Abstract
15-deoxy-Δ12,14-PGJ2 (15d-PGJ2) has been identified as an endogenous ligand for PPARγ, inducing adipogenesis in vitro. Additional roles for this molecule in the propagation and resolution of inflammation, ligation of NF-κB, and mediation of apoptosis have been proposed. However, quantitative, physiochemical evidence for the formation of 15d-PGJ2 in vivo is lacking. We report that 15d-PGJ2 is detectable using liquid chromatography–mass spectrometry–mass spectrometry at low picomolar concentrations in the medium of 3T3-L1 preadipocytes. However, despite induction of COX-2, production of PGs, including 15d-PGJ2, does not increase during adipocyte differentiation, a process unaltered by COX inhibition. 15d-PGJ2 is detectable as a minor product of COX-2 in human urine. However, its biosynthesis is unaltered during or after COX activation in vivo by LPS. Furthermore, the biosynthesis of 15d-PGJ2 is not augmented in the joint fluid of patients with arthritis, nor is its urinary excretion increased in patients with diabetes or obesity. 15d-PGJ2 is not the endogenous mediator of PPARγ-dependent adipocyte activation and is unaltered in clinical settings in which PPARγ activation has been implicated.
Introduction
The PPAR nuclear hormone transcription factors heterodimerize with retinoic acid receptors to regulate expression of distinct gene cassettes (1, 2). Although three PPAR isotypes (α, δ, and γ) have been well characterized, the identity of their natural ligands remains controversial (3–6). PPARγ is expressed predominantly in adipose tissue, colon and macrophages, and to a lesser extent, in vascular smooth muscle cells (1–3). Among the genes regulated by PPARγ, many are involved in lipid metabolism, including lipoprotein lipase (LPL), adipocyte fatty acid binding protein (aP2), acyl-CoA synthase, and fatty acid transport protein (2, 3). Synthetic PPARγ agonists promote the maturation of fibroblasts to adipocytes in vitro (2, 3) and one class of such agonists, the glitazones, has been used in the treatment of diabetes. In addition, synthetic PPARγ agonists promote uptake of modified lipids by macrophages (7, 8) and may retard atherogenesis in mice (9). The increasing convergence of insulin resistance, obesity, and cardiovascular morbidity in the metabolic syndrome has further focused attention on the molecular mechanisms of PPARγ activation.
PGs are formed through the sequential actions of COXs and a variety of PG synthases, which are expressed with some tissue specificity (10, 11). PGD2is formed abundantly in the brain (12) and in mast cells (13). It undergoes spontaneous dehydration to PGJ2 in vitro, a reaction enhanced by albumin-induced catalysis, which yields a number of additional derivatives, including 15-deoxy-Δ12,14-PGJ2 (15d-PGJ2) (14, 15). This compound, like other PGs (16), can be actively transported into cells (17). In contrast to conventional PGs, PGJ2 and its derivatives possess a highly reactive cyclopentenone (CP) ring (18), which permits them to ligate nuclear receptors and to modify intracellular signaling molecules. CP-PGs exhibit biologic properties in vitro of potential relevance to inflammation, cellular proliferation, and differentiation (18, 19).
In addition to its effects on adipocyte maturation, 15d-PGJ2 has been implicated in the PPARγ-dependent propagation (20) and resolution (21) of inflammation, and in the fibrotic reaction to activation of protease-activated receptor 2 by mast cell tryptase (22). Alternatively, 15d-PGJ2 induces apoptosis and growth inhibition in vitro via ligation of NF-κB (23) and/or IκB kinase (24) by its reactive CP moiety.
Despite much discussion of the biologic activities of 15d-PGJ2, there is actually scant physicochemical evidence for its formation in vivo (25). A commonly used immunoassay for the compound was withdrawn from the market (26). Dehydration and isomerization products of PGD2 metabolism have been measured in humans (27). However, the concentrations of 15d-PGJ2 that activate PPARγ or ligate proteins in the NF-κB signaling pathway in vitro typically range from 2.5 to100 μM (5), whereas biologically active concentrations of conventional exogenous PGs are generally in the low picomolar range (28, 29). Thus, although in vitro albumin-catalyzed dehydration of PGD2 yields levels of 15d-PGJ2detectable by gas chromatography-mass spectrometry (GC-MS), 15d-PGJ2 has not been detected by physiochemical methods in vivo (27, 30). Similarly, isolated cells exposed to PGD2 in vitro form detectable amounts of both PGJ2 and a distinct CP derivative, 9-deoxy-Δ9,12-(E)-PGD2 (Δ12-PGJ2), but not 15d-PGJ2(26, 31). Δ12-PGJ2 has been detected in the urine by immunoassay (31), but PGJ2 has never been detected as a urinary metabolite of PGD2. Thus, discordance exists between the commonly reported role of 15d-PGJ2 as a PPAR agonist and evidence for its formation in vivo. We addressed the hypothesis that it was formed in vivo by developing a specific quantitative mass spectrometric assay for 15d-PGJ2. We investigated its role in adipocyte maturation and determined whether its biosynthesis was altered in clinical settings in which PPAR activation has been implicated.
Methods
Materials.
Arachidonic acid (peroxide free), all unlabeled and [2H]-labeled PG standards, [3H]15d-PGJ2 and COX antibodies (polyclonal, rabbit), indomethacin, NS-398 ciglitazone, and bisphenol A diglycidyl ether (BADGE), a synthetic PPARγ antagonist (32), were obtained from Cayman Chemical (Ann Arbor, Michigan, USA). The details of the various isomers of the 15d-PGJ2 were described previously (26). [18O2]-labeled PGJ2 standard was synthesized from PGJ2 using the method of Wescott et al. (33). Murine monoclonal PPARγ (E-8) antibody was purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, California, USA). Reagents for quantification of triacylglycerol (TAG) were provided in an L-Type TG H Enzyme Color A Kit purchased from Wako Chemicals USA Inc. (Richmond, Virginia, USA). Oil red O, insulin, dexamethazone, 3-isobuthyl-1-methylxanthine (IBMX), and ibuprofen were obtained from Sigma-Aldrich (St. Louis, Missouri, USA).
Cellular incorporation of 15d-PGJ2.
One micromolar of 15d-PGJ2 containing 0.5 μCi [3H]15d-PGJ2 was incubated with confluent 3T3-L1 cells cultured in six-well plates (2 ml per well). The persistence of 15d-PGJ2 in the medium was estimated by liquid scintillation counting. The cells were washed at 48 hours with PBS and harvested with 0.05 % trypsin. The 15d-PGJ2 in the wash liquid and cell pellet was estimated in the same manner.
Cell culture and adipocyte differentiation assay.
The 3T3-L1 cells (ATCC, Manassas, Virginia, USA) were maintained in DMEM with 10% fetal calf serum under nondifferentiating and differentiating conditions as described previously (34). Two days after cell confluence, cells were cultured in differentiation medium (DM) (day 0), supplemented with 10 μg/ml insulin, 1 μM dexamethasone, and 0.5 mM IBMX for 2 days and switched to a post-DM, which was supplemented only with 10 μg/ml insulin. Medium was collected from the cells at sequential 24-hour intervals for determinations of PGs. Cells were collected from parallel experiments for protein and mRNA analysis. The 3T3-F442A cells (obtained from Howard Green, Harvard School of Medicine, Boston, Massachusetts, USA) were maintained and induced to differentiate as previously described (35).
PG extraction.
Samples (5.0 ml urine or cell-free tissue culture medium or 500 μl synovial fluid) were spiked with 1.0 ng [2H4]15d-PGJ2 and extracted on C18 solid phase extraction (SPE) cartridges. The cartridges were washed with 25% ethanol in water and eluted with ethyl acetate. Some samples for 15d-PGJ2 were subjected to silica gel TLC after SPE, using a mobile phase of methanol/ethyl acetate/formic acid (95:5:0.1). Silica bands (10 mm) were extracted with ethyl acetate. The cells were washed twice with PBS for extraction of intracellular 15d-PGJ2 (36), harvested in 0.6 ml PBS with 1 ng [2H4]15d-PGJ2, and then freeze-thawed twice in liquid N2. Chloroform (1 ml) and methanol (2 ml) were added, followed by vigorous vortexing and further addition of 1 ml each chloroform and water. The organic phase was taken for the analysis.
PG analysis.
Liquid chromatography–mass spectrometry–mass spectrometry (LC-MS-MS) analyses were performed on a MicromassQuattro Ultima triple quadrupole analyzer (Macromass, Beverly, Massachusetts, USA) operated in multiple reaction monitoring (MRM) mode. The collision cell was maintained at 2 × 10–3 T with argon. A coaxial electrospray ionization (ESI) probe interfaced the LC-MS-MS with dual LC-10ADVP syringe pumps (Shimadzu, Kyoto, Japan). HPLC separations used Hypersil C18-BD, 3 μm, 150 × 2-mm columns (Thermo Hypersil-Keystone, Bellefonte, Pennsylvania, USA). Linear gradients were performed from water (solvent A) and acetonitrile/methanol, 95:5 (solvent B), both containing 0.005% acetic acid adjusted to pH 5.7 with ammonium hydroxide. A flow rate of 200 μl/min was used for all assays.
A gradient of 20% to 60% B in 30 minutes was used for 15d-PGJ2 analysis. Transitions monitored were m/z 315.2 → 271.0 for 15d-PGJ2 and m/z 319.2 → 275.0 for [2H4]15d-PGJ2. MS-MS settings were as follows: collision energy 13 eV, capillary 3 kV, source 70°C, and desolvation 250°C.
All other PGs were prepared similarly, except that the SPE columns were washed with 5% methanol in water. GC-MS analysis for PGE2(37), 6-keto-PGF1α(38), 2,3-dinor-6-keto-PGF1α(PGI-M) (39), and 2,3-dinor TxB2 (40), and LC-MS analysis of 8,12-iso-iPF2α-VI (39) were performed as previously described. A gradient of 20% to 40% B in 15 minutes at a flow rate of 200 μl/min was used for ESI-LC-MS-MS detection of PGE2. MS-MS data were generated using the following settings: cone 1.0 V, collision energy 18 eV, capillary 2.5 kV, source 150°C, and desolvation 150°C. Transitions monitored were m/z 351.2 → 271.0 for PGE2 and m/z 355.2 → 275.0 for the [2H4]PGE2. The methoxime (MO) derivative of 6-keto-PGF1α, was formed by drying the sample after SPE and incubating it overnight with 20 μl of a 0.5% solution of MO-HCl in pyridine. The HPLC gradient was 10–50% B in 18 minutes. The MS settings were as follows: collision energy 22 eV, capillary 2.5 kV, source 70°C, and desolvation 200°C. Transitions monitored were m/z 398.2 > 306.0 for 6-keto-PGF1α-MO and m/z 402.2 > 310.0 for [2H4]6-keto-PGF1α-MO.
Oil red O staining and TAG assay.
Cells were washed twice with PBS, fixed with 10% formaldehyde, and stained with Oil red O. Cellular TAG was extracted as previously described (41) with an additional overnight incubation in isopropanol. Total TAG was determined using a TAG assay kit (Wako Chemicals USA Inc.).
Preparation of whole cell extracts, gel electrophoresis, and immunoblotting.
Cell contents were extracted by sonication into lysis buffer (50 mM Tris-HCl, pH 7.4, 100 μM EDTA, 100 μM EGTA, 1% NP-40, 0.1% SDS, 0.1% deoxycholic acid) containing protease inhibitor cocktail tablets (Complete; Roche, Nutley, New Jersey, USA). Total cellular protein (20 μg) was separated by electrophoresis on NuPAGE 4–12% Bis-Tris Gel (Invitrogen, Carlsbad, California, USA) and transferred to nitrocellulose membrane.
Antibodies for COX-1 and COX-2 (Cayman Chemical) were used at 1:500 and 1:1000 dilutions, respectively. Reagents for blocking, incubation, washing, and chemiluminescent-detection (WesternBreeze; Invitrogen) were used.
RNA preparation and analysis.
RNA isolation and reverse transcription were performed with TRIzol Reagent (Invitrogen) and Superscript RT (Invitrogen). Twenty micrograms of total RNA were separated by electrophoresis in a 1% agarose gel and transferred to a nylon membrane, Hybond N+ (Amersham Biosciences, Piscataway, New Jersey, USA) for Northern analysis. The cDNA probes for aP2, PPARγ, and actin were radiolabeled with [α-32P]dCTP using a random prime-labeling system, Rediprime II (Amersham) and hybridization was performed at 42°C in Rapid-hyb buffer (Amersham).
Clinical studies.
The Institutional Review Board and the General Clinical Research Center (GCRC) Advisory Board of the University of Pennsylvania approved the clinical study protocols. Urine samples for the inhibitor studies were collected before and after 6 days of treatment with aspirin (81 mg), ibuprofen (400 mg) three times a day, or celecoxib (200 mg twice daily), each in six healthy volunteers. Volunteers were 18–35 years of age, within 30% of ideal body weight, and had an unremarkable medical history and physical examination and routine hematology and biochemistry. Smokers and subjects with any bleeding disorders, allergy to aspirin or other NSAIDs, and history of any gastrointestinal or cerebrovascular disease were excluded. Subjects abstained from aspirin or other NSAIDs for at least 2 weeks before enrollment. The samples for urinary analysis of PGs were obtained in a study of bacterial LPS administration to healthy volunteers performed in the GCRC (39). Samples for the diabetic studies were obtained from a group of 200 maturity-onset diabetic patients (ages 35–75), without cardiovascular disease or symptoms, who were recruited to a cross-sectional observational study of risk factors for coronary atherosclerosis as measured by coronary artery calcification at electron beam computed tomography. Age, sex, and ethnically matched nonsmoking diabetic patients in the lowest and highest weight quartiles were selected for study. All abstained from aspirin, NSAIDs, COX-2 inhibitors, and PPAR agonists for at least 4 weeks before and during the study.
Statistics.
Data are expressed as the mean ± SD of the mean. Data were subject to ANOVA and subsequent pairwise analysis, using two-tailed t tests, as appropriate.
Results
Incorporation of 15d-PGJ2 into cells.
We first examined the stability of 15d-PGJ2 in aqueous phase. When 1 μM or 10 μM of [2H4]15d-PGJ2 was incubated in a 24-hour “spent” cell-free medium, the spike was 100% recoverable from the medium at 48 hours (data not shown). When 3T3-L1 fibroblast cells were incubated with 1 μM of [3H]15d-PGJ2, no decrease in 3H in the medium was observed over 48 hours and only 0.5% of the initial counts were recovered from the cell pellet (Figure 1a).
Figure 1.
COX-dependent biosynthesis of 15d-PGJ2 by 3T3-L1 preadipocytes. (a) Cellular incorporation of 15d-PGJ2. 3T3-L1 cells were incubated with 15d-PGJ2 mixed with [3H]15d-PGJ2 for 48 hours. Vertical bars represent dpm in the medium at the times indicated and in the cell wash and cell pellet after 48 hours. (b) Mass spectrum of standard 15d-PGJ2. Spectrum obtained by NI-ESI-LC-MS-MS. For LC-MS-MS conditions, see Methods section. (c) Substrate enhanced, COX-dependent formation of PGs. Stimulation of 15d-PGJ2 and PGE2 formation in 3T3-L1, 1-day preconfluent cells, pretreated with 15 μM arachidonic acid (AA) alone for 1 hour and in the presence of the nonselective COX inhibitor, indomethacin (Indo) (3 μM), or the COX-2 selective inhibitor, NS-398 (0.4–10 μM) in serum-free medium. Formation of PGE2 and 15d-PGJ2 were analyzed 3 hours after the stimulation. *P < 0.05 versus no inhibitors; †P < 0.01. (d) Substrate-enhanced COX proteins. Western blot analysis for COX-2 and COX-1 in 3T3-L1 1-day preconfluent cells after pretreatment with 15 μM AA.
Generation of 15d-PGJ2 by 3T3-L1 preadipocytes.
The 3T3-L1 fibroblast cells may be induced to differentiate to adipocytes by PPARγ ligands or by transient exposure to DM. We sought initially 15d-PGJ2 in lipid extracts from the medium of these cells before their maturation to adipocytes, using negative ion (NI)-ESI-LC-MS-MS. A mass chromatogram of the cell medium revealed peaks corresponding to a parent ion (m/z 315.2) and an associated collision-induced transition ion (m/z 271.0) characteristic of 15d-PGJ2 (Figure 1b).
15d-PGJ2 was detectable (0.64 ± 0.05 pg/ml per 3 hours; ∼2 pM) in the culture medium without serum under subconfluent conditions. Synthesis of substantially greater amounts of PGE2 was also evident (0.98 ± 0.13 ng/ml per 3 hours; ∼3 nM) in the same medium. Supplementation of the cells with substrate arachidonic acid (15 μM) further stimulated formation of both PGs (22.5 ± 3.1 pg/ml per 3 hours; ∼71 pM for 15d-PGJ2, and 18.3 ± 1.8 ng/ml per 3 hours; ∼52 nM for PGE2) (Figure 1c) and induced a transient upregulation of COX-2 protein expression (Figure 1d). COX-1 protein was unaltered by arachidonate treatment (Figure 1d). The nonselective COX inhibitor, indomethacin (3 μM), depressed arachidonate-induced stimulation of 15d-PGJ2 by 84% and PGE2 by 99%. The COX-2 selective inhibitor, NS-398 (IC80s for COX-2 and COX-1 are 1.0 μM and 65 μM, respectively) depressed these PGs in a dose-dependent manner (Figure 1c), establishing that substrate enriched 3T3-L1 cells can form picomolar concentrations of 15d-PGJ2 predominantly via the COX-2 isoform.
Low concentrations (1.0 ± 0.4 nM) of intracellular 15d-PGJ2 were also detectable in the lipid extract under subconfluent conditions. Intracellular 15d-PGJ2 was evaluated after glutathione depletion to assess the possibility that thiol conjugation might account for the apparently low levels of 15d-PGJ2. Glutathione depletion was achieved by exposure of cells to the γ-glutamylcysteine synthetase, DL-buthionine-sulfoximine 6 mM (42) for 24 hours. This treatment failed to increase detectably intracellular 15d-PGJ2 when compared with vehicle (1.1 ± 0.3 nM vs. 1.0 ± 0.4 nM).
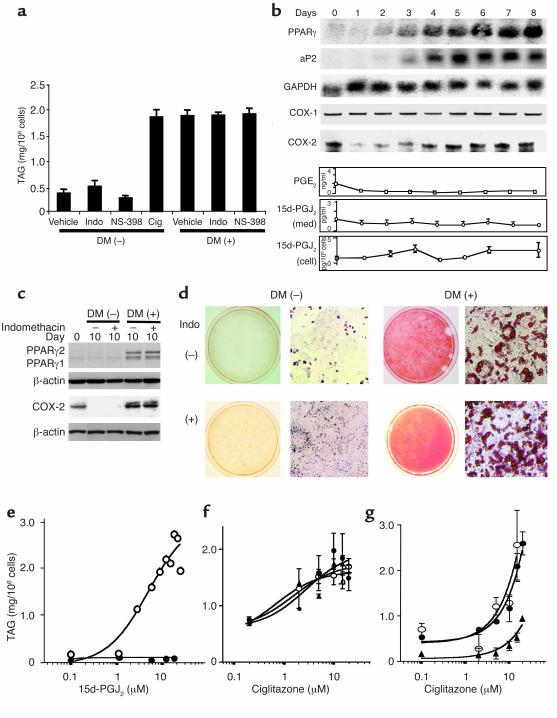
The role of 15d-PGJ2 in PPARγ-mediated adipocyte differentiation was investigated using 3T3-L1 fibroblasts induced to differentiate by transient exposure to DM or a PPARγ agonist. The extent of 3T3-L1 differentiation to mature adipocytes was assessed by measurement of cellular TAG (Figure 2a), by appearance of adipocyte-specific markers such as PPARγ and aP-2 (Figure 2b and c), and by Oil red O staining of accumulated lipids (Figure 2d). Comparable induction was achieved by treatment with DM or the PPARγ agonist, ciglitazone (15 μM), as reflected by TAG accumulation (1.9 ± 0.1 vs. 1.9 ± 0.2 mg/106 cells; Figure 2a) and by PPARγ and aP2 expression (Figure 2b and c) by day 10.
Figure 2.
3T3-L1 cells were treated with DM or ciglitazone (15 μM) to initiate differentiation and were evaluated on days 8 or 10. (a) COX inhibition fails to modulate DM-induced adipocyte differentiation. The extent of TAG formation was estimated in cells differentiated with DM, ciglitazone (Cig), or DM in combination with indomethacin (3 μM) or NS-398 (10 μM). (b) PG formation is not enhanced during adipogenesis. Products during adipogenesis, as determined by Northern blotting (PPARγ, aP2, GAPDH), RT-PCR (COX-1), ribonuclease protection assay (COX-2) analysis, and LC-MS-MS (PGE2, 15d-PGJ2) in the medium (med) and cells. (c) Inhibition of PG formation by indomethacin fails to modulate the induction of adipocyte protein expression by DM. (d) Inhibition of PG formation fails to influence accumulation of lipid induced by DM. Oil red O staining of cells illustrates lipid accumulation in untreated cells versus cells treated with DM ± indomethacin. (e) 15d-PGJ2 drives adipogenesis in a PPARγ-dependent manner. Cells were treated with 15d-PGJ2 (0.1–15 μM) for 2 days in the absence (open circle) or presence (filled circle) of the PPARγ antagonist BADGE (100 μM). (f) Low concentrations of 15d-PGJ2 do not amplify the effect of ciglitazone. Cells were differentiated with ciglitazone alone (filled circle) or in combination with 1 μM (open circle) or 0.1 μM 15d-PGJ2 (filled triangle). (g) COX inhibition fails to modulate the enhancement of ciglitazone-dependent differentiation by DM. Cells were incubated to differentiate by adding ciglitazone alone (filled triangle) or in combination with DM (filled circle) or DM + indomethacin (open circle).
The expression of COX-1 mRNA and protein are unaltered during the course of DM-induced differentiation of 3T3-L1 cells to mature adipocytes (Figure 2b). COX-2 mRNA, however, is depressed initially on addition of DM (Figure 2b). After removal of DM (on day 2), COX-2 mRNA is progressively upregulated, and marked protein expression is evident by day 10 (Figure 2c), coincident with adipocyte maturation. Analysis of medium in differentiated adipocytes for PGE2, 6-keto PGF1α (the hydrolysis product of PGI2), PGF2α, and 15d-PGJ2 established that the predominant products were PGE2 and PGI2 (Table 1). PGJ2 was undetectable. The concentrations of PGE2, 6-keto PGF1α, and 15d-PGJ2 all declined sharply after the cells reached confluence on day –2 (data not shown). Addition of DM resulted in a further detectable reduction in formation of these PGs, coincident with the transient reduction in expression of COX-2 (Figure 2b; 6-keto PGF1α not shown). However, synthesis of all three products remained depressed, despite consequent upregulation of COX-2 during adipocyte differentiation. The relative amounts of the three PGs, PGE2, 6-keto-PGF1α, and 15d-PGJ2, in mature adipocytes in excess of the background levels in the medium collected over 24 hours averaged 0.3 ng/ml (∼0.9 nM), 0.16 ng/ml (∼0.4 nM), and 1.4 pg/ml (∼4 pM), respectively (Table 1).
Table 1.
LC-MS-MS determination of PG levels in the medium of 3T3-L1 cells differentiated with DM
Indomethacin (3 μM) and NS-398 (10 μM), were added daily to 3T3-L1 cells along with DM and post-DM to suppress PG formation. Neither the extent nor the rate of their maturation to adipocytes induced by DM was affected, as evaluated by accumulation of TAG (Figure 2a), expression of PPARγ (Figure 2c), or total lipid accumulation (Figure 2d).
NSAIDs have been shown to bind and activate PPARα and PPARγ in vitro (43) at high concentrations. The EC50 for indomethacin in a standard lipogenesis assay, using C3H10T1/2 mouse mesenchymal stem cells, is 80 μM (43). Concentrations of indomethacin exceeding those necessary for maximal COX inhibition, drove adipocyte maturation, as reflected by TAG accumulation (data not shown). This effect was amplified in the presence of DM and was inhibited by the PPARγ specific antagonist, BADGE (not shown) (42). However, indomethacin, when used at a concentration sufficient to inhibit COX activity, did not induce significant differentiation of 3T3-L1 cells as characterized by PPARγ expression (Figure 2c) or TAG accumulation (Figure 2d). Thus, although high concentrations of a COX inhibitor can drive differentiation in a PPARγ-dependent manner, concentrations of the same compound sufficient to inhibit completely PG production fail to induce differentiation or to modify the rate of differentiation induced by DM.
Given the very low levels of 15d-PGJ2 that we identified in preadipocytes, we sought to characterize the concentration of added eicosanoid necessary to drive adipocyte maturation by acting as a PPARγ ligand. The EC50 for response to 15d-PGJ2, measured by TAG accumulation, was 4.5 μM and this response was antagonized by pretreatment with PPARγ antagonist (Figure 2e). We also addressed the possibility that lower concentrations of 15d-PGJ2 might enhance effects of an alternative PPARγ agonist by ligating a coactivator molecule (44). The ability of ciglitazone to induce adipocyte maturation was unaltered by adding 15d-PGJ2 (0.1 μM and 1.0 μM) (Figure 2f). Similarly, COX inhibition failed to modulate differentiation induced by ciglitazone, consistent with its failure to modulate a response to DM (Figure 2g).
In summary, 15d-PGJ2 is formed by 3T3-L1 cells. However, despite upregulation of COX-2, synthesis of this trivially abundant PG is unaltered during adipocyte differentiation, and suppression of its formation by COX inhibition fails to influence differentiation. Although addition of exogenous 15d-PGJ2 can drive adipocyte maturation by ligating PPARγ, the concentrations required (∼5 μM) exceed those of the endogenous PG released into the medium (∼5 pM) and the intracellular levels (∼1 nM) by orders of magnitude.
Endogenous 15d-PGJ2 in human urine.
Estimation of urinary metabolite excretion is a classical and sensitive approach to the study of PG biosynthesis (45). A representative chromatogram from human urine is shown in Figure 3a. A dominant endogenous peak at m/z 315.2 co-elutes from reversed phase separation with the internal standard [2H4]15d-PGJ2 and is associated with two independent transition ions (m/z 271.5 and 202.5), also characteristic of 15d-PGJ2. Although four distinct isomers of 15d-PGJ2 are identified in the spike, only the major isomer is detected in urine (additional urinary peaks at m/z 315.2 > 271.5 have not been identified, but are believed to be isoprostanes [iPs]). Urinary 15d-PGJ2 in healthy volunteers was 6.3 ± 2.7 pg/mg creatinine (n = 21) with similar levels in males (6.1 ± 3.4 [n = 11]) and females (6.6 ± 1.6 [n = 10]) (P > 0.05). The stability of PGD2 and PGJ2, known to dehydrate partially to 15d-PGJ2 in aqueous solutions, was examined by adding synthetic PGD2 and PGJ2 to urine and observing their disappearance over 24 hours with the concomitant formation of 15d-PGJ2. Although both PGs are unstable in urine over 24 hours, only PGJ2 is a significant source of 15d-PGJ2 (Figure 3b). However, PGJ2 was undetectable in urine, even when samples were collected and extracted into organic solvent within 15 minutes of voiding, thus excluding its rapid dehydration to 15d-PGJ2 ex vivo (Figure 3c).
Figure 3.
15d-PGJ2 in human urine. (a) Mass chromatogram of 15d-PGJ2 in human urine. Representative chromatogram obtained by NI-ESI- MRM. Healthy human volunteers abstained from NSAID use for more than 5 days before urine collection. Asterisks denote isomers of [2H4]15d-PGJ2 present in the internal standard. The major detectable isomer is identified by shading (b) Formation of 15d-PGJ2 by dehydration of PGJ2 or PGD2. Unlabeled PGD2 or PGJ2 (0.2 ng/ml) was added to human urine at T = 0 hour. Aliquots (5.0 ml) were analyzed at the indicated time points for loss of spike and concomitant formation of 15d-PGJ2. The numbers above bars represent amount (%) of added PG remaining at the corresponding time point. (c) Failure to detect endogenous PGJ2 in freshly voided urine. Representative chromatogram of PGJ2 in urine. NI-ESI- MRM of urine samples (5.0 ml + 1.0 ng [18O2]-PGJ2) that were SPE extracted within 15 minutes of voiding. HPLC mobile phase was 30% CH3CN (isocratic). Other parameters were identical to those used for 15d-PGJ2. The PGJ2 peak is shaded. Estimation of the threshold of PGJ2 detection was based on the least integratable peak.
We studied the impact of a mixed COX inhibitor, aspirin (81 mg/d for 6 days), on excretion of 15d-PGJ2, along with major urinary metabolites of thromboxane (Tx) (2,3-dinor-TxB2) (40) and PGI2 (PGI-M) (39) and the F2 iP, 8,12-iso-iPF2α-VI (46). Aspirin, as expected, had no effect on excretion of the free radical catalyzed iP, but depressed the Tx metabolite, which derives largely from COX-1, and to a lesser extent, PGI-M, which derives largely from COX-2. Excretion of 15d-PGJ2 was also depressed by aspirin to an extent (mean 33%) similar to that observed with the PGI2 (mean 44%) rather than with the Tx (mean 81%) metabolite (47) (Figure 4a). We assessed the contribution of the two COX isoforms to 15d-PGJ2 biosynthesis by measuring the comparative impact of a nonspecific inhibitor (ibuprofen) with that of a selective COX-2 inhibitor (celecoxib), as described previously. Urinary excretion of 15d-PGJ2 was depressed from 7.0 ± 2.7 to 1.7 ± 1.3 (P < 0.05) by ibuprofen and to 1.2 ± 0.7 (P < 0.01) by celecoxib. The similar effects of both drugs (Figure 4b) suggest that COX-2 is the dominant source of 15d-PGJ2 biosynthesis under physiologic conditions in humans.
Figure 4.
Biosynthesis of 15d-PGJ2 in humans. (a) Varied impact of COX inhibition on PG metabolites and a auto-oxidative product of arachidonic acid. Urine collections were obtained before and after aspirin (81 mg/d × 6 days) as described in the Methods section. The effects of a COX inhibitor on 15d-PGJ2 formation are compared with markers of COX-dependent (Tx-M and PGI-M) and COX-independent, free radical catalyzed isoprostane (8,12-iso-iPF2α-VI). Tx-M = 2,3-dinor TxB2. *P < 0.05. (b) COX-2 is the dominant source of 15d-PGJ2 biosynthesis in humans. 15d-PGJ2 levels in control volunteers (n = 21) were compared with those treated with the COX-2 selective NSAID celecoxib (n = 6) or nonselective ibuprofen (n = 5) (800 mg), administered acutely 30 minutes before urine collection. *P < 0.05; **P < 0.01. (c) Biosynthesis of PGI2, but not 15d-PGJ2, is altered during an acute inflammatory response in humans. Volunteers (n = 6) received LPS (4 ng/kg). PGI-M (2,3-dinor 6-keto-PGF1α) was measured at multiple time points and is plotted as the mean ± SD (dotted line). For 15d-PGJ2 measurements, urine was collected before LPS treatment (–2 to 0 hours) and at time points corresponding to peak inflammatory (4–6 hours) and resolution (16–24 hours) phases of response. Each individual is represented by a unique symbol that is conserved across time points. Median levels are indicated by horizontal lines. (d) Biosynthesis of 15d-PGJ2 is unaltered in diabetes or obesity. 15d-PGJ2 levels in healthy individuals are shown in comparison to obese (BMI ≥ 30) and nonobese (BMI < 30) patients with noninsulin-dependent type 2 diabetes. Median levels are indicated by horizontal lines.
Biosynthesis of 15d-PGJ2 in human inflammation.
15d-PGJ2 has been proposed both as a propagator of the inflammatory response (20) and a contributor to the resolution of inflammation (21, 24). Administration of LPS to humans is associated with expression of both COX isoforms ex vivo and marked PG formation coincident with a flu-like response (39). This response is associated with a marked increase in urinary excretion of PGI-M, a Tx metabolite and distinct iPs, as we previously described (39). Although 15d-PGJ2 was detectable in the urine of these individuals, there was no significant alteration in biosynthesis during or after the induction of an acute inflammatory response, despite associated expression of both COX isoforms ex vivo (39) and augmented biosynthesis of other PGs such as PGI-M (Figure 4c). We further assessed the role of 15d-PGJ2 in inflammation by examining the profile of PGs present in synovial fluid of patients with arthritis (Table 2). Although PGE2 was evident, 15d-PGJ2 fell below the limit of detection. Finally, we sought altered biosynthesis of 15d-PGJ2 in two conditions, type 2 diabetes and obesity, in which its role as an endogenous ligand for PPARγ has been suggested (3, 48). Although 15d-PGJ2 was detectable in the urine of all patients, biosynthesis of this PG was unaltered in patients with diabetes, irrespective of coincident obesity compared with healthy individuals (Figure 4d).
Table 2.
Clinical characteristic of patients and analyses of PGs in synovial fluid
In summary, despite its detection at low levels in urine, alterations in the biosynthesis of 15d-PGJ2 were not observed in diverse settings in which its role as an endogenous ligand for PPAR has been implicated.
Discussion
Interest has centered on oxidized lipids as potential agonists for nuclear receptors (49). In particular, several features of 15d-PGJ2 have rendered it attractive as a candidate endogenous ligand for PPARγ. First, it activates PPARγ-dependent signaling systems more potently than other fatty acids that have been studied. Second, a representative synthetic CP-PG, Δ12-PGJ2, is actively transported into the nucleus in a time- and temperature-dependent manner with Michaelis-Menten kinetics, suggestive of carrier-mediated active transport (16, 17). Finally, formation of immunoreactive 15d-PGJ2 has been detected during the propagation and resolution of inflammation (21) and in settings associated with PPARγ activation (22). However, the reported concentrations of 15d-PGJ2 necessary to activate PPARγ (2.5–100 μM) in such studies greatly exceed those (pM) associated with the biologic activity of conventional PGs (28), the molecular characteristics of discrete transporters or PG-like receptors with high affinity for PGJ2 or its 15-deoxy metabolite remain to be identified, the reported immunoreactive levels are high and the immunoassay used most commonly for detection of 15d-PGJ2 (21, 24) has been withdrawn.
We sought to investigate the actual biosynthesis of 15d-PGJ2 by developing a specific and sensitive approach based on mass spectrometry. We detected very small amounts of 15d-PGJ2 formed within and released by fibroblasts in vitro. This was augmented by provision of arachidonate substrate, which itself upregulated expression of COX-2 (50), apparently the dominant source of 15d-PGJ2 formation. However, the quantities of 15d-PGJ2 formed are very small by comparison with conventional PGs (26, 27).
We focused on its role in 3T3-L1 fibroblasts, where its role as a PPARγ ligand was first suggested (51). 3T3-L1 fibroblasts are stimulated to differentiate into adipocytes by the addition of DM or a synthetic PPARγ agonist, such as ciglitazone. 3T3-L1 cells do make 15d-PGJ2, and COX-2 is among the proteins upregulated during differentiation induced by DM. However, despite increased expression of the enzyme, no differences in formation of 15d-PGJ2 or the more abundant conventional PGs, PGE2 and PGI2 were observed during differentiation. Furthermore, addition of an NSAID or a selective COX-2 inhibitor, at doses shown to suppress PG formation, failed to alter induction of differentiation by either DM or ciglitazone. Consistent results (data not shown) were obtained in a second murine fibroblast cell line, 3T3-F442A. The failure of indomethacin to inhibit completely the production of 15d-PGJ2 could represent the contribution of auto-oxidative products, as previously described (52).
Characterizing the dose-related effects of the NSAIDs was important as concentrations higher than those necessary to achieve maximal inhibition of PG formation drive adipocyte maturation by ligation of PPARγ. As reported previously, exogenous 15d-PGJ2 can also function as a PPARγ agonist (41); however, the EC50 for this response, 4.5 μM, exceeds endogenous concentrations by orders of magnitude. Lower concentrations also failed to modulate the response to ciglitazone or DM, as might be expected if it bound to a PPARγ coactivator protein (53).
The intrinsic reactivity of the highly electrophilic α,β-unsaturated ketone moiety of 15d-PGJ2 somewhat complicates estimates of its availability in biologic systems. It readily cross-links in vitro with many biomolecules (23). The reactivity of the CP ring has been best characterized for another, the CP-PG, Δ12-PGJ2 (54, 55). Thiscompound undergoes inactivating conjugation with glutathione and L-cysteine both in vitro and in vivo (54, 55). When [3H]-Δ12-PGJ2 is injected into the rat, only 10% of the infused material is recovered in the urine, whereas 90% is concentrated in the bile (55). Consistent with these observations, addition of albumin reduces the potency of exogenously added 15d-PGJ2 to activate PPARγ in transfected cells by roughly one order of magnitude (56).
These observations may contribute, in part, to the marked discrepancy between the concentrations of exogenous 15d-PGJ2 necessary to activate PPARγ-dependent differentiation and the endogenous concentrations actually detected. However, free intracellular levels remained orders of magnitude below those necessary to drive differentiation. Indeed, conjugation of 15d-PGJ2 to glutathione only attains steady state after 2 hours in vitro (57), whereas PGs typically exert their actions within minutes in biologic milieux. Consistent with this discordance between in vitro observation and in vivo plausibility, glutathione depletion failed to elevate intracellular levels of 15d-PGJ2. Furthermore, although the recovery of intracellular [3H]15d-PGJ2 almost doubled after cleavage of potential chemical bonds with biomolecular thiols by treatment briefly with alkali (16), it remained less than 2 nM, far below that necessary to activate PPAR.
Estimation of PGs and their metabolites in urine has proven to be a sensitive approach to the detection of minor and transient alterations in their biosynthesis (58). The 15d-PGJ2 that we detected does reflect endogenous biosynthesis of the compound. Administration of COX inhibitors depressed excretion of the PG. Furthermore, although PGD2 can dehydrate spontaneously to 15d-PGJ2 in urine in vitro, this was a most unlikely source of artifact. Rapid extraction of freshly voided urine into organic solvent, which would stabilize excreted PGJ2, failed to permit detection of PGJ2 in human urine. Unsurprisingly, 15d-PGJ2 has eluded detection by less sensitive approaches using mass spectrometry in the past (27). There has been a recent brief report of its detection in human urine as a pentafluorobenzyl (PFB) derivative using GC-MS (25). However, these results are controversial. We found that a contaminating peak co-eluted with the PFB derivative of authentic 15d-PGJ2 and resisted chromatographic separation (data not shown). The comparable effects of selective inhibitors of COX-2, traditional NSAIDs, and aspirin on urinary 15-PGJ2, suggest that COX-2 was the dominant source of formation of this PG in vitro and in vivo.
Biosynthesis of 15d-PGJ2 is not increased in a variety of settings in which PPARγ activation by its endogenous ligand(s) has been implicated. We have previously reported that LPS administration to human volunteers results in transient flu-like systemic symptoms that are ameliorated by prior inhibition of COX-2 (39). Coincident with the symptoms, expression of both COX isoforms is detectable ex vivo, whereas biosynthesis of the conventional COX products, including TxA2, is markedly augmented (39). Despite this evidence of marked and functionally relevant COX activation and the putative role of 15d-PGJ2-dependent PPARγ ligation in the propagation of inflammation (20, 59), no alteration in the small quantities of urinary 15d-PGJ2 that we detected was observed. Similarly, despite the putative role of COX-2–derived 15d-PGJ2 in the resolution of inflammation (60), no alteration in biosynthesis occurred as symptoms resolved. The efficacy of COX-2 inhibitors and NSAIDs in the treatment of arthritis (61, 62) attests to the relevance of COX products in the mediation of pain and inflammation in this disease. Despite the ready detection of PGE2 in synovial fluid obtained from patients with arthritis who were not taking NSAIDs or related drugs, 15d-PGJ2 was undetectable. Finally, PPARγ activation by its endogenous ligand(s) has been implicated in both obesity and diabetes. We failed to detect any alteration in 15d-PGJ2 biosynthesis in patients with diabetes, with or without concurrent obesity.
In summary, 15d-PGJ2 is formed in small amounts by COX-2 in fibroblasts in vitro and is detectable as a minor product of COX-2 in human urine. It does not mediate PPARγ-dependent adipocyte maturation. Its biosynthesis is also unaltered in diverse settings of inflammation in humans in which its roles as a PPARγ ligand and a modulator of NF-κB signaling have been implicated.
Acknowledgments
We thank Issam Mardini and Ralph Schumacher for the kind provision of clinical samples. We also thank Mitchell Lazar, Claire M. Steppan, and Jane Glick for helpful advice and Helen Zou for technical assistance. This work was supported in part by a National Research Fellowship Award (DK-09942 to L.C. Bell-Parikh), a Banyu Fellowship Award in Cardiovascular Medicine (to T. Ide), and grants HL62250, HL61364 and MO1RR00040 from the National Institutes of Health.
Footnotes
See the related Commentary beginning on page 828.
Peter McNamara’s present address is: Phenomix Corp., La Jolla, California, USA.
L. Chastine Bell-Parikh and Tomomi Ide contributed equally to this work.
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: 15-deoxy-Δ12,14-PGJ2 (15d-PGJ2); lipoprotein lipase (LPL); adipocyte fatty acid binding protein (aP2); cyclopentenone (CP); gas chromatography-mass spectrometry (GC-MS); 9-deoxy-Δ9,12-(E)-PGD2 (Δ12-PGJ2); bisphenol A diglycidyl ether (BADGE); triacylglyceride (TAG); 3-isobuthyl-1-methylxanthine (IBMX); differentiation medium (DM); solid phase extraction (SPE); liquid chromatography–mass spectrometry–mass spectrometry (LC-MS-MS); multiple reaction monitoring (MRM); electrospray ionization (ESI); 2,3-dinor-6-keto-PGF1α (PGI-M); isoprostane (iP); thromboxane (Tx); methoxime (MO); negative ion (NI); General Clinical Research Center (GCRC); pentafluorobenzyl (PFB).
References
- 1.Lazar MA. Progress in cardiovascular biology: PPAR for the course. Nat. Med. 2001;7:23–24. doi: 10.1038/83301. [DOI] [PubMed] [Google Scholar]
- 2.Rangwala SM, Lazar MA. Transcriptional control of adipogenesis. Annu. Rev. Nutr. 2000;20:535–559. doi: 10.1146/annurev.nutr.20.1.535. [DOI] [PubMed] [Google Scholar]
- 3.Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature. 2000;405:421–424. doi: 10.1038/35013000. [DOI] [PubMed] [Google Scholar]
- 4.Rosen ED, Spiegelman BM. PPARγ: a nuclear regulator of metabolism, differentiation, and cell growth. J. Biol. Chem. 2001;276:37731–37734. doi: 10.1074/jbc.R100034200. [DOI] [PubMed] [Google Scholar]
- 5.Narumiya S, FitzGerald GA. Genetic and pharmacological analysis of prostanoid receptor function. J. Clin. Invest. 2001;108:25–30. doi:10.1172/JCI200113455. doi: 10.1172/JCI13455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi Y, Hon M, Evans RM. The peroxisome proliferator-activated receptor delta, an integrator of transcriptional repression and nuclear receptor signaling. Proc. Natl. Acad. Sci. U. S. A. 2002;99:2613–2618. doi: 10.1073/pnas.052707099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith JD, et al. Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein E. Proc. Natl. Acad. Sci. U. S. A. 1995;92:8264–8268. doi: 10.1073/pnas.92.18.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tontonoz P, Nagy L, Alvarez JG, Thomazy VA, Evans RM. PPARγ promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 1998;93:241–252. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- 9.Hsueh WA, Law RE. PPARγ and atherosclerosis: effects on cell growth and movement. Arterioscler. Thromb. Vasc. Biol. 2001;21:1891–1895. doi: 10.1161/hq1201.100261. [DOI] [PubMed] [Google Scholar]
- 10.Hoffmann A, et al. Purification and chemical characterization of β-trace protein from human cerebrospinal fluid: its identification as prostaglandin D synthase. J. Neurochem. 1993;61:451–456. doi: 10.1111/j.1471-4159.1993.tb02145.x. [DOI] [PubMed] [Google Scholar]
- 11.Jakobsson PJ, Thoren S, Morgenstern R, Samuelsson B. Identification of human prostaglandin E synthase: a microsomal, glutathione-dependent, inducible enzyme, constituting a potential novel drug target. Proc. Natl. Acad. Sci. U. S. A. 1999;96:7220–7225. doi: 10.1073/pnas.96.13.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Narumiya S, Ogorochi T, Nakao K, Hayaishi O. Prostaglandin D2 in rat brain, spinal cord and pituitary: basal level and regional distribution. Life Sci. 1982;31:2093–2103. doi: 10.1016/0024-3205(82)90101-1. [DOI] [PubMed] [Google Scholar]
- 13.Murray JJ, et al. Release of prostaglandin D2 into human airways during acute antigen challenge. N. Engl. J. Med. 1986;315:800–804. doi: 10.1056/NEJM198609253151304. [DOI] [PubMed] [Google Scholar]
- 14.Fitzpatrick FA, Wynalda MA. Albumin-catalyzed metabolism of prostaglandin D2. Identification of products formed in vitro. J. Biol. Chem. 1983;258:11713–11718. [PubMed] [Google Scholar]
- 15.Kikawa Y, Narumiya S, Fukushima M, Wakatsuka H, Hayaishi O. 9-Deoxy-Δ9, Δ12-13,14-dihydroprostaglandin D2, a metabolite of prostaglandin D2 formed in human plasma. Proc. Natl. Acad. Sci. U. S. A. 1984;81:1317–1321. doi: 10.1073/pnas.81.5.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Narumiya S, Ohno K, Fukushima M, Fujiwara M. Site and mechanism of growth inhibition by prostaglandins. III. Distribution and binding of prostaglandin A2 and Δ12-prostaglandin J2 in nuclei. J. Pharmacol. Exp. Ther. 1987;242:306–311. [PubMed] [Google Scholar]
- 17.Narumiya S, Ohno K, Fujiwara M, Fukushima M. Site and mechanism of growth inhibition by prostaglandins. II. Temperature-dependent transfer of a cyclopentenone prostaglandin to nuclei. J. Pharmacol. Exp. Ther. 1986;239:506–511. [PubMed] [Google Scholar]
- 18.Straus DS, Glass CK. Cyclopentenone prostaglandins: new insights on biological activities and cellular targets. Med. Res. Rev. 2001;21:185–210. doi: 10.1002/med.1006. [DOI] [PubMed] [Google Scholar]
- 19.Colville-Nash PR, Gilroy DW. COX-2 and the cyclopentenone prostaglandins — a new chapter in the book of inflammation? Prostaglandins Other Lipid Mediat. 2000;62:33–43. doi: 10.1016/s0090-6980(00)00074-5. [DOI] [PubMed] [Google Scholar]
- 20.Shibata T, et al. 15-deoxy-Δ12,14-prostaglandin J2. A prostaglandin D2 metabolite generated during inflammatory processes. J. Biol. Chem. 2002;277:10459–10466. doi: 10.1074/jbc.M110314200. [DOI] [PubMed] [Google Scholar]
- 21.Gilroy DW, et al. Inducible cyclooxygenase may have anti-inflammatory properties. Nat. Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 22.Frungieri MB, Weidinger S, Meineke V, Kohn FM, Mayerhofer A. Proliferative action of mast-cell tryptase is mediated by PAR2, COX2, prostaglandins, and PPARγ: possible relevance to human fibrotic disorders. Proc. Natl. Acad. Sci. U. S. A. 2002;99:15072–15077. doi: 10.1073/pnas.232422999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Straus DS, et al. 15-deoxy-Δ12,14-prostaglandin J2 inhibits multiple steps in the NF-κB signaling pathway. Proc. Natl. Acad. Sci. U. S. A. 2000;97:4844–4849. doi: 10.1073/pnas.97.9.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rossi A, et al. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IκB kinase. Nature. 2000;403:103–108. doi: 10.1038/47520. [DOI] [PubMed] [Google Scholar]
- 25.Thevenon C, Guichardant M, Lagarde M. Gas chromatographic-mass spectrometric measurement of 15-deoxy-Δ12,14-prostaglandin J2, the peroxisome proliferator-activated receptor gamma ligand, in urine. Clin. Chem. 2001;47:768–770. [PubMed] [Google Scholar]
- 26.Maxey KM, Hessler E, MacDonald J, Hitchingham L. The nature and composition of 15- deoxy-Δ12,14-prostaglandin J2. Prostaglandins Other Lipid Mediat. 2000;62:15–21. doi: 10.1016/s0090-6980(00)00072-1. [DOI] [PubMed] [Google Scholar]
- 27.Liston TE, Roberts LJ., 2nd Metabolic fate of radiolabeled prostaglandin D2 in a normal human male volunteer. J. Biol. Chem. 1985;260:13172–13180. [PubMed] [Google Scholar]
- 28.FitzGerald GA, Brash AR, Falardeau P, Oates JA. Estimated rate of prostacyclin secretion into the circulation of normal man. J. Clin. Invest. 1981;68:1272–1275. doi: 10.1172/JCI110373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patrono C, et al. Estimated rate of thromboxane secretion into the circulation of normal humans. J. Clin. Invest. 1986;77:590–594. doi: 10.1172/JCI112341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberts LJ, 2nd, Sweetman BJ. Metabolic fate of endogenously synthesized prostaglandin D2 in a human female with mastocytosis. Prostaglandins. 1985;30:383–400. doi: 10.1016/0090-6980(85)90114-5. [DOI] [PubMed] [Google Scholar]
- 31.Hirata Y, et al. Occurrence of 9-deoxy-Δ9,Δ12-13,14-dihydroprostaglandin D2 in human urine. J. Biol. Chem. 1988;263:16619–16625. [PubMed] [Google Scholar]
- 32.Wright HM, et al. A synthetic antagonist for the peroxisome proliferator-activated receptor gamma inhibits adipocyte differentiation. J. Biol. Chem. 2000;275:1873–1877. doi: 10.1074/jbc.275.3.1873. [DOI] [PubMed] [Google Scholar]
- 33.Westcott JY, Clay KL, Murphy RC. Preparation of oxygen-18-labeled lipoxygenase metabolites of arachidonic acid. Biomed. Mass Spectrom. 1985;12:714–718. doi: 10.1002/bms.1200121208. [DOI] [PubMed] [Google Scholar]
- 34.Xue JC, Schwarz EJ, Chawla A, Lazar MA. Distinct stages in adipogenesis revealed by retinoid inhibition of differentiation after induction of PPARγ. Mol. Cell Biol. 1996;16:1567–1575. doi: 10.1128/mcb.16.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Djian P, Phillips M, Green H. The activation of specific gene transcription in the adipose conversion of 3T3 cells. J. Cell Physiol. 1985;124:554–556. doi: 10.1002/jcp.1041240327. [DOI] [PubMed] [Google Scholar]
- 36.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 37.Knott I, et al. Routine prostaglandin assay by GC-MS in multiwell tissue culture plates: application to human synoviocytes and chondrocytes. Anal. Biochem. 1993;210:360–365. doi: 10.1006/abio.1993.1208. [DOI] [PubMed] [Google Scholar]
- 38.Kunapuli P, Lawson JA, Rokach JA, Meinkoth JL, FitzGerald GA. Prostaglandin F2α (PG F2α) and the isoprostane, 8, 12-iso-isoprostane F2α-III, induce cardiomyocyte hypertrophy. Differential activation of downstream signaling pathways. J. Biol. Chem. 1998;273:22442–22452. doi: 10.1074/jbc.273.35.22442. [DOI] [PubMed] [Google Scholar]
- 39.McAdam BF, et al. Effect of regulated expression of human cyclooxygenase isoforms on eicosanoid and isoeicosanoid production in inflammation. J. Clin. Invest. 2000;105:1473–1482. doi: 10.1172/JCI9523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng Y, et al. Role of prostacyclin in the cardiovascular response to thromboxane A2. Science. 2002;296:539–541. doi: 10.1126/science.1068711. [DOI] [PubMed] [Google Scholar]
- 41.McCloskey HM, Rothblat GH, Glick JM. Incubation of acetylated low-density lipoprotein with cholesterol-rich dispersions enhances cholesterol uptake by macrophages. Biochim. Biophys. Acta. 1987;921:320–332. doi: 10.1016/0005-2760(87)90033-6. [DOI] [PubMed] [Google Scholar]
- 42.Ueda S, et al. Redox regulation of caspase-3(-like) protease activity: regulatory roles of thioredoxin and cytochrome c. J. Immunol. 1998;161:6689–6695. [PubMed] [Google Scholar]
- 43.Lehmann JM, Lenhard JM, Oliver BB, Ringold GM, Kliewer SA. Peroxisome proliferator-activated receptors α and γ are activated by indomethacin and other non-steroidal anti-inflammatory drugs. J. Biol. Chem. 1997;272:3406–3410. doi: 10.1074/jbc.272.6.3406. [DOI] [PubMed] [Google Scholar]
- 44.Oberfield JL, et al. A peroxisome proliferator-activated receptor γ ligand inhibits adipocyte differentiation. Proc. Natl. Acad. Sci. U. S. A. 1999;96:6102–6106. doi: 10.1073/pnas.96.11.6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.FitzGerald GA, Pedersen AK, Patrono C. Analysis of prostacyclin and thromboxane biosynthesis in cardiovascular disease. Circulation. 1983;67:1174–1177. doi: 10.1161/01.cir.67.6.1174. [DOI] [PubMed] [Google Scholar]
- 46.Li H, et al. Quantitative high performance liquid chromatography/tandem mass spectrometric analysis of the four classes of F2-isoprostanes in human urine. Proc. Natl. Acad. Sci. U. S. A. 1999;96:13381–13386. doi: 10.1073/pnas.96.23.13381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Braden GA, Knapp HR, FitzGerald GA. Suppression of eicosanoid biosynthesis during coronary angioplasty by fish oil and aspirin. Circulation. 1991;84:679–685. doi: 10.1161/01.cir.84.2.679. [DOI] [PubMed] [Google Scholar]
- 48.Walczak R, Tontonoz P. PPARadigms and PPARadoxes: expanding roles for PPARγ in the control of lipid metabolism. J. Lipid Res. 2002;43:177–186. [PubMed] [Google Scholar]
- 49.Davies SS, et al. Oxidized alkyl phospholipids are specific, high affinity peroxisome proliferator-activated receptor γ ligands and agonists. J. Biol. Chem. 2001;276:16015–16023. doi: 10.1074/jbc.M100878200. [DOI] [PubMed] [Google Scholar]
- 50.Barry OP, Pratico D, Lawson JA, FitzGerald GA. Transcellular activation of platelets and endothelial cells by bioactive lipids in platelet microparticles. J. Clin. Invest. 1997;99:2118–2127. doi: 10.1172/JCI119385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kliewer SA, et al. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor γ and promotes adipocyte differentiation. Cell. 1995;83:813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 52.Chen Y, Morrow JD, Roberts LJ., 2nd Formation of reactive cyclopentenone compounds in vivo as products of the isoprostane pathway. J. Biol. Chem. 1999;274:10863–10868. doi: 10.1074/jbc.274.16.10863. [DOI] [PubMed] [Google Scholar]
- 53.Ge K, et al. Transcription coactivator TRAP220 is required for PPAR γ2-stimulated adipogenesis. Nature. 2002;417:563–567. doi: 10.1038/417563a. [DOI] [PubMed] [Google Scholar]
- 54.Atsmon J, Freeman ML, Meredith MJ, Sweetman BJ, Roberts LJ., 2nd Conjugation of 9-deoxy-Δ9, Δ12(E)-prostaglandin D2 with intracellular glutathione and enhancement of its antiproliferative activity by glutathione depletion. Cancer Res. 1990;50:1879–1885. [PubMed] [Google Scholar]
- 55.Atsmon J, Sweetman BJ, Baertschi SW, Harris TM, Roberts LJ., 2nd Formation of thiol conjugates of 9-deoxy-Δ9, Δ12 (E)-prostaglandin D2 and Δ12(E)-prostaglandin D2. Biochemistry. 1990;29:3760–3765. doi: 10.1021/bi00467a023. [DOI] [PubMed] [Google Scholar]
- 56.Person EC, Waite LL, Taylor RN, Scanlan TS. Albumin regulates induction of peroxisome proliferator-activated receptor-gamma (PPARγ) by 15-deoxy-Δ12-14-prostaglandin J2 in vitro and may be an important regulator of PPARγ function in vivo. Endocrinology. 2001;142:551–556. doi: 10.1210/endo.142.2.7965. [DOI] [PubMed] [Google Scholar]
- 57.Cox B, et al. Human colorectal cancer cells efficiently conjugate the cyclopentenone prostaglandin, prostaglandin J2, to glutathione. . Biochim. Biophys. Acta. 2002; 1584:37–45. doi: 10.1016/s1388-1981(02)00267-6. [DOI] [PubMed] [Google Scholar]
- 58.Fitzgerald DJ, Roy L, Catella F, FitzGerald GA. Platelet activation in unstable coronary disease. N. Engl. J. Med. 1986;315:983–989. doi: 10.1056/NEJM198610163151602. [DOI] [PubMed] [Google Scholar]
- 59.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor γ is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 60.Ward C, et al. Prostaglandin D2 and its metabolites induce caspase-dependent granulocyte apoptosis that is mediated via inhibition of IκBα degradation using a peroxisome proliferator-activated receptor γ-independent mechanism. J. Immunol. 2002;168:6232–6243. doi: 10.4049/jimmunol.168.12.6232. [DOI] [PubMed] [Google Scholar]
- 61.Anderson GD, et al. Selective inhibition of cyclooxygenase (COX)-2 reverses inflammation and expression of COX-2 and interleukin 6 in rat adjuvant arthritis. J. Clin. Invest. 1996;97:2672–2679. doi: 10.1172/JCI118717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bombardieri S, et al. The synovial prostaglandin system in chronic inflammatory arthritis: differential effects of steroidal and nonsteroidal anti-inflammatory drugs. Br. J. Pharmacol. 1981;73:893–901. doi: 10.1111/j.1476-5381.1981.tb08743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]