adult patients with stable pain for 3–6 days after open-label titration. In this study, both CR oxycodone and CR morphine provided adequate, stable analgesia, as most of the patients reported their pain as ‘slight’ or ‘none’ at the end of the stable phases. If the results of the two periods were combined, the patients consumed significantly more escape doses and the mean pain intensities were significantly higher with respect to CR morphine compared with CR oxycodone (Heiskanen and Kalso 1997).
In another study, 100 patients with cancer pain were randomized to double-blind treatment with CR oxycodone or CR morphine every 12 h for up to 12 days. Stable analgesia was achieved in 83% of CR oxycodone and 81% of CR morphine patients in 2 days (median). Following titration to stabilize pain control, both drugs were also comparably effective in reducing pain intensity from baseline (Mucci-LoRusso et al 1998). In the third study, 32 patients with cancer pain who had achieved stable analgesia on oral opioids were randomized to either CR oxycodone or CR morphine for 7 days and then switched to the alternate drug for another 7 days. Pain intensity was measured through both a 100-mm visual analog scale (VAS) and a 5-term (0–4) categorical scale (CAT). Both pain assessments were comparable for the two drugs; mean VAS scores were 23±21 and 24±20, and CAT scores were 1.2±0.8 and 1.3±0.7 for CR oxycodone and CR morphine, respectively (Bruera et al 1998).
With regard to the above trials, crossover designs were used in two (Heiskanen and Kalso 1997; Bruera et al 1998), whereas one was a parallel group study (Mucci-LoRusso et al 1998). A total of 177 patients were included in these studies and 73% completed the study protocol. All of the studies suggest that both CR oxycodone and CR morphine provide adequate analgesia in moderate to severe cancer pain. The equianalgesic daily dose ratios of oxycodone:morphine vary from 3:4 to 1:2. The mean daily doses utilized were: CR oxycodone 148±18 mg (Heiskanen et al 2000), 101 mg (40–360 mg) (Mucci-LoRusso et al 1998), 93±114 mg (Bruera et al 1998), and for CR morphine 204±24 mg (Heiskanen et al 2000), 140 mg (60–300 mg) (Mucci-LoRusso et al 1998), and 145±204 mg (Bruera et al 1998).
The adverse effects reported by the patients were typical opioid adverse effects, with no major differences between the groups. Fewer hallucinations were reported with oxycodone, as well as less nausea and pruritus, compared with morphine (Mucci-LoRusso et al 1998) (Table 1).
Table 1.
Incidence of adverse effects (Mucci-LoRusso et al 1998)
| CR Oxycodone (n=48) | CR Morphine (n=52) | |
|---|---|---|
| n | n | |
| Constipation | 10 | 10 |
| Drowsiness | 7 | 10 |
| Nausea | 6 | 8 |
| Vomiting | 6 | 5 |
| Vertigo | 4 | 7 |
| Pruritis | 4 | 5 |
| Dry mouth | 1 | 7 |
| Hallucinations | 0 | 2 |
Abbreviations: CR, controlled release.
In addition to these data, a prospective trial by Maddocks et al demonstrated that statistically significant improvements in mental state as well as nausea and vomiting occurred following a change from morphine to oxycodone (Maddocks et al 1996).
Hagen and Babul (1997) randomized 44 patients with stable cancer pain to receive CR oxycodone or CR hydromorphone, each given every 12 h for 7 days, in a double-blind crossover study. In this study there were no significant differences between treatments in overall Visual Analogue Scale (VAS) pain intensity (VAS 28±4 mm vs 31±4 mm), categorical pain intensity (1.4±0.1 vs 1.5±0.1), daily rescue analgesic consumption (1.4±0.3 vs 1.6±0.3), sedation scores (24±4 mm vs 18±3 mm), nausea scores (15±3 mm vs 13±3 mm), or patient preference. Two patients experienced hallucinations on CR hydromorphone, whereas none of the patients suffered such side effects while receiving CR oxycodone.
The above-described studies lasted for about a week in each treatment arm. The long-term administration of CR oxycodone was studied by Citron et al (1998). A total of 87 patients were included and 51% of the patients completed the 3-month study. A significant but modest increase in the total daily CR oxycodone dose was observed. However, the percentage of patients reporting common opioid-related adverse effects decreased over the course of the study (Kalso 2005).
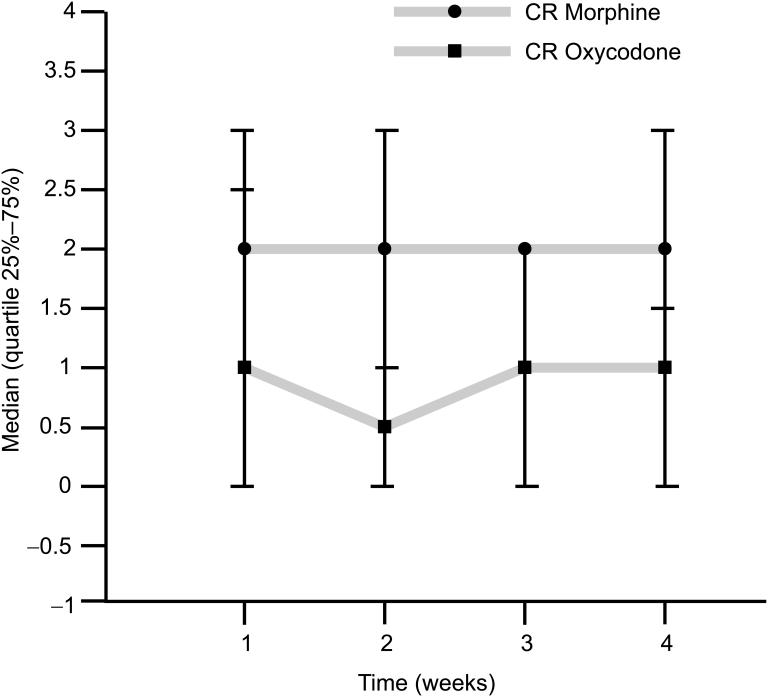
Lauretti (2003) reported opioid synergy in chronic cancer-related pain. Briefly, 22 patients received CR morphine and CR oxycodone in a crossover design involving two sequential 14-day treatment periods with IR morphine available for breakthrough pain. The requirement for breakthrough IR morphine was 38% higher in patients receiving CR morphine than in patients receiving CR oxycodone, suggesting that a synergistic analgesic interaction took place when morphine was administered to patients receiving CR oxycodone (Lauretti et al 2003) (Figure 2).
Figure 2.
Use of rescue medication. Average daily number of rescue doses of morphine (10 mg) administered (Lauretti et al 2003).
Abbreviations: CR, controlled release.
Conclusions
In summary, oxycodone is a semisynthetic opioid analgesic with a high oral-to-parenteral bioavailability and a 2-fold greater oral potency than oral morphine. It is a μ-agonist but its antinociceptive effects may also be κ-opioid receptor-mediated Like morphine and other pure agonists, there is no known ceiling to the analgesic effects of oxycodone. The pharmacokinetics are altered by gender, less by age, and significantly by impaired renal and hepatic function. CR oxycodone exhibits bi-exponential pharmacokinetics and less variable absorption than controlled-release morphine. The equianalgesic daily dose ratios of oxycodone to morphine vary from 3:4 to 1:2.
CR oxycodone combines the effectiveness and safety of oral oxycodone with the convenience of dosing every 12 h. The delivery system used in the CR oxycodone tablet matrix consists of two hydrophobic polymers, finely balanced to ensure the measured release of oxycodone. The properties of the matrix impart a bi-phasic absorption with onset of action within 1 h in most patients, followed by a more protracted phase that maintains effective blood concentrations of oxycodone over a 12 h period.
All the above-mentioned studies confirm that the efficacy of CR oxycodone in cancer-pain is at least the same as morphine, IR oxycodone, and hydromorphone. Its tolerability profile seems to be better than that of morphine and indeed there are several illustrations of a lower incidence of side-effects in the central nervous system. Further studies are necessary to clarify the possible synergistic action that oxycodone may exhibit when administered concurrently with morphine. It is therefore possible to conclude that oxycodone represents a valid first line alternative to morphine in the management of moderate to severe cancer pain.
References
- Beaver WT, Wallenstein SL, Rogers A, et al. Analgesic studies of codeine and oxycodone in patients with cancer. II. Comparisons of oral with intramuscular codeine and oral with intramuscular oxycodone. J Pharmacol Exp Ther. 1978a;207:101–8. [PubMed] [Google Scholar]
- Beaver WT, Wallenstein SL, Rogers A, et al. Analgesic studies of codeine and oxycodone in patients with cancer. I. Comparisons of oral with intramuscular codeine and oral with intramuscular oxycodone. J Pharmacol Exp Ther. 1978b;207:92–100. [PubMed] [Google Scholar]
- Bruera E, Belzile M, Pituskin E, et al. Randomized, double-blind, cross-over trial comparing safety and efficacy of oral controlled-release oxycodone with controlled-release morphine in patients with cancer pain. J Clin Oncol. 1998;16:3222–9. doi: 10.1200/JCO.1998.16.10.3222. [DOI] [PubMed] [Google Scholar]
- Citron ML, Kaplan R, Parris WV, et al. Long-term administration of controlled-release oxycodone tablets for the treatment of cancer pain. Cancer Invest. 1998;16:562–71. doi: 10.3109/07357909809032886. [DOI] [PubMed] [Google Scholar]
- Davis MP, Varga J, Dickerson D, et al. Normal-release and controlled-release oxycodone: pharmacokinetcs, pharmacodynamics and controversy. Support Care Cancer. 2003;11:84–92. doi: 10.1007/s00520-002-0385-9. [DOI] [PubMed] [Google Scholar]
- Falk E. Eukodal, ein neues Narkoticum. Munchener Medizinische Wochenschrift. 1917;20:381–4. [Google Scholar]
- Gaveriaux-Ruff C, Kieffer BL. Opioid receptors: gene structure and function. In: Stein C, editor. Opioids in pain control: basic and clinical aspects. Cambridge: Cambridge Univ Pr; 1999. pp. 1–20. [Google Scholar]
- Hagen NA, Babul N. Comparative clinical efficacy and safety of a novel controlled-release oxycodone formulation and controlled-release hydromorphone in the treatment of cancer pain. Cancer. 1997;79:1428–37. [PubMed] [Google Scholar]
- Heiskanen TE, Kalso EA. Controlled-release oxycodone and morphine in cancer related pain. Pain. 1997;73:37–45. doi: 10.1016/s0304-3959(97)00072-9. [DOI] [PubMed] [Google Scholar]
- Heiskanen TE, Ruismäki PM, Seppälä TA, et al. Morphine or oxycodone in cancer pain? Acta Oncologica. 2000;39:941–7. doi: 10.1080/02841860050215927. [DOI] [PubMed] [Google Scholar]
- Hoskin PJ, Hanks GW, Aherne GW, et al. The bioavailability and pharmacokinetics of morphine after intravenous, oral and buccal administration in healthy volunteers. Br J Clin Pharmacol. 1989;27:499–505. doi: 10.1111/j.1365-2125.1989.tb05399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiko RF, Benziger DP, Fitzmartin RD, et al. Pharmacokinetic-pharmacodynamic relationships of controlled-release oxycodone. Clin Pharmacol Ther. 1996;59:52–61. doi: 10.1016/S0009-9236(96)90024-7. [DOI] [PubMed] [Google Scholar]
- Kalso E. Oxycodone. J Pain Symptom Manage. 2005;29(5S):S47–S56. doi: 10.1016/j.jpainsymman.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Kalso E, Vainio A. Morphine and oxycodone hydrochloride in the management of cancer pain. Clin Pharmacol Ther. 1990;47:639–46. doi: 10.1038/clpt.1990.85. [DOI] [PubMed] [Google Scholar]
- Kalso E, Vainio A, Mattila MJ, et al. Morphine and oxycodone in the management of cancer pain: plasma levels determined by chemical and radioreceptor assays. Pharmacol Toxicol. 1990;67:322–8. doi: 10.1111/j.1600-0773.1990.tb00838.x. [DOI] [PubMed] [Google Scholar]
- Kaplan R, Parris WC, Citron ML, et al. Comparison of controlled-release and immediate-release oxycodone tablets in patients with cancer pain. J Clin Oncol. 1998;16:3230–7. doi: 10.1200/JCO.1998.16.10.3230. [DOI] [PubMed] [Google Scholar]
- Kirvela M, Lindgren L, Seppala T, et al. The pharmacokinetics of oxycodone in uremic patients undergoing renal transplantation. J Clin Anesth. 1996;8:13–18. doi: 10.1016/0952-8180(95)00092-5. [DOI] [PubMed] [Google Scholar]
- Klepstad P, Kaasa S, Cherny N, et al. Pain and pain treatments in European palliative care units. A cross sectional survey from the European Association for Palliative Care Research Network. Palliat Med. 2005;19:442–3. doi: 10.1191/0269216305pm1054oa. [DOI] [PubMed] [Google Scholar]
- Knapp RJ, Porreca F, Burks TF, et al. Mediation of analgesia by multiple opioid receptors. In: Hill CS, Fields WS, editors. Advances in pain research and therapy. New York: Raven Pr; 1989. pp. 247–89. [Google Scholar]
- Kress HG. International Forum on Pain medicine; May 2005; Sofia. 2005. [Google Scholar]
- Lauretti GR, Oliveira GM, Pereira NL. Comparison of sustained-release morphine with sustained-release oxycodone in advanced cancer patients. Br J Cancer. 2003;89:2027–30. doi: 10.1038/sj.bjc.6601365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy MH. Pharmacological treatment of cancer pain. N Engl J Med. 1996;335:1124–32. doi: 10.1056/NEJM199610103351507. [DOI] [PubMed] [Google Scholar]
- Maddocks I, Somogyi A, Abbott F, et al. Attenuation of morphine-induced delirium in palliative care by substitution with infusion of oxycodone. J Pain Symptom Manage. 1996;12:182–189. doi: 10.1016/0885-3924(96)00050-4. [DOI] [PubMed] [Google Scholar]
- Mandema JW, Kaiko RF, Oshlak B, et al. Characterization and validation of a pharmacokinetic model for controlled-release oxycodone. Br J Clin Pharmacol. 1996;42:747–756. doi: 10.1046/j.1365-2125.1996.00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercadante S. Opioid rotation for cancer pain: rationale and clinical aspects. Cancer. 1999;86:1856–66. doi: 10.1002/(sici)1097-0142(19991101)86:9<1856::aid-cncr30>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Mucci-LoRusso P, Berman BS, Silberstein PT, et al. Controlled-release oxycodone compared with controlled-release morphine in the treatment of cancer pain: a randomized, double-blind, parallel-group study. European J Pain. 1998;2:239–49. doi: 10.1016/s1090-3801(98)90020-9. [DOI] [PubMed] [Google Scholar]
- Parris WC, Johnson BW, Jr, Croghan MK, et al. The use of controlled-release oxycodone for the treatment of chronic cancer pain: a randomized, double-blind study. J Pain Symptom Manage. 1998;16:205–11. doi: 10.1016/s0885-3924(98)00064-5. [DOI] [PubMed] [Google Scholar]
- Poyhia R, Seppala T. Liposolubility and protein binding of oxycodone in vitro. Pharmacol Toxicol. 1994;74:23–7. doi: 10.1111/j.1600-0773.1994.tb01068.x. [DOI] [PubMed] [Google Scholar]
- Poyhia R, Vainio A, Kalso E. A review of oxycodone's clinical pharmacokinetics and pharmacodynamics. J Pain Symptom Manage. 1993;8:63–7. doi: 10.1016/0885-3924(93)90101-z. [DOI] [PubMed] [Google Scholar]
- Riley J, Ross JR, Rutter D, et al. No pain relief from morphine? Individual variation in sensitivity to morphine and the need to switch to an alternative opioid in cancer patients 2006. Support Care Cancer. 2006;14:56–64. doi: 10.1007/s00520-005-0843-2. [DOI] [PubMed] [Google Scholar]
- Ripamonti C, Dickerson ED. Strategies for the treatment of cancer pain in the new millennium. Drugs. 2001;61:955–77. doi: 10.2165/00003495-200161070-00005. [DOI] [PubMed] [Google Scholar]
- Ross F, Smith MT. The intrinsic antinociceptive effects of oxycodone appear to be κ-opioid receptor mediated. Pain. 1997;7:151–7. doi: 10.1016/S0304-3959(97)00093-6. [DOI] [PubMed] [Google Scholar]
- Salzman RT, Roberts MS, Wild J, et al. Can a controlled-release oral dose form of oxycodone be used as readily as an immediate-release form for the purpose of titrating to stable pain control? J Pain Symptom Manage. 1999;18:271–9. doi: 10.1016/s0885-3924(99)00079-2. [DOI] [PubMed] [Google Scholar]
- Stambaugh JE, Reder RF, Stambaugh MD, et al. Double-blind, randomized comparison of the analgesic and pharmacokinetic profiles of controlled- and immediate-release oral oxycodone in cancer pain patients. J Clin Pharmacol. 2001;41:500–6. doi: 10.1177/00912700122010375. [DOI] [PubMed] [Google Scholar]
- Tallgren M, Olkkola KT, Seppala T, et al. Pharmacokinetics and ventilatory effects of oxycodone before and after liver transplantation. Clin Pharmacol Ther. 1997;61:655–1. doi: 10.1016/S0009-9236(97)90100-4. [DOI] [PubMed] [Google Scholar]