Abstract
15-Deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) affects gene transcription by activating PPARγ and by covalent addition to transcription factors and signaling molecules, However, it is not known whether the high concentrations of 15d-PGJ2 required for these responses are consistent with physiological levels. A new study suggests that in vivo 15d-PGJ2 levels are actually several orders of magnitude below the levels required to induce many of the biological effects attributed to this molecule.
Over the past several years there has been increasing interest in the prostaglandin D2–derived (PGD2-derived) product 15-deoxy-Δ12,14-PGJ2 (15d-PGJ2) (Figure 1). This substance was first identified in 1983 as a degradation product of PGD2, formed after incubation for extended periods of time in the presence of albumin (1). 15d-PGJ2 attracted relatively little attention until 1995, when Forman et al. (2) and Kliewer et al. (3) independently reported in Cell that it is capable of activating the transcription factor PPARγ. This finding was of considerable interest because the endogenous ligand for PPARγ was unknown, in spite of the availability of a number of pharmacological agonists that are used in the treatment of type 2 diabetes because of their ability to enhance sensitivity to insulin. The hypothesis that 15d-PGJ2 fulfilled this role was thus very attractive, and 15d-PGJ2 is often referred to as an endogenous PPARγ ligand. For this to be correct, 15d-PGJ2 would have to act at concentrations compatible with its physiological levels. Bell-Parikh et al. now report a highly sensitive and specific assay that they have used to address this question (4).
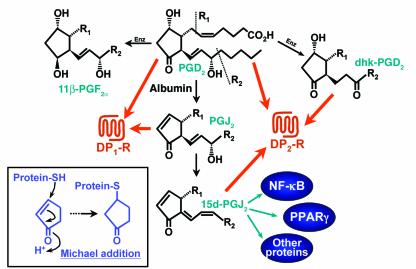
Figure 1.
Formation of 15d-PGJ2 and its effects on receptors and signaling molecules. The degradation of PGD2 to 15d-PGJ2 is enhanced by albumin. In addition to its effects on intracellular proteins, 15d-PGJ2 also activates the DP2 receptor. PGD2 can also be metabolized enzymatically (Enz) to 11β-PGF2α and 13,14-dihydro-15-keto-PGD2 (dhk-PGD2). The latter metabolite also activates the DP2 receptor. Some of the effects of 15d-PGJ2 on intracellular proteins are mediated by Michael addition to the reactive cyclopentenone ring (box). In contrast to its effect on the DP2 receptor, 15d-PGJ2 does not activate the DP1 receptor.
Conversion of PGD2 to 15d-PGJ2
The synthesis of prostaglandins is initiated by activation of phospholipase A2, resulting in the release of arachidonic acid, which is then converted by cyclooxygenases to PGH2. This unstable intermediate is converted enzymatically to a series of biologically active prostanoids, each of which has its own selective receptor(s). PGD2 is formed by the actions of hematopoietic and lipocalin-type PGD2 synthases and is metabolized enzymatically to 11β-PGF2α and 13,14-dihydro-15-keto-PGD2. Alternatively, PGD2 can undergo chemical dehydration, losing a molecule of water to form the cyclopentenone prostaglandin PGJ2. PGJ2 can then undergo further dehydration by loss of the 15-hydroxyl group, which, coupled with migration of the 13,14-double bond of PGJ2, results in the formation of 15d-PGJ2 (Figure 1). These reactions are promoted by albumin but proceed at a relatively slow rate compared to the very rapid formation of PGs from PGH2 by prostanoid synthases. There is no evidence for the enzymatic formation of any of these cyclopentenone PGs, and this lack of evidence is one of the factors that has brought into question their physiological relevance.
15d-PGJ2 as a PPARγ ligand
The two papers published in Cell in 1995 sparked considerable research on the biological effects of 15d-PGJ2. As it has been considered to be an endogenous ligand for PPARγ, 15d-PGJ2 has been employed in many studies on the role of this transcription factor and in most cases shown to have effects similar to those of synthetic PPARγ ligands. This raised the possibility that 15d-PGJ2 could be a physiological regulator of processes affected by PPARγ, including adipocyte differentiation and regulation of glucose levels (5). In addition, there has recently been considerable interest in the role of PPARγ in regulating the inflammatory response, as 15d-PGJ2 and other PPARγ agonists inhibit the expression of a variety of proteins with proinflammatory properties, including cyclooxygenase-2, iNOS, and a variety of cytokines (6). These effects appear to be mediated by inhibitory effects on various transcription factors, including NF-κB, activator protein–1 (AP-1), and signal transducer and activator of transcription–1 (STAT-1). This has led to the proposal that 15d-PGJ2 may be an anti-inflammatory mediator that plays an important role in the resolution of inflammation (7). There is also evidence that PPARγ activators may have antitumorigenic effects due to their inhibitory effects on tumor cell proliferation and angiogenesis.
Although it is clear that 15d-PGJ2 can stimulate PPARγ, the physiological and pharmacological relevance of this may be debatable for several reasons. First, the concentrations required to activate PPARγ are generally reported to be in the μM range, which is very high for prostaglandins, since they usually act at low nM concentrations and are normally formed only in small amounts. However, 15d-PGJ2 acts intracellularly, in contrast to other PGs, which act principally as extracellular mediators, interacting with receptors on the cell surface. Thus it may be argued that it is difficult to know the intracellular concentration of 15d-PGJ2. These key issues are addressed by Bell-Parikh et al. (4). Secondly, there is growing evidence that 15d-PGJ2 can induce a variety of responses independently of PPARγ, some of which would oppose those expected by activation of PPARγ. This lack of selectivity could limit the potential pharmacological usefulness of this substance.
Other effects of 15d-PGJ2
The expression of PPARγ is more restricted than is the expression of other members of the PPAR family (8), and 15d-PGJ2 has been shown to induce responses in cells devoid of this receptor (9). Some of these effects may be mediated through the covalent binding of 15d-PGJ2 to proteins. This is due to the reactive cyclopentenone ring of 15d-PGJ2, which readily reacts with substances containing nucleophilic groups such as cysteinyl thiol groups of proteins. Such reactions are termed Michael addition reactions and also occur with 2-cyclohexenone itself. Thus 15d-PGJ2, in addition to inhibiting NF-κB activation via PPARγ, has been shown to covalently bind to IκB kinase, inhibiting its activity and causing NF-κB to remain in an inactive state. 15d-PGJ2 also inhibits the binding of NF-κB subunits to DNA by a similar mechanism (10). Similarly, 15d-PGJ2 was been found to form an adduct with cysteine-184 of H-Ras in 3T3 cells, but not with either N-Ras or K-Ras, which do not contain a cysteine residue in this position (11). However, in contrast to its inhibitory effect on NF-κB, 15d-PGD2 activates H-Ras, resulting in the stimulation of MAPK and PI-3K, which appear to be responsible for the positive effect of 15d-PGJ2 on cell proliferation.
In addition to the intracellular effects of 15d-PGJ2 on transcription factors and signaling molecules, 15d-PGJ2 can also act on at least one cell membrane receptor. The parent compound, PGD2, acts through two such receptors: the DP1 receptor and the DP2 receptor, which is also known as the chemoattractant receptor–homologous molecule expressed on TH2 cells (CRTH2). We have shown that this substance activates the DP2 receptor on eosinophils with a potency (concentration of agonist that elicits 50% of the maximal response [EC50] ∼10 nM) nearly equal to that of PGD2, the main ligand for this receptor (12). This effect thus occurs at a much lower concentration than that required for activation of PPARγ and would be pro-inflammatory rather than anti-inflammatory.
Is 15d-PGJ2 biologically relevant?
15d-PGJ2 clearly induces or inhibits a variety of PPARγ-dependent and PPARγ-independent responses and is active both in vitro and in vivo. However, the high concentrations required to induce of these effects brings into question their physiological relevance, as PGs normally exhibit EC50 values in the low nM range. Nevertheless, the number of publications on 15d-PGJ2 in high-profile journals continues to increase rapidly despite the paucity of information on its concentrations in biological fluids. Measurement of 15d-PGJ2 in a physiologically relevant setting has been hampered by the lack of reliable immunoassay methods. To raise antibodies to 15d-PGJ2 and other prostaglandins, these small molecules must first be conjugated to proteins. As 15d-PGJ2 spontaneously forms such conjugates in biological milieu, it is difficult to know how much of the immunoreactive material is free 15d-PGJ2 and how much is 15d-PGJ2 or related lipids bound to proteins or glutathione. Because of such uncertainties, one commercially available immunoassay (Cayman Chemical Co., Ann Arbor, Michigan, USA) has been withdrawn from the market. Another antibody has been reported to detect relatively high levels (∼80 nM) of 15d-PGJ2 in medium from RAW264.7 macrophage-like cells stimulated with LPS (13). However, additional validation of this assay would be desirable. The gold standard for measurement of eicosanoids in biological fluids is clearly mass spectrometry, and the availability of reliable mass spectrometric methods to quantitate 15d-PGJ2 is essential to accurately estimate its biological levels as well as to validate immunological assays. In this issue of the JCI, Bell-Parikh et al. (4) report a highly sensitive liquid chromatography/tandem mass spectrometry assay for this substance. The authors show that although 3T3-L1 fibroblasts can produce a very small amount of 15d-PGJ2 (∼5 pM), its production does not increase during differentiation of these cells to adipocytes. Furthermore, the amounts of 15d-PGJ2 released into the medium are more than five orders of magnitude lower than the amounts required for PPARγ-dependent differentiation of these cells into adipocytes, and the concentration of cell-associated 15d-PGJ2 (∼1 nM) is over three orders of magnitude lower. The levels of 15d-PGJ2 excreted in the urine were also found to be very low compared to the levels of other PGs and were not altered in diabetes or following administration of LPS. 15d-PGJ2 was barely detectable in synovial fluid. These results provide strong evidence that, although 15d-PGJ2 can be produced in vivo, its levels are far too low to be compatible with a role for this substance as an endogenous activator of PPARγ. Furthermore, because of its lack of selectivity for PPARγ, 15d-PGJ2 may be of limited usefulness in defining the role of this receptor.
Conclusions
The study by Bell-Parikh et al. (4), using a highly sensitive and selective assay, suggests that the amounts of 15d-PGJ2 generated in vivo are insufficient for it to play a physiological role in modulating most of the responses, including activation of PPARγ, that it has been shown to induce at μM concentrations in vitro. There are only limited instances in which 15d-PGJ2 has been demonstrated to act at low (1–10) nM levels, such as activation of eosinophils though the DP2 receptor (12) and stimulation of tumor cell proliferation (14). Further information on the ability of certain cell types such as macrophages (13) to produce 15d-PGJ2, using mass spectrometric techniques such as those described in the current issue, would seem important in determining whether it could possibly affect such processes. The usefulness of 15d-PGJ2 at a pharmacological level is also rather questionable, as it has been shown to elicit a variety of responses, many of them in opposition to one another. For example, it has been reported to be antiangiogenic (15), yet also to induce VEGF synthesis (16), and it both induces and prevents proliferation of tumor cells (17). Because of its reactivity, 15d-PGJ2, like cyclopentenone itself, can react with proteins and either inhibit or enhance their activity. Therefore, if used at sufficiently high concentrations, it will certainly induce or inhibit responses. While this in itself may be of interest, it does not necessarily add to our understanding of physiologically relevant regulatory mechanisms.
Footnotes
See the related article beginning on page 945.
Conflict of interest: The author has declared that no conflict of interest exists.
Nonstandard abbreviations used: prostaglandin D2 (PGD2); 15-deoxy-Δ12,14-PGJ2 (15d-PGJ2); the concentration of agonist that elicits 50% of the maximal response (EC50).
References
- 1.Fitzpatrick FA, Wynalda MA. Albumin-catalyzed metabolism of prostaglandin D2. Identification of products formed in vitro. J. Biol. Chem. 1983;258:11713–11718. [PubMed] [Google Scholar]
- 2.Forman BM, et al. 15-Deoxy-Δ12,14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR-γ. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 3.Kliewer SA, et al. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor-γ and promotes adipocyte differentiation. Cell. 1995;83:813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 4.Bell-Parikh LC, et al. Biosynthesis of 15-deoxy-Δ12,14-PGJ2 and the ligation of PPARγ. J. Clin. Invest. 2003;112:945–955. doi:10.1172/JCI200318012. doi: 10.1172/JCI18012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy GJ, Holder JC. PPAR-γ agonists: therapeutic role in diabetes, inflammation and cancer. Trends Pharmacol. Sci. 2000;21:469–474. doi: 10.1016/s0165-6147(00)01559-5. [DOI] [PubMed] [Google Scholar]
- 6.Daynes RA, Jones DC. Emerging roles of PPARs in inflammation and immunity. Nat. Rev. Immunol. 2002;2:748–759. doi: 10.1038/nri912. [DOI] [PubMed] [Google Scholar]
- 7.Gilroy DW, et al. Inducible cyclooxygenase may have anti-inflammatory properties. Nat. Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 8.Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-α, -β, and -γ in the adult rat. Endocrinology. 1996;137:354–366. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- 9.Chawla A, et al. PPAR-γ dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nat. Med. 2001;7:48–52. doi: 10.1038/83336. [DOI] [PubMed] [Google Scholar]
- 10.Straus DS, et al. 15-Deoxy-Δ12,14-prostaglandin J2 inhibits multiple steps in the NF-κB signaling pathway. Proc. Natl. Acad. Sci. U. S. A. 2000;97:4844–4849. doi: 10.1073/pnas.97.9.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliva JL, et al. The cyclopentenone 15-deoxy-Δ12,14-prostaglandin J2 binds to and activates H-Ras. Proc. Natl. Acad. Sci. U. S. A. 2003;100:4772–4777. doi: 10.1073/pnas.0735842100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monneret G, Li H, Vasilescu J, Rokach J, Powell WS. 15-Deoxy-Δ12,14-prostaglandins D2 and J2 are potent activators of human eosinophils. J. Immunol. 2002;168:3563–3569. doi: 10.4049/jimmunol.168.7.3563. [DOI] [PubMed] [Google Scholar]
- 13.Shibata T, et al. 15-deoxy-Δ12,14-prostaglandin J2. A prostaglandin D2 metabolite generated during inflammatory processes. J. Biol. Chem. 2002;277:10459–10466. doi: 10.1074/jbc.M110314200. [DOI] [PubMed] [Google Scholar]
- 14.Chinery R, et al. Prostaglandin J2 and 15-deoxy-Δ12,14-prostaglandin J2 induce proliferation of cyclooxygenase-depleted colorectal cancer cells. Cancer Res. 1999;59:2739–2746. [PubMed] [Google Scholar]
- 15.Xin X, Yang S, Kowalski J, Gerritsen ME. Peroxisome proliferator-activated receptor γ ligands are potent inhibitors of angiogenesis in vitro and in vivo. J. Biol. Chem. 1999;274:9116–9121. doi: 10.1074/jbc.274.13.9116. [DOI] [PubMed] [Google Scholar]
- 16.Bamba H, Ota S, Kato A, Kawamoto C, Fujiwara K. Prostaglandins up-regulate vascular endothelial growth factor production through distinct pathways in differentiated U937 cells. Biochem. Biophys. Res. Commun. 2000;273:485–491. doi: 10.1006/bbrc.2000.2969. [DOI] [PubMed] [Google Scholar]
- 17.Clay CE, et al. Magnitude of peroxisome proliferator-activated receptor-γ activation is associated with important and seemingly opposite biological responses in breast cancer cells. J. Investig. Med. 2001;49:413–420. doi: 10.2310/6650.2001.33786. [DOI] [PubMed] [Google Scholar]