Abstract
Sunitinib malate is a novel oral multitargeted tyrosine kinase inhibitor with antitumor and antiangiogenic activities. Sunitinib was recently approved in first-line treatment for patients with advanced renal cell carcinoma (RCC) and for the treatment of patients with gastrointestinal stromal tumors (GIST) after disease progression or intolerance to imatinib mesylate therapy. We report the very interesting results of the phase II trials after cytokin failure and of the randomized recent trial of sunitinib versus cytokin-based therapy in first-line treatment for patients with metastatic RCC, as well as the promising results of the recent trials on patients with GIST after disease progression or intolerance to imatinib mesylate therapy. Oral sunitinib demonstrates a high level of efficacy with acceptable tolerability with the 50 mg daily for 4 weeks followed by 2 weeks off schedule; a continuous schedule could be of interest. Hypertension and asthenia are the most common side effects with sunitinib. Regardless of these encouraging results, studies investigating sunitinib in first-line treatment (for patients with GIST), adjuvant and neoadjuvant settings are awaited, as well as trials using sunitinb in combination with chemotherapy or other targeted therapies. Clinical trials investigating sunitinib in other tumor types are ongoing.
Keywords: sunitinib, renal cell carcinoma, GIST, review, targeted therapy
Introduction
Tyrosine kinase receptors, including platelet-derived growth factor receptors (PDGFRs), fibroblast growth factor receptors, and vascular endothelial growth factor receptors (VEGFRs) and their ligands, have been shown to play important roles in tumor growth and angiogenesis (Kerbel and Folkman 2002). Inhibition of VEGF signaling through the use of antibodies (Yang et al 2003; Ferrara et al 2004; Willett et al 2004) or VEGFR antagonists has demonstrated potent antitumor effects that might be used to circumvent resistance to classical anticancer agents (Shaheen et al 2001; Bergers et al 2003; Ahmad and Eisen 2004). Recently, the humanized anti-VEGF monoclonal bevacizumab antibody in combination with chemotherapy was associated with an increased survival in patients with advanced colon cancer (Hurwitz et al 2004).
Sunitinib (sunitinib malate; SU11248; SUTENT™; Pfizer Inc, New York, NY) is a novel oral multitargeted tyrosine kinase inhibitor with antitumor and antiangiogenic activities (Figure 1). Sunitinib has been identified as a potent inhibitor of VEGFR-1, VEGFR-2, fetal liver tyrosine kinase receptor 3 (FLT3), KIT (stem-cell factor [SCF] receptor), PDGFRα, and PDGFRβ in both biochemical and cellular assays (Abrams, Lee, et al 2003; Mendel et al 2003). In vitro, sunitinib inhibited the growth of cell lines driven by VEGF, SCF, and PDGF and induced apoptosis of human umbilical vein endothelial cells (Mendel et al 2003). In vivo, sunitinib caused bone marrow depletion and effects in the pancreas in rats and monkeys, as well as adrenal toxicity in rat (microhemorrhage). In monkeys, a slight increase in arterial blood pressure and QT interval were reported at higher doses (Faivre et al 2006). Sunitinib exhibited dose- and time-dependent antitumor activity in mice, potently repressing the growth of a broad variety of human tumor xenografts (Abrams, Lee, et al 2003; Mendel et al 2003; Murray et al 2003; Morimoto et al 2004; Yee et al 2004).
Figure 1.
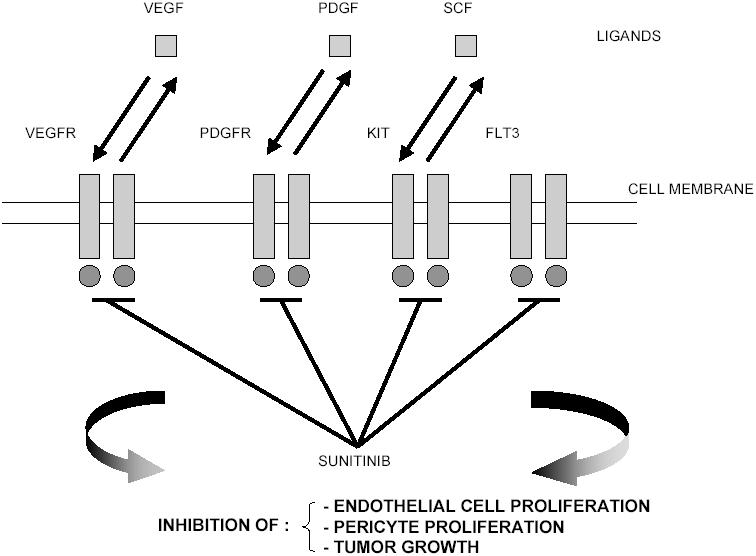
Mechanism of action of sunitinib.
Abbreviations: FLT3, fetal liver tyrosine kinase receptor 3; KIT, stem cell factor receptor; PDGF, platelet-derived growth factor; PDGFR, platelet-derived growth factor receptors; SCF stem-cell factor; VEGF, vascular endothelial growth factor;VEGER, vascular endothelial growth factor receptors.
In vitro metabolism studies demonstrated that sunitinib was primarily metabolized by cytochrome CYP3A4, resulting in formation of a major, pharmacologically active N-desethyl metabolite, SU012662. This metabolite was shown to be equipotent to the parent compound in biochemical tyrosine kinase and cellular proliferation assays, acting toward VEGFR, PDGFR, and KIT (Baratte et al 2004). SU012662 was the major plasma metabolite in mice, rats, and monkeys in vivo. SU012487 (an N-oxide metabolite) was the major metabolite in dog but was infrequently observed in human. Radiolabeled orally administrated sunitinib in preclinical species was primarily excreted in the feces.
Pharmacokinetic and pharmacodynamic data from animal studies showed that target plasma concentrations of sunitinib plus SU012662 capable of inhibiting PDGFRβ and VEGFR-2 phosphorylation were established in the range of 50 to 100 ng/mL (Abrams, Lee, et al 2003; Abrams, Murray, et al 2003; Mendel et al 2003; Murray et al 2003). Interestingly, those data were consistent with those observed in patients with acute myeloid leukemia in whom exposure to sunitinib led to a sustained inhibition of FLT3 phosphorylation in blast cells (O’Farrell et al 2003). Although initial studies were planned to provide continuous administration, the 4-week-on, 2-week-off schedule was selected at the request of the Food and Drug Administration (FDA) and other global regulatory agencies to allow patients to recover from potential bone marrow and adrenal toxicity observed in animal models.
Sunitinib was recently approved for the treatment of advanced renal cell carcinoma (RCC) and for the treatment of gastrointestinal stromal tumors (GIST) after disease progression or intolerance to imatinib mesylate therapy.
Sunitinib in the treatment of renal cell carcinoma
Renal cell carcinoma accounts for 3% of all adult cancer. Approximately one third of patients have distant metastases at presentation. Twenty five percent to 50% of patients treated by nephrectomy for localized disease develop metastatic disease (Lam et al 2005). Approximately 50% of patients with metastases at presentation will survive less than one year and 10% will survive for over 5 years (Motzer et al 2004). RCC is usually highly resistant to chemotherapy and radiotherapy. Standard first-line treatment for metastatic disease was immunotherapy with interferon-alpha and/or interleukin-2, achieving 6% to 20% response rates (Rohrmann et al 2005).
Renal cell carcinoma displays several histological types. The most common type of RCC is clear-cell RCC, accounting for 75% of cases (Motzer et al 1996). RCC are strongly associated with the von Hippel-Lindau (VHL) gene inactivation. RCC is a highly vascular tumor arising from epithelial elements of nephrons. An early event during the evolution of RCC is loss of function of the VHL gene (Latif et al 1993). In familial VHL-related RCC, the inheritance pattern is autosomal dominant (Latif et al 1993). The VHL gene is involved in the hypoxia-inducible pathway (Patel et al 2006). Specifically, the VHL gene product ubiquitinates transcriptional factor hypoxia-inducible factor (HIF)-1α (Kamura et al 2000). Physiologically, HIF-1 complex (a heterodimer composed of α and β subunits) regulates the expression of several genes in response to hypoxic stress (Wang and Semenza 1993). Human cells respond to hypoxic conditions through a series of pathways, many of which are mediated by HIF-1. In addition to regulation by VHL complex, HIF-1 activity is regulated by growth factor and cell adhesion pathways. HIF-1 binds to a variety of additional transcriptional cofactors, forming a preinitiation complex of proteins that ultimately activates transcription of hypoxia-inducible genes including VEGF leading to angiogenesis, epidermal growth factor receptor (EGFR) leading to cell growth, PDGF, and erythropoietin (Bardos and Aschcroft 2004). Phenotypically, RCC is a highly vascular tumor, with increased VEGF level, which growth could be stimulated by factors produced through the HIF-1 pathway. Consequently, the inhibition of VEGF and PDGF signaling pathways may reverse, in part, the physiologic consequences of losing VHL protein function and may inhibit tumor progression.
In the early phase I trial by Faivre and colleagues (2006), twenty eight patients received sunitinib doses ranging from 50 mg every other day to 150 mg daily. Dose-limiting toxicities reported at the maximum-tolerated doses ≥75 mg daily were reversible grade 3 fatigue, grade 3 hypertension, and grade 2 dermatitis. Therefore, the recommended dose was 50 mg daily for 4 weeks, followed by two weeks off per 6 weeks. Interestingly, 3 patients with metastatic RCC had a sustained objective response, lasting 28, 36, and 54 weeks (Faivre et al 2006).
This observation was the basis of the initiation of a large phase II trial including 63 patients with metastatic RCC who had failed cytokine-based therapy. Patients were treated with sunitinib monotherapy 50 mg daily for 4 weeks, followed by 2 weeks off (Motzer, Michaelson, et al 2006). Fifty five patients (87%) had clear cell histology. Only four patients (6%) had achieved objective response to prior cytokine-based therapy. Median duration of treatment with sunitinib was 9 months. Twenty five of 63 patients (40%) achieved partial response and 17 patients (28%) had stable disease lasting more than 3 months (Table 1). Twenty four responders had clear cell histology and one had a papillary cell type. Responding lesions included sites of local recurrence and lymphatic, hepatic, pulmonary, bone, and adrenal metastases. Median time to progression was 8.7 months and median overall survival was 16.4 months. The most commonly reported treatment-related grade 3 adverse events were fatigue (11%), nausea (3%), and diarrhea (3%) (Table 2). However, it is important to note that sunitinib may be considered as a maintenance treatment and that the grading system is designed mainly for chemotherapies that are usually not administered over prolonged periods. Thus the impact of fatigue, stomatitis, and other toxicities may be substantially underestimated. The most frequently reported grade 3 or 4 laboratory abnormalities were transient and asymptomatic elevated lipase (21%), neutropenia (13%), and anaemia (10%). Dose reductions were performed in 22 patients (35%) from 50 mg to 37.5 mg daily, and the dose for two of these patients was further reduced to 25 mg daily. The most common reasons for dose reduction were asymptomatic hyperlipasemia or hyperamylasemia and fatigue. Circulating proteins that may represent potential biomarkers of angiogenic activity were measured. At the end of each cycle, VEGF levels were frequently increased, while sVEGFR-2 (a soluble variant of VEGFR-2) levels usually decreased. A trend was observed towards a larger proportional increase in VEGF levels and decrease in sVEGFR-2 levels in patients having objective response as compared with those with stable disease or progression (Deprimo et al 2005). The observed median time to progression in this study (8.7 months) compares favorably with the median timesof 2.4 months for treatment in second line therapy at the Memorial Sloan-Kettering Cancer Center (Motzer et al 2004) and 2.5 months for treatment with placebo after cytokin failure in a phase II trial (Yang et al 2003).
Table 1.
Efficacy of sunitinib in trials with patients treated for renal cell carcinoma and GIST
| RCC | GIST | ||||
|---|---|---|---|---|---|
| Supportive (Motzer, Michaelson, et al 2006) (n = 63) | Pivotal (Motzer, Rini, et al 2006) (n = 106) | Phase III (Motzer, Hutson, et al 2006) (n = 374) | Phase I/II (Maki et al 2005) (n = 97) | Phase III (Demetri et al 2005) (n = 207) | |
| ORR | 25 (40%) | 46 (34%) | 103 (31%) | 8 (8%) | 17 (8%) |
| SD | 18 (28%) | 24 (29%) | 160 (48%) | 36 (37%) | 37 (18%) |
| TTP | 8.7 months | 8.3 months | 11 months | 7.8 months | 6.3 months |
| OS | 16.4 months | NR | NR | 19.8 months | NR |
Abbreviations: GIST, gastrointestinal stromal tumors; NR, not reached; ORR, overall response rate; OS, overall survival; RCC, renal cell carcinoma; SD, stable disease;TTP, time to progression.
Table 2.
Grade 3 or 4 toxicity of sunitinib in trials with patients treated for renal cell carcinoma and GIST
| RCC | GIST | ||||
|---|---|---|---|---|---|
| Supportive (Motzer, Michaelson, et al 2006) (n = 63) | Pivotal (Motzer, Rini, et al 2006) (n = 106) | Phase III (Motzer, Hutson, et al 2006) (n = 374) | Phase I/II (Maki et al 2005) (n = 97) | Phase III (Demetri et al 2005) (n = 207) | |
| Nonhematological | |||||
| Fatigue | 11% | 8% | 7% | 10% | 7% |
| Diarrhea | 3% | 3% | 5% | 7% | 4% |
| Nausea | 3% | 0% | 3% | 4% | 1% |
| Dermatitis | 2% | 5% | 5% | 7% | 5% |
| Stomatitis | 2% | 5% | 1% | 3% | NA |
| Asymptomatic lipase increase | 21% | 15% | 4% | 13% | NA |
| Hypertension | 2% | 6% | 8% | 17% | 4% |
| Hematological | |||||
| Neutropenia | 13% | 13% | 11% | NA | 8% |
| Anemia | 10% | 6% | 3% | NA | 4% |
| Thrombocytopenia | 0% | 6% | 8% | NA | 5% |
Abbreviations: GIST, gastrointestinal stromal tumor; NA, not available; RCC, renal cell carcinoma.
A second phase II trial was conducted to confirm the antitumor activity and safety observed in the first phase II trial (Motzer, Rini, et al 2006). One hundred and six patients with metastatic clear cell RCC were included. All have had prior nephrectomy and failed first-line cytokin-based therapy. Median duration of treatment was 5 months. Thirty six patients (34%) had partial response (Table 1). Thirty patients (29%) had stable disease lasting more than 3 months. The most commonly reported treatment-related grade 3 adverse events were fatigue (8%), hypertension (6%), stomatitis (5%), dermatitis (5%), and diarrhea (3%) (Table 2). The most frequently reported grade 3 or 4 laboratory abnormalities were asymptomatic elevated lipase (15%), neutropenia (16%), thrombocytopenia (6%), and anaemia (6%). Dose reductions were performed in 17 patients (16%) from 50 mg to 37.5 mg daily, and the dose for six of these patients was further reduced to 25 mg daily. The most common reasons for dose reduction were stomatitis and fatigue.
These outstanding results have led to a phase III trial comparing sunitinib with interferon-α as first-line therapy in patients with metastatic RCC (Motzer, Hutson, et al 2006). Patients were randomized to receive sunitinib 50 mg daily for 4 weeks, with 2 weeks off (n = 375) or subcutaneous injection 9 MU three times weekly interferon-α (n = 375). Objective response rate in the sunitinib arm and in the interferon-α arm were respectively 31% and 6% (p < 0.000001) (Table 1). Median time to progression was significantly longer in the sunitinib arm (11 vs 5 months) (p < 0.000001), as well as median overall survival (p = 0.02), although median overall survival has not yet been reached in either group. The most commonly reported treatment-related grade 3 or 4 adverse events were fatigue (7% vs 11% with interferon-α), diarrhea (5% vs 0% with interferon-α), and dermatitis (5% vs 0% with interferon-α) (Table 2). The most frequently reported grade 3 or 4 laboratory abnormalities were asymptomatic elevated lipase (4% vs 2% with interferon-α), neutropenia (11% vs 7% with interferon-α), and thrombocytopenia (8% vs 0% with interferon-α). Thirty patients (8%) withdrew from the study due to adverse event on sunitinib arm versus 49 patients (13%) on interferon-α arm. This study is the first phase III trial demonstrating a clearly clinical superiority over immunotherapy, with a favorable toxicity profile. Thus, sunitinib becomes the new standard in first-line treatment in patients with metastatic RCC.
Before sunitinib, other targeted therapies had been studied in metastatic RCC. Sorafenib, an oral multitargeted tyrosine kinase inhibitor, had also been studied in metastatic RCC. Sorafenib was identified as a potent inhibitor of VEGFR-2, VEGFR-3, FLT3, PDGFRβ, and KIT. Results with sorafenib are consistent with sunitinib results (Escudier et al 2005; Ratain et al 2005). Interestingly, the partial response rate induced by sorafenib is slightly lower to that of sunitinib, and the stable disease rate is similar. The relatively lower partial response rate associated with sorafenib may be related to the differential binding affinity of tyrosine kinase receptors including VEGFR-2 and PDGFRβ that is thought to be weaker for sorafenib as compared with that of sunitinib (Fabian et al 2005). So far, single agent clinical trials in RCC patients with either monoclonal antibodies or single molecules that block EGFR tyrosine kinase activity (eg, with erlotinib) have been disappointing (Yang et al 2003).
Sunitinib in the treatment of GIST
Approximately 85% of patients with GIST display activating mutations of KIT (Heinrich et al 2003; Corless et al 2004). Another 5% to 7% patients have activating mutations of PDGFRα (Heinrich et al 2003; Coreless et al 2005). Current standard of care for unresectable or malignant GIST is imatinib mesylate, a KIT and PDGFRα inhibitor (Blanke and Corless 2005). Approximately 12% to 14% of patients have primary resistance to imatinib (Demetri et al 2002; Van Glabbeke et al 2005). Furthermore, more than 40% of patients who initially responded to imatinib develop secondary imatinib resistance after a median of 18 to 26 months of treatment (Verweij et al 2004). Sunitinib had previously shown promising results in one patient with GIST failing prior therapy with imatinib mesylate in the phase I trial (Faivre et al 2006).
An open label multicenter phase I/II study of sunitinib in 97 patients with GIST showing progression or intolerance under imatinib mesylate therapy confirmed the recommended dose for sunitinib as 50 mg daily during 4 weeks with 2 weeks off (Maki et al 2005). Median time to progression was 7.8 months, and median overall survival 19.8 months. Thirty two patients (45%) had partial responseor stable disease lasting more than 6 months (Table 1). The most commonly reported treatment-related grade 3 or 4 adverse events were fatigue (10%), hypertension (17%), and asymptomatic lipase increase (13%) (Table 2). Tumor determination of KIT and PDGFRα mutations identified that median time to progression, as well as median overall survival, were significantly longer in patients with primary KIT exon 9 mutations or wild type versus primary KIT exon 11 mutations (14.3 and 13.8 vs 5.1 months, respectively).
A double-bind, placebo-controlled, multicenter, randomized phase III trial confirmed the efficacy and safety of sunitinib as second line therapy in 312 patients with GIST showing disease progression or intolerance under imatinib mesylate therapy (Demetri et al 2005). Patients were randomized in a 2:1 ratio to receive sunitinib 50 mg daily for 4 weeks, with 2 weeks off (n = 207) or placebo (n = 105). Objective response rate in the sunitinib arm and in the placebo arm were 8% and 0%, respectively (Table 1). Median time to progression was significantly longer in the sunitinib arm (6.3 vs 1.5 months), as well as median overall survival (hazard ratio, 0.491), although median overall survival has not yet been reached in either group. Fifty nine patients in the placebo group crossed over to sunitinib therapy due to disease progression. Ten percent had subsequent partial responses, suggesting that the optimal therapeutic effect of sunitinib may be observed when it was administered in the early disease phase. The most commonly reported treatment-related grade 3 or 4 adverse events were fatigue (7% vs 3% with placebo) and dermatitis (5% vs 0% with placebo) (Table 2). The most frequently reported grade 3 or 4 laboratory abnormalities were neutropenia (8% vs 0% with placebo) and thrombocytopenia (5% vs 0% with placebo).
Conclusions and future directions
Sunitinib has demonstrated a high level of efficacy with acceptable tolerability in both metastatic RCC and in GIST after disease progression or intolerance to imatinib mesylate therapy, leading to the recent first joint FDA approval for use in these 2 indications. Sunitinib also shows promising activity in other tumor such as breast cancer (Miller et al 2005), colorectal cancer (Lenz et al 2006), advanced non-small cell lung cancer (Socinski et al 2006), and neuroendocrine tumors (Kulke et al 2005). Phase II trials in patients with hepatocarcinoma and other solid tumors are ongoing.
The actual requested schedule for sunitinib administration is 6-week cycles of 4 weeks on treatment followed by 2 weeks off. The hypothesis that a potential tumor reactivation of tumor cells during the off-treatment period could occur has led to study continuous schedules. Phase II trials in patients with cytokine refractory metastatic RCC (De Mulder et al 2006), and imatinib-resistant GIST (George et al 2006) were performed in a continuous dose of 37.5 mg daily of sunitinib, and demonstrated that this continuous schedule is well tolerated. In this case, only few patients required treatment breaks or dose reduction.
Sunitinib has shown very promising results when used alone. Studies including other compounds with antiangiogenic properties such as bevacizumab have shown that the best efficacy was observed when combined to “classical” chemotherapy (Hurwitz et al 2004). Thus, combinations of sunitinib with these types of agents are ongoing. Another way to improve the antitumor activity could be to block different signaling pathways to overlap potential resistances to sunitinib. The combination of sunitinib and gefitinib, an EGFR inhibitor, has been tested in a phase I trial in patients with metastatic RCC (Ronnen et al 2006). The combination was well tolerated. The efficacy is being assessed in a phase II trial. Moreover, temsirolimus, a specific inhibitor of mammalian target of rapamycin (mTOR) that regulates cell growth and angiogenesis through the PI3K/AKT/mTOR pathway, has shown a very promising activity in first-line treatment compared with cytokin-based therapy in metastatic RCC (Hudes et al 2006). The combination of sunitinib with temsirolimus could be of great interest, particularly in RCC.
The results of sunitinib in GIST patients indicate that sunitinib has a greater efficacy in patients with primary KIT exon 9 mutations or wild type status than in those with primary KIT exon 11 mutations, suggesting that patients with KIT exon 9 mutations or wild type should receive sunitinib as first-line treatment.
Hypertension and asthenia are the most common side effects reported at the recommended doses of sunitinib. They have been observed with several other antiangiogenic therapies (Yang et al 2003; Ahmad and Eisen 2004; Willett et al 2004).
The majority of patients treated with sunitinib for metastatic RCC or GIST have stable disease with prolonged progression free survival, albeit response rates are sometimes low. Responding patients generally exhibit evidence of early tumor necrosis during the first treatment cycles. The size of the lesions is often stable. Most of the time, although central necrosis appears on computed tomography (CT) scan, contrast enhancement peripheral area persists, suggesting that viable tumor cells are not destroyed with sunitinib. When progression occurs, it is usually observed from the peripheral area of the tumor. These observations rise the question of the relevance of the RECIST criteria for the assessment of response to sunitinib, and in general for targeted therapies. Previous clinical studies suggest that [18F]fluorodeoxyglucose positron-emission tomography could be useful for the assessment of response in detecting early inhibition of intratumoral metabolic activity (Toner et al 2003). Measurement of CT scan density seems also to be of interest.
Considering the great efficacy of sunitinib in advanced RCC and GIST, trials to test the efficacy of sunitinib in the adjuvant or neoadjuvant setting for patients with high risk of recurrence tumors are greatly awaited.
Sunitinib belongs to a new class of multitargeted compounds that have demonstrated a high level of efficacy in metastatic RCC and GIST after disease progression or intolerance to imatinib mesylate therapy. Sunitinib malate has now been recognized as the standard treatment in first-line for patients with metastatic RCC. Sunitinib shows also promising activity in other types of cancer. Studies in adjuvant and neoadjuvant settings are awaited, as well as trials exploring the combination of sunitinb with chemotherapy or other targeted therapies.
References
- Abrams TJ, Lee JB, Murray LJ, et al. SU11248 inhibits KIT and platelet-derived growth factor receptor beta in preclinical models of human small cell lung cancer. Mol Cancer Ther. 2003;2:471–8. [PubMed] [Google Scholar]
- Abrams TJ, Murray LJ, Pesenti E, et al. Preclinical evaluation of the tyrosine kinase inhibitor SU11248 as a single agent and in combination with “standard of care” therapeutic agents for the treatment of breast cancer. Mol Cancer Ther. 2003;2:1011–21. [PubMed] [Google Scholar]
- Ahmad T, Eisen T. Kinase inhibition with BAY 43–9006 in renal cell carcinoma. Clin Cancer Res. 2004;10(18 Pt 2):6388S–92S. doi: 10.1158/1078-0432.CCR-040028. [DOI] [PubMed] [Google Scholar]
- Baratte S, Sarati S, Frigerio E, et al. Quantitation of SU1 1248, an oral multi-target tyrosine kinase inhibitor, and its metabolite in monkey tissues by liquid chromatograph with tandem mass spectrometry following semi-automated liquid-liquid extraction. J Chromatogr A. 2004;1024(1–2):87–94. doi: 10.1016/j.chroma.2003.10.085. [DOI] [PubMed] [Google Scholar]
- Bardos JL, Aschcroft M. Hypoxia-inducible factor-1 and oncogenic signalling. Bioessays. 2004;26:262–9. doi: 10.1002/bies.20002. [DOI] [PubMed] [Google Scholar]
- Bergers G, Song S, Meyer-Morse N, et al. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003;111:1287–95. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanke CD, Corless CL. State-of-the art therapy for gastrointestinal stromal tumors. Cancer Invest. 2005;23:274–80. doi: 10.1081/cnv-200055972. [DOI] [PubMed] [Google Scholar]
- Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol. 2004;22:3813–25. doi: 10.1200/JCO.2004.05.140. [DOI] [PubMed] [Google Scholar]
- De Mulder PH, Roigas J, Gillessen S, et al. A phase II study of sunitinib administered in a continuous daily regimen in patients with cytokine refractory metastatic renal cell carcinoma (mRCC). American Society of Clinical Oncology 42th Annual Meeting; 2–6 June; Atlanta, USA. 2006. [DOI] [PubMed] [Google Scholar]
- Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–80. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- Demetri DG, van Oosterom A, Blackstein M, et al. Phase 3 multicenter, randomized, double-bind, placebo-controlled trial of SU11248 in patients following failure of imatinib for metastatic GIST. American Society of Clinical Oncology 41st Annual Meeting; 13–17 May; Orlando, Florida, USA. 2005. [Google Scholar]
- Deprimo SE, Bello CL, Smeraglia J, et al. Soluble protein biomarkers of pharmacodynamic activity of the multitargeted tyrosine kinase inhibitor SU11248 in patients with metastatic renal cell carcinoma. 96th Annual Meeting of the American Association for Cancer Research; 16–21 April; Anaheim, California, USA. 2005. [Google Scholar]
- Escudier B, Szczylik C, Eisen T, et al. Randomized Phase III trial of the Raf kinase and VEGFR inhibitor sorafenib (BAY 43–9006) in patients with advanced renal cell carcinoma (RCC). American Society of Clinical Oncology 41st Annual Meeting; 13–17 May; Orlando, Florida, USA. 2005. [Google Scholar]
- Fabian MA, Biggs WH, Treiber DK, et al. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotechnol. 2005;23:329–36. doi: 10.1038/nbt1068. [DOI] [PubMed] [Google Scholar]
- Faivre S, Delbaldo C, Vera K, et al. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. 2006;24:25–35. doi: 10.1200/JCO.2005.02.2194. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Hillan KJ, Gerber HP, et al. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- George S, Casali PG, Blay J, et al. Phase II study of sunitinib administered in a continuous daily dosing regimen in patients (pts) with advanced GIST. American Society of Clinical Oncology 42th Annual Meeting; 2–6 June; Atlanta, USA. 2006. [Google Scholar]
- Hainsworth JD, Sosman JA, Spigel DR, et al. Treatment of metastatic renal cell carcinoma with a combination of bevacizumab and erlotinib. J Clin Oncol. 2005;23:7889–96. doi: 10.1200/JCO.2005.01.8234. [DOI] [PubMed] [Google Scholar]
- Heinrich MC, Corless MC, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342–9. doi: 10.1200/JCO.2003.04.190. [DOI] [PubMed] [Google Scholar]
- Hudes G, Carducci M, Tomczak P, et al. A phase 3, randomized, 3-arm study of temsirolimus (TEMSR) or interferon-alpha (IFN) or the combination of TEMSR+IFN in the treatment of first-line, poor-risk patients with advanced renal cell carcinoma (adv RCC). American Society of Clinical Oncology 42th Annual Meeting; 2–6 June; Atlanta, USA. 2006. [Google Scholar]
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- Kamura T, Sato S, Iwai K, et al. Activation of HIF1alpha ubiquitination by a reconstituted von Hippel-Lindau (VHL) tumor suppressor complex. Proc Natl Acad Sci U S A. 2000;97:10430–5. doi: 10.1073/pnas.190332597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nat Rev Cancer. 2002;2:727–39. doi: 10.1038/nrc905. [DOI] [PubMed] [Google Scholar]
- Kulke M, Lenz HJ, Meropol NJ, et al. A phase 2 study to evaluate the efficacy and safety of SU11248 in patients (pts) with unresectable neuroendocrine tumors (NETs). American Society of Clinical Oncology 41st Annual Meeting; 13–17 May; Orlando, Florida, USA. 2005. [Google Scholar]
- Lam JS, Leppert JT, Belldegrun AS, et al. Novel approaches in the therapy of metastatic renal cell carcinoma. World J Urol. 2005;23:202–12. doi: 10.1007/s00345-004-0466-0. [DOI] [PubMed] [Google Scholar]
- Latif F, Tory K, Gnarra J, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993;260:1317–20. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- Lenz H, Marshall J, Rosen L, et al. Phase II trial of SU11248 in patients with metastatic colorectal cancer (MCRC) after failure of standard chemotherapy. American Society of Clinical Oncology Gastrointestinal 4th Annual Meeting; 27–29 January; San Fransisco, USA. 2006. [Google Scholar]
- Maki RG, Fletcher JA, Heinrich MC, et al. SU11248 in patients with imatinib-resistant GIST : results from a continuation trial. American Society of Clinical Oncology 41st Annual Meeting; 13–17 May; Orlando, Florida, USA. 2005. [Google Scholar]
- Mendel DB, Laird AD, Xin X, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9:327–37. [PubMed] [Google Scholar]
- Miller KD, Burstein HJ, Elias AD, et al. Phase II study of SU11248, a multitargeted receptor tyrosine kinase inhibitor (TKI), in patients (pts) with previously treated metastatic breast cancer (MBC). San Antonio Breast Cancer Symposium 28th Annual Meeting; 8–11 December; San Antonio, Texas, USA. 2005. [Google Scholar]
- Morimoto AM, Tan N, West K, et al. Gene expression profiling of human colon xenograft tumors following treatment with SU11248, a multitargeted tyrosine kinase inhibitor. Oncogene. 2004;23:1618–26. doi: 10.1038/sj.onc.1207268. [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Bander NH, Nanus DM, et al. Renal-cell carcinoma. N Engl J Med. 1996;335:865–75. doi: 10.1056/NEJM199609193351207. [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Bacik J, Schwartz LH, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol. 2004;22:454–63. doi: 10.1200/JCO.2004.06.132. [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Rini BI, Bukowski RM, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA. 2006;295:2516–24. doi: 10.1001/jama.295.21.2516. [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Michaelson MD, Redman BG, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Hutson TE, Tomczak MD, et al. Phase III randomized trial of sunitinib malate (SU11248) versus interferon-alfa (IFN-α) as first-line systemic therapy for patients with metastatic renal cell carcinoma (mRCC). American Society of Clinical Oncology 42st Annual Meeting; 2–6 June; Atlanta, USA. 2006. [Google Scholar]
- Murray LJ, Abrams TJ, Long KR, et al. SU11248 inhibits tumor growth and CSF-1R-dependent osteolysis in an experimental breast cancer bone metastasis model. Clin Exp Metastasis. 2003;20:757–66. doi: 10.1023/b:clin.0000006873.65590.68. [DOI] [PubMed] [Google Scholar]
- O’Farrell AM, Foran JM, Fiedler W, et al. An innovative phase I clinical study demonstrates inhibition of FLT3 phosphorylation by SU11248 in acute myeloid leukemia patients. Clin Cancer Res. 2003;9:5465–76. [PubMed] [Google Scholar]
- Patel PH, Chaganti RSK, Motzer RJ. Targeted therapy for metastatic renal cell carcinoma. Br J Cancer. 2006;94:614–19. doi: 10.1038/sj.bjc.6602978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratain MJ, Eisen T, Stadler WM, et al. Final findings from a Phase II, placebo-controlled, randomized discontinuation trial (RDT) of sorafenib (BAY 43–9006) in patients with advanced renal cell carcinoma (RCC). American Society of Clinical Oncology 41st Annual Meeting; 13–17 May; Orlando, Florida, USA. 2005. [Google Scholar]
- Rohrmann K, Staehler M, Haseke N, et al. Immunotherapy in metastatic renal cell carcinoma. World J Urol. 2005;23:196–201. doi: 10.1007/s00345-004-0470-4. [DOI] [PubMed] [Google Scholar]
- Ronnen EA, Kondagunta GV, Lau C, et al. A phase I study of sunitinib malate (SU11248) in combination with gefitinib in patients with metastatic renal cell carcinoma (mRCC). American Society of Clinical Oncology 42th Annual Meeting; 2–6 June; Atlanta, USA. 2006. [Google Scholar]
- Shaheen RM, Tseng WW, Davis DW, et al. Tyrosine kinase inhibition of multiple angiogenic growth factor receptors improves survival in mice bearing colon cancer liver metastases by inhibition of endothelial cell survival mechanisms. Cancer Res. 2001;61:1464–8. [PubMed] [Google Scholar]
- Socinski MA, Novello S, Sanchez JM, et al. Efficacy and safety of sunitinib in previously treated, advanced non-small cell lung cancer (NSCLC): Preliminary results of a multicenter phase II trial. American Society of Clinical Oncology 42th Annual Meeting; 2–6 June; Atlanta, USA. 2006. [Google Scholar]
- Toner GC, Mitchell PL, De Boer R, et al. PET imaging study of SU11248 in patients with advanced malignancies. American Society of Clinical Oncology 39th Annual Meeting; 31 May–3 June; USA. 2003. [Google Scholar]
- Van Glabbeke M, Verweij J, Casali PG, et al. Initial and late resistance to imatinib in advanced gastrointestinal stromal tumors are predicted by different prognostic factors: a European Organisation for Research and Treatment of Cancer-Italian Sarcoma Group-Australasian Gastrointestinal Trials Group study. J Clin Oncol. 2005;23:5795–804. doi: 10.1200/JCO.2005.11.601. [DOI] [PubMed] [Google Scholar]
- Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumors with high-dose imatinib: randomised trial. Lancet. 2004;364:1127–34. doi: 10.1016/S0140-6736(04)17098-0. [DOI] [PubMed] [Google Scholar]
- Wang GL, Semenza GL. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci U S A. 1993;90:4304–8. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett CG, Boucher Y, di Tomaso E, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–7. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–34. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee KW, Schittenhelm M, O’Farrell AM, et al. Synergistic effect of SU11248 with cytarabine or daunorubicin on FLT3 ITD-positive leukemic cells. Blood. 2004;104:4202–9. doi: 10.1182/blood-2003-10-3381. [DOI] [PubMed] [Google Scholar]


