Abstract
Although blood transfusions are important for patients with anemia, chronic transfusions inevitably lead to iron overload as humans cannot actively remove excess iron. The cumulative effects of iron overload lead to significant morbidity and mortality, if untreated. Although the current reference standard iron chelator deferoxamine has been used clinically for over four decades, its effectiveness is limited by a demanding therapeutic regimen that leads to poor compliance. Deferasirox (Exjade®, ICL670, Novartis Pharma AG, Basel, Switzerland) is a once-daily, oral iron chelator approved for the treatment of transfusional iron overload in adult and pediatric patients. The efficacy and safety of deferasirox have been established in a comprehensive clinical development program involving patients with various transfusion-dependent anemias. Deferasirox has a dose-dependent effect on iron burden, and is as efficacious as deferoxamine at comparable therapeutic doses. Deferasirox therapy can be tailored to a patient’s needs, as response is related to both dose and iron intake. Since deferasirox has a long half-life and is present in the plasma for 24 hours with once-daily dosing, it is unique in providing constant chelation coverage with a single dose. The availability of this convenient, effective, and well tolerated therapy represents a significant advance in the management of transfusional iron overload.
Keywords: Exjade, deferasirox, transfusional iron overload, effective
Iron chelation therapy
It is well known that red blood cell transfusions are a vital, life-saving treatment for many patients with chronic anemias, including β-thalassemia, myelodysplastic syndromes (MDS), and sickle cell disease (SCD). Since every unit of transfused blood contains 200–250 mg of iron and the human body has no mechanism to actively excrete excess iron, cumulative iron overload is an inevitable consequence of chronic transfusion therapy (Porter 2001a).
During normal iron homeostasis, circulating iron is bound to transferrin, a dedicated iron-binding protein with a high affinity for ferric (Fe3+) iron. When a state of iron overload occurs, the capacity for transferrin to bind iron is exceeded and ‘free’ or nontransferrin-bound iron (NTBI) is formed; the presence of NTBI has been shown to correlate with the appearance of oxidation products and reduced plasma antioxidant capacity (De Luca et al 1999; Cighetti et al 2002). Labile plasma iron (LPI), one form of NTBI, is redox-active and can be taken up by liver, cardiac, and endocrine cells through uptake mechanisms that are independent of the transferrin receptor (Cabantchik et al 2005). It is thought that LPI provides an estimation of the total levels of labile iron loaded within cells. Excess iron in parenchymal tissues can cause serious clinical sequelae, such as cardiac failure, liver disease, diabetes, and eventual death (Ishizaka et al 2002; Cunningham et al 2004). Without treatment, the prognosis for patients with iron overload is poor (Brittenham et al 1994).
As such, the primary aim of iron chelation therapy is to bind to and remove iron from the body at a rate that is either equal to the rate of transfusional iron input (maintenance therapy) or greater than iron input (reduction therapy). This suggests that a therapy which allows flexible dosing is required. It has been established that iron chelation therapy reduces the risk for developing co-morbidities and improves patient survival during more than 40 years of clinical experience with the current reference standard chelator deferoxamine (DFO) (Desferal®, Novartis Pharma AG, Basel, Switzerland) (Brittenham et al 1994; Olivieri et al 1994; Modell et al 2000). Another aim of chelation therapy is to provide constant, 24-hour protection from the harmful effects of toxic iron (ie, NTBI), since gaps in chelation therapy result in iron reloading and further tissue damage. The direct capture of LPI has been suggested as a way to avoid the dangerous accumulation of cellular iron and to prevent resultant adverse consequences (Cabantchik et al 2005). However, 24-hour chelation coverage is not possible with ‘standard’ DFO as it is a large molecule with a short half-life (20–30 minutes) and plasma levels decline rapidly after infusion (Porter et al 1996; Porter 2001b). In one study, levels of LPI rebounded as soon as the DFO infusion was stopped (Cabantchik et al 2005). DFO administration also requires slow parenteral infusion over an 8–12-hour period, five to seven times per week (at a standard dose of 20–60 mg/kg/day) (Porter 2001b). The demands of this regimen have a significant impact on compliance, meaning that a large number of patients do not gain the full benefits of therapy and therefore die prematurely (Brittenham et al 1994; Wonke 2001). One study has demonstrated that the probability of survival to at least 25 years of age in poorly chelated patients with β-thalassemia major was just one-third that of patients who were well chelated with DFO (Brittenham et al 1994). The number of days a patient was receiving chelation was more important than the overall dose, indicating that it is essential to maximize the length of exposure to chelation therapy.
Deferiprone (Ferriprox®, Apotex, Toronto, ON, Canada), a three-times daily oral iron chelator (at a standard dose of 75 mg/kg/day), is currently available in a number of countries outside the USA and Canada for the second-line treatment of iron overload in adult patients with thalassemia major for whom DFO therapy is contraindicated or inadequate (Hoffbrand et al 2003; Apotex 2004). Deferiprone has a half-life of 3–4 hours and, like DFO, it is therefore unable to provide 24-hour chelation coverage; LPI levels have been shown to rebound in between doses (Cabantchik et al 2005). Use of deferiprone is limited to second-line therapy primarily due to the occurrence of side effects such as arthropathy, neutropenia and, rarely, agranulocytosis (al Refaie et al 1995; EMEA 2005). In addition, data on its use in pediatric patients are limited.
Development of deferasirox
Deferasirox (Exjade®, ICL670) was developed in response to the clear need for a convenient, effective and well-tolerated iron chelating agent. The development process began in 1993, when Novartis (Basel, Switzerland) produced over 700 iron chelating compounds with high affinity and selectivity for iron. Using a computational chemistry-based model, the bis-hydroxyphenyl-triazole class showed the most promise, as it combined the relevant iron chelating attributes with the potential to synthesize various derivatives. Approximately 40 compounds with this structure were synthesized. Just one molecule, known as ‘ICL670’ or deferasirox, passed this test and subsequently entered a rigorous clinical development program in 1998.
Deferasirox: properties and administration
Deferasirox is a tridentate iron chelator, meaning that two molecules are required to form a stable complex with each iron (Fe3+) atom. The active molecule (ICL670) is highly lipophilic and 99% protein-bound. The key chelation properties of deferasirox are:
High and specific affinity for Fe3+ (approximately 14 and 21 times greater than its affinity for copper [Cu2+] and zinc [Zn2+], respectively [Steinhauser et al 2004])
Oral bioavailability
Highly efficient and efficacious
Effective at multiple doses; allowing flexible regimens
Long half-life (8–16 hours); allowing once-daily dosing
Generally well tolerated
The long half-life means that deferasirox can be taken once a day (standard dose of 20–30 mg/kg/day). Tablets should be completely dispersed by stirring in water, orange juice, or apple juice until a fine suspension is obtained; this oral formulation means that deferasirox is easy-to-use for pediatric patients. Any residue should be resuspended in a small volume of liquid and swallowed to avoid introducing variability in bioavailability. For the same reason, deferasirox should be taken on an empty stomach at least 30 minutes before food (Exjade PI 2005).
Preclinical studies
In vivo animal pharmacology
As demonstrated in various animal models, deferasirox is rapidly absorbed, can efficiently and selectively mobilize iron from various tissues such as hepatocytes and cardiomyocytes, and can promote iron excretion (Nick et al 2002, 2003). As the deferasirox-iron complex is relatively inert, it is excreted in the feces rather than being redistributed (Nick et al 2002). In an iron overloaded rat model, the efficiency of deferasirox (ie, the ratio of iron excreted to the theoretical maximum of iron that could be bound by the dose given) was high—18% at doses of 50 mg/kg and 100 mg/kg—compared with both subcutaneous DFO (3%–4%) and deferiprone (~2%) (Nick et al 2003).
Safety/toxicology studies
Iron is a physiologically important element with a number of very important roles (eg, in erythropoiesis, oxygen transport, and DNA synthesis), therefore the potential for deferasirox to affect normal iron absorption was evaluated. A rat model demonstrated that deferasirox does not affect normal homeostatic uptake of dietary iron. This means that the deferasirox-iron complex does not permeate the mucosal cell layer. Further evidence of this was obtained using the Caco2 cell monolayer model (Nick et al 2002, 2003). Deferasirox was generally well tolerated across a wide range of toxicology studies, and no toxicities prohibitive for use in humans were identified. The kidney (tubular region) was the primary organ affected by iron overload in both rats and marmosets, with the severity of effects being dependent on the iron loading status of the animals.
Effect on iron burden in animals
The ability of deferasirox to reduce iron burden has been demonstrated in a number of animal models, producing significant reductions in liver iron concentration (LIC) and demonstrating greater efficacy than DFO and significantly greater efficacy than deferiprone (Nick et al 2002, 2003). As cardiac failure is a primary cause of morbidity and mortality in patients with transfusional iron overload, it is important that an iron chelator is able to remove iron from the heart. A study in rat heart cell cultures initially demonstrated the ability of deferasirox to remove iron directly from iron-loaded myocardial cells (Hershko et al 2001). More recently, a fluorescence study in living cells clearly demonstrated that deferasirox can access and chelate intracellular iron in cardiomyocytes (Glickstein et al 2005). The relative efficacy of deferasirox, deferiprone, and DFO has been evaluated in an iron-loaded gerbil model (Wood et al 2005). Deferasirox and deferiprone were equally effective, decreasing cardiac iron levels by 20.5% and 18.6%, respectively, although deferasirox was significantly more effective for reducing liver iron levels. This observation suggests that deferasirox may be effective in multiple areas of the body. Both drugs were significantly more effective than DFO, which did not reduce iron levels in this study, although this was most likely due to the mode of administration used. Preliminary data show that deferasirox is also effective for removing excess cardiac iron, as measured by an improvement in myocardial T2*, over 1 year of treatment in human thalassemia patients (Eleftheriou et al 2006). To date, this improvement in T2* has been maintained over 2 years of deferasirox treatment. As such, DFO and deferiprone appear effective for removing cardiac iron (Borgna-Pignatti et al 2006; Pennell et al 2006), as does deferasirox (Eleftheriou et al 2006).
Clinical evaluation
Deferasirox is currently approved in many countries, including the USA, Switzerland, and Europe, for the treatment of chronic transfusional iron overload in adult and pediatric patients (Exjade PI 2005). These approvals were obtained based on the results from a comprehensive series of studies, the largest ever undertaken for an iron chelating agent, that enrolled over 1000 patients with a wide range of transfusion-dependent anemias (Figure 1). As many patients require transfusion therapy from childhood, a large number of pediatric patients were enrolled to investigate efficacy and safety of deferasirox in this important population.
Figure 1.
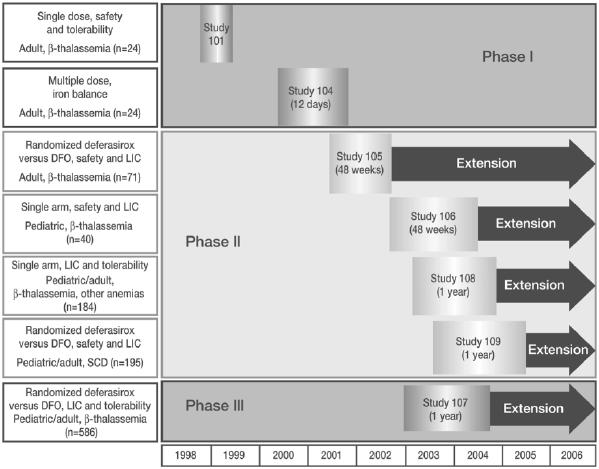
Deferasirox clinical trial program.
Abbreviations: DFO, deferoxamine; LIC, liver iron concentration; SCD, sickle cell disease.
Pharmacokinetic profile of deferasirox
In Study 101 it was shown that the serum concentration of deferasirox is proportional to the dose administered (Figure 2) (Galanello et al 2003). This study also demonstrated that unbound deferasirox had a mean half-life of 11–19 hours depending on dose, which supports the once-daily dosing regimen used throughout the clinical trial program. Deferasirox plasma levels were also shown to be maintained within the therapeutic range over a 24-hour period (20 mg/kg/day: peak levels ~60–100 µmol/L, trough levels ~15–20 µmol/L), providing constant gap-free chelation coverage with a single daily dose (Nisbet-Brown et al 2003).
Figure 2.
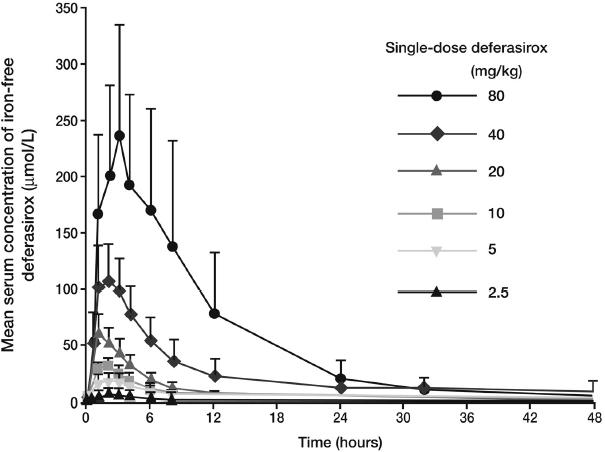
Mean plasma concentrations (+ SD) of deferasirox are proportional to dose. Copyright © 2003. Reprinted with permission from Galanello R, Piga A, Alberti D, et al. 2003. Safety, tolerability, and pharmacokinetics of ICL670, a new orally active iron-chelating agent in patients with transfusion-dependent iron overload due to beta-thalassemia. J Clin Pharmacol, 43:565–72.
The pharmacokinetic (PK) profile of deferasirox has been evaluated in patients with different ethnic backgrounds, where no significant differences were observed, and also in pediatric patients with β-thalassemia major after administration of single and multiple oral doses (Study 106). The results in pediatric patients showed no differences in maximum concentration, area under the curve, and half-life between children (aged <12 years) and adolescents (aged ≥12 years), although exposure to deferasirox was approximately 20%–30% lower than that previously observed in adult β-thalassemia patients (Galanello et al 2006). These PK characteristics demonstrate that deferasirox is suitable for once-daily oral administration in pediatric patients. It is interesting to note that patients aged <6 years had significantly lower exposure to deferasirox than other pediatric patients, which may provide an additional margin for safety and tolerability.
Effect on iron burden in humans
The proof-of-concept for deferasirox in humans was achieved by a dose escalation study in patients with β-thalassemia and transfusional iron overload that demonstrated good efficacy and a chelation efficiency of up to 20.5% (Study 104) (Nisbet-Brown et al 2003). This study identified an effective dose between 20–30 mg/kg/day, similar to what had been previously observed in marmosets. Data from a subsequent 1-year, Phase II comparative study in transfusion-dependent β-thalassemia patients (Study 105) provided the first evidence that deferasirox efficacy was comparable with DFO (Piga et al 2006). In this study, a single daily dose of deferasirox 20 mg/kg/day was as effective as DFO 40 mg/kg/day for removing iron.
Pivotal data have been recently published from a large-scale, randomized Phase III trial comparing deferasirox (n = 296) with subcutaneous DFO (n = 290) in regularly transfused adult and pediatric patients with transfusion-dependent β-thalassemia (Study 107) (Cappellini et al 2006). Dose was assigned according to baseline LIC; those with baseline LIC <7 mg Fe/g dry weight (dw) received deferasirox 5 or 10 mg/kg/day or DFO <25–<35 mg/kg, and those with baseline LIC ≥7 mg Fe/g dw received deferasirox 20 or 30 mg/kg/day or DFO 35–≥50 mg/kg. Baseline LIC values were very high in this population, despite the fact that most patients were receiving DFO therapy prior to study entry and were judged to be suitable for continued treatment, highlighting the significant unmet medical need in this population. As the study design allowed patients receiving DFO at study entry to continue on their current dose even if it was higher than would have been assigned by the protocol, patients with LIC values <7 mg Fe/g dw received lower doses of deferasirox relative to DFO as compared with patients with LIC ≥7 mg Fe/g dw. The net result was that doses were not comparable in the lower deferasirox dose ranges (Table 1).
Table 1.
Protocol-assigned and actual doses used during Study 107
| Deferasirox, mg/kg/day | ||||
| Protocol assigned dose | 5 | 10 | 20 | 30 |
| Actual mean dose ± SD | 6.2 ± 1.6 | 10.2 ± 1.2 | 19.4 ± 1.7 | 28.2 ± 3.5 |
| DFO, mg/kg/day | ||||
| Protocol assigned dose | <25 | 25–<35 | 35–<50 | ≥50 |
| Actual mean dose ± SD | 33.9 ± 9.9 | 36.7 ± 9.2 | 42.4 ± 6.6 | 51.6 ± 5.8 |
| Ratio of actual deferasirox: DFO doses | 1:5.5 | 1:3.6 | 1:2.2 | 1:1.8 |
Abbreviations: DFO, deferoxamine; SD, standard deviation.
Due to this disproportionate dosing, the primary endpoint (a nonparametric ‘success’ or ‘failure’ endpoint that was prospectively defined for each dose group) was not met in the overall population. However, there was still a clear demonstration of dose-dependent iron excretion (Figure 3). Furthermore, in patients with baseline LIC values ≥7 mg Fe/g dw (69%) where a deferasirox versus DFO dose relationship of 1:2 was maintained; similar changes in iron burden were achieved in both treatment groups. It was clear that iron balance was achieved at the recommended deferasirox starting dose of 20 mg/kg/day, and a significant reduction in iron burden was observed at 30 mg/kg/day (Table 2) (Cappellini et al 2006). Overall, changes in LIC, serum ferritin and body iron balance were consistent during the study and correlated well with one another.
Figure 3.
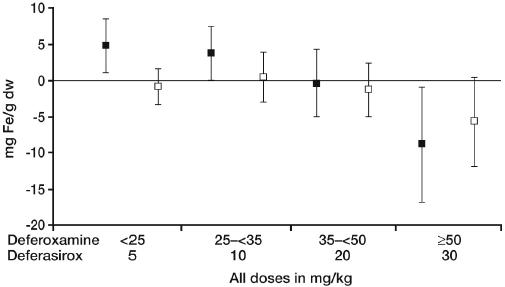
Dose-dependent change in LIC with deferasirox treatment [▪ Deferasirox; □ DFO]. Copyright © 2006. Reprinted with permission from Cappellini MD, Cohen A, Piga A, et al. 2006. A Phase III study of deferasirox (ICL670), a once-daily oral iron chelator, in patients with β-thalassemia. Blood, 107:3455–62.
Table 2.
Change in serum ferritin and iron excretion/intake ratio after 1-year of deferasirox treatment
| Median deferasirox dose, mg/kg/day | ||||
|---|---|---|---|---|
| 5 | 10 | 20 | 30 | |
| Change in serum ferritin*, μg/L | 1189 ± 700 | 833 ± 817 | −36 ± 817 | −926 ± 1416 |
| Ratio iron excretion/intake* | 0.58 ± 0.33 | 0.67 ± 0.37 | 1.02 ± 0.40 | 1.67 ± 0.72 |
Note: Mean ± Standard deviation.
Pooled data from across the deferasirox clinical trial program have demonstrated that the response to deferasirox is not only dependent on dose, but also on the rate of transfusional iron intake while on study (Cohen et al 2005; Greenberg et al 2005). Although the impact of transfusion rate was underestimated in these studies, it did enable a comparison of various transfusion rates at each dose. As shown in Figure 4, this led to the definition of some general guidance on deferasirox dosing:
10 mg/kg/day maintains iron balance in patients with low transfusional requirements (<2 units of blood/month)
20 mg/kg/day maintains or reduces iron balance in patients with low and intermediate requirements (2–4 units of blood/month)
30 mg/kg/day decreases iron balance in most patients, irrespective of transfusional requirements
Figure 4.
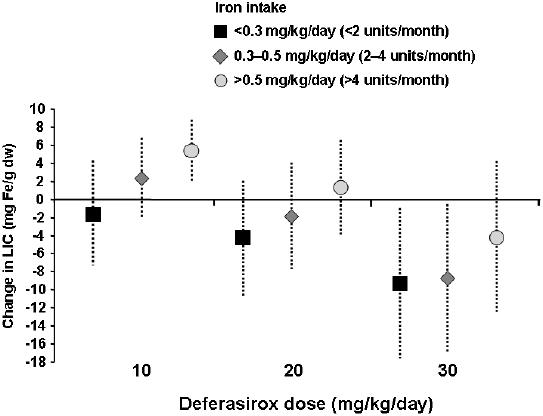
Deferasirox demonstrates dose- and transfusion-related changes in LIC (pooled data).
Abbreviations: LIC, liver iron concentration.
Deferasirox dosing can therefore be tailored to meet a patient’s need based on transfusional requirements, severity of iron overload, and treatment goal (ie, maintenance or reduction of body iron levels). As deferasirox has high and stable efficiency across doses (approximately 30% [Porter et al 2005]), 5 mg/kg/day is able to effectively remove iron but is insufficient to maintain or reduce iron balance in regularly transfused patients.
Efficacy in a range of transfusion-dependent anemias
As complications of iron overload have been best studied in β-thalassemia, this population of patients was the main focus of the deferasirox clinical trial program. However, a number of regularly transfused patients with different chronic anemias have also been enrolled, including MDS, SCD, and Diamond-Blackfan anemia (DBA). Given that the pathogenesis of chronic iron overload may vary across different transfusion-dependent anemias, it was important to investigate whether efficacy results were comparable, so that response to therapy could be monitored and treatment tailored to meet the needs of each individual patient. Preliminary data from patients with MDS and DBA (Study 108), as well as SCD (Study 109), demonstrate significant, dose-dependent effects on LIC and serum ferritin over the 1-year treatment period, which were similar to results seen in thalassemia patients (Gattermann et al 2005; Tchernia et al 2005; Vichinsky, Fischer, Fung, et al 2005). The National Comprehensive Cancer Network (NCCN) has recently published updated clinical practice guidelines for MDS, recognizing the importance of constant chelation coverage and introducing deferasirox into its treatment recommendations (Greenberg et al 2006). Importantly, deferasirox has also been shown to be effective in patients who were previously inadequately chelated with DFO (due to poor tolerance or compliance, or contraindications), suggesting that deferasirox is effective in both chelation-naïve and chelation-experienced patients.
Constant chelation coverage and impact on toxic iron levels
As discussed previously, deferasirox has a long half-life and plasma levels are maintained within the therapeutic range over the entire 24-hour period. This is important as it means that deferasirox is able to provide 24-hour chelation coverage with a single oral dose. In contrast, chelation coverage with other available iron chelators is limited to periods of drug exposure only. For example, plasma levels of DFO rapidly fall below detectable levels thus limiting chelation coverage to the duration of the infusion only (Porter et al 1996). As such, levels of toxic LPI are at times poorly controlled with DFO (Cabantchik et al 2005). This is also true for deferiprone monotherapy, with toxic LPI rebounding significantly between doses (Cabantchik et al 2005). Levels are more fully controlled with sequential combination therapy, although this regimen must be prescribed 7 days/week in order to provide constant chelation coverage. As deferasirox is convenient and taken once daily, it is continually present in the circulation and provides chelation coverage 24 hours/day, 7 days/week, thereby providing greater control of LPI. This has been investigated as part of a deferasirox clinical trial program. Preliminary data in patients with β-thalassemia demonstrate that daily trough levels of deferasirox are sufficient to maintain suppression of LPI levels, suggesting that deferasirox is able to control LPI for 24 hours with a single dose (Daar et al 2006). After 4 weeks of treatment with deferasirox (20 mg/kg/day), peak LPI levels observed just before deferasirox dosing were significantly decreased compared with baseline and were close to normal levels, indicating a sustained reduction in LPI.
Safety and tolerability profile
Deferasirox has been shown to be generally well tolerated in adults and children with different chronic anemias. Of 652 patients who received deferasirox in the core clinical trials (Studies 106, 107, 108, and 109), none experienced drug-related neutropenia or agranulocytosis, which were serious adverse events (AEs) observed during treatment with other chelators (Piga et al 2004; Gattermann et al 2005; Cappellini et al 2006). Sporadic cases were observed, but were considered by the investigators to be due to the underlying condition rather than deferasirox therapy.
The most frequent AEs reported during chronic treatment with deferasirox include transient mild-to-moderate gastrointestinal disturbances (~26% of patients) and transient mild-to-moderate skin rash (~7% of patients). These events rarely required drug discontinuation and many resolved spontaneously. Mild, non-progressive increases in serum creatinine (generally within upper limit of normal [ULN]) were observed in 34% of patients, although these are not currently thought to be clinically significant as they were temporary and reversible. There were no cases of moderate to severe renal insufficiency or renal failure and no patients permanently discontinued therapy due to creatinine rises in the core, 1-year studies. Increases above the ULN were observed in 2% of patients with β-thalassemia major and 16% with other anemias, including geriatric patients whose baseline creatinine levels were close to the ULN. Extension studies are ongoing to collect long-term data on these increases in serum creatinine. Two patients (2/703; 0.3%) experienced elevated alanine aminotransferase levels (>5 × ULN) for which an association with deferasirox treatment could not be ruled out.
Deferasirox is also generally well tolerated in children as young as 2 years of age, with a safety profile similar to that observed in adults (Piga et al 2004). To date, sexual and physical development have proceeded normally during treatment with deferasirox (Forni et al 2005).
Patients have been followed in the extension phases of the clinical trials for over 2 years; AE data from up to 5 years’ deferasirox treatment will eventually be collected.
Patients prefer deferasirox to DFO
It is generally recognized that ‘real world’ treatment compliance cannot be effectively assessed in the clinical trial setting. As such, two deferasirox studies in patients with β-thalasse-mia and SCD evaluated actual patient feedback in the form of patient-reported outcomes (Cappellini et al 2005; Vichinsky, Fischer, Pakbaz, et al 2005). Most patients who had previously received DFO were more satisfied with deferasirox therapy than DFO (85.15% vs 38.7% in β-thalassemia and 84.3% vs 23.7% in SCD, respectively), and found it to be more convenient than DFO (92.7% vs 11.3% in β-thalassemia and 79.5% vs 18.4% in SCD, respectively). These findings are supported by the impact of chelation therapy on normal daily activities, with less time being lost each month due to therapy with deferasirox than DFO (1.5–2.8 vs 11.1–11.7 hours in β-thalassemia and 2–3 versus 6–28 hours in SCD, respectively). Superior satisfaction with, and convenience of, deferasirox therapy versus DFO may translate into actual patient compliance and increase the effectiveness of chelation therapy, optimizing the outcome of iron-overloaded transfu-sion-dependent patients.
Conclusions
Iron overload, a cumulative toxicity, is an inevitable consequence of chronic transfusion therapy. With the value of transfusion therapy in various chronic anemias becoming more widely appreciated, its use is likely to increase in the future. Although iron levels can be controlled with DFO therapy, poor compliance with the demanding therapeutic regimen in many patients and a lack of constant chelation coverage significantly limits its effectiveness, leaving many patients exposed to the toxic effects of iron overload. Convenient, effective and well-tolerated chelation therapy with oral deferasirox is likely to be a significant development in the treatment of transfusional iron overload due to its ability to provide constant chelation coverage and the potential to improve compliance. In addition, this novel agent may allow treatment to be initiated at an earlier stage in the disease course due to its excellent efficacy and tolerability profile combined with a convenient administration regimen.
References
- al Refaie FN, Hershko C, Hoffbrand AV, et al. Results of long-term deferiprone (L1) therapy: a report by the International Study Group on Oral Iron Chelators. Br J Haematol. 1995;91:224–9. doi: 10.1111/j.1365-2141.1995.tb05274.x. [DOI] [PubMed] [Google Scholar]
- Apotex. 2004. Ferriprox package insert.
- Borgna-Pignatti C, Cappellini MD, De Stefano P, et al. Cardiac morbidity and mortality in deferoxamine- or deferiprone-treated patients with thalassemia major. Blood. 2006;107:3733–7. doi: 10.1182/blood-2005-07-2933. [DOI] [PubMed] [Google Scholar]
- Brittenham GM, Griffith PM, Nienhuis AW, et al. Efficacy of deferoxamine in preventing complications of iron overload in patients with thalassemia major. N Engl J Med. 1994;331:567–73. doi: 10.1056/NEJM199409013310902. [DOI] [PubMed] [Google Scholar]
- Cabantchik ZI, Breuer W, Zanninelli G, et al. LPI-labile plasma iron in iron overload. Best Pract Res Clin Haematol. 2005;18:277–87. doi: 10.1016/j.beha.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Cappellini MD, Bejaoui M, Agaoglu L, et al. Patient satisfaction with deferasirox (Exjade®, ICL670) an oral form of chelation therapy versus deferoxamine an infused chelation therapy [abstract] Blood. 2005;106:2704. [Google Scholar]
- Cappellini MD, Cohen A, Piga A, et al. A Phase III study of deferasirox (ICL670), a once-daily oral iron chelator, in patients with beta-thalas-semia. Blood. 2006;107:3455–62. doi: 10.1182/blood-2005-08-3430. [DOI] [PubMed] [Google Scholar]
- Cighetti G, Duca L, Bortone L, et al. Oxidative status and malondialdehyde in beta-thalassaemia patients. Eur J Clin Invest. 2002;32(Suppl 1):55–60. doi: 10.1046/j.1365-2362.2002.0320s1055.x. [DOI] [PubMed] [Google Scholar]
- Cohen A, Masera G, Zoumbos N, et al. Effect of iron intake on control of body iron in patients with thalassemia major treated with deferasirox (Exjade®, ICL670) [abstract] Blood. 2005;106:822. [Google Scholar]
- Cunningham MJ, Macklin EA, Neufeld EJ, et al. Complications of beta-thalassemia major in North America. Blood. 2004;104:34–9. doi: 10.1182/blood-2003-09-3167. [DOI] [PubMed] [Google Scholar]
- Daar S, Taher A, Pathare A, et al. Deferasirox (Exjade® ICL 670) provides 24-hour protection from labile plasma iron (LPI), in iron overloaded β-thalassaemia patients previously chelated with mono- or combination therapy [abstract] Haematologica. 2006;91(Suppl 1):31. [Google Scholar]
- De Luca C, Filosa A, Grandinetti M, et al. Blood antioxidant status and urinary levels of catecholamine metabolites in beta-thalassemia. Free Radic Res. 1999;30:453–62. doi: 10.1080/10715769900300491. [DOI] [PubMed] [Google Scholar]
- Eleftheriou P, Tanner M, Pennell D, et al. Response of myocardial T2* to oral deferasirox monotherapy for 1 year in 29 patients with transfusion-dependent anaemias; a subgroup analysis [abstract] Haematologica. 2006;91(Suppl 1):999. [Google Scholar]
- [EMEA] European Medicines Agency. Ferriprox European Public Assessment Report, Scientific Discussion. 2005 www emea eu int.
- [Exjade PI] Novartis Pharmaceuticals Corporation. Exjade (deferasirox) Prescribing information [online] 2005 Accessed on 20 February 2007. URL: http://www.exjade.com.
- Forni GL, Piga A, Galanello R, et al. Growth and sexual development in pediatric patients treated over 48 weeks with ICL670, a once-daily oral iron chelator [abstract] Ped Blood Cancer. 2005;44:1106. [Google Scholar]
- Galanello R, Piga A, Alberti D, et al. Safety, tolerability, and pharmacokinetics of ICL670, a new orally active iron-chelating agent in patients with transfusion-dependent iron overload due to beta-thalassemia. J Clin Pharmacol. 2003;43:565–72. [PubMed] [Google Scholar]
- Galanello R, Piga A, Forni GL, et al. Phase II clinical evaluation of deferasirox (Exjade, ICL670), a once-daily oral chelating agent, in paediatric patients with β-thalassaemia major. Haematologica. 2006;91:1343–51. [PubMed] [Google Scholar]
- Gattermann N, Cazzola M, Greenberg P, et al. The efficacy and tolerability of ICL670, a once-daily oral iron chelator, in patients with myelodysplastic syndrome (MDS) and iron overload. Leuk Res. 2005;29(Suppl 1):S67. [Google Scholar]
- Glickstein H, Ben El R, Shvartsman M, et al. Intracellular labile iron pools as direct targets of iron chelators. A fluorescence study of chelator action in living cells. Blood. 2005;106:3242–50. doi: 10.1182/blood-2005-02-0460. [DOI] [PubMed] [Google Scholar]
- Greenberg P, Dine G, Ganser A, et al. Deferasirox (Exjade®, ICL670) demonstrates dose-related effects on body iron levels related to transfusional iron intake in transfusion-dependent anemia [abstract] Blood. 2005;106:2694. [Google Scholar]
- Greenberg PL, Baer MR, Bennett JM, et al. Myelodysplastic syndromes clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2006;4:58–77. [PubMed] [Google Scholar]
- Hershko C, Konijn AM, Nick HP, et al. ICL670A: a new synthetic oral chelator: evaluation in hypertransfused rats with selective radioiron probes of hepatocellular and reticuloendothelial iron stores and in iron-loaded rat heart cells in culture. Blood. 2001;97:1115–22. doi: 10.1182/blood.v97.4.1115. [DOI] [PubMed] [Google Scholar]
- Hoffbrand AV, Cohen A, Hershko C. Role of deferiprone in chelation therapy for transfusional iron overload. Blood. 2003;102:17–24. doi: 10.1182/blood-2002-06-1867. [DOI] [PubMed] [Google Scholar]
- Ishizaka N, Saito K, Mitani H, et al. Iron overload augments angiotensin II-induced cardiac fibrosis and promotes neointima formation. Circulation. 2002;106:1840–6. doi: 10.1161/01.cir.0000031161.77536.02. [DOI] [PubMed] [Google Scholar]
- Modell B, Khan M, Darlison M. Survival in beta-thalassaemia major in the UK: data from the UK Thalassaemia Register. Lancet. 2000;355:2051–2. doi: 10.1016/S0140-6736(00)02357-6. [DOI] [PubMed] [Google Scholar]
- Nick H, Acklin P, Lattmann R, et al. Development of tridentate iron chelators: from desferrithiocin to ICL670. Curr Med Chem. 2003;10:1065–76. doi: 10.2174/0929867033457610. [DOI] [PubMed] [Google Scholar]
- Nick H, Wong A, Acklin P, et al. ICL670A: preclinical profile. Adv Exp Med Biol. 2002;509:185–203. doi: 10.1007/978-1-4615-0593-8_10. [DOI] [PubMed] [Google Scholar]
- Nisbet-Brown E, Olivieri NF, Giardina PJ, et al. Effectiveness and safety of ICL670 in iron-loaded patients with thalassaemia: a randomised, double-blind, placebo-controlled, dose-escalation trial. Lancet. 2003;361:1597–602. doi: 10.1016/S0140-6736(03)13309-0. [DOI] [PubMed] [Google Scholar]
- Olivieri NF, Nathan DG, MacMillan JH, et al. Survival in medically treated patients with homozygous beta-thalassemia. N Engl J Med. 1994;331:574–8. doi: 10.1056/NEJM199409013310903. [DOI] [PubMed] [Google Scholar]
- Pennell DJ, Berdoukas V, Karagiorga M, et al. Randomized controlled trial of deferiprone or deferoxamine in beta-thalassemia major patients with asymptomatic myocardial siderosis. Blood. 2006;107:3738–44. doi: 10.1182/blood-2005-07-2948. [DOI] [PubMed] [Google Scholar]
- Piga A, Galanello R, Forni GL, et al. Randomized phase II trial of deferasirox (Exjade, ICL670), a once-daily, orally-administered iron chelator, in comparison to deferoxamine in thalassemia patients with transfusional iron overload. Haematologica. 2006;91:873–80. [PubMed] [Google Scholar]
- Piga A, Galanello R, Foschini ML, et al. Once-daily treatment with the oral iron chelator ICL670 (Exjade®): Results of a Phase II study in pediatric patients with β-thalassemia major [abstract] Blood. 2004;104:3614. [Google Scholar]
- Porter J, Borgna-Pignatti C, Baccarani M, et al. Iron chelation efficiency of deferasirox (Exjade®, ICL670) in patients with transfusional hemosiderosis [abstract] Blood. 2005;106:2690. [Google Scholar]
- Porter JB, Abeysinghe RD, Marshall L, et al. Kinetics of removal and reappearance of non-transferrin-bound plasma iron with deferoxamine therapy. Blood. 1996;88:705–13. [PubMed] [Google Scholar]
- Porter JB. Practical management of iron overload. Br J Haematol. 2001a;115:239–52. doi: 10.1046/j.1365-2141.2001.03195.x. [DOI] [PubMed] [Google Scholar]
- Porter JB. Deferoxamine pharmacokinetics. Semin Hematol. 2001b;38:63–8. doi: 10.1016/s0037-1963(01)90061-7. [DOI] [PubMed] [Google Scholar]
- Steinhauser S, Heinz U, Bartholoma M, et al. Complex formation of ICL670 and related ligands with Fe III and Fe II. Eur J Inorg Chem. 2004;21:4177–92. [Google Scholar]
- Tchernia G, Vichinsky E, Jeng M, et al. The once-daily oral iron chelator ICL670 is well tolerated and effective in treating transfusional iron overload in Diamond-Blackfan anaemia patients. Haematologica. 2005;90(Suppl 2):192. [Google Scholar]
- Vichinsky E, Fischer R, Fung E, et al. A randomized, controlled Phase II trial in sickle cell disease patients with chronic iron overload demonstrates that the once-daily oral iron chelator deferasirox (Exjade®, ICL670) is well tolerated and reduces iron burden [abstract] Blood. 2005;106:313. [Google Scholar]
- Vichinsky E, Fischer R, Pakbaz Z, et al. Satisfaction and convenience of chelation therapy in patients with sickle cell disease (SCD): comparison between deferasirox (Exjade®, ICL670) and deferoxamine (DFO) [abstract] Blood. 2005;106:2334. [Google Scholar]
- Wonke B. Clinical management of beta-thalassemia major. Semin Hematol. 2001;38:350–9. doi: 10.1016/s0037-1963(01)90029-0. [DOI] [PubMed] [Google Scholar]
- Wood JC, Otto-Duessel M, Gonzales I, et al. ICL670 removes cardiac iron in a gerbil model of iron overload [abstract] Blood. 2005;106:2695. [Google Scholar]


