Abstract
Symbicort SMART® (Symbicort Maintenance and Reliever Therapy) represents a new and unique way of treating patients with moderate-to-severe asthma, ie, those patients who require combination treatment with an inhaled corticosteroid and a long-acting inhaled β2-agonist. Symbicort SMART enables patients to use only one inhaler, the budesonide-formoterol combination inhaler, for both maintenance and reliever therapy. The maintenance dose is adjustable, but should be a minimum of two doses per day which can be administered as two doses once daily or as one dose twice daily. It is important that the temporary reliever medication includes not only a bronchodilator but also an antiinflammatory drug because worsening of asthma includes not only more airway narrowing, but also an increase in airway inflammation. The Symbicort SMART concept therefore ensures that the patient gets an antiinflammatory drug at the time of the first signs of asthma worsening. Clinical results show that Symbicort SMART prolongs the time to the first severe asthma exacerbation, reduces the rate of exacerbations, and maintains day-to-day asthma control at a reduced load of corticosteroids (inhaled plus systemic) when compared with higher fixed maintenance doses of combination inhalers. Symbicort SMART consequently offers a more effective and simple approach to asthma management for physicians and patients. Symbicort SMART is also easier for the patient as only one inhaler is required. The positive results with Symbicort SMART can be explained by the early as-needed use on the inhaled corticosteroid component, which puts out the early flames of inflammation, together with the interaction between the β2-agonist, formoterol, and the inhaled corticosteroid, budesonide.
Keywords: asthma control, combination therapy, inhaled corticosteroid, long-acting β2-agonist, Symbicort SMART® (Symbicort Maintenance and Reliever Therapy)
Introduction
Patients suffering from asthma dislike having their disease out of control. Fear of exacerbations, emergency room visits, and hospitalizations are a reality. The introduction of effective asthma medications, such as inhaled corticosteroids (ICS), leukotriene modifiers, and long-acting bronchodilators, have given physicians and patients the therapeutic tools for effective treatment but surveys demonstrate that patients’ asthma control falls far short of the goals set out in asthma management programmes (Rabe et al 2000, 2004; Haughney et al 2004; Hyland and Ståhl 2004; Partridge et al 2006).
Patients’ perception of asthma control also differs from their actual asthma control (Rabe et al 2000). In a recent study of over 3000 patients from 11 countries treated with ICS with or without an inhaled long-acting β2-agonist bronchodilator (LABA), it was apparent that asthma control is rarely achieved (Partridge et al 2006). Using an asthma control questionnaire (ACQ6) (Juniper et al 1999, 2005), 51% of patients were found to have uncontrolled asthma, 21% were poorly controlled, and only 28% had well controlled asthma. This was despite the fact that 61% of patients were using a fixed combination of an ICS and a LABA and an additional 9% used the same medications via separate inhalers. Furthermore, 74% of patients reported a daily use of reliever medication and one or more exacerbations had occurred during the last year in 51% of these patients. At the time of deteriorating, asthma, patients tended to increase their dose of ICS late and the mean increase was less than two-fold. This was despite the fact that patients wanted to achieve immediate relief and were willing to self-manage their disease. It is thus obvious that the current management situation too often results in a major negative impact on patients’ health-related quality of life.
The discrepancy between asthma control and available treatment strategies may depend upon a variety of different factors. For example, poor compliance may result in inadequate maintenance therapy, management guidelines may not have been implemented, and the degree of disease severity may have been misinterpreted. All these factors can lead to insufficient medication at time of asthma worsening.
Worsening of asthma is not only a gradual change in airway calibre but is primarily, and more importantly, an increase in underlying airway inflammation. In a study of patients with stable asthma requiring ICS at daily doses ≥800 µg of beclomethasone or equivalents, mild exacerbations were induced by reducing the ICS treatment to 200 µg of budesonide per day (Jatakanon et al 2000). This study demonstrated that an increase in the number of sputum eosinophils and an increase in exhaled nitric oxide, which both are markers of airway inflammation, occurred before worsening of symptoms was reported. Therefore, maintaining asthma control during a phase of asthma deterioration and preventing exacerbations, such as at times of a viral infection or increased exposure to allergens or irritants, requires an increase in antiinflammatory medication in addition to more frequent use of reliever bronchodilators.
Treatment approaches
The 1-year FACET (Formoterol and Corticosteroids Establishing Therapy) study demonstrated how exacerbations can be avoided. Significant reductions in the rate of severe asthma exacerbations were seen by adding formoterol to a low (100 µg bid) or a four times higher dose (400 µg bid) of the ICS budesonide compared with the budesonide doses alone (Pauwels et al 1997). It was also shown that the four times higher budesonide dose resulted in significantly fewer severe exacerbations than low dose budesonide. Similarly, in patients with mild persistent asthma who were not sufficiently well controlled on an ICS alone, the addition of formoterol to a low dose of budesonide significantly reduced the rate of asthma exacerbations (O’Byrne et al 2001).
Combination inhalers were developed based on the results of the above-mentioned studies with an ICS and a LABA administered in separate inhalers. The first clinical study showed at least similar efficacy when the same doses were administered via one or two inhalers (Zetterström et al 2001). Later studies with ICS/LABA combinations administered via one or two separate inhalers have shown clear advantages for the single inhaler (Angus et al 2005).
Common clinical practice is to recommend doubling the dose of ICS at times of asthma worsening. This is probably not the most appropriate recommendation as clinical studies have shown that doubling the ICS dose is not enough for preventing asthma exacerbations (Garrett et al 1998; FitzGerald et al 2004; Harrison et al 2004). The ICS dose increase needs to be higher. Other studies have shown that in many patients a temporary increase in ICS dose on top of a lower maintenance dose at the time of deterioration is as effective in maintaining asthma control and preventing exacerbations as a higher maintenance dose (Foresi et al 2000).
Strategies involving the use of an ICS only as needed without maintenance therapy during asthma deterioration have been evaluated in patients with mild persistent asthma (Boushey et al 2005). Although some patients are well controlled on as-needed therapy alone, and no difference in exacerbation rates was seen between regular and intermittent treatment with budesonide in the study, this treatment approach has been justifiably criticized (O’Byrne 2005). It appears that patients with mild persistent asthma remain best controlled when they use regular maintenance medication with an antiinflammatory drug (Haahtela et al 1994). This was also seen in the study by Boushey and colleagues (2005) where regular treatment with budesonide was demonstrated to be superior to intermittent medication with regard to asthma-free days, lung function, and markers of inflammation. However, the daily maintenance dose of the ICS can be quite low (Haahtela et al 2006).
The message from all these different treatment strategies is clear. In the case of asthma worsening it is not sufficient to increase the bronchodilator dose: the inhaled antiinflammatory medication must also be appropriately increased. Without this, there is a great risk that patients have to be treated with systemic corticosteroids for at least 1–2 weeks and sometimes for much longer times. With this in mind it is of interest to review the published Symbicort SMART literature, ie, a treatment approach with budesonide-formoterol administered both as maintenance and reliever therapy ensures that the patient gets an antiinflammatory treatment at every time of the first signs of asthma worsening.
Combination of an inhaled long-acting β2-agonist and an inhaled corticosteroid
Patients with mild persistent asthma, ie, those with regular symptoms but a normal airway function, are usually well controlled on a medication consisting of an ICS as maintenance therapy and a rapid-acting β2-agonist used as needed. Patients not adequately controlled with ICS alone benefit from the addition of a LABA (NIH GINA 2006). An ICS and a LABA can be administered via separate inhalers or in a combination product. Two combination products are available: budesonide-formoterol (Symbicort®, AstraZeneca Ltd, Lund, Sweden), and salmeterol-fluticasone (Seretide™/Advair™, GlaxoSmithKline, Uxbridge, UK). They are both effective and safe therapies, but differ in two aspects. Inhaled formoterol has a rapid onset of action, similar to that of the short- and rapid-acting β2-agonist salbutamol (Seberová and Andersson 2000), whereas the onset of bronchodilation (Palmqvist et al 1997) and reversal of methacholine-induced bronchoconstriction (Politiek et al 1999) with salmeterol are significantly slower. Similarly, both the onset of bronchodilation (Palmqvist et al 2001) and the reversal of methacholine-induced bronchoconstriction (van der Woude et al 2004) are significantly faster with budesonide-formoterol compared with salmeterol-fluticasone. The systemic activity of formoterol is as short-acting as with salbutamol or terbutaline (Tötterman et al 1998; Seberová and Andersson 2000; Malolepszy et al 2001). Consequently the risk of side effects with repeated dosing of formoterol is no different to that with repeated administration of salbutamol or terbutaline. Furthermore, it is often forgotten that single doses of budesonide exhibit relevant antiinflammatory effects. A single dose of budesonide has been shown to decrease sputum eosinophil levels significantly (Gibson et al 2001) and the level of nitric oxide in exhaled air was also significantly reduced six hours after a single dose of nebulized budesonide to children with acute asthma (Tsai et al 2001). An increase in sputum eosinophils and in the amount of nitric oxide in exhaled air are predictive for the loss of asthma control (Jakatonon et al 2000). Already single doses of budesonide-formoterol thus favorably affect an early inflammation.
Both components of budesonide-formoterol exhibit a dose-response (Ellul-Micallef and Johansson 1983; Ringdal et al 1998) and very high doses of both have been found to be safe and well tolerated (Busse et al 1998; Tötterman et al 1998; Malolepszy et al 2001; Boonsawat et al 2003; Rubinfeld et al 2006). This allows patients an easy way to increase the number of daily doses of both budesonide and formoterol without changing inhaler devices in the event of worsening asthma. On the other hand, the systemic activity of salmeterol is as long-lasting as that of the bronchodilation and therefore additional doses cannot be given without causing more side effects (Bennett and Tattersfield 1997; Guhan et al 2000). Increasing the dose of salmeterol above 50 µg increases tremor and other side effects, but does not improve airway function (Ullman and Svedmyr 1988; Palmqvist et al 1999). Consequently, during asthma worsening patients on salmeterol-fluticasone must add the ICS via another inhaler or change, if possible, from one combination strength of salmeterol-fluticasone to another.
Due to the properties of budesonide-formoterol, this review will focus on a new smarter way of using this combination in the treatment of patients with moderate-to-severe asthma. Budesonide-formoterol is available in two strengths for the treatment of asthma: 80/4.5 µg and 160/4.5 µg per dose (delivered doses corresponding to 100/6 µg and 200/6 µg metered doses). A higher strength, 320/9 µg per dose, is mainly used for the treatment of chronic obstructive pulmonary disease.
Adjustable maintenance therapy with budesonide-formoterol
As described above the clinical pharmacology profile of budesonide-formoterol allows the daily dose to be adjusted in response to disease severity without changing inhaler. A series of clinical studies have been presented evaluating the clinical value of adjusting the dose of budesonide-formoterol compared with a fixed daily dose (Ställberg et al 2003; FitzGerald et al 2003; Leuppi et al 2003; Aalbers et al 2004; Buhl et al 2004; Canonica et al 2004; Ind et al 2004; Holt et al 2005). The general principle for these randomized comparisons has been that patients with moderate severe asthma were first stabilized on two doses twice daily of budesonide-formoterol. Patients randomized to fixed dosing continued on two doses twice daily while monitoring peak flow, recording night-time awakenings, and use of β2-agonists to determine when medical checks and treatment with oral steroids were required in a situation of severe asthma worsening. Patients randomized to the adjustable dosing arm and fulfilling stability requirements had their daily dose reduced to one dose twice daily. In the case of asthma worsening this dose was increased to four doses twice daily for 1–2 weeks. When stability criteria were again achieved patients reduced their dose to one in the morning and one in the evening. In the majority of the cited studies, the total burden of corticosteroids (ICS for maintenance therapy plus oral corticosteroids during exacerbations) was found to be significantly reduced compared with treatment with higher fixed dosing of ICS. However, measures of asthma control, eg, asthma symptoms, use of reliever medication, airway function, and night-time awakenings due to asthma, were not significantly different between groups randomized to fixed or adjustable maintenance treatment of budesonide-formoterol.
The take-home message from this programme is in line with the long-time experiences gained with ICS: at the time of asthma worsening the steroid dose must be increased in order to avoid exacerbations and hospitalizations. This can now also be done by temporarily quadrupling the maintenance dose of budesonide-formoterol. The total burden of corticosteroids was significantly lower compared with fixed dosing of ICS/LABA combination products.
Budesonide-formoterol as both maintenance and reliever therapy
Considering the fact that an asthma exacerbation is the combination of an increase in both inflammation and broncho-constriction, it seemed logical to investigate the usefulness of budesonide-formoterol as both maintenance and reliever therapy. This treatment approach has been coined Symbicort SMART® (Symbicort Maintenance and Reliever Therapy) (Gibson 2005) and means that patients are taking defined maintenance doses of budesonide-formoterol and additional doses as needed instead of a short-acting reliever. There are currently seven published studies evaluating the effectiveness of this treatment approach.
In initial 6- and 12-month Symbicort SMART studies, two doses of budesonide-formoterol were given once daily with additional doses as needed and compared with a higher dose of budesonide alone with terbutaline used as needed (Scicchitano et al 2004; Rabe, Pizzichini, et al 2006). As expected airway function improved when a LABA was added, but, more importantly, the risk of severe asthma exacerbations was reduced despite the fact that patients in the Symbicort SMART treatment arms used less steroids. In both studies total asthma symptom scores, symptom-free days, as-needed medication-free days, and asthma-control days, ie, a night and day with no asthma symptoms, no use of reliever medication, and no asthma-related night-time awakenings, were statistically significantly lower in the Symbicort SMART groups. In one of the studies (Rabe, Pizzichini, et al 2006) nighttime awakenings did not reach the level of statistical significance (p = 0.065).
The Symbicort SMART approach was more rigorously tested in a 12-month study in patients with moderate-to-severe asthma (n = 2760) where Symbicort SMART (twice daily maintenance treatment with budesonide-formoterol 80/4.5 µg plus additional doses as needed; patients aged 4–11 years old inhaled the medication once daily in the evening) was compared with a four times higher dose of budesonide alone with terbutaline as needed. Patients in a third treatment arm used a fixed dose of budesonide-formoterol with terbutaline as needed (O’Byrne et al 2005). Symbicort SMART therapy improved airway function significantly more than the two other treatments. It prolonged the time to the first severe asthma exacerbation (p < 0.001) resulting in a 45%–47% lower exacerbation risk versus budesonide-formoterol in fixed dosing (hazard ratio [HR] 0.55, 95% confidence interval [CI] 0.44, 0.67) or four times higher budesonide dose (HR 0.53, 95% CI 0.43, 0.65) (Figure 1). These results in favor of Symbicort SMART were achieved despite lower steroid use in that group. The adult patients used mean doses of budesonide of 240 µg, 160 µg, and 640 µg in the Symbicort SMART, budesonide-formoterol fixed dosing, and higher budesonide dose groups, respectively, and in addition oral steroids on 1255, 2918, and 2577 days, respectively, during the entire study period. In children (n = 341) similar results were seen (Bisgaard et al 2006), where the budesonide doses were 126 µg, 80 µg, and 320 µg per day and the number of days with oral steroids were 32, 230, and 141, respectively. Compared with budesonide-formoterol fixed dosing plus terbutaline as needed, the Symbicort SMART treatment resulted in statistically significantly lower use of reliever medication, night-time asthma symptoms and awakenings, but not in less daytime symptoms, symptom-free days, reliever-free days, or asthma control days.
Figure 1.
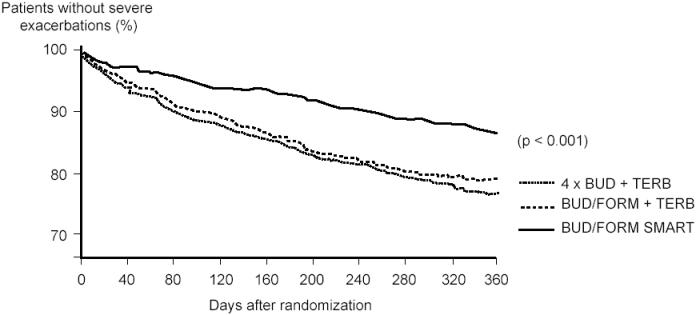
Time to first severe asthma exacerbation. The Symbicort SMART approach resulted in a 45% reduction compared with budesonide-formoterol maintenance therapy with terbutaline as needed, and in a 47% reduction compared with the four times higher dose of budesonide maintenance dose and terbutaline used as needed. American Thoracic Society copyright © 2005. Data reproduced with permission from O’Byrne PM, Bisgaard H, Godard PP, et al. 2005. Budesonide-formoterol combination therapy as both maintenance and reliever medication in asthma. Am J Respir Crit Care Med, 171:129–36. Official journal of the American Thoracic Society.
Abbreviations: BUD, budesonide; FORM, formoterol;TERB, terbutaline.
To reflect clinical practice, another 12-month study compared Symbicort SMART with salmeterol-fluticasone in adjustable doses plus salbutamol as needed. Adjustment of salmeterol-fluticasone was performed by changing inhalers based on actual asthma control as assessed by the physician (Vogelmeier et al 2005). The Symbicort SMART concept, as per the above studies, resulted in a 25% lower risk of having a first asthma exacerbation (p = 0.01) (Figure 2). The cumulative risk of having a severe exacerbation was 22% lower in the Symbicort SMART group (p < 0.05) with 0.24 events per patient per year in the Symbicort SMART group compared with 0.31 events in the salmeterol-fluticasone group. The difference in risk of having a severe exacerbation was true for all types of exacerbations based on unscheduled visits initiated by the patients, hospitalizations, and emergency room treatments as well as courses of oral steroids. The difference was statistically significant even when unscheduled visits as a cause for exacerbations were excluded. The overall mean use of ICS was very similar in the two groups: budesonide 562 µg as maintenance dose and additional 91 µg per day as needed (total daily dose 653 µg) compared with 583 µg per day of fluticasone. Oral steroids had to be given for 1980 days in the Symbicort SMART group compared with 2978 days in the salmeterol-fluticasone group. Thus a clear oral steroid sparing effect was seen in the Symbicort SMART study group. Asthma control was evaluated using the asthma control questionnaire, ACQ5 (Juniper et al 1999). No statistically significant difference was found between the treatment groups.
Figure 2.
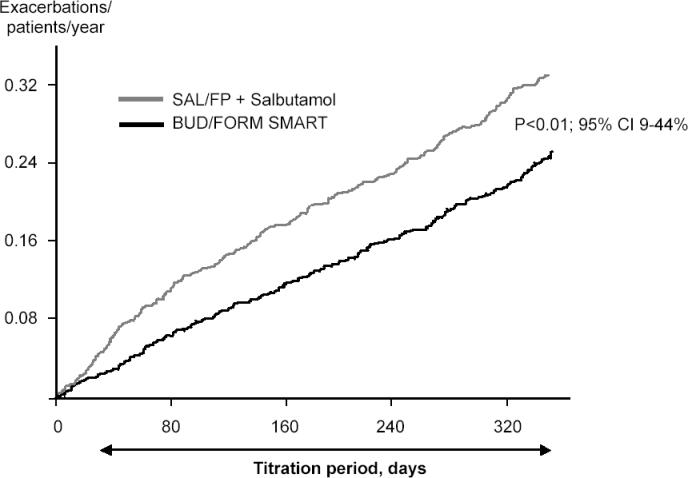
The cumulative rate of severe exacerbations in a study comparing budesonide-formoterol as maintenance and reliever therapy (Symbicort SMART®) compared with salmeterol-fluticasone in adjustable dosing and salbutamol used as needed. Symbicort SMART reduced the exacerbation rate by 22% (p < 0.01). Copyright © 2005. Reproduced with permission from Vogelmeier C, D’Urzo A, Pauwels R, et al. 2005. Budesonide-formoterol maintenance and reliever therapy: an effective asthma treatment option? Eur Respir J, 26:819–28.
Abbreviations: BUD/FORM, budesonide-formoterol; SAL/FP, salmeterol-fluticasone.
An open-label study evaluated whether a one dose once-daily maintenance treatment with Symbicort could be used with sustained efficacy when additional doses were allowed to be taken as needed. Patients with moderate-to-severe asthma (n = 491) were randomized to 6 months’ treatment with Symbicort SMART 160/4.5 µg once or twice daily with additional doses as needed, or to two doses twice daily of 160/4.5 µg plus formoterol 4.5 µg as needed (Lundborg et al 2005). Thus the patients received one, two, or four regular doses per day of budesonide-formoterol. The primary variables of efficacy were the changes in a modified ACQ5 (Juniper et al 1999) and morning peak expiratory flow (PEF). No differences between the groups were found in ACQ5 scores. The exacerbation rates were also similar in the three groups, but the study was not powered for detection of differences in exacerbation rates. Morning PEF was higher in the group receiving the high maintenance dose of budesonide-formoterol compared with the once and twice daily Symbicort SMART groups (differences 13 L/min and 9 L/min, respectively; p < 0.002). No difference was seen in asthma control days between the twice daily Symbicort SMART group and the twice higher budesonide-formoterol fixed dose group. However, the once daily Symbicort SMART group showed a significant decrease in asthma control days compared with the two other groups. Compared with higher fixed-dose budesonide-formoterol plus formoterol the use of budesonide-formoterol was 30%–40% lower in the Symbicort SMART groups. Treatment costs were significantly lower in the Symbicort SMART groups compared with the higher fixed dose group of budesonide-formoterol.
As one single maintenance dose of Symbicort SMART per day resulted in a decrease in asthma control days, such a low maintenance treatment should not be recommended for patients with moderate persistent asthma. Loss of asthma control was also demonstrated in a study comparing salmeterol-fluticasone with an adjustable maintenance dose of budesonide-formoterol forcing 82% of patients in the latter group to titrate their maintenance dose down from four to one inhalation per day (FitzGerald et al 2005). Such a low maintenance dose is not in agreement with treatment recommendations for patients with moderate severe asthma (NIH GINA 2004). However, in contrast to the study by FitzGerald and colleagues (2005), loss of exacerbation control was not seen with only one dose of Symbicort SMART. This may indicate that the Symbicort SMART approach, when pushed to a very low dose, as in the one dose once-daily arm in the study by Lundborg and colleagues (2005), allows patients to respond immediately to any breakthrough symptoms with an effective reliever therapy, thus reducing any danger of exacerbations compared with adjustable maintenance regimens that rely on detecting a deterioration in control over several days before increasing maintenance therapy.
To investigate the importance of the ICS component given as needed medication within the Symbicort SMART concept a 12-month, three-arm, double-blind, randomized study was performed (Rabe, Atienza, et al 2006). All patients (n = 3394) received identical twice-daily maintenance doses of budesonide-formoterol 160/4.5 µg, but the reliever medication consisted of terbutaline, formoterol, or budesonide-formoterol. The time to the first severe asthma exacerbation was longer with as-needed budesonide-formoterol versus formoterol (p = 0.005) and longer with formoterol versus terbutaline (p = 0.005) (Figure 3). The rate of severe exacerbations was 19, 29, and 37 per 100 patients per year with budesonide-formoterol, formoterol, and terbutaline, respectively, as as-needed medication. The ratios of budesonide-formoterol versus formoterol was 0.67 (p < 0.0001), budesonide-for-moterol versus terbutaline 0.52 (p < 0.0001), and formoterol versus terbutaline 0.78 (p = 0.00012). Thus the results show a significant benefit of adding budesonide to the as-needed medication compared with both formoterol and a traditional short-acting β2-agonist.
Figure 3.
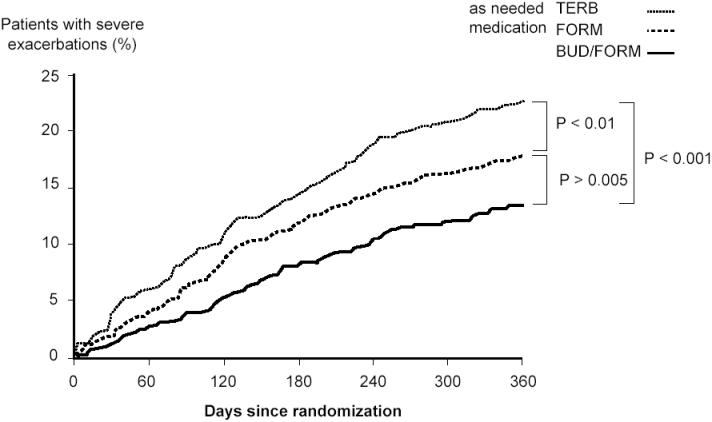
Time to the first severe asthma exacerbation. All patients used similar doses of budesonide-formoterol, as maintenance therapy. The as-needed medication was budesonide-formoterol (Symbicort SMART), formoterol or terbutaline. The risk of having a severe exacerbation was reduced by 27% with Symbicort SMART compared with formoterol and by 45% compared with terbutaline as as-needed medication. Copyright © 2006. Reproduced with permission from Rabe KF, Atienza T, Magyar P, et al. 2006. Effect of budesonide in combination with formoterol for reliever therapy in asthma exacerbations: a randomised controlled, double-blind study. Lancet, 368:744–53.
Abbreviations: BUD/FORM, budesonide-formoterol; FORM, formoterol;TERB, terbutaline.
A recent 6-month study compared Symbicort SMART (160/4.5 µg twice daily plus as needed) with the highest, fixed maintenance dose of budesonide-formoterol (320/9 µg twice daily, but with terbutaline used as needed), and with an equally high fixed dose of salmeterol-fluticasone (25/125 µg two doses twice daily, and with terbutaline as needed) (Kuna et al 2006). A total of 3335 patients aged 12 years or older were included. Indicators of asthma control, lung function, symptoms, and night-time awakenings improved similarly in all three groups. Symbicort SMART prolonged the time to the first severe asthma exacerbation compared with the higher fixed doses of budesonide-formoterol or salmeterol-fluticasone and was associated with 28% and 39% fewer exacerbations, respectively (p < 0.01 for both). The mean exacerbation rates were 12, 16, and 19 per 100 patients during the 6-month study period in the Symbicort SMART, higher dose budesonide-formoterol, and salmeterol-fluticasone groups, respectively. Patients in the Symbicort SMART group used more than 25% less ICS than patients in the fixed dose groups.
Safety and tolerability of budesonide-formoterol
The safety and tolerability of the monocomponents of the combination inhaler, budesonide and formoterol, have been well established during their long-term use. Budesonide for inhalation is approved in daily doses up to 1600 µg per day. A temporary increase in dose above this level may be reflected in a temporary suppression of the hypothalamic-pituitary-adrenal (HPA) axis but without long-term deleterious effects. It should also be remembered that the alternative to using a higher dose of budesonide in the event of asthma worsening usually consists of administration of systemic corticosteroids, which, from a safety point of view, is a less desirable alternative.
Similarly, inhaled formoterol can be given safely in very high doses to patients with acute airway obstruction (Malolepszy et al 2001; Boonsawat et al 2003; Rubinfeld et al 2006). In one study in patients with acute severe asthma, patients received 90 µg formoterol or 10 mg terbutaline via Turbuhaler® (AstraZeneca, Lund, Sweden) over 180 minutes. The bronchodilatatory capacity was similar between formoterol and terbutaline, but formoterol had significantly less effect on pulse rate and serum potassium levels (Malolepszy et al 2001). Compared with inhaled salbutamol, formoterol appears to have an improved ratio between effects on forced expiratory volume in one second (FEV1) and systemic activity measured as decrease in serum potassium (Rosenborg et al 2000, 2002).
The combination product (budesonide-formoterol) has been found to be safe and well tolerated during long-term use (Rosenhall et al 2002, 2003) and in high doses (Ankerst et al 2003).
How does the budesonideformoterol combination work so well?
The precise molecular mechanisms for the interaction between budesonide and formoterol are still not fully understood. From a simple point of view, it can be seen that budesonide influences the chronic asthmatic inflammation by affecting eosinophils and by reducing the number of surface mast cells in the airways thereby reducing the amount of released bronchoconstrictive mediators. Formoterol, on the other hand, influences processes not affected by steroids: stabilization of mast cell membranes, relaxation of smooth muscle cells, inhibition of capillary plasma leak, and sensory nerve activation. These combined effects of budesonideformoterol are thus complementary and additive (Barnes 2002, 2007).
Formoterol increases the translocation of glucocorticoid receptors from the cytoplasm into the cell nucleus where the steroid exerts its antiinflammatory effects (Usmani et al 2005). The presence of LABA also influences the recycling of glucocorticoid receptors by prolonging their stay in the nucleus and thereby prolonging steroid antiinflammatory efficacy (Haque et al 2006). Furthermore, formoterol also inhibits the release of interleukin (IL)-8 from epithelial cells thus affecting the neutrophils, which are present in patients with more severe asthma (Maneechotesuwan et al 2005). Finally, the interaction between antigen-presenting cells and effector cells in the asthmatic inflammatory process is mediated via GATA-3, a transcription factor that regulates the synthesis of Th2 cytokines. GATA-3 is strongly inhibited by corticosteroids (Maneechotesuwan et al 2002).
These mechanisms show that there is a useful molecular interaction between budesonide and formoterol, which can be either additive or synergistic. Clinically the additive effects are obvious. The synergy has been well demonstrated in vitro, but not adequately in vivo. These interactions have been described in further detail elsewhere (Barnes 2002, 2007; Miller-Larsson and Selroos 2006). A schematic picture of the interaction is shown in Figure 4.
Figure 4.
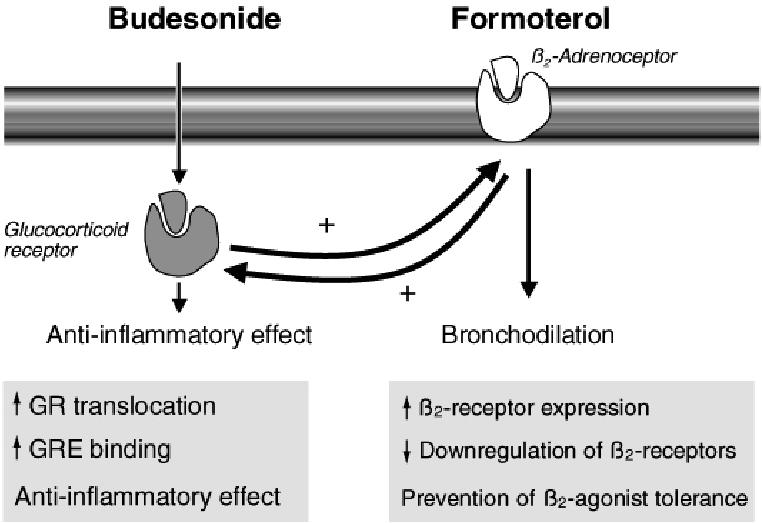
Interaction between budesonide and the long-acting β2-agonists (LABA) formoterol. Budesonide has antiinflammatory effects but also increases the numbers of β2-receptors, whereas formoterol, as well as inducing direct bronchodilation, acts on glucocorticoid receptors (GR) to increase the antiinflammatory effects of budesonide and increases the translocation of budesonide into the cell nucleus. Copyright © 2002. Modified with permission from Barnes PJ. 2002. Scientific rationale for inhaled combination therapy with long-acting β2-agonists and corticosteroids. Eur Respir J, 19:182–91.
Abbreviations: GRE, glucocorticoid response element.
It is known that signs of increased airway inflammation precede an increase in symptoms and airway obstruction during asthma worsening (Jatakonon et al 2000). An important clinical component of the Symbicort SMART approach is therefore the immediate increase in ICS dose and dosing frequency that happens when budesonide-formoterol is used also as needed. This was well demonstrated in the study by Rabe, Atienza, et al (2006). At the same time, the rapid-acting formoterol component counteracts any increase in airway smooth muscle contraction.
Patient perspectives
From the INSPIRE study (Partridge et al 2006), involving 3047 patients from 11 European countries, it was clear that a high proportion of patients (89%) had experienced episodes of asthma worsening during the preceding year despite being prescribed effective medications. The patients were selected for the study by physicians and they were all using ICS with or without LABA (approximately one third used an ICS alone). Also, patients defined as having well-controlled asthma by the ACQ6 (Juniper et al 1999, 2005) were found to have had a significant number of asthma deteriorations in the previous year. An encouraging finding was that patients were able to detect the periods of worsening early and were willing and interested in altering their medication themselves to obtain control of their asthma. Patients wanted a treatment that gives immediate relief from symptoms but were concerned about taking too much medication when they felt well. This could be interpreted as a preference for varying therapy according to the individual’s actual need but also for reducing the dose of maintenance therapy to the lowest necessary dose that provides effective asthma control. In this study there was a large number of patients who self-managed their medication as advocated in asthma management guidelines (NIH GINA 2006) and wanted to be taught to adjust their medication without having to see a physician.
Surveys also show that patients with asthma prefer simple treatment regimens, preferring treatments with as few drugs or inhalers as possible (Hyland and Ståhl 2004). When asked about ICS, many patients expressed concern about this class of drugs and wanted to use the lowest possible dose.
In a recent study using the discrete choice modelling technique it was evident that patients would like to be treated in a way that avoids hospitalizations and emergency room visits. Everyone appreciates a regimen resulting in as few symptoms as possible (Haughney et al 2006).
Based on this knowledge, it appears that the Symbicort SMART approach could be suitable for many patients with moderate-to-severe asthma, thereby fulfilling their wishes of a simple therapy within the framework of a simple and clear self-management programme.
Of course, there may be patients who from a medical point of view are less suitable for the Symbicort SMART concept. These include, for example, habitual users of bronchodilators and poor perceivers of asthma symptoms. It is therefore important that patients are well informed about the maximum number of additional doses of budesonide-formoterol that can be taken on single days and over a number of consecutive days. There may also be patients who simply do not want to adjust their medication based on a self-management plan.
Conclusions
ICS and LABA have complementary effects, and patients with asthma not adequately controlled with ICS alone benefit from the addition of a LABA (NIH GINA 2006). ICS exhibit a rapid suppression on airway inflammation. Formoterol, the LABA in the budesonide-formoterol combination product (Symbicort), relaxes airway smooth muscles but also inhibits release of mast cell mediators and has inhibitory effects on plasma exudation and neutrophilic inflammation. ICS and LABA can be administered via two separate inhalers but using a combination inhaler appears more convenient and may improve compliance. Using a low dose of budesonideformoterol during periods of good asthma control, and temporarily quadrupling the dose for one to two weeks when symptoms have developed and a decrease in airway function can be recognized, has been found to prevent the rate and severity of asthma exacerbations but at a lower total corticosteroid dose over time.
Formoterol also has a fast onset of action and can therefore be used as rescue therapy in asthma. Markers of inflammation, such as nitric oxide in exhaled air and number of eosinophils in sputum, increase at times of asthma worsening, and an increase in ICS dose is therefore also required. In addition to maintenance treatment the budesonide-formoterol combination product has therefore also been evaluated as rescue therapy. This treatment approach, Symbicort SMART, has been found useful in patients with asthma not adequately controlled on ICS alone. Symbicort SMART has not been investigated in controlled studies in comparison with adjustable maintenance therapy. Compared with much higher fixed doses of combination products, the rate and severity of exacerbations have been significantly reduced, and with lower total doses of corticosteroids over time while maintaining the same good asthma control.
When comparing adjustable maintenance dosing of budesonide-formoterol with Symbicort SMART, from the patients’ perspective it seems obvious that they prefer effective treatment at the first signs of asthma worsening and do not like to wait until a level of predefined symptoms and decrease in airway function have developed. The study with three identical Symbicort SMART arms, but with different rescue treatments, terbutalin, formoterol, or budesonide-formoterol (Rabe, Atienza, et al 2006), clearly showed the advantage of using a rescue therapy containing an ICS. Therefore, for the vast majority of patients who require combination treatment with an ICS and a LABA the Symbicort SMART approach appears clinically useful and advantageous. Thus Symbicort SMART is also a smart way to manage asthma from the patients’ perspective.
Acknowledgments
Publication support for this manuscript was provided by AstraZeneca, Lund, Sweden. Dr Selroos is a consultant for AstraZeneca R&D, Lund, Sweden, and has share options in AstraZeneca.
References
- Aalbers R, Backer V, Kava KK, et al. Adjustable maintenance dosing with budesonide-formoterol compared with fixed-dose salmeterol-fluticasone in moderate to severe asthma. Curr Med Res Opin. 2004;20:225–40. doi: 10.1185/030079903125002928. [DOI] [PubMed] [Google Scholar]
- Angus R, Reagon R, Cheesbrough A. Short-acting β2-agonist and oral corticosteroid use in asthma patients prescribed either concurrent beclomethasone and long-acting β2-agonist or salmeterol-fluticasone propionate combination. Int J Clin Pract. 2005;59:156–62. doi: 10.1111/j.1742-1241.2005.00455.x. [DOI] [PubMed] [Google Scholar]
- Ankerst J, Persson G, Weibull E. Tolerability of a high dose of budesonide-formoterol in a single inhaler in patients with asthma. Pulm Pharmacol Ther. 2003;16:147–51. doi: 10.1016/S1094-5539(03)00004-X. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Scientific rationale for inhaled combination therapy with long-acting β2-agonists and corticosteroids. Eur Respir J. 2002;19:182–91. doi: 10.1183/09031936.02.00283202. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Scientific rationale for using a single inhaler for asthma control. Eur Respir J. 2007;29:587–95. doi: 10.1183/09031936.00080306. [DOI] [PubMed] [Google Scholar]
- Bennett JA, Tattersfield AE. Time course and relative dose potency of systemic effects from salmeterol and salbutamol in healthy subjects. Thorax. 1997;52:458–64. doi: 10.1136/thx.52.5.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisgaard H, LeRoux P, Bjåmer D, et al. Budesonide-formoterol maintenance plus reliever therapy: a new strategy in pediatric asthma. Chest. 2006;130:1733–43. doi: 10.1378/chest.130.6.1733. [DOI] [PubMed] [Google Scholar]
- Boonsawat W, Charoenratanakul S, Pothiratana C, et al. Formoterol (Oxis®) Turbuhaler® as a rescue therapy compared with salbutamol by pMDI plus spacer in patients with acute severe asthma. Respir Med. 2003;97:1067–74. doi: 10.1016/s0954-6111(03)00139-2. [DOI] [PubMed] [Google Scholar]
- Boushey HA, Sorkness CA, King TS, et al. Daily versus as needed corticosteroids for mild persistent asthma. N Engl J Med. 2005;352:519–28. doi: 10.1056/NEJMoa042552. [DOI] [PubMed] [Google Scholar]
- Buhl R, Kardos P, Richter K, et al. The effect of adjustable dosing with budesonide-formoterol on health-related quality of life and asthma control compared with fixed dosing. Curr Med Res Opin. 2004;20:1209–29. doi: 10.1185/030079903125004303. [DOI] [PubMed] [Google Scholar]
- Busse WW, Chervinsky P, Condemi J, et al. Budesonide delivered by Turbuhaler is effective in a dose-dependent fashion when used in the treatment of adult patients with chronic asthma. J Allergy Clin Immunol. 1998;101:457–63. doi: 10.1016/S0091-6749(98)70353-7. [DOI] [PubMed] [Google Scholar]
- Canonica GW, Castellani P, Cazzola M, et al. Adjustable maintenance dosing with budesonide-formoterol in a single inhaler provides effective asthma symptom control at a lower dose than fixed maintenance dosing. Pulm Pharmacol Ther. 2004;17:239–47. doi: 10.1016/j.pupt.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Ellul-Micallef R, Johansson S-A. Acute dose-response studies in bronchial asthma with a new corticosteroid, budesonide. Br J Clin Pharmacol. 1983;115:419–22. doi: 10.1111/j.1365-2125.1983.tb01524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald JM, Sears MR, Boulet L-P, et al. Adjustable maintenance dosing with budesonide-formoterol reduces asthma exacerbations compared with traditional fixed dosing: a five-month multicentre Canadian study. Can Respir J. 2003;10:427–34. doi: 10.1155/2003/298039. [DOI] [PubMed] [Google Scholar]
- FitzGerald JM, Becker A, Sears MR, et al. Doubling the dose of budesonide versus maintenance treatment in asthma exacerbations. Thorax. 2004;59:550–6. doi: 10.1136/thx.2003.014936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald JM, Boulet L-P, Follows RMA. The CONCEPT trial: a 1-year, multicenter, randomized, double-blind, double-dummy comparison of a stable dosing regimen of salmeterol-fluticasone propionate with an adjustable maintenance dosing regimen of formoterol/budesonide in adults with persistent asthma. Clin Ther. 2005;27:393–406. doi: 10.1016/j.clinthera.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Foresi A, Morelli MC, Catena E. Low-dose budesonide with the addition of an increased dose during exacerbations is effective in long-term asthma control. Chest. 2000;117:440–6. doi: 10.1378/chest.117.2.440. [DOI] [PubMed] [Google Scholar]
- Garrett J, Williams S, Wong C, et al. Treatment of acute exacerbations with an increased dose of inhaled steroid. Arch Dis Child. 1998;79:12–17. doi: 10.1136/adc.79.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson PG, Saltos N, Fakes K. Acute anti-inflammatory effects of inhaled budesonide in asthma: a randomized controlled trial. Am J Respir Crit Care Med. 2001;163:32–6. doi: 10.1164/ajrccm.163.1.9807061. [DOI] [PubMed] [Google Scholar]
- Gibson PG. Teaching old drugs new tricks: asthma therapy adjusted by patient perception or noninvasive markers. Eur Respir J. 2005;25:397–9. doi: 10.1183/09031936.05.00002805. [DOI] [PubMed] [Google Scholar]
- Guhan AR, Cooper S, Oborne J, et al. Systemic effects of formoterol and salmeterol : a dose-response comparison in healthy subjects. Thorax. 2000;55:650–6. doi: 10.1136/thorax.55.8.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haahtela T, Järvinen M, Kava T, et al. Effects of reducing or discontinuing inhaled budesonide in patients with mild asthma. N Engl J Med. 1994;331:700–5. doi: 10.1056/NEJM199409153311103. [DOI] [PubMed] [Google Scholar]
- Haahtela T, Tuomisto LE, Pietinalho A, et al. A 10 year asthma programme in Finland: major change for the better. Thorax. 2006;61:663–70. doi: 10.1136/thx.2005.055699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque RA, Johnson M, Adcock IM, et al. Addition of salmeterol to fluticasone prolongs retention of glucocorticoid receptors within the nucleus of BEAS-2B cells and enhances downstream glucocorticoid effects. Proc Am Thorac Soc. 2006;3:A78. [Google Scholar]
- Harrison TW, Oborne J, Newton S, et al. Doubling the dose of inhaled corticosteroid to prevent asthma exacerbations: randomised controlled trial. Lancet. 2004;363:271–5. doi: 10.1016/s0140-6736(03)15384-6. [DOI] [PubMed] [Google Scholar]
- Haughney J, Barnes G, Partridge M, et al. The living and breathing study: a study of patients’ views of asthma and its treatments. Prim Care Resp J. 2004;13:28–35. doi: 10.1016/j.pcrj.2003.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughney J, Fletcher M, Wolfe S, et al. Features of asthma management: quantifying the patient’s perspective using discrete choice modelling. Eur Respir J. 2006;28(suppl 50):122s. [Google Scholar]
- Holt S, Ryder-Lewis S, Masoli M, et al. Fixed and adjustable dose asthma action plans based on combination therapy: a pilot study. Respirology. 2005;10:497–503. doi: 10.1111/j.1440-1843.2005.00741.x. [DOI] [PubMed] [Google Scholar]
- Hyland ME, Ståhl E. Asthma treatment needs: a comparison of patients’ and health care professionals’ perceptions. Clin Ther. 2004;26:2141–52. doi: 10.1016/j.clinthera.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Ind PW, Haughney J, Price D, et al. Adjustable and fixed dosing with budesonide-formoterol via a single inhaler in asthma patients: the ASSURE study. Respir Med. 2004;98:464–75. doi: 10.1016/j.rmed.2003.07.010. [DOI] [PubMed] [Google Scholar]
- Jatakanon A, Lim S, Barnes PJ. Changes in sputum eosinophils predict loss of asthma control. Am J Respir Crit Care Med. 2000;161:64–72. doi: 10.1164/ajrccm.161.1.9809100. [DOI] [PubMed] [Google Scholar]
- Juniper EF, O’Byrne PM, Guyatt GH, et al. Development and validation of questionnaire to measure asthma control. Eur Respir J. 1999;14:902–7. doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
- Juniper EF, Svensson K, Mörk A-C, et al. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med. 2005;99:553–8. doi: 10.1016/j.rmed.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Kuna P, Peters J, Buhl R. Budesonide-formoterol as maintenance and reliever therapy reduces asthma exacerbations versus a higher maintenance dose of budesonide-formoterol or salmeterol-fluticasone. Eur Respir J. 2006;28(suppl 50):205s. [Google Scholar]
- Leuppi JD, Salzberg M, Meyer L, et al. An individualized, adjustable maintenance regimen of budesonide-formoterol provides effective asthma symptom control at a lower overall dose than fixed dosing. Swiss Med Wkly. 2003;133:302–9. doi: 10.4414/smw.2003.10229. [DOI] [PubMed] [Google Scholar]
- Lundborg M, Wille S, Bjermer L, et al. Maintenance plus reliever budesonide-formoterol compared with a higher maintenance dose of budesonide-formoterol plus formoterol as reliever in asthma: an efficacy and cost-effectiveness study. Curr Med Res Opin. 2005;22:809–21. doi: 10.1185/030079906X100212. [DOI] [PubMed] [Google Scholar]
- Malolepszy J, Böszörményi Nagy G, Selroos O, et al. Safety of formoterol Turbuhaler at cumulative doses of 90 g in patients with acute bronchial obstruction. Eur Respir J. 2001;18:928–34. doi: 10.1183/09031936.01.00251901. [DOI] [PubMed] [Google Scholar]
- Maneechotesuwan K, Usmani OS, Adcock IM, et al. The modulation of GATA-3 nuclear localization by fluticasone & salmeterol. Am J Respir Crit Care Med. 2002;165:A616. [Google Scholar]
- Maneechotesuwan K, Essilfie-Quaye S, Meah S, et al. Formoterol attenuates neutrophilic airway inflammation in asthma. Chest. 2005;128:1936–42. doi: 10.1378/chest.128.4.1936. [DOI] [PubMed] [Google Scholar]
- Miller-Larsson A, Selroos O. Advances in asthma and COPD treatment: combination therapy with inhaled corticosteroids and long-acting β2-agonists. Curr Pharmaceut Design. 2006;12:3261–79. doi: 10.2174/138161206778194187. [DOI] [PubMed] [Google Scholar]
- [NIH GINA] National Institutes of Health, Global Initiative for Asthma. Global strategy for asthma management and prevention [online] 2006 Accessed December 2006. URL: http://www.ginasthma.org/. NIH Publication No 02–3659, Bethesda, MD, 1995 (updated 2006)
- O’Byrne PM, Barnes PJ, Rodriguez-Rousin R, et al. Low dose inhaled budesonide and formoterol in mild persistent asthma. The OPTIMA randomized trail. Am J Respir Crit Care Med. 2001;164:1392–7. doi: 10.1164/ajrccm.164.8.2104102. [DOI] [PubMed] [Google Scholar]
- O’Byrne PM. Daily inhaled corticosteroid treatment should be prescribed for mild persistent asthma. Am J Respir Crit Care Med. 2005;172:410–12. doi: 10.1164/rccm.2505001. [DOI] [PubMed] [Google Scholar]
- O’Byrne PM, Bisgaard H, Godard PP, et al. Budesonide-formoterol combination therapy as both maintenance and reliever medication in asthma. Am J Respir Crit Care Med. 2005;171:129–36. doi: 10.1164/rccm.200407-884OC. [DOI] [PubMed] [Google Scholar]
- Palmqvist M, Persson G, Lazer L, et al. Inhaled dry-powder formoterol and salmeterol in asthmatic patients; onset of action, duration of effect and potency. Eur Respir J. 1997;10:2484–9. doi: 10.1183/09031936.97.10112489. [DOI] [PubMed] [Google Scholar]
- Palmqvist M, Ibsen T, Mellen A, et al. Comparison of the relative efficacy of formoterol and salmeterol in asthmatic patients. Am J Respir Crit Care Med. 1999;160:244–9. doi: 10.1164/ajrccm.160.1.9901063. [DOI] [PubMed] [Google Scholar]
- Palmqvist M, Arvidsson P, Beckman O, et al. Onset of bronchodilation of budesonide-formoterol vs. salmeterol-fluticasone in single inhalers. Pulm Pharmacol Ther. 2001;14:29–34. doi: 10.1006/pupt.2000.0260. [DOI] [PubMed] [Google Scholar]
- Partridge M, van der Molen T, Myrseth S-E, et al. Attitudes and actions of asthma patients on regular maintenance therapy: The INSPIRE study. BMC Pulm Med. 2006;6:13. doi: 10.1186/1471-2466-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels RA, Löfdahl C-G, Postma DS, et al. Effect of inhaled formoterol and budesonide on exacerbations of asthma. N Engl J Med. 1997;337:1405–11. doi: 10.1056/NEJM199711133372001. [DOI] [PubMed] [Google Scholar]
- Politiek MJ, Boorsma M, Aalbers R. Comparison of formoterol, salbutamol and salmeterol in methacholine-induced severe bronchoconstriction. Eur Respir J. 1999;13:988–92. doi: 10.1034/j.1399-3003.1999.13e10.x. [DOI] [PubMed] [Google Scholar]
- Rabe KF, Vermeire PA, Soriano JB, et al. Clinical management of asthma in 1999: the Asthma Insight and Reality in Europe (AIRE) study. Eur Respir J. 2000;16:802–7. doi: 10.1183/09031936.00.16580200. [DOI] [PubMed] [Google Scholar]
- Rabe KF, Adachi M, Lai CKW, et al. Worldwide severity and control of asthma in children and adults: the global Asthma Insight and Reality surveys. J Allergy Clin Immunol. 2004;114:40–7. doi: 10.1016/j.jaci.2004.04.042. [DOI] [PubMed] [Google Scholar]
- Rabe KF, Pizzichini E, Ställberg B, et al. Budesonide-formoterol in a single inhaler for maintenance and relief in mild-to-moderate asthma. A randomized, double-blind trial. Chest. 2006;129:246–56. doi: 10.1378/chest.129.2.246. [DOI] [PubMed] [Google Scholar]
- Rabe KF, Atienza T, Magyar P, et al. Effect of budesonide in combination with formoterol for reliever therapy in asthma exacerbations: a randomised controlled, double-blind study. Lancet. 2006;368:744–53. doi: 10.1016/S0140-6736(06)69284-2. [DOI] [PubMed] [Google Scholar]
- Ringdal N, Derom E, Wåhlin-Boll E, et al. Onset and duration of action of single doses of formoterol inhaled via Turbuhaler. Respir Med. 1998;92:1017–21. doi: 10.1016/s0954-6111(98)90348-1. [DOI] [PubMed] [Google Scholar]
- Rosenborg J, Bengtsson T, Larsson P, et al. Relative systemic dose potency and tolerability of inhaled formoterol and salbutamol in healthy subjects and asthmatics. Eur J Clin Pharmacol. 2000;56:363–70. doi: 10.1007/s002280000160. [DOI] [PubMed] [Google Scholar]
- Rosenborg J, Larsson P, Rott Z, et al. Relative therapeutic index between inhaled formoterol and salbutamol in asthma patients. Respir Med. 2002;96:412–17. doi: 10.1053/rmed.2002.1291. [DOI] [PubMed] [Google Scholar]
- Rosenhall L, Heinig JH, Lindqvist A, et al. Budesonide-formoterol (Symbicort®) is well tolerated and effective in patients with moderate persistent asthma. Int J Clin Pract. 2002;56:427–33. [PubMed] [Google Scholar]
- Rosenhall L, Elvstrand A, Tilling B, et al. One-year safety and efficacy of budesonide-formoterol in a single inhaler (Symbicort® Turbuhaler®) for the treatment of asthma. Respir Med. 2003;97:702–8. doi: 10.1053/rmed.2003.1504. [DOI] [PubMed] [Google Scholar]
- Rubinfeld AR, Scicchitano R, Hunt A, et al. Formoterol Turbuhaler® as reliever medication in patients with acute asthma. Eur Respir J. 2006;27:735–41. doi: 10.1183/09031936.06.00027405. [DOI] [PubMed] [Google Scholar]
- Scicchitano R, Aalbers R, Ukena D, et al. Efficacy and safety of budesonide-formoterol single inhaler therapy versus a higher dose of budesonide in moderate to severe asthma. Curr Med Res Opin. 2004;20:1403–18. doi: 10.1185/030079904X2051. [DOI] [PubMed] [Google Scholar]
- Seberová E, Andersson A. Oxis (formoterol given by Turbuhaler) showed as rapid an onset of action as salbutamol given by pMDI. Respir Med. 2000;94:607–11. doi: 10.1053/rmed.2000.0788. [DOI] [PubMed] [Google Scholar]
- Ställberg B, Olsson P, Jörgensen LA, et al. Budesonide-formoterol adjustable maintenance dosing reduces asthma exacerbations versus fixed dosing. Int J Clin Pract. 2003;57:656–61. [PubMed] [Google Scholar]
- Tötterman KJ, Huhti L, Sutinen E, et al. Tolerability of high doses of formoterol and terbutaline via Turbuhaler® for 3 days in stable asthmatic patients. Eur Respir J. 1998;12:573–9. doi: 10.1183/09031936.98.12030573. [DOI] [PubMed] [Google Scholar]
- Tsai YG, Lee MY, Yang KD, et al. A single dose of nebulized budesonide decreases exhaled nitric oxide in children with acute asthma. J Pediatr. 2001;139:433–7. doi: 10.1067/mpd.2001.116295. [DOI] [PubMed] [Google Scholar]
- Ullman A, Svedmyr N. Salmeterol, a new long acting inhaled beta2adrenoceptor agonist: comparison with salbutamol in adult asthmatic patients. Thorax. 1988;43:674–8. doi: 10.1136/thx.43.9.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usmani OS, Ito K, Maneechotesuwan K, et al. Glucocorticoid receptor nuclear translocation in airway cells after inhaled combination therapy. Am J Respir Crit Care Med. 2005;172:704–12. doi: 10.1164/rccm.200408-1041OC. [DOI] [PubMed] [Google Scholar]
- van der Woude HJ, Boorsma M, Bergqvist PBF, et al. Budesonideformoterol in a single inhaler rapidly relieves methacholine-induced moderate-to-severe bronchoconstriction. Pulm Pharmacol Ther. 2004;17:89–95. doi: 10.1016/j.pupt.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Vogelmeier C, D’Urzo A, Pauwels R, et al. Budesonide-formoterol maintenance and reliever therapy: an effective asthma treatment option? Eur Respir J. 2005;26:819–28. doi: 10.1183/09031936.05.00028305. [DOI] [PubMed] [Google Scholar]
- Zetterström O, Buhl R, Mellem H, et al. Improved asthma control with budesonide-formoterol in a single inhaler, compared with budesonide alone. Eur Respir J. 2001;18:262–8. doi: 10.1183/09031936.01.00065801. [DOI] [PubMed] [Google Scholar]


