Abstract
Tumor necrosis factor (TNF) has been implicated in a number of arthritic disease states, including rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. Adalimumab is the first fully human, high-affinity, recombinant immunoglobulin G1 (IgG1) anti-TNF monoclonal antibody. Adalimumab in combination with methotrexate or standard antirheumatic therapies, or as monotherapy, is effective in the treatment of adults with active rheumatoid arthritis who have had an inadequate response to disease-modifying antirheumatic drugs. Adalimumab is also effective in the treatment of patients with moderately to severely active psoriatic arthritis, improving both joint and skin manifestations of the disease as well as disability due to joint damage. In the Adalimumab Trial Evaluating Long-term Efficacy and Safety in Ankylosing Spondylitis (ATLAS), adalimumab significantly reduced the signs and symptoms of active ankylosing spondylitis and established a sustained clinical response in patients who had an inadequate response or intolerance to nonsteroidal antiinflammatory drug therapy. Overall, across these indications, adalimumab demonstrated a rapid onset of action, sustained efficacy with long-term treatment, and was well-tolerated, with few patients discontinuing treatment because of adverse events. The safety profile was similar to other TNF antagonists. Inhibition of TNF activity by adalimumab also significantly improved physical functioning and quality of life measures.
Keywords: Adalimumab, TNF antagonists, rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis
Introduction
Therapeutic options for the treatment of arthritic diseases, including rheumatoid arthritis (RA), psoriatic arthritis (PsA), and ankylosing spondylitis (AS), have been broadened by the development of specifically targeted biologic agents. Among these are strategies aimed at specific neutralization of the proinflammatory cytokine tumor necrosis factor (TNF). The basis for focusing on TNF as a therapeutic target comes largely from studies showing that concentrations of TNF are elevated in synovial fluid and sera of patients with active RA (Saxne et al 1988), joints and skin of PsA patients (Partsch et al 1997; Ritchlin et al 1998), and sacroiliac joint biopsy specimens from patients with AS (Braun et al 1995). Three TNF antagonists are currently available (Lee and Kavanaugh 2005) (Figure 1): infliximab, a chimeric monoclonal anti-TNF antibody; etanercept, a recombinant soluble p75 TNF-receptor-Fc fusion protein; and adalimumab, a human monoclonal anti-TNF antibody. All 3 agents have demonstrated efficacy in the treatment of RA; however, efficacy in other inflammatory arthritides varies because of differences in drug characteristics (eg, pharmacokinetics, dosing, immunogenicity, ability to block lymphotoxin or fix complement, or propensity to induce apoptosis) and disease pathophysiology (eg, role of lymphotoxin) (Haraoui 2005). This review will focus specifically on the clinical efficacy and safety of adalimumab in RA, PsA, and AS.
Figure 1.
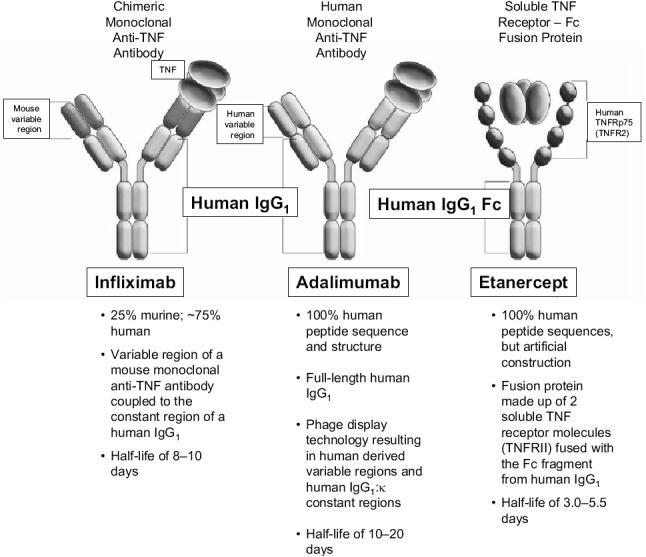
Adalimumab structure in comparison with other TNF antagonists. Copyright © 2005. Adapted from Anderson PJ. 2005. Tumor necrosis factor inhibitors: clinical implications of their different immunogenicity profiles. Semin Arthritis Rheum, 34(5 Suppl 1):19–22.
Abbreviations: IgG1, immunoglobulin G1; TNF, tumor necrosis factor.
Adalimumab
Adalimumab is the first fully human, high-affinity, recombinant immunoglobulin G1 (IgG1) anti-TNF monoclonal antibody. It is composed of human-derived heavy- and light-chain variable regions and human IgG1:κ constant regions engineered through phage display technology and produced in a Chinese hamster ovary mammalian cell line (van de Putte et al 2003). Because it is indistinguishable in structure and function from naturally occurring human IgG1, adalimumab has high selectivity and affinity for TNF; suitability for long-term chronic administration with a low degree of immunogenicity, with or without concomitant methotrexate (MTX) use; a low incidence of allergic reactions; and a half-life comparable to that of IgG1, allowing every-other-week dosing for patient convenience. Adalimumab is currently indicated for reducing the signs and symptoms, inducing major clinical response, inhibiting the progression of joint damage, and improving physical function in adult patients with moderately to severely active RA, as well as for reducing the signs and symptoms of active arthritis in patients with PsA. At this time, the manufacturer of adalimumab has submitted a supplemental Biologics License Application with the US Food and Drug Administration and a Type II Variation to the European Medicines Agency (EMEA) seeking an indication for the treatment of AS (AL 2005). The Committee for Medicinal Products for Human Use, the scientific committee of the EMEA, granted a positive opinion recommending approval of adalimumab for the treatment of severe active AS based on results from recent trials.
Adalimumab exerts its therapeutic effects by blocking the interaction of TNF with the p55 and p75 cell surface TNF receptors. It does not bind to other cytokines, such as lymphotoxin, and interleukins (IL), such as IL-1 (van de Putte et al 2003; Humira® PC 2005; Humira PI 2005). TNF is a potent osteogenic cytokine and the central mediator of inflammation and joint destruction underlying RA, PsA, and AS pathology. Produced in the setting of inflammation, TNF induces receptor activator of NF-κB ligand (RANKL) expression on the surface of stromal/osteoblast cells (Thomson et al 1987). Clinical studies have shown that in patients with RA, TNF blockade is able to attenuate cartilage and bone destruction (Lipsky et al 2000a; Klareskog et al 2004). This may be attributed in part to the downregulation of serum matrix metalloproteinase (MMP)-1 and MMP-3 following anti-TNF therapy, as well as downregulating the role of TNF in osteoclast maturation and activation (Brennan et al 1997; Catrina et al 2002, 2006).
Pharmacodynamics
In patients with active RA, adalimumab has the following pharmacodynamic effects:
Acute-phase reactants of inflammation (C-reactive protein, fibrinogen, and erythrocyte sedimentation rate) are rapidly decreased (Humira PI 2005).
Serum concentrations of IL-1, IL-6, IL-8, and granulocyte-macrophage colony-stimulating factor are reduced (Choy and Panayi 2001).
Systemic concentrations of IL-1β messenger RNA, IL-1 receptor antagonist, and IL-6 are decreased within 24 hours and remain below baseline concentrations through Day 14 (Barrera et al 2001).
Significant reductions from baseline in concentrations of MMP-1, MMP-3, pro-MMP-1, and pro-MMP-3 and other markers of cartilage and synovium turnover occur (eg, oligomeric matrix protein, intracellular adhesion molecule-1 [ICAM-1], human cartilage glycoprotein-39) (den Broeder et al 2002; Weinblatt et al 2003).
Concentrations of adhesion molecules responsible for leukocyte migration (endothelial-leukocyte adhesion molecule-1, vascular cell adhesion molecule-1, and ICAM-1) are reduced (den Broeder et al 2002; Humira PI 2005).
Importantly, normal immune function is preserved during adalimumab therapy (Kavanaugh et al 2002).
Pharmacokinetics
The key pharmacokinetic properties of adalimumab are summarized in Table 1. Linear pharmacokinetics are observed throughout the clinical dosage range, with the mean plasma concentration increasing proportionally with an increase in dosage. In healthy adults, the average bioavailability of adalimumab was 64% following a single 40 mg subcutaneous (sc) dose, and maximum plasma concentrations (4.7 μg/mL) were achieved within 5 days. In 5 patients with RA, adalimumab concentrations in the synovial fluid were 31% to 96% of those in sera. Concomitant MTX administration does not adversely affect adalimumab serum concentrations; adalimumab had an average steady-state serum concentration of 5mg/mL and 8–9 μg/mL without and with MTX, respectively. Pharmacokinetic data are not available in patients with hepatic or renal impairment (Humira PI 2005).
Table 1.
Pharmacokinetic properties of adalimumab (Humira PI 2005)
| Absolute bioavailability | 64% |
| Time to maximum concentrationa | 131 ± 56 h |
| Maximum serum concentrationa | 4.7 ± 1.6 μg/mL |
| Volume of distribution | 4.7–6.0 L |
| Steady-state trough concentration: | |
| Without concomitant MTX | ∼ 5 μg/mL |
| With concomitant MTX | ∼ 8–9 μg/mL |
| Systemic clearance | 12 mL/h |
| Terminal half-life | ∼ 2 weeks |
Note: Following a single 40 mg subcutaneous dose.
Abbreviations: MTX, methotrexate.
Therapeutic efficacy
Rheumatoid arthritis
Although there are no studies that directly compare etanercept, infliximab, or adalimumab, data from noncomparative trials suggest that all 3 agents have similar therapeutic efficacy in RA. Etanercept has demonstrated consistent responses in 5 clinical trials, 3 that studied etanercept monotherapy and 2 that studied combination etanercept/MTX therapy for periods of up to 26 weeks (Moreland et al 1997; 1999; Weinblatt et al 1999; Klareskog et al 2004). The efficacy of infliximab in combination with MTX in RA was established in 1 large clinical trial (the Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy [ATTRACT] study) (Maini et al 1999; Lipsky et al 2000a; 2000b). This review will focus on the efficacy and safety of adalimumab, which was assessed in 5 randomized, double-blind, placebo-controlled pivotal trials in patients aged 18 years or older with active RA diagnosed according to American College of Rheumatology (ACR) criteria (Table 2). Adalimumab was administered sc in combination with MTX (12.5–25mg), with other disease-modifying antirheumatic drugs (DMARDs), or as monotherapy (Humira PI 2005).
Table 2.
Summary of primary and selected secondary endpoints from pivotal and additional trials of adalimumab in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis (Furst et al 2003;Weinblatt et al 2003; Burmester et al 2004, 2005; Keystone et al 2004; van de Putte et al 2004; Breedveld et al 2005, 2006; Mease et al 2005, 2005a, 2005b; van der Heijde et al 2006)
| Trial Name | Treatmenta | N | Study Duration | Primary Endpoint(s) | Secondary Endpoints |
|---|---|---|---|---|---|
| Rheumatoid Arthritis | |||||
| ARMADA (Weinblatt et al 2003) | Adalimumab (20 mg, 40 mg, 80 mg) + MTX | 69, 67, 73 | 24 wk | ACR20: 48%, 67%, 66% vs 15% | ACR50: 32%, 55%, 43% vs 8% |
| ACR70: 10%, 27%, 19% vs 10% | |||||
| Placebo + MTX | 62 | HAQ: .0.54, .0.62, .0.59 vs .0.27 | |||
| DE019 (Keystone et al 2004) | Adalimumab (40 mg eow, 20 mg weekly) + MTX | 207, 212 | 52 wk | ΔTSS: 0.1, 0.8 vs 2.7 ACR20 at Week 24: 63%, 61% vs 30% | ACR20: 59%, 55% vs 24% ACR50: 42%, 38% vs 10% ACR70: 23%, 21% vs 5% |
| Placebo + MTX | 200 | ΔHAQ: −0.59, −0.61 vs −0.25 | |||
| DE011 (van de Putte et al 2004) | Adalimumab (20 mg eow, 20 mg weekly, 40 mg, 40 mg weekly) Placebo | 106, 112, 113, 103 110 | 26 wk | ACR20: 36%, 39%, 46%, 53% vs 19% | ACR50: 19%, 21%, 22%, 35% vs 8% ACR70: 9%, 10%, 12%, 18% vs 2% |
| Moderate EULAR response: 42%, 48%, 56%, 63% vs 26% Good EULAR response: 7%, 10%, 9%, 14% vs 4%ΔHAQ: −0.29, −0.39, −0.38, −0.49 vs−0.07 | |||||
| STAR (Furst et al 2003) | Adalimumab + DMARDs Placebo + DMARDs | 318 318 | 24 wk | AEs: no statistically significant differences between adalimumab and placebo group in serious AEs; severe or life-threatening AEs; AEs leading to withdrawal; rates of infections or serious infections | ACR20: 53% vs 35% ACR50: 29% vs 11% ACR70: 15% vs 4% |
| ReAct (Burmester et al 2004, 2005; Burmester pers comm. 2006) | Adalimumab | 6235 1251 | 12 wk | 12 Weeks 52 wk ACR20, 50, 70: 66%, 28%, 17% Moderate EULAR response: 81% Good EULAR response: 32%ΔDAS28: −2.1 DAS28 ≤2.6: 20% ΔHAQ: −0.52 | 52 Weeks ACR20, 50, 70: 67%, 45%, 24% Moderate EULAR response: 82% Good EULAR response: 38% DAS28: −2.3 DAS28 ≤2.6: 35% ΔHAQ: −0.57 |
| Early Rheumatoid Arthritis | |||||
| PREMIER (Breedveld et al 2005, 2006) | Adalimumab + MTX Adalimumab MTX | 268 274 257 | 2 y | ACR50 at 1 year: 62%, 41%, vs 46% ΔTSS at 1 year: 1.3, 3.0, vs 5.7 | At Year 2: ACR20: 69%, 49% vs 56% ACR50: 59%, 37% vs 43% ACR70: 47%, 28% vs 28% ACR90: 27%, 9% vs 13% ΔTSS: 1.9, 5.5 vs 10.4 DAS28 <2.6: 49%, 25% vs 25% ACR70 maintained ≥6 months: 49%, 25% vs 27% |
| Psoriatic Arthritis | |||||
| ADEPT (Mease et al 2005, 2005a, 2005b) | Adalimumab Placebo | 151 162 | 24 weeks | ACR20 at Week 12: 58% vs 14% ΔmTSS at Week 24: −0.2 vs 1.0 | At Week 24: ACR20: 57% vs 15% ACR50: 39% vs 6% ACR70: 23% vs 1% PASI 50: 75% vs 12% PASI 75: 59% vs 1% PASI 90: 42% vs 0% PGA clear or almost clear: 71% vs 12% ΔHAQ: −0.4 vs −0.1 |
| Ankylosing Spondylitis | |||||
| ATLAS (van der Heijde et al 2006) | Adalimumab Placebo | 208 107 | 12 weeks | ASAS20: 58% vs 21% | BASDAI 50: 45% vs 16% ASAS40: 41% vs 14% ASAS 5/6: 49% vs 13% Partial remission: 21% vs 4% |
Note:The dose of adalimumab is 40 mg eow unless specified otherwise.
Abbreviations: ACR, American College of Rheumatology; ADEPT, Adalimumab Effectiveness in Psoriatic Arthritis Trial; AE, adverse event; ARMADA, Anti-TNF Research Study Program of the Monoclonal Antibody Adalimumab in Rheumatoid Arthritis; ASAS, ASessment in Ankylosing Spondylitis International Working Group Improvement Criteria; ATLAS, Adalimumab Trial Evaluating Long-term Efficacy and Safety for Ankylosing Spondylitis; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; DAS28, Disease Activity Score 28; DMARDs, disease-modifying antirheumatic drugs; EULAR, European League Against Rheumatism; eow, every other week; HAQ, Health Assessment Questionnaire; mTSS, modified Total Sharp Score; MTX, methotrexate; PASI, Psoriasis Area and Severity Index; PGA, Physician's Global Assessment; ReAct, Research in Active Rheumatoid Arthritis Trial; STAR, Safety Trial of Adalimumab in Rheumatoid Arthritis;TSS, total Sharp score.
Adalimumab plus methotrexate
The ARMADA (Anti-TNF Research Study Program of the Monoclonal Antibody Adalimumab) trial evaluated 271 patients with active RA who had failed therapy with 1 to 4 DMARDs and had an inadequate response to MTX. Patients received MTX plus adalimumab 20 mg, 40 mg, or 80 mg or MTX plus placebo every other week (eow) for 24 weeks. Patients receiving adalimumab plus MTX experienced significant improvement in disease activity compared with those receiving placebo plus MTX (Table 2). Responses were rapid, with the greatest proportion of adalimumab-treated patients achieving an ACR20 response at the first scheduled visit (Week 1). Statistically greater improvements in the Health Assessment Questionnaire Disability Index (HAQ DI), in health-related quality-of-life measures (QOL) (eg, Short Form-36 Health Survey [SF-36] and Functional Assessment of Chronic Illness Therapy fatigue [FACIT-F] scores), and in concentrations of acute-phase reactants (eg, C-reactive protein) were observed for each adalimumab dose plus MTX group (Weinblatt et al 2003). Results of extension studies showed that clinical efficacy was sustained through 5 years with 76%, 64%, and 39% of patients achieving ACR20, 50, 70 responses, respectively; 52% achieving clinical remission; and 28% having no physical limitations (Weinblatt et al 2004, 2005). In addition, most patients were able to reduce corticosteroid and/or MTX dose without adversely affecting long-term efficacy (Weinblatt et al 2005).
DE019, a 52-week, randomized, double-blind study, investigated the ability of adalimumab to reduce disease activity and inhibit radiographic progression in 619 RA patients with active disease despite MTX therapy. Patients received adalimumab 40 mg eow, adalimumab 20 mg weekly, or placebo plus concomitant MTX. At Week 52, there was statistically significantly less radiographic progression, as measured by the change in total Sharp score (TSS), in patients receiving adalimumab 40 mg eow plus MTX versus the placebo group (mean change 0.1 vs 2.7, respectively); moreover, 72% of adalimumab plus MTX-treated patients had no radiographic progression from baseline. QOL and functional improvement, as measured by the SF-36 and HAQ DI, also were significantly improved in the combination group versus placebo (Keystone et al 2004). Results from an open-label extension (OLE) of this study showed that at 3 years 61% of patients had no radiographic disease progression. In addition, significant clinical responses were sustained with 58%, 42%, and 23% of patients maintaining their ACR20, 50, and 70 responses, respectively. Importantly, placebo-treated patients who had significant disease progression at 52 weeks during the randomized controlled trial experienced inhibition of radiographic progression and improved clinical responses when treated with adalimumab during the OLE (Keystone et al 2004, 2004b, 2004c).
The results of ARMADA and DE019 confirm other studies showing that TNF antagonists in combination with MTX are more effective in the relief of signs and symptoms of RA and in inhibiting radiographic progression than MTX monotherapy, even in patients with long-standing disease.
Adalimumab plus traditional DMARDs
To establish the safety and effectiveness of adalimumab in a broad RA population in real-life clinical practice, the open-label Research in Active RA (ReAct) trial was conducted. In this study, 6610 RA patients (the largest cohort of RA patients to be treated with a TNF antagonist) with active disease received adalimumab 40 mg eow in addition to their current standard DMARD for 12 weeks, providing valuable information on the drug’s performance in patients treated with various DMARDs and with previous biologic experience. At Week 12, 69% of patients achieved an ACR20 response and 83% achieved a moderate European League Against Rheumatism (EULAR) response (Table 2). These response rates were maintained over time in patients who continued adalimumab treatment for 52 weeks during an extension phase (Burmester et al 2005). Improvements in functional status as measured by the Disease Activity Score in 28 joints and the HAQ DI were also clinically significant and sustained over time. In patients who had received prior biologic therapy, the ACR20 response rate (60%) was only slightly lower than in patients who were naïve to biologic therapy (70%). Moderate (84%) and good (35%) EULAR response rates were higher in the patients who had not received prior treatment with a biologic response modifier compared with those patients who had received at least 1 biologic agent. Thus, even after treatment failure with a TNF antagonist, a significant clinical response can be achieved with a different agent (Burmester et al 2004, 2005).
The STAR (Safety Trial of Adalimumab in RA) trial assessed the safety and efficacy of adalimumab in a heterogeneous group of RA patients with persistent disease activity despite concomitant DMARD therapy. In this randomized, double-blind study, 636 RA patients receiving 0 to ≥3 DMARDs received placebo or adalimumab 40 mg eow for 24 weeks while continuing their baseline DMARDs. Patients treated with adalimumab achieved statistically superior ACR20, 50, and 70 response rates at Week 24 compared with placebo-treated patients (Table 2). There were no statistically significant differences between the adalimumab and placebo groups in their respective rates of adverse events (AEs), serious AEs, severe or life-threatening AEs, or AEs leading to withdrawal. In addition, rates of infections or serious infections did not vary among the groups, demonstrating the safety of adalimumab in patients receiving standard DMARDs (Furst et al 2003).
Monotherapy
Adalimumab monotherapy may be appropriate for patients who have not achieved adequate disease control with MTX because of poor efficacy or tolerability. DE011, a 26-week, double-blind, placebo-controlled trial, evaluated the efficacy and safety of adalimumab monotherapy in 544 RA patients with severely active disease for whom previous DMARD treatment had failed (3.7 DMARDs on average). Patients were randomized to placebo or monotherapy with 20 mg or 40 mg of adalimumab eow or weekly. Significantly better response rates were observed in patients treated with adalimumab 20 mg or 40 mg eow or weekly than in those treated with placebo after 26 weeks (Table 2). Moderate EULAR response rates also were significantly greater with adalimumab than with placebo. In addition, the 40 mg weekly dose resulted in a slightly higher ACR response than 40 mg eow. Adalimumab monotherapy achieved significant, rapid, and sustained improvements in disease activity and improved physical function in patients with severe RA refractory to DMARD treatment (van de Putte et al 2004).
Early rheumatoid arthritis
Recent studies suggest that early, aggressive combination treatment of RA achieves superior long-term outcomes (O’Dell 2002; Keystone, Furst, et al 2003; Keystone, Kavanaugh, et al 2003; Keystone, Haraoui, et al 2003; Weinblatt et al 2003; Keystone 2005). The PREMIER study was a 2-year, randomized, double-blind, active comparator-controlled trial designed to compare the efficacy and safety of adalimumab plus MTX versus adalimumab monotherapy or MTX monotherapy in 799 MTX-naïve RA patients with early (<3 years), aggressive disease (Breedveld et al 2006). It was the first head-to-head study of a TNF antagonist plus MTX vs the TNF antagonist alone and MTX alone. Patients received adalimumab 40mg eow plus MTX, adalimumab 40mg eow monotherapy, or MTX monotherapy. For all endpoints, combination therapy was superior to both MTX and adalimumab monotherapy (Table 2). At Year 2, the percentage of patients who exhibited an ACR50 response was 59% with combination therapy, 43% with MTX alone, and 37% with adalimumab monotherapy (Figure 2a). Combination therapy also yielded superior ACR20, 70, and 90 response rates at 1 and 2 years. The differences in radiographic progression for patients treated with adalimumab versus MTX alone at Year 1 were even more marked at the end of Year 2, with an accumulated TSS change of 10.4 in the MTX monotherapy arm versus 5.5 in the adalimumab monotherapy arm and 1.9 in the combination arm. Although ACR responses were comparable between the 2 monotherapy arms, there was statistically less radiographic progression with adalimumab monotherapy than with MTX monotherapy at Year 1 (3.0 vs 5.7) and Year 2 (5.5 vs 10.4) (Figure 2b). After 2 years of treatment, twice as many patients receiving combination therapy than receiving adalimumab or MTX monotherapy achieved a major clinical response and had no radiographic progression. These findings emphasize that clinical assessment of signs and symptoms of RA may not fully reflect therapeutic benefit (van der Heijde, Landewe, et al 2005). A radiographic subanalysis conducted to examine whether the frequency or severity of radiographic progression at 6 months or 2 years varies with the level of ACR response demonstrated the following (Genovese et al 2005):
Adalimumab plus MTX led to less frequent and less severe disease progression than MTX at all levels of clinical response.
ACR score was a poor predictor of radiographic efficacy; therefore, radiographic monitoring may be warranted in patients regardless of their clinical response.
Early TNF-antagonist therapy may be needed to prevent joint damage in patients with early RA, including some with a good clinical response to MTX monotherapy.
Figure 2.
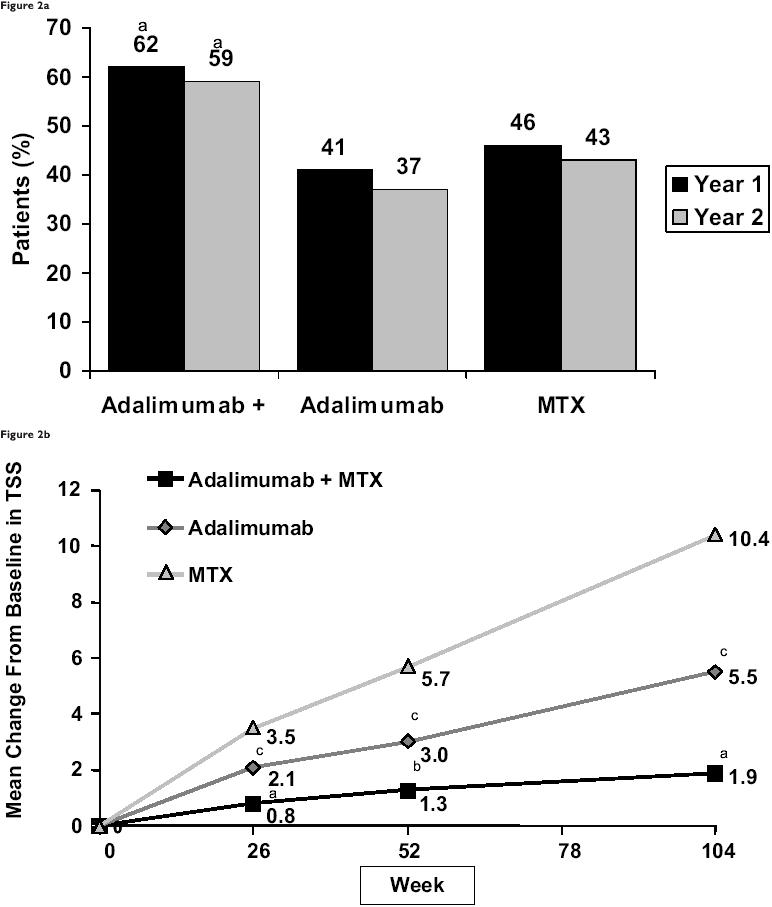
Results from the PREMIER study. (a) American College of Rheumatology 50 response at Years 1 and 2. aP<0.001 for adalimumab plus methotrexate (MTX) vs MTX alone and adalimumab plus MTX vs adalimumab alone. (b) Mean change from baseline in total Sharp scores (TSS) over time. aP<0.001 for adalimumab plus MTX vs MTX alone and adalimumab alone. bP<0.001 for adalimumab plus MTX vs MTX alone and P=0.002 for adalimumab plus MTX vs adalimumab alone. cP<0.001 for adalimumab vs MTX alone. Copyright © 2006. Reproduced with permission from Breedveld FC, Weisman MH, Kavanaugh AF, et al; for the PREMIER investigators. 2006. The PREMIER Study: combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in methotrexate-naïve patients with early, aggressive rheumatoid arthritis. Arthritis Rheum, 54:26-37.
For MTX-naïve patients with rapidly progressive, early RA, adalimumab plus MTX showed greater radiographic efficacy than MTX monotherapy across the entire spectrum of radiographic disease progression parameters; moreover, combination therapy prevented nearly all severe radiographic progression observed with MTX alone, as early as 6 months (van der Heijde, Landewe, et al 2005).
Psoriatic arthritis
Psoriatic arthritis is a chronic, inflammatory arthritis commonly associated with psoriasis. Overexpression of TNF is believed to play a key role in the pathogenic mechanisms linking psoriasis and arthritis (Mease 2005). As with clinical studies in RA, trials in PsA have shown excellent clinical results with the TNF antagonists, etanercept (Mease et al 2000, 2003), infliximab (Mease et al 2004; Antoni et al 2003; Antoni, Kavanaugh, et al 2005; Antoni, Krueger, et al 2005), and adalimumab (Mease et al 2005a, 2005b; Mease, Sharp, et al 2005), in a variety of domains, including the joints, QOL, function, and radiographic progression. These agents have also demonstrated benefit in domains more unique to PsA, such as the skin lesions of psoriasis, enthesitis, and dactylitis, highlighting the similar pathogenesis of the disease in the skin, the tendons, and the synovial membrane (Mease and Antoni 2005).
The Adalimumab Effectiveness in Psoriatic Arthritis Trial (ADEPT) evaluated the safety and efficacy of adalimumab as monotherapy or in combination with MTX in patients with moderately to severely active PsA who had failed nonsteroidal anti-inflammatory drug (NSAID) therapy. Patients (n=313) were stratified according to MTX use (yes or no) and extent of psoriasis involvement (≥3% or <3% of body surface) at baseline and were subsequently randomized to receive adalimumab 40 mg or placebo eow for 24 weeks. Significantly higher ACR and Psoriasis Area and Severity Index (PASI) response rates were observed in patients treated with adalimumab than in those treated with placebo at all time points (Table 2). At Week 24, the physician’s global assessment of psoriasis among patients with ≥3% body surface area affected resulted in ratings of the lesions as clear or almost clear in 71% of the 70 adalimumab-treated patients compared with only 12% of the 70 placebo-treated patients. Significant inhibition of structural changes on radiographs also was observed with adalimumab treatment. The mean change in the modified TSS in patients who had both baseline and Week 24 radiographs was −0.2 for patients receiving adalimumab versus 1.0 for patients receiving placebo. Adalimumab treatment also significantly improved disability (HAQ), QOL (SF-36), and fatigue (FACIT) measures in patients with moderately to severely active PsA (Mease et al 2005a). Notably, observed improvement in the skin symptoms of psoriatic disease was more dramatic with adalimumab than with etanercept or infliximab (Mease et al 2000, 2004), although the efficacy of these agents in PsA has not been studied in a head-to-head trial.
In an ongoing OLE of this trial, all continuing patients received adalimumab 40 mg eow. For patients initially treated with adalimumab, ACR and PASI response rates were maintained over 48 weeks of treatment (ACR20/50/70; 61%/46%/31% [Figure 3a]) (PASI 50/75/90; 70%/58%/46% [Figure 3b]). For patients previously receiving placebo and newly receiving adalimumab, ACR20, 50, and 70 response rates were 54%, 37%, and 21%, respectively (Figure 3a). Of 152 placebo- and 144 adalimumab-treated patients who had radiographs at baseline and 24 weeks, 141 and 133, respectively, had radiographs taken at Week 48. Mean change in modified TSS at Week 24 was maintained to Week 48 in adalimumab-treated patients (Figure 3c) (Mease et al 2005b; Mease, Sharp, et al 2005). A recent ADEPT subanalysis assessing the correlation of skin and joint responses showed that 84% of ACR50 responders and 94% of ACR70 responders also achieved a PASI 75 response (Ritchlin et al 2006).
Figure 3.
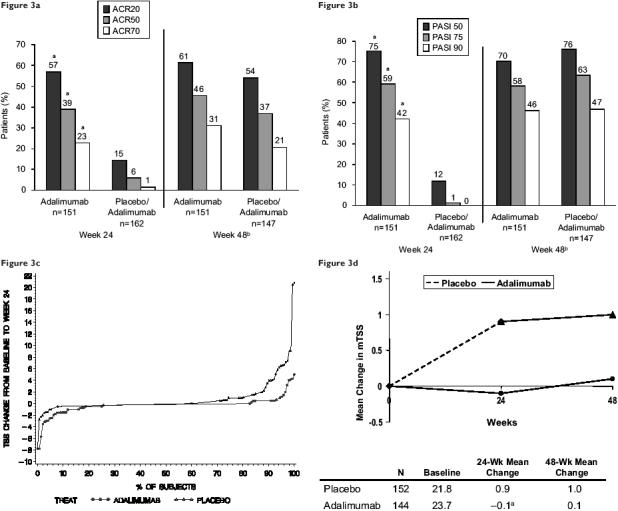
Results from ADEPT (Adalimumab Effectiveness in Psoriatic Arthritis Trial). (a) Percentage of patients with psoriatic arthritis who achieved a 20%, 50%, and 70% improvement in American College of Rheumatology (ACR) response criteria at Week 24 and Week 48. aP<0.001 placebo vs adalimumab at Week 24, adjusted for baseline methotrexate use and extent of psoriasis at baseline. bPlacebo patients received adalimumab after Week 24. Patients who prematurely discontinued prior to receiving adalimumab are not included in this analysis. (b) Percentages of patients with psoriatic arthritis and ≥3% body surface area psoriasis involvement at baseline who achieved a 50%, 75%, and 90% improvement in Psoriasis Area and Severity Index (PASI) response criteria at Week 24 and Week 48. aP<0.001 placebo vs adalimumab at Week 24, adjusted for baseline methotrexate use and extent of psoriasis at baseline. bPlacebo patients received adalimumab after Week 24. Patients who prematurely discontinued prior to receiving adalimumab are not included in this analysis. (c) Changes in modified total Sharp score (mTSS) at Weeks 24 and 48. aP<0.001 placebo vs adalimumab using a ranked analysis of covariance (Mease at al 2005, 2005a, 2005b).
Ankylosing spondylitis
Ankylosing spondylitis is a chronic inflammatory disease primarily affecting the sacroiliac joints and spine. The rationale for treating AS with a TNF antagonist is based on the observation that large amounts of TNF are expressed at the site of inflammation in patients with this disease (Braun et al 1995). Recent studies have demonstrated significant efficacy of TNF antagonist therapy for the treatment of AS (Gorman et al 2002; Brandt et al 2003; Braun et al 2002, 2003; van der Heijde, Dijkmans, et al 2005), and adalimumab received expanded approval for the treatment of severe, active AS from the European Commission in June 2006.
Adalimumab was evaluated in a 2:1, randomized, double-blind, multicenter study, ATLAS (Adalimumab Trial Evaluating Long-term Efficacy and Safety in Ankylosing Spondylitis), to assess its efficacy and safety in patients with AS (Table 2). Assessment in AS (ASAS) International Working Group criteria were significantly improved after 2 weeks of adalimumab therapy; 58% of adalimumab-treated patients achieved an ASAS20 response compared with 21% of placebo-treated patients (Figure 4a). In addition, significantly more adalimumab-treated patients had at least a 50% improvement in the Bath AS Disease Activity Index (BASDAI) score at Week 12 compared with the placebo group (45% vs 16%, respectively). Patients treated with adalimumab also achieved significantly higher ASAS5/6, ASAS40, and partial remission responses at Weeks 12 and 24 compared with placebo-treated patients (Figure 4b). Adalimumab significantly improved BASFI (Bath Ankylosing Spondylitis Functional Index) scores; at Week 12, adalimumab-treated patients had a statistically significantly greater improvement in BASFI scores (−35.8) compared with placebo-treated patients (−8.0) and a sustained response at Week 24 (−37.7 vs −8.5). Adalimumab-treated patients also experienced a significant reduction in enthesis pain, as indicated by both the Maastricht Ankylosing Spondylitis Enthesitis Score (MASES) and the enthesis question on the BASDAI. At Week 12, adalimumab-treated patients had a statistically significantly greater improvement in MASES (−2.68) compared with placebo-treated patients (−1.28) and a sustained response at Week 24 (−3.21 vs −1.55). Uniquely in this study, patients with radiographic evidence of total spinal ankylosis were allowed to enroll. Of these patients, 50% (3/6) treated with adalimumab achieved an ASAS20 response at Week 12 compared with 0% (0/5) of placebo patients. At Week 24, 66.7% (4/6) of the adalimumab patients had an ASAS20 response compared with 0% (0/5) of the placebo patients (van der Heijde et al 2006).
Figure 4.
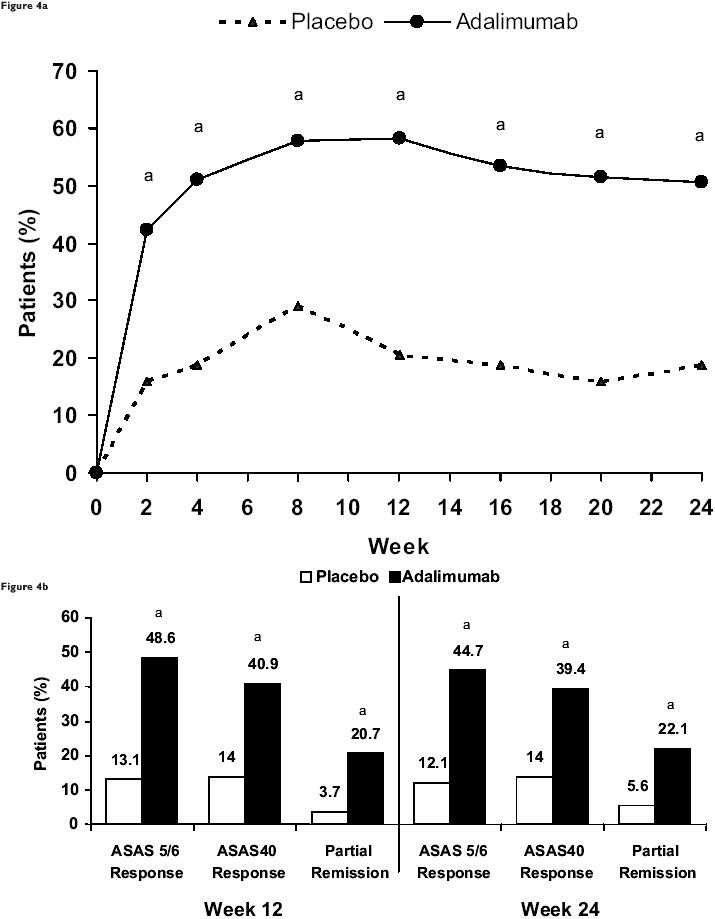
Results from ATLAS (Adalimumab Trial Evaluating Long-Term Efficacy and Safety in Ankylosing Spondylitis). (a) Proportion of patients who achieved a 20% improvement response over time according to the criteria of the ASssessment in Ankylosing Spondylitis (ASAS) Working Group. aP<0.001 vs placebo based on an intention-to-treat analysis using nonresponder imputation. (b) ASAS 5/6 criteria response, ASAS40 response, and partial remission. aP<0.001 vs placebo based on an intention-to-treat analysis using nonresponder imputation. Copyright (c) 2006. Reproduced with permission from van der Heijde D, Kivitz A, Schiff MH, et al; the Adalimumab Trial Evaluating Long-term Efficacy and Safety in Ankylosing Spondylitis Study Group. 2006. Efficacy and safety of adalimumab in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ATLAS). Arthritis Rheum, 54:2136-46.
Safety
Adalimumab has been prescribed to more than 110 000 patients worldwide (Schiff et al 2006). The safety profile has been extensively studied in randomized and long-term OLE clinical trials. Schiff and colleagues (2006) evaluated two sources of data to describe the incidences of serious adverse events (SAEs) of interest with adalimumab in RA patients: as of April 15, 2005, the RA clinical trial database included 10 050 patients, representing 12 506 patient-years (PY) of adalimumab exposure and as of June 30, 2005, an estimated 78 522 PY of adalimumab exposure were included from the US postmarketing period. Based on these 2 sources of data, adalimumab has been shown to be generally safe and well-tolerated in patients with RA. The rates of adverse events were low and stable over time (Schiff et al 2006).
Table 3 lists AEs reported by ≥5% of adalimumab-treated patients during placebo-controlled periods of RA studies (Humira PI 2005) (Table 3). Injection site reactions are the most common AE associated with adalimumab treatment across RA trials (20.3% for adalimumab vs 13.8% for placebo). Most of these reactions were mild to moderate and did not recur (Wells et al 2003). The adverse event profile and overall safety of adalimumab in PsA and AS patients are similar to that reported in patients with RA (Weinblatt et al 2003; Keystone et al 2004; van de Putte et al 2004; Mease et al 2005a, 2005b; van der Heijde et al 2006).
Table 3.
Adverse events reported by ≥5% of patients treated with adalimumab during placebo-controlled periods of rheumatoid arthritis studies (Humira PI 2005)
| Adverse event | Adalimumab 40 mg eow (n=705) (%) | Placebo (n=690) (%) |
|---|---|---|
| Respiratory | ||
| Upper respiratory infection | 17 | 13 |
| Sinusitis | 11 | 9 |
| Flu syndrome | 7 | 6 |
| Gastrointestinal | ||
| Nausea | 9 | 8 |
| Abdominal pain | 7 | 4 |
| Laboratory testsa | ||
| Laboratory test abnormal | 8 | 7 |
| Hypercholesterolemia | 6 | 4 |
| Hyperlipidemia | 7 | 5 |
| Hematuria | 5 | 4 |
| Alkaline phosphatase increased | 5 | 3 |
| Other | ||
| Injection site pain | 12 | 12 |
| Headache | 12 | 8 |
| Rash | 12 | 6 |
| Accidental injury | 10 | 8 |
| Injection site reactionb | 8 | 1 |
| Back pain | 6 | 4 |
| Urinary tract infection | 8 | 5 |
| Hypertension | 5 | 3 |
Note: Laboratory test abnormalities were reported as adverse events in European trials;
Does not include erythema and/or itching, hemorrhage, pain, or swelling.
Abbreviations: eow, every other week.
Since the introduction of TNF antagonists, which affect host defenses by modulating cellular immune responses, there has been concern about an increased risk of serious infections among TNF-antagonist–treated patients. Recent safety analyses of adalimumab in global clinical trials and US postmarketing surveillance of RA patients indicated that rates of serious infections were similar to those reported with DMARDs and other TNF antagonists across RA trials and did not increase over time (Schiff et al 2003, 2006). Across 4 pivotal trials for RA (ARMADA, DE011, DE019, and STAR) infection rates were similar to placebo, at approximately 1.0/PY (Humira PI 2005). The most frequently reported infections were upper respiratory tract infections, rhinitis, bronchitis, and urinary tract infections (Lee and Kavanaugh 2005).
There is an increased risk of tuberculosis (TB) in the context of anti-TNF therapy, which has led to the recommendation for screening for TB prior to initiation of therapy. From December 31, 2002, to June 30, 2005, postmarketing surveillance has noted 17 cases of TB among the 78 522 PY of adalimumab exposure (0.2/100 PY) (Schiff et al 2006). These data are consistent with those for other TNF antagonists. In the adalimumab RA clinical trials, prior to the initiation of routine TB screening, there were 7 cases of TB in Europe (1.3/100 PY). The introduction of routine TB screening in clinical trials has decreased the rate of TB by 75% (0.33/100 PY) (Schiff et al 2006).
Opportunistic infections have been reported with the use of all 3 TNF antagonists (Keystone 2005). These infections include histoplasmosis, listeriosis, pulmonary aspergillosis, and Pneumocystis carinii pneumonia. In patients receiving adalimumab, opportunistic infections are infrequent and involve a variety of organisms (Schiff et al 2006).
Other safety issues include autoimmune disease, demyelination disorders, and malignancies, particularly lymphoma (Lee and Kavanaugh 2005). Autoantibodies to antinuclear antigen and double-stranded DNA develop in approximately 3% to 12% of adalimumab-treated patients. The clinical implications of these antibodies remain to be defined, as progression to lupus-like illness appears to be uncommon. After 12 506 PY of adalimumab exposure, only 13 cases of systemic lupus erythematosus and lupuslike syndromes have been reported in the RA clinical trials (Schiff et al 2006).
Rare cases of neurologic AEs have been reported in association with adalimumab and other TNF antagonists. Ten cases of demyelinating diseases were observed among RA patients after 12 506 PY of exposure (0.08/100 PY); 6 were multiple sclerosis (MS) cases (Schiff et al 2006). Patients with MS have a statistically significantly higher coexistence of RA and psoriasis than matched controls, suggesting that patients with these conditions may innately be at increased risk of MS as compared with the general population (Heinzlef et al 2000; Magnano et al 2004). The true impact of TNF antagonists on the development of this disorder is unknown (Magnano et al 2004; Lee and Kavanaugh 2005).
There is some speculation that lymphomas may be associated with the use of TNF antagonists (Geborek et al 2005). Among adalimumab recipients, 15 cases of lymphoma were observed (0.12/100 PY) after 12 506 PY of exposure in RA clinical trials (Schiff et al 2006). These incidence rates do not appear, at this time, to exceed those reported in the RA population prior to the availability of TNF inhibitors. Reports in the literature suggest that RA patients are at increased risk for lymphoma compared with the general population, which may be further increased in patients with highly active disease (Isomaki et al 1978). Most patients in adalimumab clinical trials had moderately to severely active RA at trial entry, increasing their risk for lymphoma. The standardized incidence ratio of 3.19 for lymphoma in adalimumab-treated patients is consistent with that expected in RA patients naïve to TNF antagonists. Rates of lymphoma and of the previously mentioned AEs have remained stable over time (Table 4) (Schiff et al 2006). For PsA and AS trials, rates of lymphoma were 0.41 and 0.24/100 PY, respectively (Burmester et al 2006). Most analyses to date have found no association between anti-TNF therapy and non-lymphomatous cancers. However, results from a recent meta-analysis of 9 randomized clinical trials of infliximab and adalimumab suggest that treatment with TNF inhibitors may be associated with an increased risk for serious infections and malignancies (Bongartz et al 2006). In contrast, a large population-based study using data from the Swedish nationwide cancer and census registers did not find an increased risk of solid malignancies in RA patients treated with TNF antagonists (Askling et al 2005). Further evaluations are needed to assess the true risks for these important adverse events with anti-TNF therapy.
Table 4.
Rates for serious adverse events of interest reported in the Clinical Trial Safety Database (Schiff et al 2006)
| All RA trials as of 08-31-02 (E/100 PY)a | All RA trials as of 04-15-05 (E/100 PY)b | |
|---|---|---|
| Tuberculosis | 0.27 | 0.27 |
| Histoplasmosis | 0.06 | 0.03 |
| Demyelinating diseases | 0.08 | 0.08 |
| Lymphoma | 0.21 | 0.12 |
| SLE/lupuslike syndrome | 0.08 | 0.10 |
| Congestive heart failure | 0.29 | 0.28 |
Note: n=2468, 4870 PY;
n=10,050, 12,506 PY.
Abbreviations: E/100 PY, events per 100 PY; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus.
Patient-focused perspectives
Several studies have examined the effect of adalimumab therapy on health-related quality of life (HRQOL) and functional status. In both concomitant therapy and monotherapy trials, adalimumab was associated with significantly greater improvements from baseline in HRQOL measures than placebo (Weinblatt et al 2003, 2005; Keystone et al 2004; van de Putte et al 2004; Breedveld et al 2005, 2006; Mease et al 2005; Burmester pers comm. 2006). DE019, ARMADA, and STAR trials demonstrated significantly greater improvements in FACIT-F scores (Strand, Weisman, et al 2003); SF-36 domain scores for Physical Functioning, Bodily Pain, and Vitality; and SF-36 Physical and Mental Component Summary (PCS, MCS) Scores (with adalimumab 40 mg eow than with placebo (in combination with MTX or standard antirheumatic therapy) after 24 weeks (Strand, Chartash, et al 2003). Quality-adjusted life-years gained per year of treatment were 0.10, 0.15 and 0.07, respectively, with adalimumab 40 mg eow (in combination with MTX or standard antirheumatic therapy) (Strand, Weisman, et al 2003). In the ADEPT trial, disability (as measured by the HAQ DI) improved significantly among PsA patients receiving adalimumab compared with those receiving placebo through Week 24 (−0.4 ± 0.5 in the adalimumab group vs −0.1 ± 0.5 in the placebo group at Week 12). Statistically significant improvements in SF-36 PCS scores, FACIT-F scores, and Dermatologic Life-Quality Index (DLQI) scores were also observed at Week 24 in patients receiving adalimumab (Mease et al 2005a).
Conclusions
Tumor necrosis factor has been implicated in a number of arthritic disease states, including RA (Saxne et al 1988), PsA (Partsch et al 1997; Ritchlin et al 1998), and AS (Braun et al 1995). Inhibition of TNF activity by adalimumab has been shown to significantly improve signs and symptoms, function, and QOL; induce remission; and reduce objectively measured damage in patients with these conditions (Weinblatt et al 2003; Keystone et al 2004a, 2004b; 2004c; van de Putte et al 2004; Mease et al 2005a, 2005b; van der Heijde et al 2006). Adalimumab has a rapid onset of action and sustained efficacy with long-term treatment and is well-tolerated, with few patients discontinuing treatment because of adverse events (Humira PI 2005).
Place of adalimumab in therapy
Adalimumab in combination with MTX or standard antirheumatic therapies, or as monotherapy, is effective in the treatment of adults with active RA who have had an inadequate response to DMARDs. Adalimumab is also effective in the treatment of patients with moderately to severely active PsA, improving both joint and skin manifestations of the disease as well as disability due to joint damage (Mease et al 2005a). In the ATLAS trial, adalimumab significantly reduced the signs and symptoms of active AS and established a sustained clinical response in patients who had an inadequate response or intolerance to NSAID therapy (van der Heijde et al 2006). ACR treatment guidelines recommend the use of biologic agents in patients who have a contraindication to MTX therapy or who have not achieved adequate disease control with MTX because of poor efficacy or tolerability. Unlike traditional DMARDs, patients receiving adalimumab do not require routine laboratory monitoring for adverse effects. As with the other TNF antagonists, patients must be screened for infections prior to commencing therapy (TB, in particular) and monitored for the presence of infection during therapy.
References
- [AB] Abbott Laboratories Press Releases. Abbott announces US and EU regulatory submissions seeking approval of Humira® (adalimumab in ankylosing spondylitis (AS) [online] 2005 Oct 4; Accessed 3 April 2006. URL: http://www.abbott.com/news/releaseonly.cfm?id=997.
- Abbott Laboratories Press Releases. Abbott’s HUMIRAÒ (adalimumab) receives positive opinion from the European Medicines Agency for the treatment of Ankylosing Spondylitis [online] 2006 Apr 28; Accessed 24 May 2006. URL: http://www.abbott.com/news_media_center.cfm.
- Anderson PJ. Tumor necrosis factor inhibitors: clinical implications of their different immunogenicity profiles. Semin Arthritis Rheum. 2005;34(5 Suppl 1):19–22. doi: 10.1016/j.semarthrit.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Antoni C, Kavanaugh A, Kirkham B, et al. The one year results of the Infliximab Multinational Psoriatic Arthritis Controlled Trial (IMPACT): substantial efficacy on synovitis and psoriatic lesions with or without concomitant DMARD therapy. Arthritis Rheum. 2003;48:S265. [Google Scholar]
- Antoni C, Kavanaugh A, Kirkham B, et al. Sustained benefits of infliximab therapy for dermatological and articular manifestations of psoriatic arthritis: results from the infliximab multinational psoriatic arthritis controlled trial (IMPACT) Arthritis Rheum. 2005;52:1227–36. doi: 10.1002/art.20967. [DOI] [PubMed] [Google Scholar]
- Antoni C, Krueger GG, de Vlam K, et al. Infliximab improves signs and symptoms of psoriatic arthritis: results of the IMPACT 2 trial. Ann Rheum Dis. 2005;64:1150–7. doi: 10.1136/ard.2004.032268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askling J, Fored CM, Brandt L, et al. Risks of solid cancers in patients with rheumatoid arthritis and after treatment with tumour necrosis factor antagonists. Ann Rheum Dis. 2005;64:1421–6. doi: 10.1136/ard.2004.033993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera P, Joosten LA, den Broeder AA, et al. Effects of treatment with a fully human anti-tumour necrosis factor monoclonal antibody on the local and systemic homeostasis of interleukin 1 and TNF in patients with rheumatoid arthritis. Ann Rheum Dis. 2001;60:660–9. doi: 10.1136/ard.60.7.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongartz T, Sutton AJ, Sweeting MJ, et al. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies. Systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275–85. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- Brandt J, Khariouzov A, Listing J, et al. Six-month results of a double-blind, placebo-controlled trial of etanercept treatment in patients with active ankylosing spondylitis. Arthritis Rheum. 2003;48:1667–75. doi: 10.1002/art.11017. [DOI] [PubMed] [Google Scholar]
- Braun J, Bollow M, Neure L, et al. Use of immunohistologic and in situ hybridization techniques in the examination of sacroiliac joint biopsy specimens from patients with ankylosing spondylitis. Arthritis Rheum. 1995;38:499–505. doi: 10.1002/art.1780380407. [DOI] [PubMed] [Google Scholar]
- Braun J, Brandt J, Listing J, et al. Treatment of active ankylosing spondylitis with infliximab: a randomised controlled multicentre trial. Lancet. 2002;359:1187–93. doi: 10.1016/s0140-6736(02)08215-6. [DOI] [PubMed] [Google Scholar]
- Braun J, Brandt J, Listing J, et al. Long-term efficacy and safety of infliximab in the treatment of ankylosing spondylitis. Arthritis Rheum. 2003;48:2224–33. doi: 10.1002/art.11104. [DOI] [PubMed] [Google Scholar]
- Breedveld FC, Weisman MH, Kavanaugh AF, et al. The efficacy and safety of adalimumab (Humira®) plus methotrexate vs. adalimumab alone or methotrexate alone in the early treatment of rheumatoid arthritis: 1- and 2-year results of the PREMIER study [abstract] Ann Rheum Dis. 2005;64:60. [Google Scholar]
- Breedveld FC, Weisman MH, Kavanaugh AF, et al. for the PREMIER investigators The PREMIER Study: combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in methotrexate-naïve patients with early, aggressive rheumatoid arthritis. Arthritis Rheum. 2006;54:26–37. doi: 10.1002/art.21519. [DOI] [PubMed] [Google Scholar]
- Brennan FM, Browne KA, Green PA, et al. Reduction of serum matrix metalloproteinase 1 and matrix metalloproteinase 3 in rheumatoid arthritis patients following anti-tumour necrosis factor-a (cA2) therapy. Br J Rheumatol. 1997;36:643–50. doi: 10.1093/rheumatology/36.6.643. [DOI] [PubMed] [Google Scholar]
- Burmester GR, Saez IM, Malaise M, et al. Efficacy and safety of adalimumab in European clinical practice: the ReAct Trial [abstract] Ann Rheum Dis. 2004;63(Suppl 1):90. [Google Scholar]
- Burmester GR, Monteagudo Sáez I, Malaise MG, et al. Adalimumab (Humira®) is effective and safe in treating rheumatoid arthritis (RA) in real-life clinical practice: 1-year-results of the ReAct study [abstract] Arthritis Rheum. 2005;52(Suppl):S541–2. [Google Scholar]
- Burmester G, Mease PJ, Dijkmans BAC, et al. Adalimumab is safe in global clinical trials in multiple indications and reduced mortality in rheumatoid arthritis [abstract] 2006. Presented at EULAR 2006. [Citation can be updated with published reference in mid-to late-June.]
- Catrina AI, Lampa J, Ernestam, et al. Anti-tumour necrosis factor (TNF)-a therapy (etanercept) down-regulates serum matrix metalloproteinase (MMP)-3 and MMP-1 in rheumatoid arthritis. Rheumatology (Oxford) 2002;41:484–9. doi: 10.1093/rheumatology/41.5.484. [DOI] [PubMed] [Google Scholar]
- Catrina AI, af Klint E, Ernestam S, et al. Anti-tumor necrosis factor therapy increases synovial osteoprotegerin expression in rheumatoid arthritis. Arthritis Rheum. 2006;54:76–81. doi: 10.1002/art.21528. [DOI] [PubMed] [Google Scholar]
- Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001;344:328–46. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- den Broeder AA, Joosten LAB, Saxne T, et al. Long term anti-tumour necrosis factor a monotherapy in rheumatoid arthritis: effect on radiological course and prognostic value of markers of cartilage turnover and endothelial activation. Ann Rheum Dis. 2002;61:311–18. doi: 10.1136/ard.61.4.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furst DE, Schiff MH, Fleischmann RM, et al. Efficacy and safety of the fully human anti-tumour necrosis factor-monoclonal antibody, and concomitant standard antirheumatic therapy for the treatment of rheumatoid arthritis: results of STAR (Safety Trial of Adalimumab in Rheumatoid Arthritis) J Rheumatol. 2003;30:2563–71. [PubMed] [Google Scholar]
- Geborek P, Bladstrom A, Turesson C, et al. TNF blockers do not increase overall tumour risk in patients with rheumatoid arthritis, but may be associated with increased risk of lymphomas. Ann Rheum Dis. 2005;64:699–703. doi: 10.1136/ard.2004.030528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese MC, Kavanaugh AF, Cohen SB, et al. The relationship of radiographic progression to clinical response in patients with early RA treated with adalimumab (HUMIRAÒ) plus MTX or MTX alone [presentation 1178] Ann Rheum Dis. 2005;64(Suppl 1):295. [Google Scholar]
- Gorman JD, Sack KE, Davis JC. Treatment of ankylosing spondylitis by inhibition of tumor necrosis factor α. N Engl J Med. 2002;346:1349–56. doi: 10.1056/NEJMoa012664. [DOI] [PubMed] [Google Scholar]
- Haraoui B. Differentiating the efficacy of the tumor necrosis factor inhibitors. Semin Arthritis Rheum. 2005;34(Suppl 1):7–11. doi: 10.1016/j.semarthrit.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Heinzlef O, Alamowitch S, Sazdovitch V, et al. Autoimmune diseases in families of French patients with multiple sclerosis. Acta Neurol Scand. 2000;101:36–40. doi: 10.1034/j.1600-0404.2000.101001036.x. [DOI] [PubMed] [Google Scholar]
- [HUMIRA PI] HUMIRA® (adalimumab) Prescribing Information. North Chicago, IL, 60064 USA: Abbott Laboratories; 2005. October 2005. [Google Scholar]
- [HUMIRA PC] HUMIRA® (adalimumab) Summary of Product Characteristics. North Chicago, IL, 60064 USA: Abbott Laboratories; 2005. [Google Scholar]
- Isomaki HA, Hakulinen T, Joutsenlahti U. Excess risk of lymphomas, leukemia and myeloma in patients with rheumatoid arthritis. J Chronic Dis. 1978;31:691–6. doi: 10.1016/0021-9681(78)90071-1. [DOI] [PubMed] [Google Scholar]
- Kavanaugh A, Grenwald M, Zizic T, et al. Treatment with adalimumab (D2E7) does not affect normal immune responsiveness [abstract] Arthritis Rheum. 2002;45(Suppl 9):S132. [Google Scholar]
- Keystone EC. Safety of biologic therapies—an update. J Rheumatol. 2005;32(Suppl):8–12. [PubMed] [Google Scholar]
- Keystone E, Furst DE, Kavanaugh AF, et al. Subgroup analysis of radiographic progression in RA patients treated with adalimumab [abstract] Ann Rheum Dis. 2003;62(Suppl 1):169. [Google Scholar]
- Keystone E, Kavanaugh AF, Fischkoff S. Response to adalimumab in patients with early versus late rheumatoid arthritis (RA) [abstract] Ann Rheum Dis. 2003;62(Suppl 1):170. [Google Scholar]
- Keystone EC, Kavanaugh AF, Perez JL, Spencer-Green GT. Adalimumab (Humira) plus methotrexate provides sustained improvements in physical function over 2 years in treatment of patients with rheumatoid arthritis [abstract] Ann Rheum Dis. 2004;64(Suppl 1):278. [Google Scholar]
- Keystone EC, Kavanaugh AF, Sharp JT, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy. Arthritis Rheum. 2004a;50:1400–11. doi: 10.1002/art.20217. [DOI] [PubMed] [Google Scholar]
- Keystone EC, Kavanaugh AF, Sharp JT, et al. Radiographic inhibition of structural damage sustained in patients with long-standing rheumatoid arthritis following 2 years of treatment with adalimumab (Humira) plus methotrexate [abstract] Ann Rheum Dis. 2004b;64(Suppl 1):277. [Google Scholar]
- Keystone EC, Kavanaugh AF, Sharp JT, et al. Radiographic inhibition of structural damage sustained in patients with long-standing rheumatoid arthritis following 3 years of treatment with adalimumab (Humira) plus methotrexate [abstract] Arthritis Rheum. 2004c;50(Suppl):S189. (Poster 371) [Google Scholar]
- Keystone E, Haraoui B, Bykerk VP. Role of adalimumab in patients with early versus late rheumatoid arthritis (RA) [abstract] Clin Exp Rheumatol. 2003;21(5 Suppl 31):S198–9. [PubMed] [Google Scholar]
- Klareskog L, van der Heijde D, de Jager JP, et al. TEMPO (Trial of Etanercept and Methotrexate with Radiographic Patient Outcomes) study investigators. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomized controlled trial. Lancet. 2004;363:675–81. doi: 10.1016/S0140-6736(04)15640-7. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Kavanaugh A. Adalimumab for the treatment of rheumatoid arthritis. Therapy. 2005;2:13–21. [Google Scholar]
- Lipsky PE, van der Heijde DM, St Clair EW, et al. the Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group Infliximab and methotrexate in the treatment of rheumatoid arthritis. N Engl J Med. 2000a;343:1594–602. doi: 10.1056/NEJM200011303432202. [DOI] [PubMed] [Google Scholar]
- Lipsky PE, van der Heijde DM, St Clair EW, et al. 102-wk clinical and radiologic results from the ATTRACT trial: a 2 year, randomized, controlled, phase 3 trial of infliximab (RemicadeÒ) in pts with active RA despite MTX [abstract] Arthritis Rheum. 2000b;43:S269. [Google Scholar]
- Magnano M, Robinson WH, Genovese MC. Demyelination and the use of TNF inhibition. Clin Exp Rheumatol. 2004;22(5 Suppl 35):S134–40. [PubMed] [Google Scholar]
- Maini R, St Clair EW, Breedveld F, et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomized phase III trial. Lancet. 1999;354:1932–9. doi: 10.1016/s0140-6736(99)05246-0. [DOI] [PubMed] [Google Scholar]
- Mease P. TNF-α inhibitors in psoriatic arthritis. In: Gordon KB, Ruderman EM, editors. Psoriasis and psoriatic arthritis: an integrated approach. Heidelberg, Germany: Springer-Verlag; 2005. pp. 223–34. [Google Scholar]
- Mease PJ, Antoni CE. Psoriatic arthritis treatment: biological response modifiers. Ann Rheum Dis. 2005;64(Suppl II):ii78–82. doi: 10.1136/ard.2004.034157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mease PJ, Gladman DD, Ritchlin CT, et al. the ADEPT Study Group Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial. ADEPT. Arthritis Rheum. 2005a;52:3279–89. doi: 10.1002/art.21306. [DOI] [PubMed] [Google Scholar]
- Mease PJ, Gladman DD, Ritchlin CT, et al. Clinical efficacy and safety of adalimumab for psoriatic arthritis: 48-week results of ADEPT [abstract] Arthritis Rheum. 2005b;52(Suppl):S215. [Google Scholar]
- Mease PJ, Goffe BS, Metz J, et al. Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomized trial. Lancet. 2000;356:385–90. doi: 10.1016/S0140-6736(00)02530-7. [DOI] [PubMed] [Google Scholar]
- Mease P, Kavanaugh A, Krueger G, et al. Infliximab improves psoriasis regardless of arthritis response in patients with active psoriatic arthritis: results from IMPACT 2 trial. Arthritis Rheum. 2004;50:S616. [Google Scholar]
- Mease PJ, Ruderman EM, Kivitz AJ, et al. Continued efficacy and safety of etanercept (ENBRELÒ) in patients with psoriatic arthritis and psoriasis. Arthritis Rheum. 2003;48(9S):(Abstract 343)S169. [Google Scholar]
- Mease P, Sharp J, Ory P, et al. Inhibition of joint destruction in PsA with adalimumab: 48-week results of ADEPT [abstract] Arthritis Rheum. 2005;52(Suppl):S631. [Google Scholar]
- Moreland LW, Baumgartner SW, Schiff MH, et al. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N Engl J Med. 1997;337:141–7. doi: 10.1056/NEJM199707173370301. [DOI] [PubMed] [Google Scholar]
- Moreland LW, Schiff MH, Baumgartner SW, et al. Etanercept therapy in rheumatoid arthritis: a randomized, controlled trial. Ann Intern Med. 1999;130:478–86. doi: 10.7326/0003-4819-130-6-199903160-00004. [DOI] [PubMed] [Google Scholar]
- O’Dell JR. Treating rheumatoid arthritis early: a window of opportunity? Arthritis Rheum. 2002;46:283–5. doi: 10.1002/art.10092. [DOI] [PubMed] [Google Scholar]
- Partsch G, Steiner G, Leeb BF, et al. Highly increased levels of tumor necrosis factor-a and other proinflammatory cytokines in psoriatic arthritis synovial fluid. J Rheumatol. 1997;24:518–23. [PubMed] [Google Scholar]
- Ritchlin C, Haas-Smith SA, Hicks D, et al. Patterns of cytokine production in psoriatic synovium. J Rheumatol. 1998;25:1544–52. [PubMed] [Google Scholar]
- Ritchlin CT, Mease PJ, Sasso EH. Correlation of skin and joint responses in psoriatic arthritis: ADEPT subanalysis. Poster presentation at the Spring Symposium of the European Academy of Dermatology and Venereology (EADV); February 9–12, 2006; Lapland, Finland. 2006. [Google Scholar]
- Saxne T, Palladino MA, Jr, Heinegard D, et al. Detection of tumor necrosis factor alpha but not tumor necrosis factor beta in rheumatoid arthritis synovial fluid and serum. Arthritis Rheum. 1988;31:1041–5. doi: 10.1002/art.1780310816. [DOI] [PubMed] [Google Scholar]
- Schiff MH, Burmester GR, Kent J, et al. Safety analyses of adalimumab (HUMIRAÒ) in global clinical trials and US postmarketing surveillance of patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65:889–94. doi: 10.1136/ard.2005.043166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff M, van de Putte LB, Breedveld FC, et al. Rates of infection in adalimumab rheumatoid arthritis clinical trials [abstract] Ann Rheum Dis. 2003;62(Suppl 1):184. [Google Scholar]
- Strand V, Weisman MH, Nichol MB, et al. Adalimumab improves health-related quality of life in rheumatoid arthritis patients [abstract no. 99] Arthritis Rheum. 2003;48(9 Suppl):S402. [Google Scholar]
- Strand V, Chartash E, Sengupta N, et al. Improvement in health-related quality of life, health utility, and fatigue in patients with active rheumatoid arthritis (RA) on adalimumab (Humiraä, Abbott) therapy [abstract no. SAT0246] Ann Rheum Dis. 2003;62(Suppl 1):356. [Google Scholar]
- Thomson BM, Mundy GR, Chambers TJ. Tumor necrosis factors a and b induce osteoblastic cells to stimulate osteoclastic bone resorption. J Immunol. 1987;138:775–9. [PubMed] [Google Scholar]
- van der Heijde D, Landewe RBM, Keystone EC, et al. Adalimumab (HUMIRA®) plus MTX prevents nearly all severe radiographic progression observed with methotrexate monotherapy in early, aggressive rheumatoid arthritis. Arthritis Rheum. 2005;Suppl:S110. (Poster Presentation 207) [Google Scholar]
- van der Heijde D, Dijkmans B, Geusens P, et al. Efficacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ASSERT) Arthritis Rheum. 2005;52:582–91. doi: 10.1002/art.20852. [DOI] [PubMed] [Google Scholar]
- van der Heijde D, Kivitz A, Schiff MH, et al. the Adalimumab Trial Evaluating Long-term Efficacy and Safety in Ankylosing Spondylitis Study Group Efficacy and safety of adalimumab in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ATLAS) Arthritis Rheum. 2006;54:2136–46. doi: 10.1002/art.21913. [DOI] [PubMed] [Google Scholar]
- van de Putte LBA, Salfeld J, Kaymakcalan Z. Adalimumab. In: Moreland LW, Emery P, editors. TNF-inhibition in the treatment of rheumatoid arthritis. London: Martin Dunitz; 2003. pp. 71–93. [Google Scholar]
- van de Putte LB, Atkins C, Malaise M, et al. Efficacy and safety of adalimumab as monotherapy in patients with rheumatoid arthritis for whom previous disease modifying antirheumatic drug treatment has failed. Ann Rheum Dis. 2004;63:508–16. doi: 10.1136/ard.2003.013052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinblatt ME, Keystone EC, Furst DE, et al. Adalimumab, a fully human anti-tumor necrosis factor a monoclonal antibody for the treatment of RA in patients taking concomitant methotrexate. The ARMADA trial. Arthritis Rheum. 2003;48:35–45. doi: 10.1002/art.10697. [DOI] [PubMed] [Google Scholar]
- Weinblatt ME, Keystone EC, Furst DE, et al. The ARMADA trial: sustained efficacy and long-term safety of adalimumab (Humira) plus methotrexate over 3 years in patients with long-standing rheumatoid arthritis [abstract] Ann Rheum Dis. 2004;64(Suppl 1):295. [Google Scholar]
- Weinblatt ME, Keystone EC, Furst DE, et al. Long term efficacy and safety of adalimumab plus methotrexate in patients with rheumatoid arthritis in the ARMADA trial [abstract] Arthritis Rheum. 2005;52(Suppl):S563 (Poster 1497). doi: 10.1136/ard.2005.044404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinblatt ME, Kremer JM, Bankhurst AD, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor: Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999;340:253–9. doi: 10.1056/NEJM199901283400401. [DOI] [PubMed] [Google Scholar]
- Wells AF, Kupper H, Fischkiff S, et al. Injection site reactions in adalimumab rheumatoid arthritis (RA) pivotal clinical trials [abstract]. Ann Rheum Dis. 2003;62(Suppl 1):411. [Google Scholar]


