Abstract
T lymphocytes react minimally with nonactivated endothelial cells (ECs). However, natural killer (NK) lymphocyte interactions with resting ECs are rapid, avid, and result in endothelial activation and/or cytotoxicity. The molecular basis for these interactions and EC sensitivity to NK-mediated lysis is unclear. To address the EC-specific nature of NK sensitivity, we used syngeneic human umbilical vein ECs, dermal microvascular ECs, dermal fibroblasts, and B lymphoblastoid cell lines in calcein-AM retention NK assays with allogeneic NK effector cells and found the EC lines consistently more NK-sensitive. Because NK inhibitory receptors are engaged by membrane major histocompatibility complex (MHC) I molecules and MHC I-deficient targets are NK-sensitive, we investigated the quantitative levels of membrane MHC I on the panel of syngeneic lines. Highly sensitive ECs expressed similar (or higher) levels of membrane MHC I than their syngeneic NK-resistant counterparts. Pretreatment of ECs with γ interferon (IFN-γ) conferred protection against NK-mediated lysis, with much more rapid kinetics (2–6 hr) than those required for membrane MHC I hyperinduction (>8 hr). These kinetics are consistent with induction of transporter associated with antigen processing (TAP) expression and function. As opposed to NK-resistant cell lines, TAP-1 was undetectable in resting ECs. Recombinant expression of the TAP inactivator ICP47 by adenoviral-mediated transduction was used to selectively inhibit IFN-γ-mediated EC TAP function. ICP47 expression abrogated EC cytoprotection conferred by IFN-γ. We demonstrate a relationship between both basal and induced TAP-1 expression/function and EC sensitivity to NK-mediated cytotoxicity. We discuss the influence of an induced MHC I-associated peptide repertoire on vascular vulnerability to cytotoxic lymphocytes.
The endothelium plays a prominent role in recruitment and emigration of circulating lymphocytes into sites of inflammation and immune responses (1). Natural killer (NK) cells make up to 30% of circulating peripheral blood lymphocytes and their role in providing the first line of defense against viral infections and tumors has been established (2). Endothelial cells (ECs) can be primary targets of immunologic injury, leading to vasculopathy and organ dysfunction. T lymphocytes react minimally with resting endothelium (3). In contrast, we have previously shown that NK cells adhere avidly to and activate resting allogeneic ECs and have the ability to efficiently lyse ECs in vitro (4, 5). The molecular basis for this endothelial sensitivity to NK-mediated lysis is unclear.
There has been a longstanding observation of an inverse correlation between the expression of major histocompatibility complex (MHC) class I molecules on target cells and their susceptibility to NK-mediated lysis (6, 7). The lack of indiscriminate killing by NK cells reflects the expression of specialized NK inhibitory receptors (NKIRs) that recognize allelic forms of MHC class I molecules (for review, see ref. 8). These receptors are clonally distributed (9), and upon engagement, negative signals are generated that inhibit the cytotoxic process (10–12). Certain allele products, such as HLA-A2, are not protective (7). It remains unclear whether the mere quantity of target membrane MHC class I molecules determines the level of sensitivity or whether the “quality” and nature of processed antigenic peptides presented by these class I molecules plays a major inhibitory role.
Activation of target cells with gamma interferon (IFN-γ) can confer resistance to targets that are otherwise highly sensitive to NK-mediated lysis (13, 14). Given the profound effect of IFN-γ on MHC I expression and the inhibitory signals generated by target cell MHC I molecules upon NKIR engagement, it appears likely that this protective effect of IFN-γ is mediated through augmentation of membrane MHC I. However, there have been reports of IFN-γ-mediated protection in the absence of increased surface MHC I levels (15–17). IFN-γ affects peptide antigen processing not only by enhancing transcription of MHC I heavy chain and β2-microglobulin genes but also by inducing expression of the proteasome subunits LMP7, LMP2, and MECL-1, which replace the “housekeeping” subunits X, Y, and Z, respectively (18). In addition, IFN-γ up-regulates the 20S proteasome activator PA28α subunit (19) and transporter associated with antigen processing (TAP) expression and activity (20, 21), thereby altering their catalytic activity. These IFN-γ-mediated events may alter the nature of MHC I molecules expressed on target cell membranes.
In this work, we use a panel of syngeneic cell lines and demonstrate a discordance between membrane MHC I levels and sensitivity to NK-mediated lysis, with ECs exhibiting the greatest degree of NK sensitivity despite expressing normal MHC I levels. IFN-γ treatment converts ECs to NK-resistant cells with much more rapid kinetics than those required for increasing membrane MHC I levels. We use an adenovirus-based transduction system to express ICP47 in ECs. The recombinant expression of ICP47, which binds to and inactivates TAP complexes, abrogates the IFN-γ-mediated protection. We thus demonstrate a relationship between both basal and induced TAP-1 expression/function and EC sensitivity to NK-mediated lysis. We discuss this apparent “defect” in peptide antigen processing in human ECs and its contribution to endothelial susceptibility to injury by circulating NK lymphocytes.
MATERIALS AND METHODS
Antibodies and Reagents.
Recombinant IFN-γ, EC growth supplement, and dispase were obtained from Collaborative Biomedical Products (Bedford, MA). The following mAb-containing ascites fluid (A) or purified Abs (P) were used for panning or flow cytometry: anti-CD3 (clones 7D6 and OKT-3, A), anti-CD4 (clone S1.1, A), and anti-CD8 (clone S3.5, A) were made available by E. Engleman (Stanford University); anti-β2-microglobulin (clone BBM.1, A), anti-HLA-B5 (clone 4D12, A), anti-HLA-B7 (clone BBM7.1, A), and anti-HLA-A2 (clone BBM7.2, A) were purchased from the American Tissue Culture Collection; anti-pan-HLA-B alleles (clone 4E, P) is a mAb with broad reactivity to HLA-B alleles (22); anti-HLA-C (clone L31, A) was as described (23); anti-class-I HLA (clone W6/32, P), anti-VCAM-1 (clone E1/6, A), and anti-intercellular cell adhesion molecule 1 (ICAM-1) (clone R6.5, A) were provided by P. Parham (Stanford University), R. Rothlein (Boehringer Ingelheim, Ridgefield, CT), and M. Bevilacqua (University of California, San Diego), respectively.
Cell Culture.
Single-donor human umbilical vein endothelial cells (HUVECs) were harvested as described (24) and always maintained as single-donor lines in medium supplemented with fetal bovine serum (FBS, 15%), EC growth supplement (25 μg/ml), and heparin (50 μg/ml) and used within the fifth passage. Stimulation with IFN-γ and all other assays were performed in EC growth supplement/heparin-free medium. Syngeneic human microvascular ECs (MVECs) were established as described (3) with modifications. Preputial skin from newborns, from whom the umbilical cords were obtained, were treated with dispase (100%) for 1 hr at 37°C, after which the MVECs were extruded and cultured in medium supplemented with FBS (8%), human peripartum serum (2%), 3-isobutyl-1-methylxanthine (IBMX, 33 mM) and dibutyryl cAMP (DBcAMP, 0.5 mM). Cells were identified as ECs by morphological analysis using light microscopy and immunofluorescence techniques as described (3). All assays were performed in IBMX- and DBcAMP-free medium. Autologous fibroblasts were also isolated from preputial skin as described (3) and cultured in IBMX/DBcAMP-free MVEC medium. Syngeneic B lymphoblastoid cell lines (B-LCLs) were established from autologous cord blood by Epstein–Barr virus transformation with 395–8 Marmoset supernatant. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll/Hypaque gradient centrifugation of either leukocyte-enriched products or whole blood samples obtained from healthy adult donors. NK-cell-enriched cultures were established by maintaining PBMCs in the presence of irradiated (2,000 rad; 1 rad = 0.01 Gy) RPMI-8866 cells for 2–6 weeks as described (25). NK cells were isolated by negative immunoselection with a panning technique, using a combination of mAbs anti-CD3, anti-CD4, and anti-CD8. NK cells obtained by this method were >98% CD56+ CD16+ and less then 1% CD3+ as detected by cytofluorographic analysis using a FACSort cytometer (Becton Dickinson). Purified NK cells were “rested” and maintained in RPMI medium supplemented with FBS (10%) for at least 18 hr prior to cytotoxicity assays. Alternatively, freshly isolated resting NK cells were obtained from normal leukopheresis as described (26), routinely yielding >97% CD3−, CD56+, CD16+ NK lymphocyte populations.
Flow Cytometry.
Lymphocytes or ECs were harvested and stained either directly with conjugated Abs or with primary then fluorophore-conjugated secondary Abs, and 5,000 cells were analyzed per sample on a FACSort.
Cytotoxicity Assays.
Cytotoxicity analysis was performed by using calcein-AM retention assays, as described (27). Briefly, adherent confluent target cells plated in gelatin-coated microtiter plates at a concentration of 2 × 104 cells per well were washed once with PBS and labeled with 8 μM calcein-AM (Molecular Probes) in serum- and phenol red-free medium (GIBCO/BRL) for 40 min at 37°C. B-LCLs were labeled with 5 μM calcein-AM, at a concentration of 2 × 106 cells per ml, washed once with PBS, and distributed into U-bottom microtiter plates at a concentration of 2 × 104 cells per well. Labeling efficiency was initially assessed by using a Cytofluor II fluorescence plate reader (Perseptive Biosystems, Framingham, MA). Effector cells were added at different effector/target (E/T) ratios in quadruplicate. Phenol red-free medium was added to a six-well set of target cells for estimation of retention of calcein-AM in medium alone. Maximal lysis was determined by solubilizing six wells of target cells in lysis buffer (50 mM sodium borate/0.1% Triton X-100, pH 9.0). After the indicated time (2–3 hr) of incubation at 37°C, the assays were terminated by washing the plates twice and the remaining fluorescence was read. Percent specific cytotoxicity was calculated as follows: % cytotoxicity = [(retention experimental well − retention maximal lysis)/(retention in medium − retention maximal lysis)] × 100. Retention values were calculated by normalizing measured fluorescence with initial labeling of the same well.
Recombinant Adenoviral Transduction of EC.
The recombinant adenovirus expressing ICP47 (AdICP47–1) and the “null” adenovirus, used as control (Add1E1), were provided by D. Johnson (Oregon Health Sciences, Portland, OR). The construction of AdICP47–1 and Add1E1 has been described in detail (28). The recombinant adenovirus expressing β-galactosidase (LacZ) was provided by R. Crystal (Cornell University). ECs were infected at a multiplicity of infection of 200 in a small volume of high glucose DMEM supplemented with 5% FBS, glutamine, and antibiotics for 1 hr at 37°C. Fresh endothelial growth medium (see above) was then added for additional 14 hr at 37°C before functional experiments. Despite prior reports of EC activation induced by adenoviral infection (29, 30), membrane, ICAM-1, and MHC I levels, were identical in uninfected, control virus-infected, and ICP47-transduced HUVECs.
Immunoblotting and Immunoprecipitation.
ICP47 was detected in transduced cells by Western blotting and ECL using rabbit anti-ICP47 antibodies (28), provided by D. Johnson. For TAP-1 immunoprecipitation, cells were metabolically labeled for 4 hr with Trans-35S-label (ICN) and lysed in TBS (10 mM Tris⋅HCl, pH 7.4/150 mM NaCl) containing 1% Triton X-100, 1 mM EDTA, 0.02% sodium azide, 0.2 mM phenylmethylsulfonyl fluoride, and a protease inhibitor mixture [leupeptin (1 μg/ml)/chymostatin (1 μg/ml)/pepstatin (1 μg/ml)/1 mM benzamidine/killikrein inhibitor (10 units/ml)/aprotinin (1 μg/ml)]. Label incorporation was determined by trichloroacetic acid precipitation as described (31). Total protein amounts were determined by using the Bio-Rad Bradford method. Equal amounts of 35S-labeled proteins were subjected to immunoprecipitation with rabbit anti-TAP-1 antibodies. After SDS/PAGE and autoradiography, densitometric analysis was done on a Computing Densitometer, model 300E (Molecular Dynamics).
RESULTS
Comparative NK Sensitivity and MHC I Expression in a Panel of Syngeneic Cell Lines.
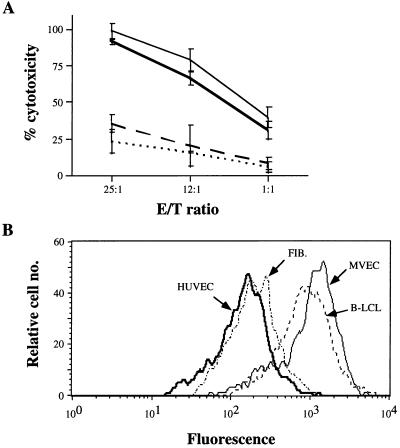
We have demonstrated (3, 4) that EC are sensitive to NK-mediated lysis. To determine whether there exists a preference or specificity toward ECs in this regard, HUVECs, MVECs, fibroblasts, and B-LCLs, all isolated from the same donor, were used as targets in a 3-hr cytotoxicity assay. Fig. 1A demonstrates that both EC types were highly sensitive NK targets, whereas the syngeneic B-LCLs and fibroblasts were relatively resistant. In fact, the measured cytotoxicity against ECs (>90% at a 25:1 E/T ratio) is nearly identical to that detected when standard MHC I-deficient NK targets, such as K562, were analyzed (data not shown). The relative hierarchy of sensitivity was highly reproducible when multiple NK cell–target combinations were used.
Figure 1.
MHC I expression and NK sensitivity of a panel of syngeneic cell types. (A) Calcein-AM-loaded HUVECs (thick solid line), MVECs (solid line), fibroblasts (dashed line), and B-LCLs (dotted line) isolated from the same donor were used as targets in a calcein-AM retention NK assay, with allogeneic NK effector cells at the noted E/T ratios. Results are representative of three experiments, each with different NK–target allocombinations. (B) The indicated cells, all isolated from the same donor, and corresponding to those used as targets in A, were analyzed for total membrane MHC I expression by flow cytometry using mAb w6/32. Five thousand gated events were analyzed per sample.
Because of the well-described inverse relationship between NK sensitivity and membrane MHC I levels, target-cell class I levels were determined by flow cytometry. Fig. 1B demonstrates that the absolute level of total MHC I membrane expression does not correlate with resistance to NK-mediated lysis. That is, syngeneic fibroblasts and B-LCLs express nearly identical MHC I levels to those of HUVECs and MVECs, respectively, yet both non-EC types were NK-resistant. MVECs, the most NK-sensitive of all the syngeneic cells, express the highest level of membrane MHC I (Fig. 1B), demonstrating that features of ECs other than total membrane class I levels confer their sensitivity to NK-mediated lysis. Anti-HLA-A2 (for HLA-A2+ cell lines) and anti-pan-HLA-B antibodies were also used to determine whether sensitivity correlated with the amount of membrane MHC I encoded at specific loci/alleles. The pattern was identical to that seen with W6/32. That is, HLA-A2 and -B expression were greater in MVECs and B-LCLs, yet MVECs and HUVECs were, by far, the most NK-sensitive targets, as shown above. HLA-C staining, using mAb L31 (23), revealed that only B-LCLs are minimally reactive, possibly due to antibody cross-reactivity with membrane Epstein–Barr virus antigens.
We have previously shown that the β2 integrin–ICAM-1 adhesion pathway plays a major role in NK–EC interactions (26), and ICAM-1 expression correlates with target sensitivity to NK-mediated lysis in some experimental systems (32). All four syngeneic cell types expressed the same level of basal ICAM-1, as determined by superimposed flow cytometry-generated fluorescence histograms (data not shown). Thus, the differential NK sensitivities cannot be explained by varying ICAM-1 levels.
IFN-γ-Induced Protection Against NK-Mediated EC Lysis.
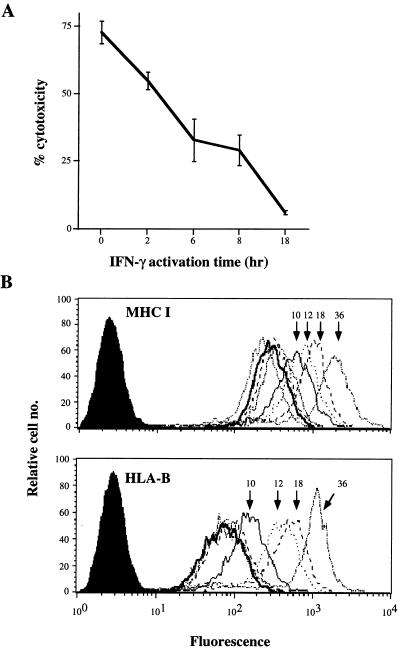
As described for other cell types (13, 14), pretreatment of ECs with IFN-γ (100 units/ml) conferred resistance to NK-mediated cytotoxicity. Fig. 2A demonstrates a representative experiment, using the HUVEC line H637 as a target. Protection by IFN-γ was observed at the earliest time point evaluated, that of a 2-hr activation (which includes the cytotoxicity assay). A brief exposure to IFN-γ, followed by removal of the cytokine from the medium, achieved the same effect as maintaining IFN-γ in the EC culture for the duration of the activation (data not shown). In contrast to these rapid kinetics, no changes in MHC I membrane expression levels were detected within 8 hr of EC stimulation by IFN-γ. The basal levels of H637 total MHC I and HLA-B were 280 and 70 mean channel fluorescence units, respectively (Fig. 2B). After 10 or more hours of IFN-γ stimulation, membrane MHC I levels gradually rose, reaching mean channel fluorescence values in excess of 1,000 after 36 hr.
Figure 2.
Induced HUVEC resistance to NK-mediated lysis by IFN-γ. (A) Calcein-AM-loaded HUVECs were used as targets in a calcein-AM retention NK assay with allogeneic NK effectors at 10:1 E/T ratio. HUVECs were untreated (0 hr) or IFN-γ (100 units/ml)-pretreated for the indicated times. Results are representative of eight experiments, each with different NK–HUVEC allocombinations. (B) HUVECs used in the cytotoxicity assay above were analyzed in parallel for total membrane MHC I (mAb w6/32) or HLA-B (mAb 4E) expression by flow cytometry. Cells were untreated (heavy line) or treated for 2, 4, or 8 hr (curves overlapping with 0-hr curve) or for the indicated times with IFN-γ (100 units/ml) before harvesting for analysis. Five thousand gated events were analyzed per sample.
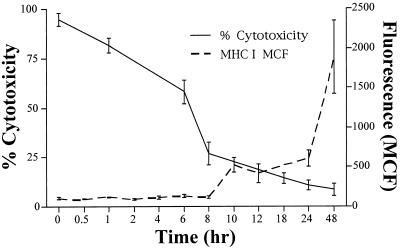
The kinetics of IFN-γ-mediated EC MHC I induction and protection against NK-mediated lysis were compared by combining data from eight parallel cytotoxicity and flow cytometry assays, using eight NK–EC allocombinations. Fig. 3 confirms, as noted above, that IFN-γ induces EC protection against NK-mediated lysis much more rapidly than detectable increases in MHC I can be observed.
Figure 3.
Comparative kinetics of IFN-γ-induced cytoprotection and membrane MHC I hyperinduction. Calcein-AM retention NK assay and flow cytometry of membrane MHC I from eight experiments (eight different allocombinations) were combined and displayed graphically. Fluorescence values correspond to mean channel fluorescences (MCF) at the indicated IFN-γ treatment times. Values are the mean ± SEM from the combined data.
The Role of TAP in IFN-γ-Mediated Resistance.
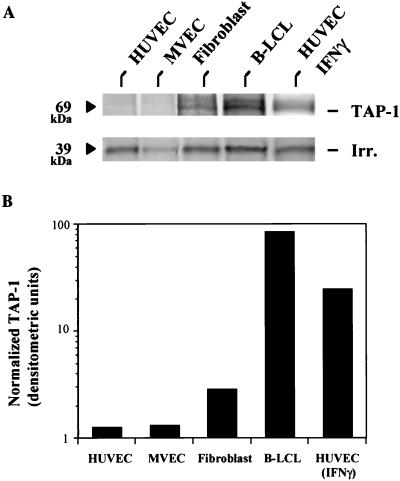
Although absolute EC MHC I levels do not change as rapidly as NK sensitivity in response to IFN-γ, there are several critical steps in the MHC I assembly and membrane expression pathway that are potentially enhanced by IFN-γ and may play a role in the nature of the expressed MHC I complexes. Peptide transport from the cytosol to the endoplasmic reticulum lumen is one such step. TAP-1 and TAP-2 levels are minimal in resting HUVECs, but both mRNA and protein are induced rapidly by IFN-γ (O.A. and J.R.B., unpublished observation and ref. 21). To determine whether basal levels of TAP-1 correlate with NK sensitivity, the four syngeneic cell types were metabolically radiolabeled followed by TAP-1 immunoprecipitation. Volume of cell lysate used per sample for the immunoprecipitation was determined by measured total protein recovered and 35S incorporation. Fig. 4A demonstrates easily detectable TAP-1 immunoprecipitated from fibroblasts, B-LCLs, and IFN-γ-treated HUVECs, which was absent from resting HUVECs and MVECs. Fig. 4B displays the densitometric analysis, normalized for a nonspecific irrelevant 39-kDa band (seen also TAP-1-negative cell line immunoprecipitations and was not IFN-γ-modulated) observed throughout all experimental samples. The TAP doublet represents coimmunoprecipitated TAP-1 and TAP-2 (69 and 72 kDa, respectively), as described (33). This supports the concept that NK sensitivity relates to TAP function, i.e., expression of MHC I loaded with TAP-dependent peptides.
Figure 4.
TAP-1 expression in a panel of syngeneic cell lines. HUVECs, MVECs, fibroblasts, B-LCLs, and IFN-γ-treated (for 18 hr) HUVECs, all isolated from the same donor, were 35S-labeled for 4 hr. Upon cell lysis, equal amounts of labeled protein extracts (based on total protein and acid-precipitable counts) were used for immunoprecipitation. (A) The 69-kDa immunoprecipitated TAP-1 band and an irrelevant (39 kDa) band seen in are in all lanes. (B) The densitometric analysis for A, with normalization to the irrelevant band.
To evaluate whether the induction of TAP function is a required critical event in the noted cytokine-mediated resistance, HUVECs were transduced with an adenoviral construct encoding the herpes simplex viral protein ICP47, which specifically binds and inactivates TAP-1/TAP-2 dimers (34). The ICP47 gene in this E1/E3-deleted construct is under the control of the cytomegalovirus promoter, thereby blocking the expression of other viral proteins (28) and minimizing unwanted effects on antigen processing. As controls, HUVECs were infected with either null (vector without insert) or LacZ-containing virus. Western blot analysis demonstrated the presence of the 9-kDa ICP47 protein in the ICP47-transduced cells, which was undetectable in the null virus-infected cells (data not shown). As described, the transduction efficiency was very high, with positive β-galactosidase staining observed in >95% of LacZ-transduced cells (data not shown).
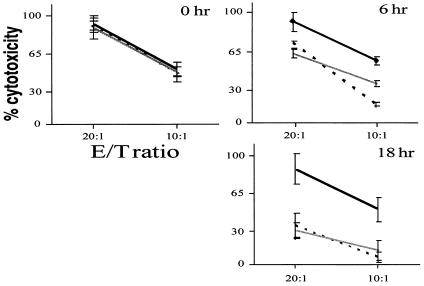
As expected, Western blot analysis demonstrated that ICP47 expression has no effect on IFN-γ-mediated TAP-1 protein induction (data not shown). Thus, any observed effect would be a consequence of functional inhibition of the transporter complex. To directly address this, HUVECs infected with ICP47, LacZ, or null virus were used as targets in a 3-hr cytotoxicity assay with allogeneic NK effector cells. No differences were detected in the NK sensitivity of resting ECs (no IFN-γ) infected with AdICP47 or control viruses (Fig. 5, 0 hr). Because ICP47 inhibits TAP function, this further suggests that basal EC TAP is minimal and not functionally involved in the determining resting EC sensitivity (or resistance) to NK-mediated lysis. After 6 hr of EC stimulation with cytokine, the protection induced by IFN-γ is completely abrogated in the ICP47-transduced cells but persists in null and LacZ virus-infected cells (Fig. 5, 6 hr). As noted above, this 6-hr time preceded any detectable increase in the level of membrane MHC I (data not shown), suggesting that induced active peptide transport plays a critical role in IFN-γ-mediated protection, even without augmenting total class I levels. The inhibitory effect of ICP47 expression was more dramatic with an 18-hr IFN-γ stimulation, after which cytotoxicity in the LacZ-infected cells was minimally detectable (6% at a 10:1 E/T ratio), whereas ICP47-transduced cells were lysed as in the non-IFN-γ-treated cells (50% at a 10:1 E/T ratio) (Fig. 5, 18 hr). The more pronounced difference at 18 hr is likely a consequence of both enhanced TAP function and increased total membrane MHC I levels (data not shown). However, despite such an increase in both total membrane MHC I and HLA-B in the ICP47-transduced cells, the long-term (18 hr) IFN-γ treatment could not induce NK-resistance in these cells.
Figure 5.
Effect of ICP47 on IFN-γ-mediated HUVEC cytoprotection. HUVECs were infected with ICP47 (heavy line), LacZ (broken line), or null (shaded line) adenovirus, loaded with calcein-AM, and used as targets in NK assays with allogeneic NK effector cells. Transduced HUVECs were either untreated (0 hr) or IFN-γ-treated (100 units/ml) for the indicated times.
DISCUSSION
According to the “missing-self” theory of immunosurveillance initially proposed by Kärre (35), NK cells efficiently lyse MHC I-deficient targets. The characterization of NK inhibitory receptors specific for MHC I has shed considerable light on the molecular mechanisms governing NK cell–target interactions. The endothelium is a unique target, because it is continuously exposed to circulating lymphocytes, of which up to 30% are NK cells. NK cell–EC interactions that control allorecognition and lytic mechanisms could be primarily involved in the acute or chronic injury of allograft vessels.
In this study, we demonstrate that, of several target cells tested, ECs are the most sensitive to NK-mediated lysis. Within an experiment, the target cells used were all isolated from the same donor, thus controlling for the target MHC haplotype. Moreover, the effector cells used were polyclonal in their class I specificity and NKIR expression (O.A. and J.R.B., unpublished observation) and were tested in numerous E/T allocombinations, representing a wide spectrum of NKIR–MHC I interactions. The differential sensitivities of the target cells were not simply a manifestation of their MHC I membrane levels, as these two target cell features were discordant. Rather, there was a correlation between basal NK sensitivity and lack of TAP-1 expression, as detected by quantitative immunoprecipitations of TAP-1 from metabolically labeled target cells. This suggests that the qualitative nature of membrane MHC peptide complexes is at least as critical as the quantitative level of surface class I to engage and activate inhibitory receptors and confer target cell protection. The control of TAP expression in a cell type/tissue-specific fashion and, in particular, the cis elements and transregulatory factors that dictate the lack of basal expression and inducibility in EC have not been explored.
Another indication that the inherent NK sensitivity of ECs is not merely the result of insufficient surface MHC I expression comes from IFN-γ activation experiments. The kinetics of IFN-γ-induced EC protection against NK-mediated lysis are reproducibly much more rapid than those for hyperinducing membrane MHC I levels. The protective kinetics are consistent with the induction of functional TAP complexes. TAP complexes consist of two polypeptides, TAP-1 and -2, which combine to form a heterodimeric member of a family of integral membrane transporters that bear a transmembrane peptide channel and a cytosolic ATP binding cassette. Endogenous proteins are proteolytically degraded and the product peptides can be transported by TAP across the endoplasmic reticulum membrane (36). These peptides can then associate with MHC I heavy chain–β2-microglobulin complexes, promoting stabilization, transport, and expression on the cell membrane (37). TAP preferentially transports peptides of 8–10 amino acids, with some further specificity (38) (see below). The kinetics of TAP induction in human ECs have recently been demonstrated (21). HUVEC TAP-1 and -2 mRNA and proteins are rapidly induced by IFN-γ (onset within 3 hr). This is consistent with the cytoprotection rapidly achieved in IFN-γ-treated HUVECs, further substantiating that it is the nature of the membrane MHC I–peptide complexes, rather than the quantity, that is critical for NKIR engagement and NK resistance.
To confirm that TAP induction is critically involved in the conversion of ECs from NK sensitive to resistant, a strategy to selectively inhibit TAP was used. Expression of recombinant proteins in HUVECs is generally fraught with inconsistent transfection efficiencies of plasmid DNA. Viral infection and gene transduction has been much more successfully achieved, by using retrovirus (39)- and adenovirus (40)-based systems. To this end, we expressed ICP47 by HUVEC infection with recombinant adenovirus (serotype 5) and assessed whether this protein, which is a 9-kDa herpes simplex virus-encoded protein that binds and inactivates TAP complexes, could inhibit the IFN-γ-mediated protection against NK-mediated lysis. Adenoviral gene products, in particular those encoded in the E1A conserved region 1 (41), can, in of themselves, inhibit IFN-γ-mediated cytoprotection. However, all the viral constructs used in these experiments were E1 (and E3)-deleted, and the recombinant protein (ICP47 or LacZ) was driven by the cytomegalovirus promoter, bypassing the expression of other adenoviral gene products. The inactivation of TAP through ICP47 expression abrogated the protective effect of IFN-γ. This further substantiates that a basal “TAP deficiency” state in human ECs results in NK sensitivity and that a rapid TAP induction is required for induced resistance.
Although prevention of TAP function inhibits IFN-γ-mediated cytoprotection, other IFN-γ-regulatable genes/molecules may also be critically involved in this phenomenon. Because absolute membrane MHC I levels do not dictate NK sensitivity in this system, other elements that affect MHC I–peptide antigen processing and the qualitative nature of MHC I–peptide complexes may be important determinants. Upon stimulation of various antigen-presenting cells with IFN-γ, two constitutive subunits of the proteolytic 20S proteasome are replaced by MHC-encoded subunits LMP2 and LMP7. The PA28 regulator of this proteasome complex, which promotes dual protein cleavage and markedly enhances the production of antigenic peptide MHC ligands, is also IFN-γ-regulated (42). The combination of LMP2, LMP7, and PA28 within the complex markedly alters the quality of antigenic peptides produced (43), dramatically increasing the spectrum of generated peptides. It is noteworthy that a recent report described augmented efficiency of cytotoxic T lymphocyte-mediated lysis in the context of a 3-fold increase in target PA28 expression, in the absence of increases in surface MHC I (19). Any or all of these regulatory steps may be critically lacking in basal resting human ECs and integrally involved in protection conferred by IFN-γ.
On the basis of NKIR–target MHC I interactions, immune surveillance by NK cells is regulated by allotype-specific recognition. When HLA class I allele products are expressed in TAP-deficient cell lines, specific peptides can both rescue membrane MHC I expression and inhibit NK-cell-mediated lysis (44). Other peptides, in particular those with lysine at position 8 (P8), are not inhibitory. The exact effect of the peptide and the nature of its interaction with inhibitory receptors remains unclear. A recent report demonstrated that peptides derived from either glutamic acid decarboxylase (GAD) or coxsackie virus may or may not have inhibitory activity against NK2-specific clones, when loaded onto HLA-Cw7, the NKIR2-reactive inhibitory MHC I (45). Because GAD and coxsackie virus have both been associated with autoimmune diabetes mellitus, it was postulated that the exact sequence of MHC I-loaded peptides is a critical determinant in promoting NKIR-mediated inhibition and that an auto-peptide antigen that is not protective could allow establishment of a tissue destruction cycle, manifested as autoimmunity.
Whereas the anchor side chain of the peptide C-terminal amino acid (usually P9) is in the F pocket of the binding groove, the P8 side chain points upward. This has been proposed as an explanation of the critical nature of this amino acid position, in that it has the potential to interfere with NKIR–MHC interactions, and/or mimic an MHC I residue that is nonprotective (45). The link between the specific nature of MHC I-associated peptides and the TAP dependence demonstrated in this report is not clear. That is, the substrate specificity of the TAP transporter, although biased for peptides that can bind to MHC I molecules (9- to 12-amino acid peptides with a compatible C-terminal residue), is not obviously directed at those peptides that will more efficiently allow a productive NKIR engagement by MHC I, thereby promoting inhibition. Studies to determine the “protective peptide repertoire” induced by IFN-γ in human EC are currently underway in our laboratory. We have recently demonstrated that cytoprotection is conferred on otherwise NK-sensitive, resting HUVEC, when these cells are pulsed with peptides isolated from syngeneic IFN-γ-treated HUVECs (O.A. and J.R.B., unpublished observation). This further suggests that a class of IFN-γ-induced peptides can contribute to NK resistance in ECs.
As noted above, endothelial sensitivity to NK-mediated lysis could have substantial relevance in the setting of vascularized allografts. Furthermore, NK cells have the potential to damage autologous vascular endothelium. Self MHC I expressed on highly sensitive NK targets may not be sufficiently protective, especially in the setting of activated NK cells. For example, an antiviral NK cell response in lymphoid tissue may expose normal endothelium to NK-mediated damage. The production of IFN-γ by activated NK cells would confer cytoprotection on the surrounding ECs, focusing the immune response on virus-infected antigen-presenting cells and sparing the vascular supply of the vital tissue or organ.
In summary, we have demonstrated that human ECs are exquisitely sensitive NK targets and that their sensitivity relates to a basal insufficiency in MHC I–peptide antigen processing. IFN-γ can correct this defect, at least in part by augmenting TAP expression and function. Further studies on other components of endothelial MHC I assembly, peptide antigen processing, and induced protective peptide repertoires should provide further insight into the peptide specificity of NKIR engagement and potential immune-mediated vascular injury by this subset of cytotoxic lymphocytes.
Acknowledgments
We are grateful to David Johnson for kindly providing AdICP47 and to Albert Deisseroth and members of his laboratory for assistance in adenoviral culture. We thank Louise Benson and Gwen Davis for assistance with cell culture, Rita Girdzis for performing leukopheresis, and Dana Brenckle for help with manuscript preparation. We also express gratitude to the Milford General Hospital Delivery Room nursing staff for obtaining umbilical cords, cord blood, and foreskins and the Yale Pheresis Unit for assisting with leukopac preparation. This work was supported by National Institutes of Health Grant HL43331 (J.R.B.), the Howard Hughes Medical Institute (P.C.), and European Community Grant BMH4-CT95–0875 and the Consiglio Nazionale delle Ricerche Biotechnology Project (R.P.). J.R.B. is a Raymond and Beverly Sackler Foundation Scholar. E.A.H. is supported by the National Institutes of Health Medical Scientist Training Program.
ABBREVIATIONS
- EC
endothelial cell
- NK
natural killer
- MHC
major histocompatibility complex
- IFN-γ
γ interferon
- NKIR
NK inhibitory receptor
- HUVEC
human umbilical vein endothelial cell
- MVEC
microvascular endothelial cell
- AM
tetrakis(acetoxymethyl) ester
- TAP
transporter associated with antigen processing
- LMP
low molecular mass polypeptide
- FBS
fetal bovine serum
- B-LCL
B lymphoblastoid cell line
- E/T
effector/target
- ICAM-1
intercellular cell adhesion molecule 1
References
- 1.Springer T A. Annu Rev Physiol. 1995;57:827–872. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- 2.Karre K. Immunol Rev. 1997;155:5–9. doi: 10.1111/j.1600-065x.1997.tb00935.x. [DOI] [PubMed] [Google Scholar]
- 3.Bender J R, Pardi R, Karasek M A, Engleman E G. J Clin Invest. 1987;79:1679–1688. doi: 10.1172/JCI113007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bender J R, Pardi R, Engleman E. Proc Natl Acad Sci USA. 1990;87:6949–6953. doi: 10.1073/pnas.87.18.6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watson C A, Petzelbauer P, Zhou J, Pardi R, Bender J R. J Immunol. 1995;154:3222–3233. [PubMed] [Google Scholar]
- 6.Piontek G E, Taniguchi K, Ljunggren H G, Gronberg A, Kiessling R, Klein G, Karre K. J Immunol. 1985;135:4281–4288. [PubMed] [Google Scholar]
- 7.Storkus W J, Salter R D, Alexander J, Ward F E, Ruiz R E, Cresswell P, Dawson J R. Proc Natl Acad Sci USA. 1991;88:5989–5992. doi: 10.1073/pnas.88.14.5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lanier L L. Immunity. 1997;6:371–378. doi: 10.1016/s1074-7613(00)80280-0. [DOI] [PubMed] [Google Scholar]
- 9.Gumperz J E, Valiante N M, Parham P, Lanier L L, Tyan D. J Exp Med. 1996;183:1817–1827. doi: 10.1084/jem.183.4.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Binstadt B A, Brumbaugh K M, Dick C J, Scharenberg A M, Williams B L, Colonna M, Lanier L L, Kinet J P, Abraham R T, Leibson P J. Immunity. 1996;5:629–638. doi: 10.1016/s1074-7613(00)80276-9. [DOI] [PubMed] [Google Scholar]
- 11.Burshtyn D N, Scharenberg A M, Wagtmann N, Rajagopalan S, Berrada K, Yi T, Kinet J P, Long E O. Immunity. 1996;4:77–85. doi: 10.1016/s1074-7613(00)80300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olcese L, Lang P, Vely F, Cambiaggi A, Marguet D, Blery M, Hippen K L, Biassoni R, Moretta A, Moretta L, et al. J Immunol. 1996;156:4531–4534. [PubMed] [Google Scholar]
- 13.Tomita Y, Watanabe H, Kobayashi H, Nishiyama T, Tsuji S, Fujiwara M, Sato S. Cancer Immunol Immunother. 1992;35:381–387. doi: 10.1007/BF01789016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kondo S, Miyatake S, Kikuchi H, Oda Y, Iwasaki K, Ohyama K, Namba Y. Neurosurgery. 1992;31:534–540. doi: 10.1227/00006123-199209000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Reiter Z, Fischer D G, Rubinstein M. Immunol Lett. 1988;17:323–328. doi: 10.1016/0165-2478(88)90005-3. [DOI] [PubMed] [Google Scholar]
- 16.Leiden J M, Karpinski B A, Gottschalk L, Kornbluth J. J Immunol. 1989;142:2140–2147. [PubMed] [Google Scholar]
- 17.Nishimura M, Mitsunaga S, Akaza T, Mitomi Y, Tadokoro K, Juji T. Immunology. 1994;83:75–80. [PMC free article] [PubMed] [Google Scholar]
- 18.Hisamatsu H, Shimbara N, Saito Y, Kristensen P, Hendil K B, Fujiwara T, Takahashi E, Tanahashi N, Tamura T, Ichihara A, Tanaka K. J Exp Med. 1996;183:1807–1816. doi: 10.1084/jem.183.4.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groettrup M, Soza A, Eggers M, Kuehn L, Dick T P, Schild H, Rammensee H G, Koszinowski U H, Kloetzel P M. Nature (London) 1996;381:166–168. doi: 10.1038/381166a0. [DOI] [PubMed] [Google Scholar]
- 20.Min W, Pober J S, Johnson D R. J Immunol. 1996;156:3174–3183. [PubMed] [Google Scholar]
- 21.Ma W L, Lehner P J, Cresswell P, Pober J S, Johnson D R. J Biol Chem. 1997;272:16585–16590. doi: 10.1074/jbc.272.26.16585. [DOI] [PubMed] [Google Scholar]
- 22.Sadasivan B, Lehner P J, Ortmann B, Spies T, Cresswell P. Immunity. 1996;5:103–114. doi: 10.1016/s1074-7613(00)80487-2. [DOI] [PubMed] [Google Scholar]
- 23.Grassi F, Meneveri R, Gullberg M, Lopalco L, Rossi G B, Lanza P, De Santis C, Brattsand G, Butto S, Ginelli E, et al. J Exp Med. 1991;174:53–62. doi: 10.1084/jem.174.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaffe E A, Nachman R I, Becker C G, Minick R C. Circulation. 1972;46:211–253. [Google Scholar]
- 25.Perussia B, Ramoni C, Anegon I, Cuturi M C, Faust J, Trinchieri G. Nat Immun Cell Growth Regul. 1987;6:171–188. [PubMed] [Google Scholar]
- 26.Pfau S, Leitenberg D, Rinder H, Smith B R, Pardi R, Bender J R. J Cell Biol. 1995;128:969–978. doi: 10.1083/jcb.128.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lichtenfels R, Biddison W E, Schulz H, Vogt A B, Martin R. J Immunol Methods. 1994;172:227–239. doi: 10.1016/0022-1759(94)90110-4. [DOI] [PubMed] [Google Scholar]
- 28.York I A, Roop C, Andrews D W, Riddell S R, Graham F L, Johnson D C. Cell. 1994;77:525–535. doi: 10.1016/0092-8674(94)90215-1. [DOI] [PubMed] [Google Scholar]
- 29.Newman K D, Dunn P F, Owens J W, Schulick A H, Virmani R, Sukhova G, Libby P, Dichek D A. J Clin Invest. 1995;96:2955–2965. doi: 10.1172/JCI118367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerszten R E, Luscinskas F W, Ding H T, Dichek D A, Stoolman L M, Gimbrone M A, Jr, Rosenzweig A. Circ Res. 1996;79:1205–1215. doi: 10.1161/01.res.79.6.1205. [DOI] [PubMed] [Google Scholar]
- 31.Coligan J E, Gates F T d, Kimball E S, Maloy W L. Methods Enzymol. 1983;91:413–434. doi: 10.1016/s0076-6879(83)91039-x. [DOI] [PubMed] [Google Scholar]
- 32.Zamai L, Zauli G, Bavelloni A, Marmiroli S, Cataldi A, Weber G, Vitale M. Cell Immunol. 1995;164:100–104. doi: 10.1006/cimm.1995.1147. [DOI] [PubMed] [Google Scholar]
- 33.Grandea A G r, Androlewicz M J, Athwal R S, Geraghty D E, Spies T. Science. 1995;270:105–108. doi: 10.1126/science.270.5233.105. [DOI] [PubMed] [Google Scholar]
- 34.Ahn K, Meyer T H, Uebel S, Sempe P, Djaballah H, Yang Y, Peterson P A, Fruh K, Tampe R. EMBO J. 1996;15:3247–3255. [PMC free article] [PubMed] [Google Scholar]
- 35.Karre K, Ljunggren H G, Piontek G, Kiessling R. Nature (London) 1986;319:675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 36.Spies T, Bresnahan M, Bahram S, Arnold D, Blanck G, Mellins E, Pious D, DeMars R. Nature (London) 1990;348:744–747. doi: 10.1038/348744a0. [DOI] [PubMed] [Google Scholar]
- 37.Androlewicz M J, Anderson K S, Cresswell P. Proc Natl Acad Sci USA. 1993;90:9130–9134. doi: 10.1073/pnas.90.19.9130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Momburg F, Roelse J, Hammerling G J, Neefjes J J. J Exp Med. 1994;179:1613–1623. doi: 10.1084/jem.179.5.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dichek D A. Mol Biol Med. 1991;8:257–266. [PubMed] [Google Scholar]
- 40.Lemarchand P, Jaffe H A, Danel C, Cid M C, Kleinman H K, Stratford-Perricaudet L D, Perricaudet M, Pavirani A, Lecocq J P, Crystal R G. Proc Natl Acad Sci USA. 1992;89:6482–6486. doi: 10.1073/pnas.89.14.6482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Routes J M. J Immunol. 1993;150:4315–4322. [PubMed] [Google Scholar]
- 42.Realini C, Dubiel W, Pratt G, Ferrell K, Rechsteiner M. J Biol Chem. 1994;269:20727–20732. [PubMed] [Google Scholar]
- 43.Groettrup M, Ruppert T, Kuehn L, Seeger M, Standera S, Koszinowski U, Kloetzel P M. J Biol Chem. 1995;270:23808–23815. doi: 10.1074/jbc.270.40.23808. [DOI] [PubMed] [Google Scholar]
- 44.Correa I, Raulet D H. Immunity. 1995;2:61–71. doi: 10.1016/1074-7613(95)90079-9. [DOI] [PubMed] [Google Scholar]
- 45.Mandelboim O, Wilson S B, Vales-Gomez M, Reyburn H T, Strominger J L. Proc Natl Acad Sci USA. 1997;94:4604–4609. doi: 10.1073/pnas.94.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]