Abstract
The specialized cardiac conduction system (CCS) consists of the sinoatrial node (SAN) and the atrioventricular (AV) conduction system (AVCS), which includes proximal (AV node, bundle of His and bundle branches) and distal (Purkinje fibers) components. In four CCS marker mice [two transgenic (cGATA6|lacZ, CCS|lacZ) and two targeted gene knock-in (minK|lacZ, Hop|lacZ)] the expression of the lacZ gene (β-gal) has been reported to mark portions of the proximal and distal AVCS; the expression of this marker in the adult SAN is unknown. The primary objective of this study was to analyze the utility of these marker mice in the identification of the SAN. Intercaval and interventricular septal regions, containing all the components of the CCS, were freshly dissected from adult mice based on the anatomical landmarks and sectioned. Immunohistochemical characterization was performed with SAN markers (Cx45, HCN4), compared to the reporter expression (β-gal) and markers of the working myocardium (Cx40 and Cx43). In all four of the CCS marker mice, we found that β-gal expression is consistently observed in the proximal and distal AVCS. However, the presence of lacZ gene expression in the working myocardium outside the CCS and/or the absence of this reporter expression in the SAN prevent the effective use of these mice to identify the SAN, leading us to conclude that none of the four CCS marker mice we studied specifically mark the SAN.
Keywords: Conduction system, Connexins, Histo(patho)logy, Sinus node, Transgenic animal models
Introduction
The heterogeneous tissues of the cardiac conduction system (CCS) are responsible for initiating, maintaining and coordinating the rhythmic pumping of the heart. The components of the CCS include the sinoatrial node (SAN), the proximal (atrioventricular node and bundle of His), and distal (bundle branches and Purkinje fibers) atrioventricular conduction system (AVCS). The SAN is the impulse generating tissue or primary pacemaker of the heart. Early studies focused on the morphological and electrophysiological characteristics of the SAN and other components of the CCS (reviewed in [1, 2]). In 1883 Gaskell described the sinus venosus-atrial junction, which has the highest rate of automaticity, as the pace-making region [3]. Keith and Flack described the complete morphology of SAN 24 years later [4]. Subsequently, there have been multiple reports characterizing SAN structure, function and their interrelationships [5-7], but it been only in the past decade that a definition of the SAN based on molecular and histochemical markers was published by Boyett and colleagues [8, 9].
The SAN has been defined by anatomical, electrical, and histochemical means. Taken together, the SAN can be defined as a heterogeneous population of automatic cells in the atria near the crista-terminalis with its core positive for Cx45 and HCN4 and periphery positive for both Cx45 and Cx43[9-11]. Cx40, Cx43, Cx45 and Cx30.2 are considered the primary connexins of the heart and have specific compartmentalized expression pattern both in the conduction system and in the myocardium. Cx40, with the highest electrical conduction of the four connexins is expressed in the atria and in the bundle of His and bundle branches. Cx43, the moderate conductor is expressed in all the working myocardium. Cx45, with low conductance and is associated with SAN and AVN [12, 13]. Cx30.2 with the lowest conductance has an expression pattern similar to that of Cx45[14]. HCN4 on the other hand is a non-specific cation channel known to produce the If current, characteristic of the cardiac pacing tissue (Reviewed in[15]).
Based on the conservation of the CCS paradigm among mammals and being amenable to transgenic and recombination techniques, the mouse has emerged as an important tool for the study of the CCS. Recent reports have described genetically engineered mouse models, where a reporter gene, lacZ (gene product is β-galactosidase; β-gal), marks components of the CCS (“CCS marker” mice) [16-20]. Expression of this reporter in the various components of the AVCS has been extensively characterized in four models: 1) cGATA6|lacZ 2) minK|lacZ 3) CCS|lacZ and 4) Hop|lacZ. The purpose of the present study was to delineate the SAN-specific expression pattern of the reporter in adult hearts of these four CCS marker mice, and to determine this we compared immunohistochemical localization of SAN-specific markers (Cx45, HCN4) with the staining patterns of the β-gal reporter expression.
Materials and Methods
Conduction System Marker Mice
The previously published characteristics of the conduction system marker mice are shown in Table 1. In these mice, the morphology of the conduction system has not been reported to be affected by the insertion of the reporter gene, but the expression pattern of the reporter varies among the models. cGATA6|lacZ transgenic mice, first described by Davis and colleagues [17], harbors a fragment of the chicken GATA6 promoter (−1.5/0.0/GATA6) driving the expression of lacZ in the 129J strain of mice[21]. GATA6 belongs to the GATA family of zinc finger transcription factors and is important for regulating terminal differentiation in the heart, lung, gut and gut-derived organs. The reporter expression in the conduction system of the CCS|lacZ transgenic mouse, originally termed MC4/Eng2|lacZ and described by Logan [22], was delineated by Rentschler et al [19]. This mouse has been widely used in the study of the AVCS [23, 24]. minK|lacZ, first described by Kupershmidt and associates [18] and characterized by Kondo [6], are mice with a homologous recombination of the lacZ gene replacing the KCNE1 channel in a Swiss black background (kind gift of Dr. Dan Roden). minK (KCNE1) is a voltage-gated delayed rectifier potassium channel that is highly expressed in the heart, intestine, lungs and kidney. It acts as a β-subunit for a variety of ion channels including, but not limited to, KCNQ1, HERG and HCN1-4. HOP|lacZ mice (a kind gift of Dr. Jon Epstein), reported and characterized by Chen and Ismat [20, 25], were generated by homologous targeting of the lacZ gene into the HOP locus in 628 strain of mice. HOP is an atypical homeodomain-only transcription factor that does not bind to DNA. It negatively regulates transcription by binding and inhibiting serum response factor and by recruiting histone deacetylases.
Table 1.
| Mouse | Developmental Stage |
Genetic Manipulation |
Expression | Citation | |||||
|---|---|---|---|---|---|---|---|---|---|
| SAN | AVN | HB | BB | Purkinje | Other Regions | ||||
| cGATA6|lacZ | (Neonate) | Transgenic | − | + | + | − | − | Patchy expression in the atria and ventricular free walls |
[21] |
| CCS|lacZ | (Neonate) | Transgenic | + | + | + | + | + | Systemic venous valves | [19] |
| minK|lacZ | (E13.5-Adult) | Homologous replacement |
− | + | + | + | + | Aortic and pulmonic ring, mitral and tricuspid musculature and the junction of systemic venous tributaries and right atrium |
[18] |
| HOP|lacZ | (E16.5-Adult) | Homologous replacement |
− | + | + | + | + | Generalized expression in the atrial and ventricular myocardium |
[20] |
SAN = sinoatrial node
AVN = atrioventricular node
HB = bundle of His
BB = bundle branches (both right and left)
Dissection of the SAN
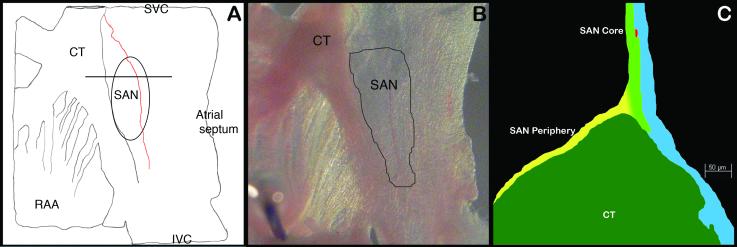
Ten adult mice per genotype (PGT) of mixed gender were humanely sacrificed according to institutional and national protocols [26], using an overdose of isofluorane (inhalation) and the beating heart was removed and placed in a tyrode-perfused chamber as previously described [27]. In brief, each active SAN was dissected using anatomical markers by sequential removal of the ventricles, great arteries, pulmonary veins and the left atrium. The right atrium was then opened along the caval axis to expose the cristaterminalis and the SAN (Fig. 1). The inter-ventricular septum and part of the right ventricle around the tricuspid annulus were retained during isolation of the AV node. The left atrium was retained in some specimens for better ease of tissue handling. The whole tissues were visualized under a Nikon SMZ1500 dissection microscope and imaged with a DXM1200F camera. Of the ten mice 2-3 mice PGT were used for X-gal staining and 4-6 mice PGT were used for immunohistochemistry.
Fig. 1.
Staining and Immunohistochemistry (IHC)
Isolated intercaval regions with or without AV nodal tissue were pinned on silicone blocks and kept in ice-cold Hanks buffer before staining with X-gal to identify the β-gal expressing tissue. Staining technique for β-gal detection, described by Hogan [28], was used for all specimens. Stained intercaval regions were photographed, embedded in OCT and cryosectioned. Sections (10-15μm) were counter-stained with Eosin or Nuclear Fast Red before microscopy. Unstained intercaval regions were cryosectioned and adjacent sections (5-8μm) were labeled using X-gal stain or antibodies against β-gal, Connexin40 (Cx40), Connexin43 (Cx43), Connexin45 (Cx45), HCN4 and VEGF-R3. Details on the antibody source, concentration and antigen are provided in Table 2, in the supplementary materials. Confocal microscopic analysis was performed on the specimens. We used the immunohistochemical definition of the adult SAN of Boyett et al [9-11], i.e., a heterogeneous population of automatic cells in the atria near the crista-terminalis with its core positive for Cx45 and HCN4 and periphery positive for both Cx45 and Cx43. Immunostained sections were then examined for the specificity of marker expression with respect to the CCS under an Axioplane LSM510 (Carl Zeiss) confocal microscope.
Table 2.
β-galactosidase expression in various tissues, among the four CCS-marker mice
| Mouse Genotype | SAN | Peri-SAN Atria | Atria | AVN |
|---|---|---|---|---|
| cGATA6|LacZ | − | + | ++ | + |
| CCS|LacZ | − | + | ++ | ++ |
| minK|LacZ | − | − | − | ++ |
| HOP|LacZ | + | + | ++ | ++ |
Results
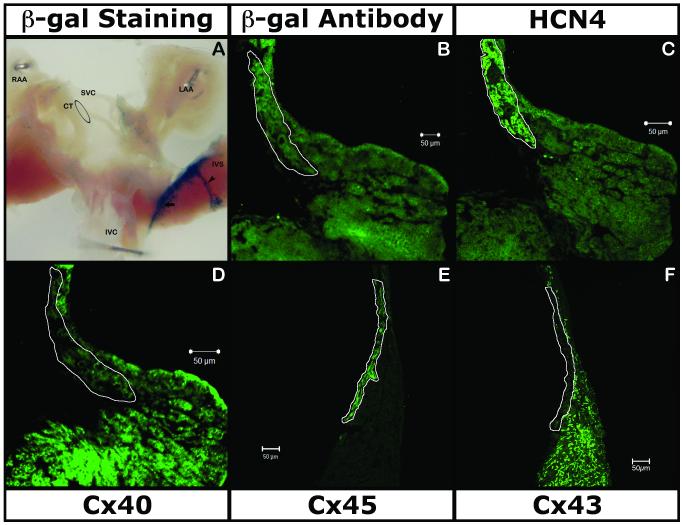
A cartoon representation of the anatomical region of the sinus venosus containing the SAN in relation to other prominent structures of the posterior right atrium is shown in Figure 1A. The outlined region in Figure 1A and 1B demarcate the potential SAN. Figure 1C shows a cartoon of the confocal image of the SAN sectioned along the plane of sectioning shown in Figure 1A. Representative pictures of whole mount X-gal-stained intercaval regions and photomicrographs of immunostained sections representing genes analyzed in the four CCS marker mice are shown in Figures 2-5. Consistent β-gal expression in the proximal and distal AVCS was found in all four studied adult models, but there was significant variability in atrial expression in mice of different genotypes. To a lesser extent, the expression varied even between mice from the same genotype (supplemental Fig. 1; supplemental Fig. 2). However, identification of the β–gal reporter outside the CCS and/or the absence of β-gal expression in the SAN prevents them from being used as effective markers for identification of adult SAN cells. The staining was considered “CCS-specific” when the marker expression was restricted only to components of the CCS and “non-CC-specific” or “generalized” when expressed anywhere outside the CCS. A description of findings in each marker mouse model is provided below and tabulated in Table 2.
Fig. 2.
Fig. 5.
cGATA6|lacZ
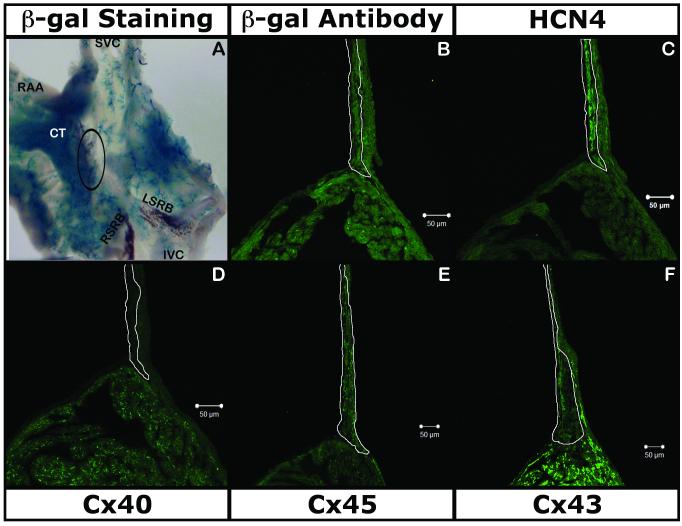
LacZ gene was strongly expressed over the entire intercaval region (Fig. 2A, 2B, supplemental Fig. 1C). X-gal staining showed β-gal activity near the SAN region along the crista-terminalis with a vascular pattern (Fig. 2A, supplemental Fig. 1C). IHC with antibody against β-gal and section X-gal staining (Fig. 2B, supplemental Fig. 1) showed expression in the atrial muscle (expressing Cx40 and Cx43; Fig. 2D & Fig. 2F respectively). The β-gal IHC and X-gal staining also confirmed the absence of the reporter in the SAN (expressing HCN4 and Cx45; Fig. 2C & 2E respectively). Examination of the sections at higher magnification and co-localization of β-gal with VEGF-R2 identified marker expression in vascular endothelium (Data not shown).
CCS|lacZ
X-gal staining of the hearts of these mice showed consistent proximal and distal AVCS (Fig. 3B inset) and patchy and variable expression of the reporter within the genotype in the intercaval region, left and right atria including the appendages (Fig. 3A & 3B, supplemental Fig. 2) similar to the pattern previously reported for neonates [19]. Section X-gal staining (Fig. 3B, supplemental Fig. 2) revealed the absence of expression of the lacZ gene in the SAN marked by HCN4 (Fig. 3C) and Cx45 (Fig. 3E) while being variably present in the adjacent myocardium marked by Cx43 and Cx40 (Fig. 3F & 3D).
Fig. 3.
minK|lacZ
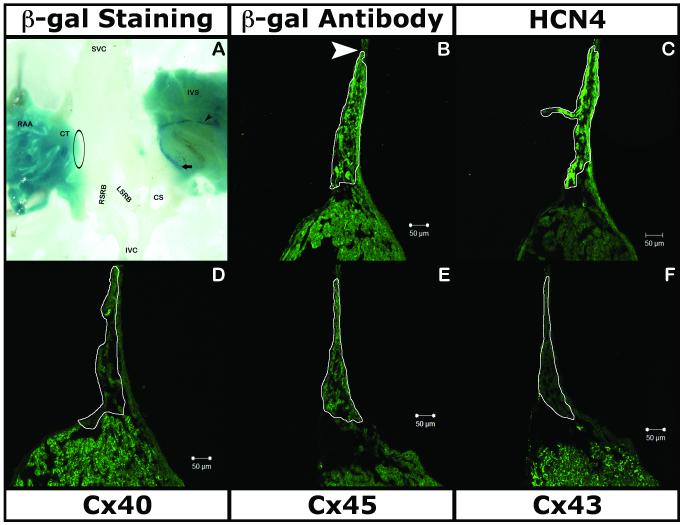
As reported by Kupershmidt [18] in neonates and adults, X-gal staining was consistent, strong and specific in the AVN, bundle of His and Purkinje fibers (Fig. 4A), indicating expression of the lacZ gene in the proximal and distal AVCS. There was no trace of expression in the SAN region with either X-gal staining (Fig. 4A) or by IHC (Fig. 4B). Immunohistochemical analysis with Cx45 (Fig. 4E), HCN4 (Fig. 4C) and Cx43 (Fig. 4F) confirmed that the reporter is not expressed in either the core or the periphery of the SAN. minK|lacZ did not show any generalized expression outside of the CCS in the atrial tissue stained by Cx40 (Fig. 4D).
Fig. 4.
Hop|lacZ
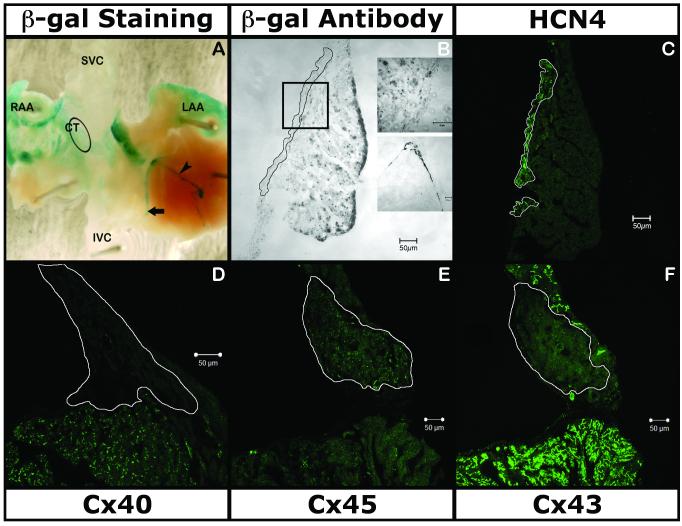
X-gal staining was consistently seen in the proximal and distal AVCS (Fig. 5A). However, staining was also seen in a generalized pattern in both the atrial appendages, crista-terminalis and in the inter-ventricular septum. IHC staining revealed the presence of the reporter gene (Fig. 5B) in the SAN (expressing HCN4 and Cx45; Fig. 5C & 5E), albeit as an extension of the expression in the myocytes of the crista-terminalis (expressing Cx43 and Cx40; Fig. 5F & 5D, respectively).
Discussion
Using immunohistochemical techniques, Boyett and colleagues provided a molecular description of the SAN as a heterogeneous population of automatic cells in the right atria at the junction of the crista-terminalis (CT) and the sinus venosus with its core positive for Cx45 and HCN4 and periphery positive for both Cx45 and Cx43. Cx40, the most abundant connexin in the atria, is not present in the SAN [9-11]. Using this approach to identify the SAN in adult CCS marker mice, we found that a reporter gene that is consistently expressed in the proximal and distal AVCS is not specifically or consistently expressed in the SAN. Thus, while the current study shows that in each of these genetically engineered mice, the reporter specifically marks some components of the CCS, they lack specific marker expression in the adult SAN.
Our results demonstrate that Hop|lacZ is expressed in the SAN, but this cannot be distinguished from generalized expression in surrounding atrial myocardium. CCS|lacZ, on the other hand, has been reported to have marker expression in the SAN region in early embryonic and neonatal time points (personal communication and [29]), but our study does not show any SAN expression in adult hearts. In some mice with this genotype, we found marker expression in the tissue around the SAN along the ICR, which in a whole mount would give an impression of β-gal expression in the SAN (supplemental Fig. 2). cGATA6|lacZ and minK|lacZ both have strong reporter expression in the proximal AVCS with no expression in the SAN. From these results it is tempting to speculate that either the SAN originates from a different primordial tissue compared to the rest of the CCS (three of the four mice don't mark the SAN) or alternatively, in later developmental stages cells in the SAN down-regulate reporter gene expression, while other components of the CCS continue to express it.
While molecular markers have facilitated study of the CCS, the question of CCS origin still remains unanswered [16, 30]. There are currently three schools of thought on the developmental origin of the CCS. In the first, it is believed that sub-populations of primitive myocardium/mesenchymal tissue in specific regions of the primitive heart are prevented from differentiating into atrial and ventricular working myocardium. These cells continue to retain their primitive phenotype and contribute to the formation of the CCS, septae and valves [31-33]. In the second model, vascular endothelium or endocardium is believed to emit paracrine signals that induce recruitment and re-differentiation of the periarteiolar working myocardium into the CCS, especially in the AVCS [29, 34-36]. Finally in a third scenario, it has been proposed that neural crest cells migrating from the posterior rhomboencephalons contribute or signal the generation of the CCS from the myocardium [37-39]. Based on existing evidence, the first two models seem more likely, but with current tools, it is not possible to resolve the differences in the three proposed models.
An ideal molecular marker, one that identifies the specific components of CCS primordia from the first cardiac contraction throughout adult life, would be a real asset in delineating the most appropriate model. While such a molecular marker model would be invaluable, the existing tools need to be evaluated for specificity and persistence throughout development and adulthood. While multiple transgenic and recombineered mouse lines have been described that express a reporter gene in various components of the CCS (reviewed in [40, 41]), details of CCS-specific expression have only been obtained in a small subset. Accurate evaluation of the existing and future conduction system marker mice will lead to better understanding of the developmental aspects of the SAN and other components of the CCS. Such understanding promises improved comprehension of the pathogenesis of conduction system disease, e.g. sick sinus syndrome, which in turn will lead to alternative therapeutic strategies, e.g. the development of biological pacemakers [42, 43].
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brooks CM, Lu H-h. The sinoatrial pacemaker of the heart. C. C. Thomas; Springfield, Ill.: 1972. [Google Scholar]
- 2.Little RC. Physiology of atrial pacemakers and conductive tissues. Futura Pub. Co.; Mount Kisco, N.Y.: 1980. [Google Scholar]
- 3.Gaskell WH. On the innervation of the heart, with special reference to the heart of the tortoise. J Physiol. 1883;4(1):43–127. doi: 10.1113/jphysiol.1883.sp000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keith A, Flack MW. The form and nature of the muscular connection between the primary divisions of the vertibrate heart. J Anat Physiol. 1907;41(1):172–89. [PMC free article] [PubMed] [Google Scholar]
- 5.Van Mierop LH, Gessener IH. The morphologic development of the sinoatrial node. The Ameriacn Journal of Cardiology. 1970 Feb;25(2):204–12. doi: 10.1016/0002-9149(70)90580-1. [DOI] [PubMed] [Google Scholar]
- 6.Kondo RP, Anderson RH, Kupershmidt S, Roden DM, Evans SM. Development of the cardiac conduction system as delineated by minK-lacZ. J Cardiovasc Electrophysiol. 2003 Apr;14(4):383–91. doi: 10.1046/j.1540-8167.2003.02467.x. [DOI] [PubMed] [Google Scholar]
- 7.Bowman LN, Jongsma HJ. Structure and Function of the sino-atrial node: a review. European Heart Journal. 1986 Feb;7(2):94–104. doi: 10.1093/oxfordjournals.eurheartj.a062047. [DOI] [PubMed] [Google Scholar]
- 8.Coppen SR, Kodama I, Boyett MR, Dobrzynski H, Takagishi Y, Honjo H, et al. Connexin45, a major connexin of the rabbit sinoatrial node, is co-expressed with connexin43 in a restricted zone at the nodal-crista terminalis border. J Histochem Cytochem. 1999 Jul;47(7):907–18. doi: 10.1177/002215549904700708. [DOI] [PubMed] [Google Scholar]
- 9.Boyett MR, Honjo H, Kodama I. The sinoatrial node, a heterogeneous pacemaker structure. Cardiovasc Res. 2000 Sep;47(4):658–87. doi: 10.1016/s0008-6363(00)00135-8. [DOI] [PubMed] [Google Scholar]
- 10.Boyett MR, Dobrzynski H, Lancaster MK, Jones SA, Honjo H, Kodama I. Sophisticated architecture is required for the sinoatrial node to perform its normal pacemaker function. J Cardiovasc Electrophysiol. 2003 Jan;14(1):104–6. doi: 10.1046/j.1540-8167.2003.02307.x. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto M, Dobrzynski H, Tellez J, Niwa R, Billeter R, Honjo H, et al. Extended atrial conduction system characterised by the expression of the HCN4 channel and connexin45. Cardiovasc Res. 2006 Aug 2; doi: 10.1016/j.cardiores.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 12.Miquerol L, Dupays L, Theveniau-Ruissy M, Alcolea S, Jarry-Guichard T, Abran P, et al. Gap junctional connexins in the developing mouse cardiac conduction system. Novartis Found Symp. 2003;250:80–98. doi: 10.1002/0470868066.ch6. discussion -109, 276-9. [DOI] [PubMed] [Google Scholar]
- 13.Lo CW. Role of gap junctions in cardiac conduction and development: insights from the connexin knockout mice. Circ Res. 2000 Sep 1;87(5):346–8. doi: 10.1161/01.res.87.5.346. [DOI] [PubMed] [Google Scholar]
- 14.Kreuzberg MM, Sohl G, Kim JS, Verselis VK, Willecke K, Bukauskas FF. Functional properties of mouse connexin30.2 expressed in the conduction system of the heart. Circ Res. 2005 Jun 10;96(11):1169–77. doi: 10.1161/01.RES.0000169271.33675.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stieber J, Hofmann F, Ludwig A. Pacemaker channels and sinus node arrhythmia. Trends Cardiovasc Med. 2004 Jan;14(1):23–8. doi: 10.1016/j.tcm.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Wessels A, Juraszek AL, Edwards AV, Burch JBE. The Development of the Cardiac Conduction System: An old story with new perspectives. In: Benson DW, Srivastava D, Nakazawa M, editors. Cardiovascular Development and Congenital Malformations: Molecular and Genetic Mechanisms. Blackwell Publishing; Malden, Mass.: 2005. pp. 101–4. [Google Scholar]
- 17.Davis DL, Edwards AV, Juraszek AL, Phelps A, Wessels A, Burch JB. A GATA-6 gene heart-region-specific enhancer provides a novel means to mark and probe a discrete component of the mouse cardiac conduction system. Mech Dev. 2001 Oct;108(12):105–19. doi: 10.1016/s0925-4773(01)00500-7. [DOI] [PubMed] [Google Scholar]
- 18.Kupershmidt S, Yang T, Anderson ME, Wessels A, Niswender KD, Magnuson MA, et al. Replacement by homologous recombination of the minK gene with lacZ reveals restriction of minK expression to the mouse cardiac conduction system. Circ Res. 1999 Feb 5;84(2):146–52. doi: 10.1161/01.res.84.2.146. [DOI] [PubMed] [Google Scholar]
- 19.Rentschler S, Vaidya DM, Tamaddon H, Degenhardt K, Sassoon D, Morley GE, et al. Visualization and functional characterization of the developing murine cardiac conduction system. Development. 2001;128(10):1785–92. doi: 10.1242/dev.128.10.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ismat FA, Zhang M, Kook H, Huang B, Zhou R, Ferrari VA, et al. Homeobox protein Hop functions in the adult cardiac conduction system. Circ Res. 2005 Apr 29;96(8):898–903. doi: 10.1161/01.RES.0000163108.47258.f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He CZ, Burch JB. The chicken GATA-6 locus contains multiple control regions that confer distinct patterns of heart region-specific expression in transgenic mouse embryos. J Biol Chem. 1997 Nov 7;272(45):28550–6. doi: 10.1074/jbc.272.45.28550. [DOI] [PubMed] [Google Scholar]
- 22.Logan C, Khoo WK, Cado D, Joyner AL. Two enhancer regions in the mouse En-2 locus direct expression to the mid/hindbrain region and mandibular myoblasts. Development. 1993 Mar;117(3):905–16. doi: 10.1242/dev.117.3.905. [DOI] [PubMed] [Google Scholar]
- 23.Moskowitz IP, Pizard A, Patel VV, Bruneau BG, Kim JB, Kupershmidt S, et al. The T-Box transcription factor Tbx5 is required for the patterning and maturation of the murine cardiac conduction system. Development. 2004 Aug;131(16):4107–16. doi: 10.1242/dev.01265. [DOI] [PubMed] [Google Scholar]
- 24.Poelmann RE, Jongbloed MR, Molin DG, Fekkes ML, Wang Z, Fishman GI, et al. The neural crest is contiguous with the cardiac conduction system in the mouse embryo: a role in induction? Anat Embryol (Berl) 2004 Aug;208(5):389–93. doi: 10.1007/s00429-004-0401-6. [DOI] [PubMed] [Google Scholar]
- 25.Chen F, Kook H, Milewski R, Gitler AD, Lu MM, Li J, et al. Hop is an unusual homeobox gene that modulates cardiac development. Cell. 2002 Sep 20;110(6):713–23. doi: 10.1016/s0092-8674(02)00932-7. [DOI] [PubMed] [Google Scholar]
- 26.Institute of Laboratory Animal Resources (U.S.) NIH publication. U.S. Dept. of Health and Human Services, Public Health Service:v; Bethesda, Md.: Committee on Care and Use of Laboratory Animals. Guide for the care and use of laboratory animals. [Google Scholar]
- 27.Dobrzynski H, Rothery SM, Marples DD, Coppen SR, Takagishi Y, Honjo H, et al. Presence of the Kv1.5 K(+) channel in the sinoatrial node. J Histochem Cytochem. 2000 Jun;48(6):769–80. doi: 10.1177/002215540004800606. [DOI] [PubMed] [Google Scholar]
- 28.Hogan B. Manipulating the mouse embryo : a laboratory manual. 2nd ed. Cold Spring Harbor Laboratory Press; Plainview, N.Y.: 1994. [Google Scholar]
- 29.Rentschler S, Zander J, Meyers K, France D, Levine R, Porter G, et al. Neuregulin-1 promotes formation of the murine cardiac conduction system. Proc Natl Acad Sci U S A. 2002 Aug 6;99(16):10464–9. doi: 10.1073/pnas.162301699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chadwick D, Goode J, Novartis Foundation . Development of the cardiac conduction system. J. Wiley; Chichester, UK ; Hoboken, NJ: 2003. [Google Scholar]
- 31.Christoffels VM, Burch JB, Moorman AF. Architectural plan for the heart: early patterning and delineation of the chambers and the nodes. Trends Cardiovasc Med. 2004 Nov;14(8):301–7. doi: 10.1016/j.tcm.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Christoffels VM, Mommersteeg MT, Trowe MO, Prall OW, de Gier-de Vries C, Soufan AT, et al. Formation of the venous pole of the heart from an Nkx2-5-negative precursor population requires Tbx18. Circ Res. 2006 Jun 23;98(12):1555–63. doi: 10.1161/01.RES.0000227571.84189.65. [DOI] [PubMed] [Google Scholar]
- 33.Hoogaars WM, Tessari A, Moorman AF, de Boer PA, Hagoort J, Soufan AT, et al. The transcriptional repressor Tbx3 delineates the developing central conduction system of the heart. Cardiovasc Res. 2004 Jun 1;62(3):489–99. doi: 10.1016/j.cardiores.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 34.Gourdie RG, Harris BS, Bond J, Edmondson AM, Cheng G, Sedmera D, et al. HisPurkinje lineages and development. Novartis Found Symp. 2003;250:110–22. discussion 22-4, 276-9. [PubMed] [Google Scholar]
- 35.Gourdie RG, Mima T, Thompson RP, Mikawa T. Terminal diversification of the myocyte lineage generates Purkinje fibers of the cardiac conduction system. Development. 1995;121(5):1423–31. doi: 10.1242/dev.121.5.1423. [DOI] [PubMed] [Google Scholar]
- 36.Takebayashi-Suzuki K, Pauliks LB, Eltsefon Y, Mikawa T. Purkinje fibers of the avian heart express a myogenic transcription factor program distinct from cardiac and skeletal muscle. Dev Biol. 2001 Jun 15;234(2):390–401. doi: 10.1006/dbio.2001.0270. [DOI] [PubMed] [Google Scholar]
- 37.Gittenberger-de Groot AC, Blom NM, Aoyama N, Sucov H, Wenink AC, Poelmann RE. The role of neural crest and epicardium-derived cells in conduction system formation. Novartis Found Symp. 2003;250:125–34. doi: 10.1002/0470868066.ch8. discussion 34-41, 276-9. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura T, Colbert MC, Robbins J. Neural crest cells retain multipotential characteristics in the developing valves and label the cardiac conduction system. Circ Res. 2006 Jun 23;98(12):1547–54. doi: 10.1161/01.RES.0000227505.19472.69. [DOI] [PubMed] [Google Scholar]
- 39.Poelmann RE, Gittenberger-de Groot AC. A subpopulation of apoptosis-prone cardiac neural crest cells targets to the venous pole: multiple functions in heart development? Dev Biol. 1999 Mar 15;207(2):271–86. doi: 10.1006/dbio.1998.9166. [DOI] [PubMed] [Google Scholar]
- 40.Wessels A, Phelps A, Trusk TC, Davis DL, Edwards AV, Burch JB, et al. Mouse models for cardiac conduction system development. Novartis Found Symp. 2003;250:44–59. discussion -67, 276-9. [PubMed] [Google Scholar]
- 41.Moorman AF, Christoffels VM, Anderson RH. Anatomic substrates for cardiac conduction. Heart Rhythm. 2005 Aug;2(8):875–86. doi: 10.1016/j.hrthm.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 42.Rosen MR. 15th annual Gordon K. Moe Lecture. Biological pacemaking: in our lifetime? Heart Rhythm. 2005 Apr;2(4):418–28. doi: 10.1016/j.hrthm.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 43.Rosen MR, Robinson RB, Brink P, Cohen IS. Recreating the biological pacemaker. Anat Rec A Discov Mol Cell Evol Biol. 2004 Oct;280(2):1046–52. doi: 10.1002/ar.a.20073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.