Abstract
Elucidation of the cellular immunopathology and cytokine profile of psoriatic arthritis (PsA), a chronic inflammatory disease associated with psoriasis, has resulted in the development of a number of novel biologic therapies. Among these biologics, tumor necrosis factor-alpha (TNF-α) inhibitors have been used successfully to treat patients suffering from rheumatoid arthritis or psoriasis. The pivotal role of TNF-α in the pathogenesis and progression of PsA suggested that anti-TNF-α agents could be effective in controlling PsA. The results from two large, randomized, double-blind, placebo-controlled trials in patients with moderate to severe PsA indicated that the anti-TNF-inhibitor, infliximab, can control both the joint and skin manifestations of the disease. This review focuses on the clinical development of infliximab as a treatment for PsA. The development of other anti-TNF-α biologics is also discussed.
Keywords: psoriatic arthritis, psoriasis, spondyloarthropathies, TNF inhibition, biologics
Introduction
Psoriatic arthritis (PsA) is a progressive and often destructive form of inflammatory arthritis that frequently occurs in psoriasis patients (Zachariae 2003). It is characterized by moderate to severe psoriatic skin lesions with chronic joint pain, swelling, and fatigue. In many cases, psoriasis symptoms may precede the arthritis component of the disease by several years. PsA can be debilitating, culminating in severe, erosive joint damage and functional impairment of individuals suffering from the disease. Reduced qualities of life, increased risk of mortality, and premature death have all been documented for patients with PsA (Wong et al 1997; Husted et al 2001; Sokoll and Helliwell 2001). This review provides an update on the clinical development of anti-tumor necrosis factor (TNF)-α agents like infliximab and other innovative therapies that can be used to treat PsA.
Clinical presentation
The coexistence of inflammatory arthritis symptoms with psoriasis has been known for many years but was not recognized as a clinical entity distinct from rheumatoid arthritis (RA) and other arthropathies until pioneering observations by Wright (1959). The condition was further codified in the 1960s and early 1970s (Blumberg et al 1964; Moll and Wright 1973b). Subsequent studies revealed that PsA shares a variety of genetic, pathogenic, and clinical features with RA and other forms of inflammatory arthritis. This has led to some confusion among clinicians when attempting to distinguish among PsA, RA, and other forms of inflammatory arthritis. Nevertheless, PsA can be distinguished from other arthropathies and, in particular RA, based on several clinically distinct features of the disease.
First, approximately 80% of patients with RA are positive for the presence of rheumatoid factor whereas 91%–94% of patients with PsA are negative for this factor (Gladman 2005). Second, PsA and RA frequently differ in the extent of joint involvement and the pattern of inflamed joints. In general, the involved joints in patients with PsA are fewer, less inflamed, contain less fluid, and exhibit less tenderness compared with those of RA patients (Gladman 1998). Furthermore, inflammation tends to be more asymmetrical in its distribution, at least in the early stages of PsA (Gladman et al 1987, 2005). Dactylitis (digit inflammation), spondylitis (spine involvement), sacroiliitis, and distal interphalangeal joint involvement are also common in PsA but frequently absent in RA (Gladman et al 1987; Fournie et al 1999). Finally, patients with PsA virtually always have psoriatic skin lesions whereas psoriasis occurs (by chance) in only 2%–3% of RA patients. Psoriatic nail lesions are very common in PsA and help to distinguish between patients who have PsA and those who have RA. Studies show that nail lesions are present in approximately 87% of PsA patients but occur in only 40%–46% of patients with uncomplicated psoriasis (Gladman et al 1986). The presence of multiple (20 or more) nail pit lesions has been used to distinguish patients with PsA from those with RA and psoriasis (Eastmond and Wright 1979).
In an attempt to refine and make the diagnostic criteria for PsA more specific, several groups proposed combining the unique clinical attributes of PsA with characteristic radiological features commonly observed with the disease. These include joint erosions, joint space narrowing, bony proliferation including periarticular and shaft periostitis, osteolysis (bone resorption) including “pencil in cup” deformity and acro-osteolysis, ankylosis spur formation and spondylitis (Moll and Wright 1973b; Gladman 1998; Wassenberg et al 2001; Ory 2003). These unique radiographic diagnostic criteria, in conjunction with increased use of newer imaging techniques such as ultrasonography and magnetic resonance imaging (MRI), have helped to improve early detection and diagnosis of PsA (Ory 2003; Ory et al 2005).
A classification scheme that recognizes five clinically distinct patterns among patient with PsA was introduced in 1973 (Table 1) (Moll and Wright 1973b). These subtypes include: 1) oligoarticular (<5 involved joints), often asymmetric; 2) polyarticular, typically more symmetric; 3) distal interphalangeal predominant; 4) spine predominant; and 5) arthritis mutilans. In this first series of patients, oligoarticular presentation was most common, but in all subsequent large series, polyarticular presentation has been most prevalent (Gladman et al 2005). Recognizing the need for a classification system based on a more systematic analysis of a large cohort of patients, Helliwell and Taylor (2005) organized a multi-center study of approximately a 1000 patients, half with PsA and half “control” patients with inflammatory arthritis, analyzed by history, physical exam, laboratory and x-ray. The classification criteria being developed will involve those aspects of the disease which yield the greatest sensitivity and specificity for diagnosis (Taylor 2006).
Table 1.
Clinical subtypes of PsA identified by Moll and Wright (1973b)
| Clinical subtypes | Disease features |
|---|---|
| Polyarticular RA-like (symmetrical) | Nail lesions; symmetrical pattern of joint involvement |
| Oligoarticular asymmetrical | <5 joints involved; asymmetrical joint involvement |
| Distal predominant pattern | Distal interphalangeal joint involvement prevalent |
| Spondylitis | Spinal involvement is predominant |
| Arthritis mutilans | Severe joint destruction |
Abbreviations: PsA, psoriatic arthritis; RA, rheumatoid arthritis.
Etiology and pathogenesis of PsA
The prevalence of PsA and its rate of occurrence have been difficult to estimate. This is largely due to the lack of a universally-agreed upon diagnostic criteria and disease classification scheme, coupled with a high frequency of misdiagnosis (Helliwell and Taylor 2005). The rate of occurrence of PsA among patients with psoriasis has also been reported to vary widely, ranging from 6% to 39% (Leonard et al 1978; Shbeeb et al 2000; Soderlin et al 2002; Salonen 2003; Zachariae 2003; Gelfand et al 2005). However, results from recent studies indicate that up to 30% of patients with psoriasis develop PsA (Salonen 2003; Zachariae 2003). PsA does not appear to be gender specific and can develop at any age, although it is most common in persons aged between 30 and 55 years (Espinoza et al 1992; Helliwell and Taylor 2005).
The factors that contribute to the development and pathogenesis of PsA are not completely understood. The onset and development of PsA appear to involve a complex interplay between genetics, environmental factors (eg, physical trauma and infection), and the immune system. Recent reports indicate that genetic factors may predispose individuals to PsA (Gladman et al 1986, 1998, 1999, 2003). In support of this, several population and twin studies showed that the risk of developing PsA was elevated among first degree and close relatives of PsA patients (Moll and Wright 1973a; Swanbeck et al 1997). A strong association has also been demonstrated between PsA disease progression and certain major histocompatibility complex (MHC) loci (Armstrong et al 1983; Gladman et al 1986, 1998, 1999, 2003; Bowcock and Cookson 2004). A number of recent studies identified a relationship between PsA and several so-called psoriatic arthritis susceptibility genes (Gladman 2003; Karason et al 2003; Rahman et al 2003; Bowcock 2005). Finally, non-genetic factors, including bacterial infections, various traumas (which lead to induction of prolonged inflammatory reactions), and immune dysregulation are also thought to contribute to the development of PsA (Langevitz et al 1990; Gladman et al 2005).
Despite the lack of a clear understanding of the factors that predispose individuals to PsA, identification of the underlying immunological components of PsA has provided insights into the pathogenesis and progression of the disease. Activated T-cells, monocytes, and pro-inflammatory cytokines, most notably TNF-α, have all been shown to play pivotal roles in the pathogenesis of both the joint and psoriatic components of PsA (Figure 1) (Gladman 1993; Costello et al 1999; Mease 2003, 2004b). It is generally agreed that T-cell activation, followed by a vigorous Th1 cytokine response, is responsible for the inflammatory reactions that characterize the skin and joint lesions observed in patients with PsA (Ritchlin et al 1998; Veale et al 2005). The key mediators of these inflammatory responses are the pro-inflammatory cytokines, TNF-α, interleukin (IL)-1, IL-6 and IL-8 (for a review see Mease and Goffe 2005). Elevated levels of these cytokines are thought to induce the proliferation and activation of synovial and epidermal fibroblasts, which results in the joint fibrosis that is characteristic of chronic PsA disease (Espinoza, Aguilar, et al 1994; Espinoza, Espinoza, et al 1994). Pro-inflammatory cytokines can also stimulate the proliferation of skin keratinocytes, which leads to further inflammation, induration, and psoriatic plaque formation (Giustizieri et al 2001). Finally, these cytokines upregulate the expression of endothelial and dendritic cell adhesion molecules, intercellular adhesion molecules-1 (ICAM-1), vascular cell adhesion molecules-1 (VCAM-1), and E-selectin, which promote the recruitment and access of activated T-cells to afflicted joints. This results in the release of additional TNF-α, which stimulates inflammation, interferes with bone formation, hinders proteoglycan synthesis and promotes joint destruction (Saklatvala 1986; Veale et al 1993, 1995; Terajima et al 1998).
Figure 1.
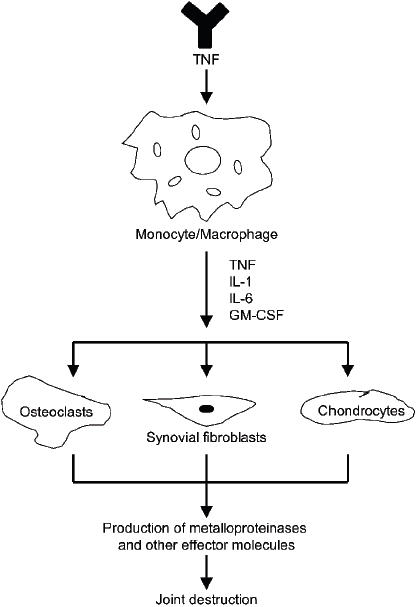
The inflammatory response cascade induced by tumor necrosis factor-α (TNF-α) manifesting in joint destruction. Copyright © 2005. Reproduced from Mease PJ, Goffe BS, Metz J, et al. 2000. Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial. Lancet, 356:385–90. TNF-α binding stimulates mononuclear phagocytes to secrete the pro-inflammatory cytokines interleukin (IL)-1, IL-6, and granulocyte macrophage–colony stimulating factor (GM-CSF).These cytokines promote recruitment of T-cells into afflicted joints and also stimulate proliferation of osteoclasts, synovial fibroblasts and chondrocytes at these sites.The resultant inflammatory responses, coupled with release of metalloproteinases and other effector molecules by activated cells, results in joint destruction.
In addition to their roles as inflammatory response regulators, IL-1 and TNF-α recently were found to play central roles in bone metabolism (Bertolini et al 1986; Nakashima et al 2003). These cytokines are thought to stimulate bone resorption (osteoclastogenesis) by upregulating the synthesis of osteoprotegerin ligand, a newly identified member of the TNF receptor family that is expressed by activated T-cells (Lacey et al 1998; Burgess et al 1999). The finding that erosive joint disease in PsA is associated with increased osteoclast precursors in the peripheral circulation suggests that the effects of IL-1 and TNF-α on bone metabolism likely contribute to the PsA-induced joint disease (Saklatvala 1986; Ritchlin et al 2003). IL-1 and TNF-α also stimulate monocytes and dendritic cells to overproduce metalloproteinases and prostaglandin E2, which mediate cartilage and collagen erosion in affected joints (Dayer et al 1985; Ritchlin et al 2003).
Over the past two decades, it has become increasingly apparent that PsA can be a serious disease with substantial morbidity and mortality when not diagnosed early or treated properly. Historically, PsA treatment has been empirically determined, drawing on therapies that have been used to treat either RA, psoriasis or both (Gladman 2003; Pipitone et al 2003). With this in mind, the type and course of treatment for patients with PsA usually depend upon the extent and severity of both skin and joint manifestations. Because a majority of PsA patients typically have psoriatic skin involvement, in addition to arthritis, the PsA therapy that is typically employed should attempt to target both the skin and joint disease.
Treatment
The medications traditionally used to treat PsA have included non-steroidal anti-inflammatory drugs (NSAIDs) and disease-modifying anti-rheumatic drugs (DMARDs), such as methotrexate, sulphasalazine and leflunomide, sometimes in combination with topical and light therapies for skin manifestations (Gladman 2003, 2005; Pipitone et al 2003; Nash and Clegg 2005). NSAID treatment helps to control arthritis pain and may work for mild inflammation in patients with PsA (Gladman 2003, 2005; Pipitone et al 2003; Nash and Clegg 2005). Mild skin involvement has been typically treated with topical medications including corticosteroid creams, tar shampoos, vitamin D creams and ultraviolet (UV) irradiation (Gladman 2003; Lebwohl et al 2005). More severe skin disease, which may be refractory to topical drug treatment, can be treated with psoralen with UVA irradiation, cyclosporine, and methotrexate (MTX) (Brockbank and Gladman 2002; Gladman 2003; Pipitone et al 2003). In cases where both skin and joint involvement are severe, systemic therapies using the DMARD agents, MTX, cyclosporine, and sulfasalazine have been traditionally used to treat PsA (Marguerie et al 2002). A thorough review of these traditional therapies is beyond the scope of this paper; detailed reviews can be found elsewhere (Nash and Clegg 2005; Lebwohl et al 2005).
Although NSAIDs and DMARDs provide some patients with substantial relief from both the dermatologic and arthritic symptoms of PsA, none of these agents has been successfully shown to control or hinder progression of the disease, as measured radiographically and there may be a high discontinuation rate due to lack of efficacy or adverse effects (Abu-Shakra et al 1995; Mader et al 1995; Rahman et al 1998; Nash and Clegg 2005). Over the past few years, the armamentarium of medications used to treat PsA has been greatly expanded to include biological agents, and several newer treatments exhibit improved efficacy over conventional therapies (Pipitone et al 2003; Gladman 2005; Mease and Antoni 2005).
Anti-TNF-α agents
Because TNF-α mediates multiple biological processes in the pathogenesis of PsA (Figure 1), a reduction in TNF-α levels was postulated to improve clinical outcomes in patients suffering from PsA. Several anti-TNF-α medications approved for treating and controlling RA were studied in patients with PsA and found to manage effectively both the psoriatic and arthritic manifestations of the disease. This resulted in the clinical development of a variety of TNF-α inhibitors, including infliximab, etanercept, adalimumab, and others, to treat PsA (Mease and Antoni 2005).
Infliximab for the treatment of PsA
Infliximab is a chimeric, human-mouse IgG1 monoclonal antibody that specifically targets and neutralizes soluble and membrane-bound TNF-α. It is approved for a variety of autoimmune diseases, including Crohn’s disease and ulcerative colitis (the only biologic approved for these indications) and moderate to severe RA (when used in combination with MTX). The drug was approved by the European Union (EU) in 2004 for use in combination with MTX to treat active and progressive PsA in patients who have responded inadequately to DMARDs. Partly related to dermatologist’s concerns about chronic methotrexate use in psoriasis patients, the inclusion criteria for the pivotal clinical trials made MTX use optional. Consequently, when infliximab was approved by the FDA for the treatment of PsA in late 2005, it was approved for monotherapy. In 2005, infliximab was approved by the EU for the treatment of moderate to severe plaque psoriasis.
Clinical development of infliximab for PsA was indicated following an open-label, compassionate-use, clinical trial conducted on 10 DMARD-refractory PsA patients. The results from this study showed that infliximab was effective, safe and well tolerated for treating both the psoriatic and joint components of PsA (Antoni et al 2002). Positive results from a second clinical study that evaluated the safety and efficacy of infliximab on patients with spondyloarthropathy, which also included patients with PsA, prompted late-stage clinical development of infliximab for PsA (Van den Bosch et al 2000). Two randomized, double-blind, placebo-controlled trials—one designated IMPACT (Infliximab Multinational Psoriatic Arthritis Controlled Trial) and a second called IMPACT II—were conducted to determine whether infliximab could be used to treat PsA (Antoni, Krueger, et al 2005; Antoni, Kavanaugh, et al 2005).
The IMPACT trial included 104 patients with PsA who were seronegative for rheumatoid factor, had at least five tender and five swollen joints, and were refractory to therapy with ≥1 DMARD (Antoni, Kavanaugh, et al 2005). Patients were required to have negative results for active or latent tuberculosis by purified protein derivative skin test and chest radiography. Patients were allowed to receive concomitant therapy with MTX, leflunomide, sulfasalazine, hydroxychloroquine, intramuscular gold, penicillamine, or azathioprine and stayed on baseline dosages of these medications throughout the study. Patients were randomized into an infliximab treatment group (52 patients at 5 mg/kg/infusion) or placebo (52 patients) for four infusions that were administered at 0, 2, 6, and 14 weeks. After week 16, patients initially assigned to receive placebo crossed over to receive infliximab 5 mg/kg every 8 weeks through week 50, while patients initially randomized to infliximab continued to receive active treatment at the same dose through week 50. The primary efficacy outcome of the randomized, double-blind portion of the study was achievement of the American College of Rheumatology criteria for 20% improvement in rheumatoid arthritis (ACR20) at 16 weeks. Main secondary endpoints included an assessment of skin involvement using the Psoriasis Area and Severity Index (PASI), and the proportion of patients who achieved ACR50 and ACR70 and the Psoriatic Arthritis Response Criteria (PsARC).
The proportion of infliximab-treated patients who achieved ACR20 at 16 weeks was 65%, which was significantly higher (p<0.001) than the response of the placebo group (10%) (Antoni, Kavanaugh, et al 2005). Moreover, 46% of infliximab-treated patients achieved an ACR50 and 29% achieved an ACR70 response, whereas none of the placebo-treated patients achieved either of these two endpoints (p<0.001). At the 16-week evaluation, 75% of infliximab-treated patients improved according to PsARC, compared with only 21% of placebo-treated patients (p<0.001). The percentage improvement in ACR20 achieved by infliximab-treated patients at week 16 was sustained through week 50 of the study (Figure 2). Moreover, crossover patients from the placebo group, who received infliximab therapy after week 16, achieved ACR20 response rates at week 50 that were comparable with those exhibited by patients who were initially placed in the infliximab treatment group. It is important to note that, at week 16, the concomitant use of DMARDs (primarily MTX) had no significant effect on the ACR20 response rate in either the infliximab-treated or placebo groups. For example, 62.5% of infliximab patients also receiving MTX achieved an ACR20 response rate at 16 weeks, as did 68% of infliximab patients not receiving MTX and 74% of patients not receiving DMARD therapy.
Figure 2.
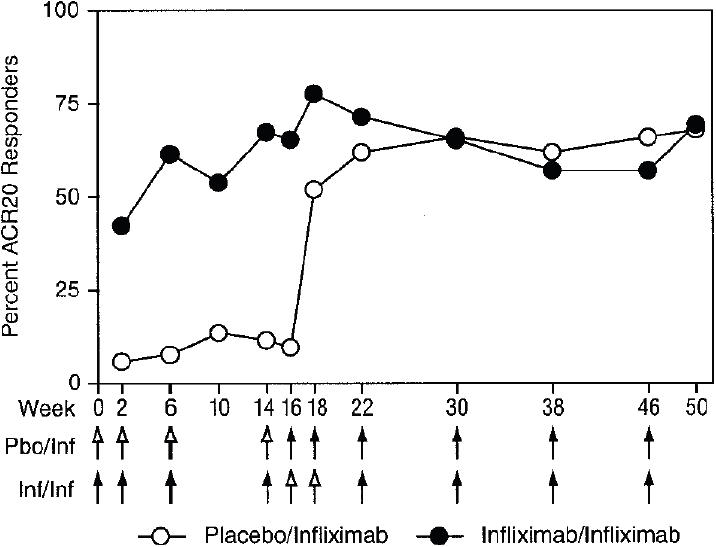
Percentages of patients achieving improvement by the American College of Rheumatology 20% (ACR20) criteria for improvement in rheumatoid arthritis through week 50. Results from the Phase III, IMPACT trial that assessed the effectiveness of infliximab for treating psoriatic arthritis. Copyright © 2005. Reproduced with permission from Antoni CE, Kavanaugh A, Kirkham B, et al. 2005. Sustained benefits of infliximab therapy for dermatologic and articular manifestations of psoriatic arthritis: results from the infliximab multinational psoriatic arthritis controlled trial (IMPACT). Arthritis Rheum, 52:1227–36. Arrows indicate weeks at which infusions were administered: open arrows denote placebo (Pbo) infusions, and solid arrows denote infusions of infliximab (Inf) 5 mg/kg.
Skin responses were measured in 39 patients (21 patients randomized to infliximab and 18 randomized to placebo), all of whom had PASI scores of ≥2.5 at baseline (Antoni, Kavanaugh, et al 2005). Among patients with baseline PASI scores of ≥2.5, 68% of infliximab-treated patients showed an improvement of ≥75% in PASI score at week 16 as compared with none of the patients in the placebo group (p<0.001; Figure 3). After crossing over to infliximab therapy, patients initially assigned to the placebo group experienced improvements in skin response that were very similar to those exhibited by patients in the infliximab treatment group. Both groups were able to maintain the skin responses until week 50 of the study.
Figure 3.
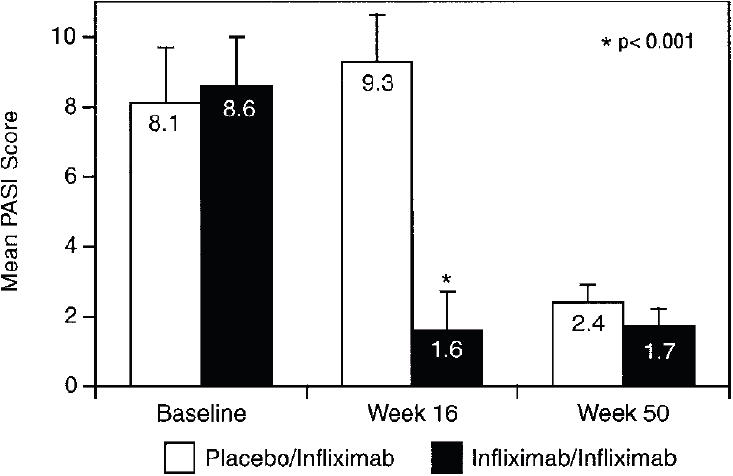
Psoriasis Area and Severity Index (PASI) scores (mean and SD) at baseline, week 16, and week 50 in patients who had a PASI score of ≥2.5 at baseline. Results from the Phase III, IMPACT trial that assessed the effectiveness of infliximab for treating PsA. Copyright © 2005. Reproduced with permission from Antoni CE, Kavanaugh A, Kirkham B, et al. 2005. Sustained benefits of infliximab therapy for dermatologic and articular manifestations of psoriatic arthritis: results from the infliximab multinational psoriatic arthritis controlled trial (IMPACT). Arthritis Rheum, 52:1227–36.
As part of the IMPACT study, hand and feet radiographs were obtained for 72/104 patients at 0 and 50 weeks (Kavanaugh, Antoni, Gladman, et al 2006). The missing patients consisted of 10 patients who discontinued the study, 8 patients who did not have any radiographic films, 3 patients who did not have a baseline film or who had an unevaluable film at baseline, and 11 patients who did not have radiographic films at 50 weeks. Total radiographic scores for these patients were determined using the PsA-modified van der Heijde-Sharp (vdH-S) method (Van der Heijde et al 2005). At baseline, the estimated mean annual rate of progression of PsA was 5.8 modified-vdH-S points per year for patients in both the placebo and treatment groups. Mean changes from baseline to week 50 in the total modified vdH-S score were −1.95 ± 0.50 for the placebo/infliximab treatment group and −1.50 ± 0.50 for the infliximab group. At week 50, 85% of patients in the placebo/infliximab group and 84% of patients in the infliximab group had no worsening in total modified vdH-S scores. These results suggest that either continued or delayed treatment with infliximab had marked inhibitory effects on radiographic PsA disease progression; however, if radiographic progression was not linear in this group of patients with PsA, it is possible that the reduced radiographic change may have been independent of treatment with infliximab.
Infliximab treatment was generally well tolerated with a discontinuation rate of only 13% over the 50 weeks of the study. There were no significant differences in the numbers or types of adverse events reported in infliximab treatment or placebo groups. Only two patients reported serious adverse events (one in the placebo group and one in the infliximab group). No patients experienced opportunistic infection, including tuberculosis, nor were there any reports of autoimmune, cytopenic or neurologic events (Antoni, Kavanaugh, et al 2005).
A second, larger randomized, double-blind IMPACT II trial was initiated to confirm the efficacy and safety of infliximab observed in the original IMPACT study (Antoni, Krueger, et al 2005). Patients were required to be prescreened for tuberculosis (TB) as in the original IMPACT study. In this trial, 200 patients with active PsA who were refractory to previous treatment were randomized to infusions of infliximab 5 mg/kg or placebo at weeks 0, 2, 6, 14, and 22. The primary measure of clinical response was the percentage of patients achieving ACR20, and secondary endpoints included PsARC, PASI, dactylitis and enthesopathy assessments. At baseline, the percentages of patients with 1 or more dactylitis digits or with enthesopathy were 41% and 35%, respectively, for placebo-treated patients and 40% and 42%, respectively, for infliximabtreated patients. At week 14, 58% of the patients who received infliximab and 11% receiving placebo achieved an ACR20 response (p<0.001). Additionally, 77% of infliximab-treated patients and 27% of placebo-treated achieved PsARC (p<0.001). Furthermore, at week 14, 64% of patients who received infliximab had at least a 75% improvement in PASI compared with only 2% of the patients receiving placebo (p<0.001). Fewer infliximab-treated patients compared with placebo had dactylitis at both week 14 (18% vs 30%; p=0.025) and week 24 (12% vs 34%, p<0.001). Also, fewer patients in the infliximab-treated group as compared with placebo-treated patients had active enthesopathy at week 14 (22% vs 34%, p=0.016) and week 24 (20% vs 37%, p=0.002). All of the therapeutic effects exhibited by infliximab were observed through the last evaluation that took place at week 24. Infliximab was generally well tolerated, with a similar incidence of adverse effects in the infliximab-treated and placebo groups. A radiographic analysis similar to that conducted in the first IMPACT study was also conducted for IMPACT II (Van der Heijde et al 2005). The results from this study corroborated the results obtained in the first IMPACT study, which suggested that treatment with infliximab had marked inhibitory effects on radiographic PsA disease progression.
The effects of infliximab on health-related quality of life (HRQoL) in patients with PsA who were enrolled in the IMPACT II study were recently reported (Kavanaugh, Antoni, Krueger, et al 2006). HRQoL was assessed using the 36-item Short Form Health Survey (SF-36) and functional disability was assessed using the Health Assessment Questionnaire (HAQ). At week 14, the mean percentage improvement from baseline in HAQ scores was 48.6% for infliximab-treated patients, compared with a worsening of 18.4% in the placebo-treated patients (p<0.001). Improvement in all eight scales of the SF-36 were greater than those in the placebo group at week 14 (p<0.001), with these benefits sustained through week 24.
Infliximab for the treatment of psoriasis
A role for TNF-α in the pathogenesis of psoriasis, along with results from the two IMPACT studies, suggested that infliximab may also provide therapeutic benefits for patients suffering from severe psoriasis. This prompted the initiation of two multicenter, randomized, double-blind and placebo-controlled Phase III studies to assess the effects of infliximab on patients with severe plaque psoriasis. The first of these studies, called SPIRIT (Study of Psoriasis with Infliximab [REMICADE] Induction Therapy) (Gottlieb et al 2004) was conducted in the US whereas the second trial, designated EXPRESS (European Infliximab for Psoriasis [REMICADE] Efficacy and Safety Study) (Reich et al 2005), was conducted in Europe.
The SPIRIT trial evaluated the efficacy, safety, and tolerability of infliximab therapy in 249 patients with severe plaque psoriasis who had previously received long-wave ultraviolet-A light (PUVA) or systemic therapy for psoriasis (Gottlieb et al 2004). These patients were randomly assigned to receive intravenous infusions of either 3.0 mg/kg (99 patients) or 5.0 mg/kg (99 patients) infliximab or placebo (51 patients) at 0, 2, and 6 weeks. The primary endpoint of the study was the proportion of patients who achieved at least 75% improvement in PASI scores (PASI 75) from baseline at week 10. At week 26, patients with a Physician Global Assessment score that indicated moderate or severe disease were eligible for a single intravenous infusion of their assigned treatment regimen to assess the safety of retreatment after a 20-week treatment-free interval. Approximately two-thirds of the patients had received prior phototherapy and 90.8% had received prior therapy with systemic agents. At week 10, 72% of patients treated with infliximab (3 mg/kg) and 88% of patients treated with infliximab (5 mg/kg) achieved 75% or greater improvement from baseline PASI 75 scores as compared with only 6% of patients treated with placebo (p<0.001). Nearly 50% of the patients treated with infliximab 5mg/kg achieved PASI 75 scores as early as week 4. Moreover, 58% of the patients experienced PASI 90 (almost complete clearance of lesions) by week 10. At week 26, 114 patients were retreated with their initial dose of study medication. Four weeks after retreatment, the percentages of patients in the 3 mg/kg, 5 mg/kg, and placebo groups who achieved a Physician’s Global Assessment (PGA) of mild, minimal, or clear (PGA <3) were 38%, 64%, and 18%, respectively. There was no significant difference in the number of adverse events reported by patients in the treatment or placebo groups, and infliximab was generally well tolerated in this study. The retreatment infusion was well tolerated with no hypersensitivity reactions reported.
The EXPRESS study evaluated the effects of infliximab on 378 patients with moderate to severe plaque psoriasis (Reich et al 2005). These patients were randomized into two groups that received infusions of either infliximab 5 mg/kg or placebo at weeks 0, 2, and 6, and then every 8 weeks up to week 46. At week 24, placebo-treated patients were permitted to crossover to infliximab treatment. Skin signs of psoriasis were assessed using PASI scores, and the primary endpoint in the study was the proportion of patients who achieved at least 75% improvement in PASI (PASI 75) from baseline to week 10. At week 10, 80% of patients treated with infliximab achieved at least 75% improvement from baseline PASI as compared with only 3% of patients in the placebo group (p<0.0001). Moreover, 57% of infliximab-treated patients, as compared with only 1% of patients in the placebo group, achieved 90% improvement in PASI. PASI 75 scores were maintained at week 24 by 82% of infliximab-treated patients and 4% of patients who received placebo (p<0.0001). At week 50, 61% of infliximab patients were able to sustain PASI 75 levels and 45% achieved PASI 90 scores. A pre-specified subanalysis of responders showed that 89% of the patients who achieved a PASI 75 score at week 10 maintained this level of response at 6 months and 65% sustained this response at 1 year. Similar to the results from the SPIRIT study, infliximab was generally well tolerated.
The effects of treatment with infliximab on HRQoL in patients enrolled in the EXPRESS study was recently published (Reich et al 2006). At week 10, patients treated with infliximab had significantly greater improvement in Dermatology Life Quality Index scores than placebo-treated patients (p<0.001). Significant improvement was also reported for all eight subscales of the SF-36 for infliximab-versus placebo-treated patients (p<0.001). The significant improvement in HRQoL persisted at week 24, and substantial benefit still remained at week 50.
Dosing of infliximab
For psoriatic arthritis, the recommended dosing of infliximab in the US and the EU is 5 mg/kg given as an intravenous infusion (Remicade package insert 2006a, 2006b). This is followed with similar doses at 2 and 6 weeks after the first infusion, then every 8 weeks thereafter. Infliximab can be used with or without MTX.
For psoriasis, the recommended dosing of infliximab in the EU is 5 mg/kg given as an intravenous infusion over a 2-hour period, followed by additional 5 mg/kg infusion doses at 2 and 6 weeks after the first infusion, then every 8 weeks thereafter (Remicade package insert 2006a). If a patient shows no response after 14 weeks (ie, after 4 doses), no additional treatment with infliximab should be given.
Other TNF-α agents
Etanercept
Etanercept is a fully human soluble TNF receptor-IgG fusion protein that binds and inactivates soluble and cell-bound TNF-α and lymphotoxin (TNF-α) (Mease et al 2004). It is approved for use in the US and Europe to treat patients with PsA and also approved to treat psoriasis patients who are candidates for phototherapy or systemic DMARD therapy. Unlike infliximab, which is administered intravenously, etanercept is given subcutaneously as a 25 mg twice weekly or 50 mg weekly treatment regimen. The efficacy of etanercept in PsA was shown in 2 randomized, double-blind, placebo-controlled clinical trials (Mease et al 2000, 2004).
Adalimumab
Adalimumab, a fully human anti-TNF monoclonal antibody, is currently approved for the treatment of RA and PsA (Keystone et al 2004). In addition to being fully humanized, which reduces the incidence of induction of neutralizing immune responses, it has the advantage of being administered subcutaneously at biweekly intervals (Gladman 2005). The effectiveness of adalimumab for treating PsA was suggested based on results from a small open-label clinical trial (Ritchlin et al 2004) and recently verified in a larger, randomized, double-blind, placebo-controlled study (ADEPT) (Mease, Gladman, Ritchlin, 2005).
Safety profiles of TNF-α antagonists Infliximab and the other TNF-α inhibitors are generally well tolerated by patients. Some of the more frequently reported side effects of all of these agents include: 1) increased risk of infection, particularly mycobacterial infections, 2) induction of anti-nuclear antibodies, 3) possible increased cancer risk, and 4) infusion/injection site hypersensitivity reactions (Braun and Sieper 2003). Injection site reactions with etanercept and adalimumab generally are mild and transient. Infusion reactions with infliximab occur infrequently and can generally be managed with slowing the infusion rate and/or medical treatment (Terajima et al 1998; Braun and Sieper 2003; Cheifetz et al 2003). It has been shown that patients with RA have a higher risk of lymphoma and leukemia than the general population; however, the risk of lymphoma and leukemia during treatment with different TNF-α antagonists is not significantly different than for RA patients in general (Askling, Fored, Baecklund, et al 2005). A similar study of the risk of solid cancers reported that RA patients have a 70% increased risk for non-melanoma skin cancer, which was increased somewhat during treatment with TNF-α antagonists (standardized incidence ratio = 3.6) (Askling, Fored, Brandt, et al 2005). Long-term studies will be required to characterize further the safety risks associated with TNF-α antagonists.
Switching among TNF-α antagonists
Although it has not been studied in patients with PsA, it has been shown in patients with RA and spondyloarthropathy that a reasonable approach to use when one TNF-α antagonist loses its effectiveness is to switch to a different agent in the same class. Observational studies have shown that a substantial number of patients who experience adverse events or lack of effectiveness with one TNF-α antagonist may benefit from a switch to a different TNF-α antagonist (Delaunay et al 2005; Wick et al 2005; Gomez-Reino and Carmona 2006).
New PsA treatments:T-cell-directed agents
Other biological therapies proposed for PsA treatment attempt to interfere with T-cell recruitment and activation, activities which are thought to play a role in the pathogenesis of PsA. Although the exact role of T-cells in PsA is poorly understood, their contribution to the disease is based on the finding that T-cells are routinely present in high numbers in the inflammatory infiltrate found in PsA-associated skin lesions and afflicted joints. Two new drugs, efalizumab and alefacept that interfere with T-cell function and effector cell interactions have been approved for psoriasis and have been preliminarily investigated for treatment of PsA.
Efalizumab is a humanized IgG1 monoclonal antibody that is thought to act by inhibiting T-cell recruitment and activation at sites of chronic inflammation. Efalizumab is effective in treating psoriasis and has been approved for this use in the US (Gordon et al 2003; Lebwohl et al 2003). Results from a recent clinical phase 2 study in PsA did not show statistically significant superiority in the efalizumab arm of the study (Mease 2004a).
Alefacept is a fully human, anti-T-cell receptor fusion protein that is thought to reduce inflammatory reactions by preventing T-cell activation and proliferation and inducing selective T-cell apoptosis (Ellis and Krueger 2001). Alefacept has been approved for use in psoriasis and has been shown to be effective in treating PsA in preliminary clinical studies (Korman and Moul 2005; Mease, Gladman, Keystone 2005, 2006). Although alefacept has shown a statistically significant improvement over placebo, the magnitude of the effect was modest.
Conclusions
Although the factors responsible for development and progression of PsA are still not completely understood, new insights into the underlying immunological mechanisms of the disease have resulted in the development of novel therapies. These include the widely-used anti-TNF-α antagonists and the newer T-cell directed agents. Both new drug classes have the potential for superior efficacy and improved tolerability profiles when compared with traditional PsA treatments. Furthermore, the biologics may possibly have a better overall safety profile than conventional treatments. Moreover, unlike older, conventional PsA treatments, these new agents may hinder or inhibit the progression of PsA and thus prevent the erosive joint damage that interferes with the quality of life of many PsA patients. Thus, infliximab, as the first FDA-approved TNF-α monoclonal antibody, may provide a significant advance in treatment for some patients with PsA.
References
- Abu-Shakra M, Gladman DD, Thorne JC, et al. Longterm methotrexate therapy in psoriatic arthritis: clinical and radiological outcome. J Rheumatol. 1995;22:241–5. [PubMed] [Google Scholar]
- Antoni C, Dechant C, Hanns-Martin Lorenz PD, et al. Open-label study of infliximab treatment for psoriatic arthritis: clinical and magnetic resonance imaging measurements of reduction of inflammation. Arthritis Rheum. 2002;47:506–12. doi: 10.1002/art.10671. [DOI] [PubMed] [Google Scholar]
- Antoni C, Krueger GG, de Vlam K, et al. Infliximab improves signs and symptoms of psoriatic arthritis: results of the IMPACT 2 trial. Ann Rheum Dis. 2005;64:1150–7. doi: 10.1136/ard.2004.032268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni CE, Kavanaugh A, Kirkham B, et al. Sustained benefits of infliximab therapy for dermatologic and articular manifestations of psoriatic arthritis: results from the infliximab multinational psoriatic arthritis controlled trial (IMPACT) Arthritis Rheum. 2005;52:1227–36. doi: 10.1002/art.20967. [DOI] [PubMed] [Google Scholar]
- Armstrong RD, Panayi GS, Welsh KI. Histocompatibility antigens in psoriasis, psoriatic arthropathy, and ankylosing spondylitis. Ann Rheum Dis. 1983;42:142–6. doi: 10.1136/ard.42.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askling J, Fored CM, Baecklund E, et al. Haematopoietic malignancies in rheumatoid arthritis: lymphoma risk and characteristics after exposure to tumour necrosis factor antagonists. Ann Rheum Dis. 2005;64:1414–20. doi: 10.1136/ard.2004.033241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askling J, Fored CM, Brandt L, et al. Risks of solid cancers in patients with rheumatoid arthritis and after treatment with tumour necrosis factor antagonists. Ann Rheum Dis. 2005;64:1421–6. doi: 10.1136/ard.2004.033993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolini DR, Nedwin GE, Bringman TS, et al. Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature. 1986;319:516–18. doi: 10.1038/319516a0. [DOI] [PubMed] [Google Scholar]
- Blumberg BS, Bunim JJ, Calkins E, et al. ARA nomenclature and classification of arthritis and rheumatism. Arthritis Rheum. 1964;7:93–7. doi: 10.1002/art.1780070113. [DOI] [PubMed] [Google Scholar]
- Bowcock AM. Understanding the pathogenesis of psoriasis, psoriatic arthritis, and autoimmunity via a fusion of molecular genetics and immunology. Immunol Res. 2005;32:45–56. doi: 10.1385/IR:32:1-3:045. [DOI] [PubMed] [Google Scholar]
- Bowcock AM, Cookson WO. The genetics of psoriasis, psoriatic arthritis and atopic dermatitis. Hum Mol Genet. 2004;13:R43–55. doi: 10.1093/hmg/ddh094. [DOI] [PubMed] [Google Scholar]
- Braun J, Sieper J. Role of novel biological therapies in psoriatic arthritis: effects on joints and skin. BioDrugs. 2003;17:187–99. doi: 10.2165/00063030-200317030-00005. [DOI] [PubMed] [Google Scholar]
- Brockbank J, Gladman D. Diagnosis and management of psoriatic arthritis. Drugs. 2002;62:2447–57. doi: 10.2165/00003495-200262170-00004. [DOI] [PubMed] [Google Scholar]
- Burgess TL, Qian Y, Kaufman S, et al. The ligand for osteoprotegerin (OPGL) directly activates mature osteoclasts. J Cell Biol. 1999;145:527–38. doi: 10.1083/jcb.145.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheifetz A, Smedley M, Martin S, et al. The incidence and management of infusion reactions to infliximab: a large center experience. Am J Gastroenterol. 2003;98:1315–24. doi: 10.1111/j.1572-0241.2003.07457.x. [DOI] [PubMed] [Google Scholar]
- Costello P, Bresnihan B, O’Farrelly C, et al. Predominance of CD8+ T lymphocytes in psoriatic arthritis. J Rheumatol. 1999;26:1117–24. [PubMed] [Google Scholar]
- Dayer JM, Beutler B, Cerami A. Cachectin/tumor necrosis factor stimulates collagenase and prostaglandin E2 production by human synovial cells and dermal fibroblasts. J Exp Med. 1985;162:2163–8. doi: 10.1084/jem.162.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaunay C, Farrenq V, Marini-Portugal A, et al. Infliximab to etanercept switch in patients with spondyloarthropathies and psoriatic arthritis: preliminary data. J Rheumatol. 2005;32:2183–5. [PubMed] [Google Scholar]
- Eastmond CJ, Wright V. The nail dystrophy of psoriatic arthritis. Ann Rheum Dis. 1979;38:226–8. doi: 10.1136/ard.38.3.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis CN, Krueger GG. Treatment of chronic plaque psoriasis by selective targeting of memory effector T lymphocytes. N Engl J Med. 2001;345:248–55. doi: 10.1056/NEJM200107263450403. [DOI] [PubMed] [Google Scholar]
- Espinoza LR, Aguilar JL, Espinoza CG, et al. Fibroblast function in psoriatic arthritis. I. Alteration of cell kinetics and growth factor responses. J Rheumatol. 1994;21:1502–6. [PubMed] [Google Scholar]
- Espinoza LR, Cuellar ML, Silveira LH. Psoriatic arthritis. Curr Opin Rheumatol. 1992;4:470–8. [PubMed] [Google Scholar]
- Espinoza LR, Espinoza CG, Cuellar ML, et al. Fibroblast function in psoriatic arthritis. II. Increased expression of beta platelet derived growth factor receptors and increased production of growth factor and cytokines. J Rheumatol. 1994;21:1507–11. [PubMed] [Google Scholar]
- Fournie B, Crognier L, Arnaud C, et al. Proposed classification criteria of psoriatic arthritis. A preliminary study in 260 patients. Rev Rhum Engl Ed. 1999;66:446–56. [PubMed] [Google Scholar]
- Gelfand JM, Gladman DD, Mease PJ, et al. Epidemiology of psoriatic arthritis in the population of the United States. J Am Acad Dermatol. 2005;53:573. doi: 10.1016/j.jaad.2005.03.046. [DOI] [PubMed] [Google Scholar]
- Giustizieri ML, Mascia F, Frezzolini A, et al. Keratinocytes from patients with atopic dermatitis and psoriasis show a distinct chemokine production profile in response to T cell-derived cytokines. J Allergy Clin Immunol. 2001;107:871–7. doi: 10.1067/mai.2001.114707. [DOI] [PubMed] [Google Scholar]
- Gladman DD. Toward unraveling the mystery of psoriatic arthritis. Arthritis Rheum. 1993;36:881–4. doi: 10.1002/art.1780360702. [DOI] [PubMed] [Google Scholar]
- Gladman DD. Psoriatic arthritis. Rheum Dis Clin North Am. 1998;24:829–44. x. doi: 10.1016/s0889-857x(05)70044-2. [DOI] [PubMed] [Google Scholar]
- Gladman DD. Effectiveness of psoriatic arthritis therapies. Semin Arthritis Rheum. 2003;33:29–37. doi: 10.1053/sarh.2002.50024. [DOI] [PubMed] [Google Scholar]
- Gladman DD. Traditional and newer therapeutic options for psoriatic arthritis: an evidence-based review. Drugs. 2005;65:1223–38. doi: 10.2165/00003495-200565090-00004. [DOI] [PubMed] [Google Scholar]
- Gladman DD, Anhorn KA, Schachter RK, et al. HLA antigens in psoriatic arthritis. J Rheumatol. 1986;13:586–92. [PubMed] [Google Scholar]
- Gladman DD, Antoni C, Mease P, et al. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005;64(Suppl 2):ii14–7. doi: 10.1136/ard.2004.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladman DD, Cheung C, Ng CM, et al. HLA-C locus alleles in patients with psoriatic arthritis (PsA) Hum Immunol. 1999;60:259–61. doi: 10.1016/s0198-8859(98)00123-2. [DOI] [PubMed] [Google Scholar]
- Gladman DD, Farewell VT, Kopciuk KA, et al. HLA markers and progression in psoriatic arthritis. J Rheumatol. 1998;25:730–3. [PubMed] [Google Scholar]
- Gladman DD, Farewell VT, Pellett F, et al. HLA is a candidate region for psoriatic arthritis. evidence for excessive HLA sharing in sibling pairs. Hum Immunol. 2003;64:887–9. doi: 10.1016/s0198-8859(03)00162-9. [DOI] [PubMed] [Google Scholar]
- Gladman DD, Shuckett R, Russell ML, et al. Psoriatic arthritis (PSA)—an analysis of 220 patients. Q J Med. 1987;62:127–41. [PubMed] [Google Scholar]
- Gomez-Reino JJ, Carmona L. Switching TNF antagonists in patients with chronic arthritis: an observational study of 488 patients over a four-year period. Arthritis Res Ther. 2006;8:R29. doi: 10.1186/ar1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon KB, Papp KA, Hamilton TK, et al. Efalizumab for patients with moderate to severe plaque psoriasis: a randomized controlled trial. JAMA. 2003;290:3073–80. doi: 10.1001/jama.290.23.3073. [DOI] [PubMed] [Google Scholar]
- Gottlieb AB, Evans R, Li S, et al. Infliximab induction therapy for patients with severe plaque-type psoriasis: a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2004;51:534–42. doi: 10.1016/j.jaad.2004.02.021. [DOI] [PubMed] [Google Scholar]
- Helliwell PS, Taylor WJ. Classification and diagnostic criteria for psoriatic arthritis. Ann Rheum Dis. 2005;64(Suppl 2):ii3–8. doi: 10.1136/ard.2004.032318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husted JA, Gladman DD, Farewell VT, et al. Health-related quality of life of patients with psoriatic arthritis: a comparison with patients with rheumatoid arthritis. Arthritis Rheum. 2001;45:151–8. doi: 10.1002/1529-0131(200104)45:2<151::AID-ANR168>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Karason A, Gudjonsson JE, Upmanyu R, et al. A susceptibility gene for psoriatic arthritis maps to chromosome 16q: evidence for imprinting. Am J Hum Genet. 2003;72:125–31. doi: 10.1086/345646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanaugh A, Antoni C, Krueger GG, et al. Infliximab improves health related quality of life and physical function in patients with psoriatic arthritis. Ann Rheum Dis. 2006;65:471–7. doi: 10.1136/ard.2005.040196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanaugh A, Antoni CE, Gladman DD, et al. The Infliximab Multinational Psoriatic Arthritis Controlled Trial (IMPACT): Results of radiographic analyses after 1 year. Ann Rheum Dis. 2006;65:1038–43. doi: 10.1136/ard.2005.045658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keystone EC, Kavanaugh AF, Sharp JT, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004;50:1400–11. doi: 10.1002/art.20217. [DOI] [PubMed] [Google Scholar]
- Korman NJ, Moul DK. Alefacept for the treatment of psoriasis: a review of the current literature and practical suggestions for everyday clinical use. Semin Cutan Med Surg. 2005;24:10–8. doi: 10.1016/j.sder.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Lacey DL, Timms E, Tan HL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–76. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- Langevitz P, Buskila D, Gladman DD. Psoriatic arthritis precipitated by physical trauma. J Rheumatol. 1990;17:695–7. [PubMed] [Google Scholar]
- Lebwohl M, Ting PT, Koo JY. Psoriasis treatment: traditional therapy. Ann Rheum Dis. 2005;64(Suppl 2):ii83–6. doi: 10.1136/ard.2004.030791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebwohl M, Tyring SK, Hamilton TK, et al. A novel targeted T-cell modulator, efalizumab, for plaque psoriasis. N Engl J Med. 2003;349:2004–13. doi: 10.1056/NEJMoa030002. [DOI] [PubMed] [Google Scholar]
- Leonard DG, O’Duffy JD, Rogers RS. Prospective analysis of psoriatic arthritis in patients hospitalized for psoriasis. Mayo Clin Proc. 1978;53:511–18. [PubMed] [Google Scholar]
- Mader R, Gladman DD, Long J, et al. Does injectable gold retard radiologic evidence of joint damage in psoriatic arthritis? Clin Invest Med. 1995;18:139–43. [PubMed] [Google Scholar]
- Marguerie L, Flipo RM, Grardel B, et al. Use of disease-modifying antirheumatic drugs in patients with psoriatic arthritis. Joint Bone Spine. 2002;69:275–81. doi: 10.1016/s1297-319x(02)00396-2. [DOI] [PubMed] [Google Scholar]
- Mease P. Psoriatic arthritis/psoriasis. In: Smolen JS, editor. Targeted Therapies in Rheumatology. London: Martin Dunitz Ltd; 2003. pp. 525–48. [Google Scholar]
- Mease P. Efalizumab in psoriatic arthritis. Toronto, Canada: International Psoriasis Society; 2004a. [Google Scholar]
- Mease P. TNFalpha therapy in psoriatic arthritis and psoriasis. Ann Rheum Dis. 2004b;63:755–8. doi: 10.1136/ard.2004.020719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mease P, Gladman D, Keystone E. Efficacy of alefacept in combination with methotrexate in the treatment of psoriatic arthritis. Ann Rheum Dis. 2005;64:324. doi: 10.1002/art.21870. [DOI] [PubMed] [Google Scholar]
- Mease P, Goffe BS. Diagnosis and treatment of psoriatic arthritis. J Am Acad Dermatol. 2005;52:1–19. doi: 10.1016/j.jaad.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Mease PJ, Antoni CE. Psoriatic arthritis treatment: biological response modifiers. Ann Rheum Dis. 2005;64(Suppl 2):ii78–82. doi: 10.1136/ard.2004.034157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mease PJ, Gladman DD, Keystone EC. Alefacept in combination with methotrexate for the treatment of psoriatic arthritis: results of a randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2006;54:1638–45. doi: 10.1002/art.21870. [DOI] [PubMed] [Google Scholar]
- Mease PJ, Gladman DD, Ritchlin CT, et al. Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2005;52:3279–89. doi: 10.1002/art.21306. [DOI] [PubMed] [Google Scholar]
- Mease PJ, Goffe BS, Metz J, et al. Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial. Lancet. 2000;356:385–90. doi: 10.1016/S0140-6736(00)02530-7. [DOI] [PubMed] [Google Scholar]
- Mease PJ, Kivitz AJ, Burch FX, et al. Etanercept treatment of psoriatic arthritis: safety, efficacy, and effect on disease progression. Arthritis Rheum. 2004;50:2264–72. doi: 10.1002/art.20335. [DOI] [PubMed] [Google Scholar]
- Moll JM, Wright V. Familial occurrence of psoriatic arthritis. Ann Rheum Dis. 1973a;32:181–201. doi: 10.1136/ard.32.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll JM, Wright V. Psoriatic arthritis. Semin Arthritis Rheum. 1973b;3:55–78. doi: 10.1016/0049-0172(73)90035-8. [DOI] [PubMed] [Google Scholar]
- Nakashima T, Wada T, Penninger JM. RANKL and RANK as novel therapeutic targets for arthritis. Curr Opin Rheumatol. 2003;15:280–7. doi: 10.1097/00002281-200305000-00016. [DOI] [PubMed] [Google Scholar]
- Nash P, Clegg DO. Psoriatic arthritis therapy: NSAIDs and traditional DMARDs. Ann Rheum Dis. 2005;64(Suppl 2):ii74–7. doi: 10.1136/ard.2004.030783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ory PA. Radiography in the assessment of musculoskeletal conditions. Best Pract Res Clin Rheumatol. 2003;17:495–512. doi: 10.1016/s1521-6942(03)00022-6. [DOI] [PubMed] [Google Scholar]
- Ory PA, Gladman DD, Mease PJ. Psoriatic arthritis and imaging. Ann Rheum Dis. 2005;64(Suppl 2):ii55–7. doi: 10.1136/ard.2004.033928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipitone N, Kingsley GH, Manzo A, et al. Current concepts and new developments in the treatment of psoriatic arthritis. Rheumatology (Oxford) 2003;42:1138–48. doi: 10.1093/rheumatology/keg363. [DOI] [PubMed] [Google Scholar]
- Rahman P, Bartlett S, Siannis F, et al. CARD15: a pleiotropic autoimmune gene that confers susceptibility to psoriatic arthritis. Am J Hum Genet. 2003;73:677–81. doi: 10.1086/378076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman P, Gladman DD, Cook RJ, et al. The use of sulfasalazine in psoriatic arthritis: a clinic experience. J Rheumatol. 1998;25:1957–61. [PubMed] [Google Scholar]
- Reich K, Nestle FO, Papp K, et al. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet. 2005;366:1367–74. doi: 10.1016/S0140-6736(05)67566-6. [DOI] [PubMed] [Google Scholar]
- Reich K, Nestle FO, Papp K, et al. Improvement in quality of life with infliximab induction and maintenance therapy in patients with moderate-to-severe psoriasis: a randomized controlled trial. Br J Dermatol. 2006;154:1161–8. doi: 10.1111/j.1365-2133.2006.07237.x. [DOI] [PubMed] [Google Scholar]
- Remicade. Malvern, PA: Centocor, Inc.; 2006a. Remicade package insert, EU. [Google Scholar]
- Remicade. Malvern, PA: Centocor, Inc.; 2006b. Remicade package insert, US. [Google Scholar]
- Ritchlin C, Anandarajaha A, Totterman S, et al. Preliminary data from a study of adalimumab in the treatment of psoriatic arthritis [abstract] Ann Rheum Dis. 2004;63:403. [Google Scholar]
- Ritchlin C, Haas-Smith SA, Hicks D, et al. Patterns of cytokine production in psoriatic synovium. J Rheumatol. 1998;25:1544–52. [PubMed] [Google Scholar]
- Ritchlin CT, Haas-Smith SA, Li P, et al. Mechanisms of TNF-alpha-and RANKL-mediated osteoclastogenesis and bone resorption in psoriatic arthritis. J Clin Invest. 2003;111:821–31. doi: 10.1172/JCI16069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saklatvala J. Tumour necrosis factor alpha stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature. 1986;322:547–9. doi: 10.1038/322547a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonen S. The EUROPSO psoriasis patient study: treatment history and satisfaction reported by 17,900 members of European psoriasis patients associations (poster). 2003.
- Shbeeb M, Uramoto KM, Gibson LE, et al. The epidemiology of psoriatic arthritis in Olmsted County, Minnesota, USA, 1982–1991. J Rheumatol. 2000;27:1247–50. [PubMed] [Google Scholar]
- Soderlin MK, Borjesson O, Kautiainen H, et al. Annual incidence of inflammatory joint diseases in a population based study in southern Sweden. Ann Rheum Dis. 2002;61:911–15. doi: 10.1136/ard.61.10.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoll KB, Helliwell PS. Comparison of disability and quality of life in rheumatoid and psoriatic arthritis. J Rheumatol. 2001;28:1842–6. [PubMed] [Google Scholar]
- Swanbeck G, Inerot A, Martinsson T, et al. Genetic counselling in psoriasis: empirical data on psoriasis among first-degree relatives of 3095 psoriatic probands. Br J Dermatol. 1997;137:939–42. [PubMed] [Google Scholar]
- Taylor W. ClASsification criteria for Psoriatic ARthritis: results from the CASPAR study. Arthritis Rheum. 2006;54:2665–73. doi: 10.1002/art.21972. [DOI] [PubMed] [Google Scholar]
- Terajima S, Higaki M, Igarashi Y, et al. An important role of tumor necrosis factor-alpha in the induction of adhesion molecules in psoriasis. Arch Dermatol Res. 1998;290:246–52. doi: 10.1007/s004030050299. [DOI] [PubMed] [Google Scholar]
- Van den Bosch F, Kruithof E, Baeten D, et al. Effects of a loading dose regimen of three infusions of chimeric monoclonal antibody to tumour necrosis factor alpha (infliximab) in spondyloarthropathy: an open pilot study. Ann Rheum Dis. 2000;59:428–33. doi: 10.1136/ard.59.6.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Heijde D, Gladman D, Kavanaugh A, et al. Infliximab inhibits progression of radiographic damage in patients with active psoriatic arthritis: 54 week results from IMPACT 2. Arthritis Rheum. 2005;52:S281. doi: 10.1002/art.22805. [DOI] [PubMed] [Google Scholar]
- Veale D, Rogers S, Fitzgerald O. Immunolocalization of adhesion molecules in psoriatic arthritis, psoriatic and normal skin. Br J Dermatol. 1995;132:32–8. doi: 10.1111/j.1365-2133.1995.tb08621.x. [DOI] [PubMed] [Google Scholar]
- Veale D, Yanni G, Rogers S, et al. Reduced synovial membrane macrophage numbers, ELAM-1 expression, and lining layer hyperplasia in psoriatic arthritis as compared with rheumatoid arthritis. Arthritis Rheum. 1993;36:893–900. doi: 10.1002/art.1780360705. [DOI] [PubMed] [Google Scholar]
- Veale DJ, Ritchlin C, FitzGerald O. Immunopathology of psoriasis and psoriatic arthritis. Ann Rheum Dis. 2005;64(Suppl 2):ii26–9. doi: 10.1136/ard.2004.031740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassenberg S, Fischer-Kahle V, Herborn G, et al. A method to score radiographic change in psoriatic arthritis. Z Rheumatol. 2001;60:156–66. doi: 10.1007/s003930170064. [DOI] [PubMed] [Google Scholar]
- Wick MC, Ernestam S, Lindblad S, et al. Adalimumab (Humira) restores clinical response in patients with secondary loss of efficacy from infliximab (Remicade) or etanercept (Enbrel): results from the STURE registry at Karolinska University Hospital. Scand J Rheumatol. 2005;34:353–8. doi: 10.1080/03009740510026887. [DOI] [PubMed] [Google Scholar]
- Wong K, Gladman DD, Husted J, et al. Mortality studies in psoriatic arthritis: results from a single outpatient clinic. I. Causes and risk of death. Arthritis Rheum. 1997;40:1868–72. doi: 10.1002/art.1780401021. [DOI] [PubMed] [Google Scholar]
- Wright V. Rheumatism and psoriasis: a re-evaluation. Am J Med. 1959;27:454–62. doi: 10.1016/0002-9343(59)90011-7. [DOI] [PubMed] [Google Scholar]
- Zachariae H. Prevalence of joint disease in patients with psoriasis: implications for therapy. Am J Clin Dermatol. 2003;4:441–7. doi: 10.2165/00128071-200304070-00001. [DOI] [PubMed] [Google Scholar]


