Abstract
The E2F transcription factors are key downstream targets of the retinoblastoma protein (pRB) tumor suppressor. We have previously shown that E2F3 plays a critical role in mediating the mitogen-induced activation of E2F-responsive genes and contributes to both the inappropriate proliferation and the p53-dependent apoptosis that arise in pRB-deficient embryos. Here we show that E2F3 also has a significant effect on the phenotype of tumor-prone Rb+/− mice. The absence of E2F3 results in a significant expansion in the life spans of these animals that correlates with a dramatic alteration in the tumor spectrum. E2F3 loss suppresses the development of the pituitary tumors that normally account for the death of Rb+/− mice. However, it also promotes the development of medullary thyroid carcinomas yielding metastases at a high frequency. This increased aggressiveness does not seem to result from any change in p53 levels or activity in these tumors. We show that, instead, E2F3 loss leads to an increase in the rate of tumor initiation. Finally, analysis of Rb+/−; E2f3+/− mice shows that this tumor-suppressive function of E2F3 is dose dependent.
The retinoblastoma gene (RB-1) was identified by its absence in early childhood retinoblastoma (6, 7, 18). Subsequent studies revealed that RB-1 is mutated in approximately one-third of all human tumors (reviewed in reference 45). To elucidate the precise role of the retinoblastoma protein (pRB) in both tumorigenesis and development, a number of mutant mouse strains have been established (reviewed in reference 26). These analyses underscore the importance of pRB both as a tumor suppressor and as a key regulator of cellular growth in normal development. Consistent with the familial cancer syndromes, mice carrying a single Rb mutant allele are highly cancer prone (12, 46). These animals develop pituitary tumors with almost complete penetrance, and a significant number also display medullary thyroid tumors (MTCs). In addition, pRB is essential for embryogenesis (1, 15, 17). The midgestation lethality of pRB-deficient embryos is accompanied by defective development of the fetal liver, erythrocytes, neurons, and lens resulting from a combination of ectopic S-phase entry and inappropriate programmed cell death. Subsequent studies have shown that the apoptosis can be either p53 dependent or p53 independent depending on the particular tissue (22, 25).
The growth-suppressive properties of pRB are thought to be largely dependent on its ability to regulate the E2F transcription factors (reviewed in references 5 and 41). The E2Fs control the expression of genes essential for cell proliferation, including key components of both the DNA replication and cell cycle machinery. pRB binds to E2F during the G1 phase of the cell cycle and inhibits the activation of E2F-dependent target genes. In response to mitogenic signals, pRB is phosphorylated by the cell cycle-dependent kinases cyclin D/cdk4 or cyclin D/cdk6 and cyclin E/cdk2, and transcriptionally active E2F is released. Notably, almost all pRB-positive tumors contain activating mutations in cycD1 or cdk4 or inactivating mutations in the cyclin-dependent kinase inhibitor p16 (reviewed in reference 39). These findings suggest that functional inactivation of pRB, and the consequent inappropriate release of E2F activity, is an essential step in tumorigenesis.
To date, six genes that encode members of the E2F family have been cloned (reviewed in references 5 and 41). Although most of these genes encode a single protein product, E2f3 has two alternative promoters that yield two distinct proteins, called E2F3a and E2F3b, which differ in their N-terminal sequences. E2F3b has only recently been identified, and its biological properties are not well understood. The remaining E2F proteins have been divided into three distinct subgroups based on significant differences in structure and function.
E2F1, -2, and -3a represent one subgroup, and they are believed to be the key downstream targets of pRB. These three E2Fs are specifically regulated by pRB and not by the pRB-related protein p107 or p130 (19). When overexpressed, E2F1, -2, and -3a are potent transcriptional activators and are each sufficient to induce quiescent cells to enter S phase (4, 21). The endogenous E2F1, -2, and -3a proteins are released from pRB during late G1 and then associate with E2F-responsive promoters just prior to the activation of E2F-responsive genes (34, 40). Taken together, these data suggest that E2F1, -2, and -3a play a key role in the induction of cellular proliferation. In agreement with this hypothesis, mouse embryonic fibroblasts that lack E2F3a and E2F3b (for simplicity, these two proteins are referred to below as E2F3) have a defect in the mitogen-induced activation of almost all known E2F-responsive genes that impairs the proliferation of both primary and transformed cells (14). Moreover, the combined loss of E2F1, E2F2, and E2F3 completely blocks cellular proliferation, suggesting that these proteins have overlapping roles in vivo (47). Finally, these “activator E2Fs” can trigger cells to undergo apoptosis through both p53-dependent (32, 38, 48) and p53-independent mechanisms (11, 31).
Analysis of E2f mutant mouse strains suggests that the activating E2Fs play both overlapping and unique roles in normal development. E2F1 and E2F2 are not required for embryonic development, but adult E2f1−/− and E2f2−/− mice each develop a unique spectrum of tissue-specific abnormalities including defined, but distinct, defects in T-lymphocyte development (8, 27, 50-52). E2f1 mutant mice are also susceptible to hematopoietic malignancies, and the tumor incidence appears to be increased by E2f2 mutation (50, 52). Tumorigenesis is not dependent on loss of the remaining wild-type E2f1 and/or E2f2 allele, and there is still considerable debate about the underlying basis for these tumor-suppressive properties (reviewed in reference 41). Initially, these properties were thought to be due to E2F1's role in the active repression of E2F-responsive genes via recruitment of pRB and associated histone deactylases. However, others have suggested that E2F1 acts as a tumor suppressor through its ability to induce apoptosis and/or its participation in a DNA damage response (20, 23, 24).
In contrast to that of the other activating E2Fs, the loss of E2F3 causes a high frequency of neonatal lethality (2, 14). In certain mixed-strain backgrounds, a small proportion of E2f3−/− mice survive to adulthood, but most die prematurely of congestive heart failure (2). Analysis of E2f1; E2f3 compound mutant mice indicates that the developmental defects arising in the E2f1 or E2f3 single-mutant mice are exacerbated by the combination of the two mutations (2). This finding strongly suggests that these genes have critical, overlapping functions in development. However, E2f3 mutation has no detectable effect on tumor incidence among E2f1 mutant mice, raising the possibility that tumor suppression is a specific property of E2f1 and E2f2 but not of E2f3 (2).
Compound Rb; E2f mutant mouse models have been used to determine how pRB's growth-suppressive properties relate to its role in the inhibition of the activating E2Fs. These studies show that the absence of either E2F1 or E2F3 greatly suppresses the ectopic S-phase entry and the p53-dependent and p53-independent apoptosis arising in pRB-deficient embryos and thereby significantly extends their life spans (42, 53). This finding suggests that E2F1 and E2F3 both make significant contributions to the phenotypic consequences of pRB deficiency. In agreement with this hypothesis, E2F1 loss has been shown to significantly reduce the development of pRB-deficient tumors (49). However, the lack of a complete rescue raised the possibility that one or more additional E2Fs might also contribute to the tumor phenotype. This notion is supported by the finding that E2F4 loss greatly suppresses the formation of tumors in Rb+/− mice by enabling p107 and p130 to bind, and presumably inhibit, both E2F1 and E2F3 (16). In this study, we have generated Rb; E2f3 compound mutant mice to assess how E2F3 contributes to the Rb mutant phenotype. Our analysis reveals an unexpected role for E2F3 in suppressing the metastasis of pRB-deficient MTCs.
MATERIALS AND METHODS
Animal maintenance and histological analysis.
The Rb and E2f3 mutant mouse strains were genotyped by using previously described PCR protocols (14, 15). Animals were sacrificed just prior to the expected time of death or as warranted by the sizes of the tumors. In some cases, animals were examined after they were found dead. Full necroscopies were performed on all animals according to standard procedures. For histology all tissues were fixed in 10% phosphate-buffered formalin or Bouin's solution and embedded in paraffin blocks, and 4- to 6-μm-thick representative sections were stained with hematoxylin and eosin. For more-detailed analysis of the pathology of thyroids and c-cell lesions as well as ensuing metastasis in lungs, sections from four to six different levels of these organs were produced. Tumor genotypes were determined by conducting semiquantitative PCR on tissues that had been isolated by using a dissection microscope.
Statistical analysis and establishment of relative tumor areas.
Survival and longevity statistics were calculated with the Excel (Microsoft) and Stata (version 6.0; Stata Corporation) programs. To establish the relative areas of pituitary tumors, low-power microscopic views of Rb mutant pituitary tumors were photographed, and a normalized grid was electronically merged with the entire tumor area. The largest pituitary carcinoma was set to 100%, and tumors of all other genotypes were measured accordingly.
Immunohistochemistry, Western blotting, and gel retardation assays.
Immunohistochemical detection of p53 (CM5; Novocastra), pRB (144011A; BD Bioscience), and calcitonin (CMC-101; Cell Marque) was performed on formalin-fixed, paraffin-embedded, 4-μm-thick sections. Antigen retrieval was performed by boiling the specimen in Trilogy (Cell Marque). Primary antibodies were detected by using a biotinylated secondary antiserum facilitated by ABC (avidin-peroxidase-biotin complex) as suggested by the manufacturer (Vector Laboratories). The specificities of anti-pRB primary antibodies were verified by using sections from the central nervous systems of wild-type and viable Rb−/− day-13.5 embryos. All stainings included a control reaction that did not contain any primary antibody. Western blotting and gel retardation assays were performed on whole-cell extracts of dissected late-stage MTCs as previously described (43).
RESULTS
Rb mutation increases the viability of E2f3−/− neonates.
It has previously been shown that E2F3 is critical for cellular proliferation and contributes to the inappropriate proliferation and apoptosis that arise in pRB-deficient embryos (14, 53). Given these observations, we hypothesized that E2F3 might contribute to the development of pRB-deficient tumors. Since the viability of the E2f3−/− mice and the severity of the tumor phenotype of the Rb+/− mice are both influenced by strain-specific modifiers (2), we conducted crosses between Rb (15) and E2f3 (14) mutant mouse strains on both a 129/Sv and a mixed (129/Sv × C57BL/6) background.
Initially, we examined the abilities of mice with the compound Rb; E2f3 mutant genotypes to survive until weaning. In the pure 129/Sv background (data not shown), we observed complete embryonic lethality of the E2f3−/− mice as previously described (2). Unfortunately, the presence of a single Rb mutant allele did not suppress the lethality of the E2f3−/− animals. However, Rb+/− and Rb+/−; E2f3+/− mice were both generated at the expected Mendelian ratio and were aged for analysis of adult phenotypes. In the mixed 129/Sv × C57BL/6 strain background, a small fraction of the E2f3−/− mice survived to weaning (2). Significantly, matings of mixed-background Rb+/−; E2f3+/− females with either Rb+/−; E2f3+/− or Rb+/−; E2f3−/− males yielded surviving E2f3−/− animals at a significantly higher frequency in the presence (25.75% ± 4.05% of the expected frequency) than the absence (11.4% ± 0.3% of the expected frequency) of one Rb mutant allele (Table 1). This result strongly suggests that a reduction in pRB levels is sufficient to overcome the requirement for E2F3 in one or more developmental processes that are essential for neonatal viability.
TABLE 1.
Rb mutation increases the viability of E2f3−/− neonates
| Cross and genotypea | No. of animals | % of expected frequency |
|---|---|---|
| Rb+/−; E2f3+/− × Rb+/−; E2f3+/− (Σ = 479 progeny) | ||
| WT | 60 | 100 |
| E2f3+/− | 110 | 104.5 |
| Rb+/− | 85 | 91.7 |
| Rb+/−; E2f3+/− | 191 | 70.8 |
| E2f3−/− | 7 | 11.7 |
| Rb+/−; E2f3−/− | 26 | 21.7 |
| Rb+/−; E2f3+/− × Rb+/−; E2f3−/− (Σ = 375 progeny) | ||
| E2f3+/− | 99 | 100 |
| Rb+/−; E2f3+/− | 206 | 104 |
| E2f3−/− | 11 | 11.1 |
| Rb+/−; E2f3−/− | 59 | 29.8 |
Genotypic analysis of the progeny was performed at weaning or at the age of 21 days. WT, wild type.
E2f3 mutation increases the life spans of the tumor-prone Rb+/− mice.
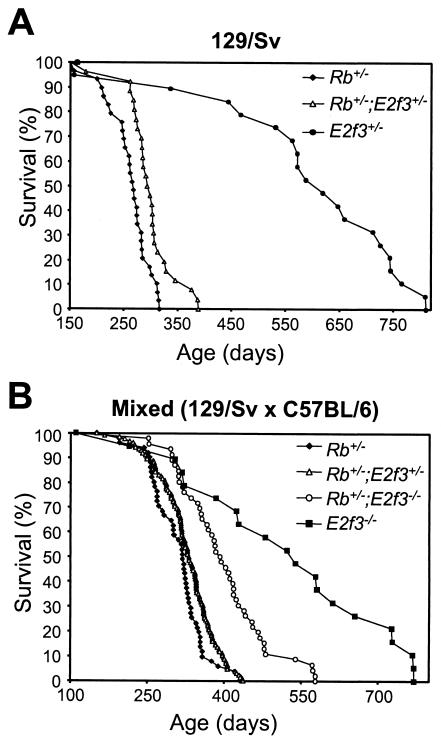
To determine whether E2F3 loss alters the viability of Rb+/− adults, we aged a large cohort of surviving neonatal E2f3; Rb mutant littermates and compared their life spans (Fig. 1; Table 2). Among the inbred 129/Sv mice, we observed a significant increase (P = 0.0008 by the log rank test) in the mean survival of Rb+/−; E2f3+/− mice (9.8 ± 1.3 months) over that of their Rb+/− littermates (8.6 ± 1.2 months). Similarly, in the mixed (129/Sv × C57BL/6) strain, the life spans of Rb+/− mice (10.2 ± 1.6 months) were progressively increased by the presence of either one (10.8 ± 1.8 months) or two (13 ± 2.6 months) E2f3 mutant alleles. Both of these changes are statistically significant (P = 0.0128 and P < 0.00001 by the log rank test, respectively). Thus, E2f3 mutation increases the viability of Rb+/− mice in a dose-dependent manner. Importantly, the Rb+/−; E2f3−/− mice could also be clearly distinguished from their E2f3−/− littermate controls; they died earlier and had no evidence of the congestive heart failure that causes the deaths of most E2f3−/− adults (2). Thus, the Rb mutation influences the viability of these Rb+/−; E2f3−/− adults.
FIG. 1.
Effect of E2f3 status on the life spans of Rb heterozygous mice. Progeny arising from Rb+/−; E2f3+/− intercrosses were aged together. Shown are log rank survival curves obtained by plotting percent survival against age. (A) In the pure 129/Sv background, the life spans of Rb+/−; E2f3+/− animals (n = 25) were significantly higher than those of Rb+/− animals (n = 28). Additionally, E2f3+/− animals (n = 19) were aged as a control. (B) In the mixed 129/Sv × C57BL/6 background, the life spans of Rb+/−; E2f3+/− (n = 125) and Rb+/−; E2f3−/− (n = 40) animals were progressively longer than those of Rb+/− (n = 50) animals but shorter than those of E2f3−/− animals (n = 18).
TABLE 2.
E2F3 loss increases life spans of Rb+/− mice in mixed (129/Sv × C57BL/6) and pure (129/Sv) genetic backgrounds
| Genetic background and genotype | No. of animals | Mean survival (months) ± SD | Survival range (days) |
|---|---|---|---|
| Mixed (129/Sv × C57BL/6) | |||
| Rb+/− | 50 | 10.2 ± 1.6 | 194 to 431 |
| Rb+/−; E2f3+/− | 125 | 10.8 ± 1.8 | 166 to 437 |
| Rb+/−; E2f3−/− | 50 | 13.0 ± 2.6 | 252 to 582 |
| E2f3−/− | 18 | 17.1 ± 5.5 | 213 to 770 |
| Pure (129/Sv) | |||
| Rb+/− | 28 | 8.6 ± 1.2 | 157 to 316 |
| Rb+/−; E2f3+/− | 25 | 9.8 ± 1.3 | 262 to 388 |
| E2f3+/− | 19 | 19.5 ± 5.3 | 157 to 810 |
E2f3 mutation suppresses the development of pituitary tumors in Rb heterozygous mutant mice.
Since E2f3 mutation extends the life spans of Rb+/− animals, we first examined whether it had any effect on either the incidence or the sizes of the intermediate-lobe pituitary tumors that are the documented cause of death for Rb heterozygotes (10, 12, 28, 35, 46). For these studies, we focused primarily on the mixed 129/Sv × C57BL/6 background, because all genotypes of interest were available for analysis. The phenotypes of the Rb+/− littermate controls were entirely consistent with previous reports. We observed melanotroph carcinomas that had arisen from the intermediate lobe of the pituitary gland (Fig. 2A and data not shown). In almost every case, the tumors had expanded to compress adjacent brain structures, causing the death of the animal. In contrast, microscopic histological examination showed that the pituitary glands of E2f3 mutant littermates were completely normal (Fig. 2A and data not shown).
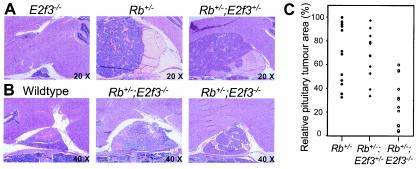
FIG. 2.
Loss of E3f3 suppresses tumor formation in the pituitary gland. (A) Hematoxylin-and-eosin-stained sections of pituitary glands from a healthy E2f3−/− animal that died at 14 months due to congestive heart failure and Rb+/− and Rb+/−; E2f3+/− mice that died at the age of 10.5 months due to the pituitary tumors. Magnification, ×20. (B) Hematoxylin-and-eosin-stained sections of pituitary glands from a healthy wild-type animal sacrificed at the age of 12 months and two Rb+/−; E2f3−/− mice that died at 10.5 and 12 months. Magnification, ×40. (C) Comparison of pituitary tumor sizes (as judged by the area of the median section) at the time of death for Rb+/− (n = 12), Rb+/−; E2f3+/− (n = 11), and Rb+/−; E2f3−/− (n = 15) mice.
At the time of death, the pituitary tumors of the Rb+/−; E2f3+/− mice were indistinguishable from those of the Rb+/− controls as judged by incidence, size, and pathological criteria (Fig. 2A and C and data not shown). Since there is a short increase in the life spans of the Rb+/−; E2f3+/− animals relative to those of the Rb+/− controls, it seems likely that these tumors develop slightly more slowly. Consistent with this hypothesis, the complete absence of E2F3 had a major effect on pituitary tumor development. Although all of the Rb+/−; E2f3−/− mice displayed pituitary carcinomas, these were significantly smaller than those detected in the Rb+/− controls (Fig. 2B and C). Indeed, in several cases, the tumor was still primarily contained within the intermediate layer of the pituitary gland, causing little disruption of the other layers (Fig. 2B). The suppression of tumor formation is particularly striking given that the Rb+/−; E2f3−/− animals live longer than their Rb+/− controls. Importantly, for about 30% of the Rb+/−; E2f3−/− animals, the pituitary tumor was ruled out as the cause of death. We therefore conclude that E2F3 acts to promote the development of pituitary tumors in Rb mutant mice.
E2f3 mutation promotes the development of pRB-deficient MTCs.
Since the pituitary tumors could not account for the deaths of all Rb+/−; E2f3−/− animals, we next examined whether E2f3 status altered the known MTC tumor phenotype of the Rb heterozygotes. Rb+/− mice are predisposed to develop c-cell hyperplasia, which subsequently progresses into MTCs(10, 12, 30, 46). Depending on the specific recombinant mouse mutant used and the genetic background, the incidence of these tumors ranges from 50 to 90%. In our study, 56% of the Rb+/− cohort displayed either c-cell hyperplasia or MTCs (Fig. 3A). Consistent with previous reports, the MTCs were mostly small, predominantly unilateral, and nonaggressive and showed signs of necrosis. In contrast, 10 E2f3−/− and 15 E2f3+/− littermates that were aged together with our Rb+/− cohort showed no signs of c-cell abnormalities (data not shown).
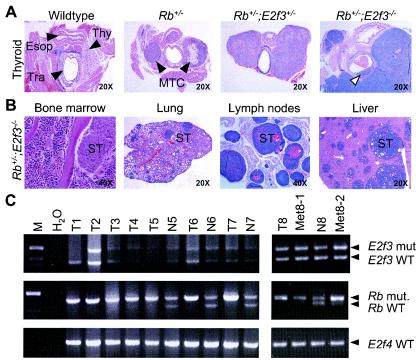
FIG. 3.
E2f3 mutation promotes thyroid tumor development. (A) Hematoxylin-and-eosin-stained sections of thyroids from a healthy control wild-type animal sacrificed at the age of 10.5 months and of MTCs derived from Rb+/−, Rb+/−; E2f3+/−, and Rb+/−; E2f3−/− mice that died at the ages of 10.5, 11, and 12 months, respectively. Arrowheads indicate the locations of the thyroid (Thy), trachea (Tra), and esophagus (Esop) in the wild-type control. The open arrowhead highlights the infiltration of the tumor into the intratracheal space in an Rb+/−; E2f3−/− animal. (B) Cross-section of metastatic tumor growth in the bone marrow, lungs, lymph nodes, and livers of four different Rb+/−; E2f3−/− animals. Selected sites of secondary tumor growth (ST) are indicated. (C) PCR-based LOH analysis of DNA extracted from freshly isolated biopsy specimens from either MTCs (T), metastases (Met), or matching normal tissues (N) derived from either Rb+/− (T1) or Rb+/−; E2f3+/− (T2 to T8; Met8-1 and Met8-2) mice alongside a water-only control reaction (H2O). All of the tumors showed LOH for Rb but not for E2f3. An E2f4-specific PCR was used to confirm equal input of DNA. WT, wild type; mut., mutant.
Significantly, the mutation of E2f3 dramatically increased the development of these tumors. In the Rb+/−; E2f3+/− animals, MTCs with almost complete penetrance (98%) were detected. Moreover, almost all of these compound mutants had bilateral rather than unilateral tumors, and these were much larger than those observed in the Rb+/− controls (Fig. 3A). In many cases, these tumors had invaded adjacent structures and were palpable in the living mice (Fig. 3A). In a subset of the Rb+/−; E2f3+/− animals, the tumor had begun to compress the trachea and impede breathing. In these mice, the pituitary and c-cell tumors were both sufficiently severe to be considered the cause of death. The relatively small difference in the time of death between Rb+/− (10.2 ± 1.6 months) and Rb+/−; E2f3+/− (10.8 ± 1.8 months) mice strongly suggests that the increase in MTC development is induced by the change in E2f3 dosage rather than by the change in life span.
The aggressiveness of this particular tumor located in the thyroid was further increased in the Rb+/−; E2f3−/− mice. MTCs were detected at a high frequency (92%), and the vast majority were bilateral and showed extensive infiltration into adjacent structures (Fig. 3A). In several animals (3 of 38), the tumors were found to have traversed the muscle and cartilage layers and invaded into tracheal space (Fig. 3A). Histologically, the MTCs of the Rb+/−; E2f3−/− mice also appeared much more aggressive than those of their Rb+/− and Rb+/−; E2f3+/− littermates, as evidenced by a significant increase in the nuclear/cytoplasmic ratio (data not shown). Importantly, these highly aggressive MTCs and/or the resulting metastases (see below) could account for the death of the subset of Rb+/−; E2f3−/− mice that had insignificant pituitary tumors. Thus, in the same animal, E2f3 mutation suppresses tumor development in the pituitary gland while promoting the development of c-cell tumors.
E2f3 mutation promotes metastasis of pRB-deficient MTCs in a dose-dependent manner.
In a significant fraction of the Rb+/−; E2f3−/− mice (37.5%), we observed metastasis of the MTC (Fig. 3B; Table 3). These secondary tumors were easily observed in the liver and lungs upon macroscopic inspection. Further histopathological analyses revealed the presence of metastases in numerous other organs including the kidneys, intestines, lymph nodes, and adrenal glands, and more importantly, in the marrow of various bones. Importantly, metastases were detected in Rb+/−; E2f3−/− mice that died at the earliest (e.g., 252 days) as well as the latest time points (e.g., 582 days), indicating that the onset of metastasis was not merely a result of life span extension. In comparison, only a small fraction of the Rb+/− controls (9%) developed metastases, and these were primarily located in the lungs (Table 3). Interestingly, there was also an increase in the frequency of metastasis in the Rb+/−; E2f3+/− mice (23%), although these were also mostly present in the lungs and liver and merely a few animals developed secondary tumors in numerous organs comparable to those in Rb+/−; E2f3−/− mice (Table 3). Additionally, the onset of metastasis was detected despite the fact that these animals were similar in age to the Rb+/− mice (Table 2). Taken together, these data show that E2F3 acts to suppress the development of the pRB-deficient thyroid tumor and to inhibit its metastasis.
TABLE 3.
Loss of E2F3 promotes metastasis of pRB-deficient MTCs to distant organs
| Organ in which metastases were present | No. of animals with metastasis/total no. of animals
|
||
|---|---|---|---|
| Rb+/− | Rb+/−; E2f3+/− | Rb+/−; E2f3−/− | |
| Lung | 3/36 | 9/46 | 12/40 |
| Liver | 1/36 | 4/46 | 11/40 |
| Kidney | 0/36 | 3/46 | 6/40 |
| Lymph nodes | 1/36 | 2/46 | 4/40 |
| Bone (marrow) | 0/8 | 0/10 | 3/12 |
| Intestines | 0/36 | 3/46 | 3/40 |
| Adrenal | 1/30 | 2/40 | 1/35 |
| Stomach | 0/36 | 1/46 | 1/40 |
| Heart | 0/36 | 2/46 | 1/40 |
| Testis | 0/36 | 1/46 | 1/40 |
| Prostate | 0/36 | 0/46 | 1/40 |
| Mammary | 0/36 | 1/46 | 1/40 |
| Total | 4/45 (8.9%) | 12/52 (23.1%) | 15/40 (37.5%) |
We wanted to establish whether the increased aggressiveness of MTCs and ensuing metastasis in Rb+/−; E2f3+/− mice required inactivation of the wild-type E2f3 allele. To address this issue, we microdissected late-stage MTCs from mice of the various Rb; E2f3 genotypes and performed semiquantitative PCR to screen for loss of heterozygosity (LOH) of both the Rb and E2f3 genes. In agreement with the results of a previous analysis (28), we observed LOH for Rb in all of the tumors derived from Rb+/−, Rb+/−; E2f3+/−, and Rb+/−; E2f3−/− animals (Fig. 3C and data not shown), confirming that our tumor samples are of sufficient purity to detect LOH. In contrast, we consistently detected the wild-type E2f3 allele in tumors derived from the 8 Rb+/− and 15 Rb+/−; E2f3+/− mice (Fig. 3C and data not shown). Importantly, there was no evidence of LOH for E2f3 in either the primary thyroid tumors or any of the 10 metastatic lesions that we examined. We therefore conclude that E2F3 is acting in a dose-dependent manner to suppress the development and the metastatic growth of MTCs in mice.
Rb; E2f3 mutant animals display several novel tumorigenic lesions.
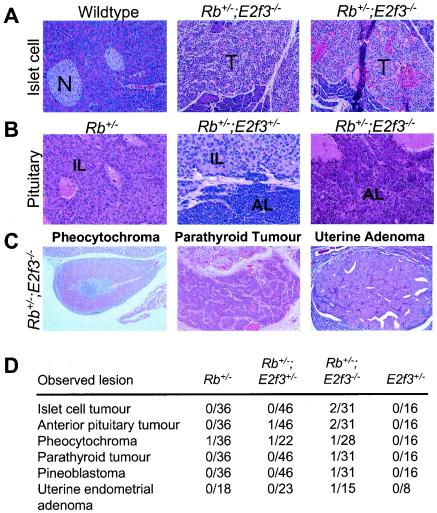
In addition to the change in the MTC phenotype, our necropsy studies showed that the Rb; E2f3 compound mutant mice also developed novel tumorigenic lesions at a low frequency (Fig. 4). We detected tumors that had clearly initiated in the anterior lobe of the pituitary gland in both an Rb+/−; E2f3−/− and an Rb+/−; E2f3+/− animal. In the latter case, the anterior-lobe tumor had developed alongside, but clearly independently from, the typical intermediate-lobe pituitary tumor. The remaining, novel tumors were all specifically detected in the Rb+/−; E2f3−/− and not the Rb+/−; E2f3+/− mice. First, two animals developed islet cell tumors that were large, highly vascularized, and nonnecrotic. Although this incidence is low, histological examination revealed a significant incidence of islet cell hyperplasia in the majority of Rb+/−; E2f3−/− animals that is clearly distinct from the background level of occasional hyperplastic islet cells that arise in many ageing animals of this specific genetic background. Finally, we also observed one parathyroid tumor and one pineoblastoma in two independent Rb+/−; E2f3−/− animals. Interestingly, islet cell tumors, parathyroid tumors, and pineoblastomas are never observed in the Rb+/− controls (10, 12, 28, 35, 46; this study) but have been detected previously in Rb+/−; p53+/− and Rb+/−; p53−/− mutant mice (44, 46). The presence of these novel tumor types in the Rb+/−; E2f3−/− mice reinforces our conclusion that E2F3 can act to suppress tumor formation in the mouse.
FIG. 4.
Additional, novel tumor types arise in Rb+/−; E2f3−/− mice. (A) Comparison of normal islets (N) in a wild-type mouse with islet-cell tumors (T) from two Rb+/−; E2f3−/− mice. Magnification, ×20. (B) Histological appearance of a typical Rb mutant intermediate-lobe (IL) pituitary tumor versus that of pituitary tumors from two different Rb+/−; E2f3−/− mice who had tumors initiating in both the intermediate lobe and the anterior lobe (AL) or solely in the anterior lobe of the pituitary gland. Magnification, ×40. (C) A pheocytochroma, a parathyroid tumor, and a uterine endometrial adenoma detected in different Rb+/−; E2f3−/− mice. (D) Incidences of various lesions in Rb+/−, Rb+/−; E2f3+/−, Rb+/−; E2f3−/−, and E2f3+/− mice. Magnification, ×40.
E2f3 mutation does not alter p53 levels or activity in pRB-deficient tumors.
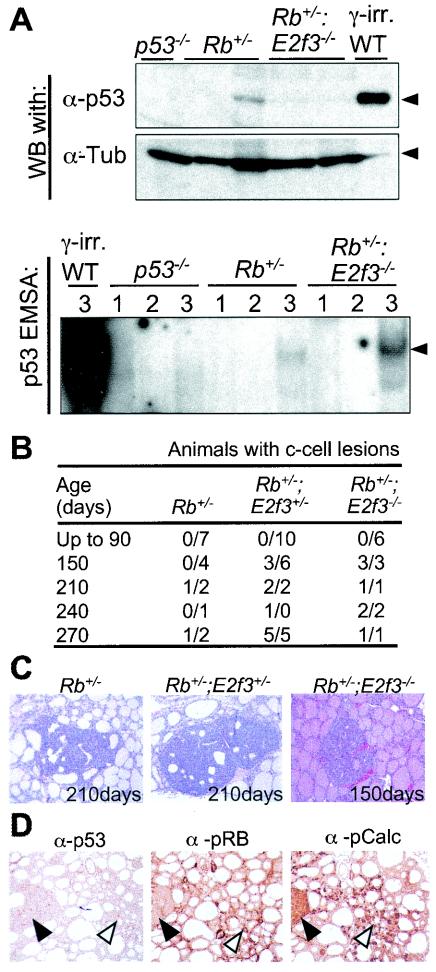
Inhibition of apoptosis is often a critical event in tumor development, and p53, or its upstream regulators, is a frequent target for mutation (reviewed in reference 9). It has previously been shown that E2f3 contributes to the induction of p53-dependent apoptosis arising in pRB-deficient embryos (53), and we now find that Rb+/−; E2f3−/− mice develop a similar spectrum (although not the same incidence) of novel tumors as Rb+/−; p53+/− and Rb+/−; p53−/− animals. These observations suggested that E2f3 mutation might promote the development of pRB-deficient tumors by reducing the activation of p53 and therefore reducing the need to functionally inactivate this protein. To test this hypothesis, we first compared p53 protein levels in size-matched, late-stage MTCs derived from 8 Rb+/−, 12 Rb+/−; E2f3+/−, and 9 Rb+/−; E2f3−/− animals (Fig. 5A and data not shown). Regardless of genotype, p53 was expressed at very low levels in most of the tumor samples. Although a small fraction of the tumor samples (2 of 8 Rb+/−, 3 of 12 Rb+/−; E2f3+/−, and 1 of 9 Rb+/−; E2f3−/− samples) expressed slightly higher levels of p53, we did not observe any significant differences between the various genotypes (Fig. 5A and data not shown).
FIG. 5.
Further characterization of tumors derived from Rb; E2f3 mice. (A) (Upper panels) Levels of p53 protein in MTCs derived from Rb+/− versus Rb+/−; E2f3−/− mice were assayed by Western blotting (WB) of whole-cell extracts derived from various tumors. Whole-cell lysates from wild-type mouse embryonic fibroblasts that had been subjected to 1 Gy of γ-irradiation (γ-irr. WT) and a lymphoma derived from a p53−/− mouse (p53−/−) were used as positive and negative controls, respectively. β-Tubulin was used as a loading control. α-p53, antibody against p53; α-Tub, antibody against β-tubulin. (Lower panel) The same cell extracts were also screened for the presence of p53 activity by EMSAs. The samples were incubated with a mutant competitor (lanes 1), a wild-type competitor (lanes 2), and/or a mutant competitor that included a p53-specific antibody (lanes 3). Arrowhead indicates the p53-DNA complex. (B) Numbers of Rb+/−, Rb+/−; E2f3+/−, and Rb+/−; E2f3−/− animals that had c-cell lesions or developed MTCs at the indicated ages according to histological analysis of serial sections of thyroids. (C) There was a considerable difference in the sizes of tumors in the various Rb; E2f3 genotypes. For example, thetumor in a 210-day-old Rb+/− animal was smaller than that in an age-matched Rb+/−; E2f3+/− mouse and closely resembled that of a 150-day-old Rb+/−; E2f3−/− animal. Magnification, ×40. (D) Analysis of p53, pRB, and pCalc (calcitonin gene product) expression in early c-cell adenomas of Rb+/−; E2f3−/− mice by immunohistochemistry. Solid arrowheads highlight the presence of low levels of p53 and high levels of pCalc, but the absence of pRB expression, in early adenomas. Open arrowheads indicate expression of p53, pRB, and pCalc in c-cells of the thyroid. Magnification, ×40.
To determine whether the p53 protein was functionally active, we used electrophoretic mobility shift assays (EMSAs) to screen for p53 DNA binding activity. For the majority of tumor samples, including those with the highest levels of p53, there was a direct correlation between p53 protein levels and DNA binding activity (Fig. 5A and data not shown). This was also true of the metastases that were assayed (data not shown). Although we cannot rule out the possibility that a low level of mutant p53 is present in a subset of these tumors, our data suggest that the majority of the MTCs and their metastases still maintain a low level of functional wild-type p53. These data suggest that the functional inactivation of p53 is not a prerequisite for the development or metastasis of the MTCs in Rb+/− mice. Thus, there is little reason to believe that E2F3 loss promotes the development of these tumors through the regulation of p53.
E2f3 mutation promotes the initiation of the MTC.
We next tested whether E2f3 status affected the development of pRB-deficient MTCs by influencing the time of onset. To address this question, Rb+/−, Rb+/−; E2f3+/−, and Rb+/−; E2f3−/− littermates were sacrificed at the age of 90, 150, 210, 240, or 270 days and their thyroids were screened for the presence of hyperplastic c-cell lesions or early tumors by serial sectioning (Fig. 5C and data not shown). Up to the age of 90 days, there was no evidence of lesions in any of the animals examined. However, by 150 days we observed significant differences in both the frequency and the size of tumors among the three genotypes. While tumors were not detected in the Rb+/− controls (0 of 4), they were present in half of the Rb+/−; E2f3+/− (3 of 6) and all of the Rb+/−; E2f3−/− (3 of 3) animals that we examined. However, two of the lesions in the Rb+/−; E2f3−/− mice were larger than those arising in their Rb+/−; E2f3+/− littermates (data not shown). C-cell tumors were first detected in Rb+/− mice at the age of 210 days. Importantly, these were much smaller than those arising in age-matched Rb+/−; E2f3+/− mice and were similar in size to the tumors of Rb+/−; E2f3−/− mice at the age of just 150 days. Immunohistochemistry confirmed that the early tumorigenic lesions of Rb+/−; E2f3−/− mice had all the hallmarks of pRB-deficient MTCs (Fig. 5D). The tumor stained positive for calcitonin, a specific marker of c-cells. Moreover, pRB was clearly expressed in the c-cells but not in the MTC, confirming that its loss is required for tumorigenesis. Finally, in agreement with our analysis of late-stage tumors, we detected low-level p53 staining in the early MTCs derived from Rb+/−; E2f3−/− mice and also in those from Rb+/− and Rb+/−; E2f3+/− mice (Fig. 5D and data not shown). Taken together, these data indicate that E2f3 acts in a dose-dependent manner to suppress the initiation of MTCs.
DISCUSSION
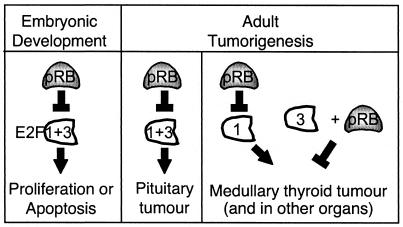
An E2f3 mutant mouse strain in which expression of E2F3a and E2F3b is disrupted has been described previously, and it has been shown that E2F3 contributes to the induction of cellular proliferation in both wild-type and pRB-deficient cells (14, 53). In this study, we have used Rb; E2f3 compound mutant mice to determine how E2F3 contributes to the development of pRB-deficient tumors. This analysis reveals considerable interplay between E2F3 and pRB in both normal development and tumorigenicity (Fig. 6).
FIG. 6.
Relative roles of pRB and E2F3 in both development and tumorigenesis.
E2F3 and pRB act in opposition to one another in normal development.
With regard to development, all of our findings support a simple model in which pRB and E2F3 act in direct opposition to one another (Fig. 6). Our data clearly show that mutation of a single Rb allele is sufficient to increase the fraction of surviving E2f3−/− neonates two- to threefold. Moreover, it also completely suppresses the congestive heart failure that is responsible for the deaths of most E2f3−/− adults (2; this study). This directly complements the previous finding that E2f3 mutation suppresses the inappropriate proliferation and apoptosis in pRB-deficient embryos and thereby greatly extends their life spans (37, 53). Significantly, many of the properties of E2F3 in normal development are shared by E2F1. The phenotypes of Rb−/−; E2f3−/− and Rb−/−; E2f1−/− embryos are similar to one another (42, 53). Moreover, the analysis of E2f1; E2f3 compound mutant mice shows that the developmental and age-related defects of the individual E2f1 or E2f3 mice are exacerbated by the mutation of the other E2f gene (2). Together, these data indicate that E2F1 and E2F3 play critical, overlapping roles in the development and maintenance of a variety of tissues and that pRB opposes the action of these factors. Importantly, this regulation appears to be critically dependent on the appropriate balance of pRB and E2F proteins, since a reduction in Rb or E2f gene dosage can either disrupt or restore development.
E2f3 acts as either an oncogene or a tumor suppressor in different Rb mutant tumors.
In contrast to their roles in developmental regulation, the relative roles of pRB and E2F3 in tumorigenesis appear highly complex. It is well documented that the retinoblastoma gene behaves as a classic tumor suppressor. Humans or mice carrying a germ line Rb mutation develop tumors with complete penetrance, and tumor development is accompanied by LOH (reviewed in reference 26). One significant difference is in the spectrum of tumors: retinoblastoma in humans versus pituitary and c-cell carcinomas in mice. In this study, we show that E2F3 loss in mice has a differential effect on the development of these specific tumor types. In the pituitary gland, the absence of E2F3 significantly suppressed tumor formation, resulting in an extension of life span. Thus, E2f3 acts as an oncogene in this tissue. In contrast, in c-cells, E2F3 loss clearly increased tumorigenicity, leading to the formation of highly aggressive carcinomas that metastasized to form secondary tumors within a wide variety of other tissues. Indeed, these MTCs and/or their metastases, and not pituitary tumors, were responsible for the deaths of a significant fraction of the Rb+/−; E2f3−/− animals. This represents the first evidence that E2F3 can collaborate with pRB to act as a tumor suppressor in vivo. This conclusion is supported by the finding that Rb+/−; E2f3−/− mice develop additional types of tumors that are not observed in the Rb+/− controls. Thus, in different tissues of a single animal, E2f3 acts as either an oncogene or a tumor suppressor (Fig. 6). This underscores the importance of tissue-specific roles for E2F3 and raises questions about the underlying mechanism of these opposing activities. Clearly, since the E2f3 mutation affects expression of both E2F3a and E2F3b, it is entirely possible that these opposing effects result from differential activities of the two E2F isoforms.
Before further discussion of potential mechanisms, it is important to consider what we now know about the tumorigenic properties of the various E2F family members. The concept that E2f genes could function as tumor suppressors was originally deduced from the analysis of E2f1 mutant mice (49, 50). This early study showed that both E2f1+/− and E2f1−/− mice had an increased propensity to develop tumors with late onset and low penetrance (50). It was subsequently shown that E2f2 mutant mice are tumor prone and that the combined mutation of E2f1 and E2f2 increases both the incidence and onset of tumorigenesis (52). Thus, at least in certain tissues, E2F1 and E2F2 have similar, additive roles in the suppression of tumors. In contrast, we have found that the mutation of E2f3, either alone or in combination with mutation of E2f1, or E2f4 has no detectable effect on tumorigenesis (2, 13). This initially suggested that tumor suppression might be a specific property of E2f1 and E2f2 but not of E2f3 or E2f4.
Significantly, the tumorigenic properties of the individual E2f genes appear quite different when analyzed in the context of the Rb+/− mutant background. In this setting, E2f1 clearly displays oncogenic, not tumor-suppressive properties. E2F1 loss suppresses the development of pRB-deficient brain tumors in a large T-antigen transgenic mouse model (29) and the formation of both pituitary and thyroid tumors in Rb+/− mice, yielding a dramatic extension of life span (49). In contrast, E2F3 loss has opposing effects on pituitary tumors (suppressing) and thyroid tumors (promoting), indicating that it is behaving as both an oncogene and a tumor suppressor. The phenotype of the Rb+/−; E2f4−/− mice adds to this complexity. E2f3 mutation suppresses the pituitary and thyroid tumors arising in Rb+/− mice more effectively than the loss of any other E2F tested to date (16). However, molecular analyses suggest that this suppression occurs via an indirect mechanism in which the absence of E2F4 allows its associated pocket proteins, p107 and p130, to bind, and presumably suppress the activity of, E2F1 and E2F3 (16). Thus, it appears that the complete loss of E2F3 has a different effect on the tumor spectrum of Rb+/− mice than restoration of the normal pocket protein regulation of the endogenous E2F1 and E2F3. We cannot rule out the possibility that the consequences of E2F3 loss result from changes in the regulation or activity of the remaining E2F proteins. However, together these observations show that the activating E2Fs (E2F1, -2, and -3) all have the ability to either promote or suppress tumorigenicity depending on the setting.
It is widely believed that the oncogenic activity of the activating E2Fs results from the known, shared (cell-autonomous) role of these proteins in the transcriptional activation of E2F-responsive genes and the induction of cellular proliferation (14, 47). In contrast, there is still considerable debate about the underlying basis for the E2Fs' tumor-suppressive activity. Several models, all of which are cell autonomous, have been proposed to account for the E2Fs' tumor-suppressive activity. One of the most popular is that this results in the known ability of the E2Fs to activate p53-dependent apoptosis (32, 38, 48). Since apoptosis requires a higher threshold level of E2F activity than the induction of cellular proliferation, this offers a simple explanation for the E2fs' ability to act as an oncogene (low levels induce proliferation) or a tumor suppressor (high levels induce apoptosis). Our analysis of Rb; E2f3 mutant tumors provides some insight into this model. Initially, our observation that Rb+/−; E2f3−/− animals develop the same novel tumor types as Rb+/−; p53+/− and Rb+/−; p53−/− mutant mice seemed to support it. Perhaps E2F3 loss promoted tumor development by reducing the requirement to inactivate p53-dependent apoptosis. However, our further analysis did not support this conclusion. Regardless of whether the MTCs and their metastases were isolated from Rb+/−, Rb+/−; E2f3+/−, or Rb+/−; E2f3−/− mice, the majority retained a low level of functional p53 protein. Thus, p53 inactivation is not a prerequisite for the development of MTCs or subsequent metastases, and E2f3 status does not appear to alter its frequency. This suggests that, at least in this specific tumor type, E2f3 does not exert its tumor suppressor effect by altering the need to inactivate p53. Obviously, this does not rule out the possibility that the apoptosis mechanism is operating in other tumor types, including the additional, rare tumors arising in Rb+/−; E2f3−/− mice that phenocopy those arising in Rb+/−; p53 mutant animals.
Importantly, our analysis of staged tumors strongly suggests that E2f3 status affects the rate of tumor onset. Specifically, tumorigenic lesions arise at a progressively earlier time in the thyroids of Rb+/−; E2f3−/−, Rb+/−; E2f3+/−, and Rb+/− mice. This observed acceleration of tumor onset is consistent with three other models of the E2Fs' tumor suppressor function. First, since the activating E2Fs bind pRB family members and their associated histone deacetylases, it is widely believed that they can participate in the repression of E2F-responsive genes (for an example, see reference 34). Second, E2F1 has also been implicated as a component of the ATM/ATR-Nbs1/Mre11 damage response pathway (20, 23, 24). Finally, it has recently been reported that E2F3 loss can lead to centrosome amplification, mitotic spindle defects, and aneuploidy (36). Significantly, the latter two mechanisms have been linked primarily to E2F1 and E2F3, respectively. This raises the possibility that the activating E2Fs might suppress tumor formation through distinct mechanisms.
The tumor-suppressive properties of E2f1 and E2f3 do have at least one, unusual characteristic in common. Analysis of tumors derived from E2f1+/− mice showed that there is no evidence of LOH of E2f1 (2, 50, 52). This is entirely consistent with the finding that E2f1+/− and E2f1−/− mice have similar spectra, incidences, and kinetics of tumor formation, and it strongly suggests that a reduction in E2F1 levels is sufficient to negate its tumor-suppressive properties. Here we show that the development of pRB-deficient thyroid tumors is promoted by the heterozygous mutation of E2f3 and that the primary and secondary tumors retain the wild-type E2f3 allele (in a mixed background). This is further supported by the fact that heterozygous mutation of E2f3 also increases the incidence of MTCs in Rb+/− mutant mice on a pure 129/Sv background (data not shown). Thus, in a manner analogous to that of E2f1, a mere reduction in E2f3 dosage is sufficient to impair tumor suppression. This is not a unique phenomenon, but it has been ascribed to only a small number of tumor suppressors (reviewed in references 3 and 33). Thus, the finding that this is a shared property of E2f1 and E2f3 supports the notion that they may suppress tumor formation via similar mechanisms.
Interestingly, the tumorigenicities of Rb+/−; E2f3+/− and Rb+/−; E2f3−/− animals are not equivalent. Mutation of the second E2f3 allele increases the aggressiveness of the MTC, broadens the tissue spectrum of the resulting metastases, and allows the formation of novel tumor types. There are two possible explanations for these different tumor phenotypes. E2f3 could simply act to suppress tumors in a dose-dependent manner. Alternatively, the heterozygous and homozygous mutations of E2f3 could promote tumorigenesis via different mechanisms. Indeed, it seems possible that E2f3 haploinsufficiency could promote tumor formation through a mechanism that is shared with E2F1 while complete loss of E2F3 might act through a unique mechanism, for example, centrosome amplification. Obviously, additional experiments will be required to address all potential models.
Regardless of the underlying mechanism, it now seems clear that the activating E2Fs (E2F1, -2, and -3) all display similar abilities to promote or suppress tumorigenicity. However, the observed behavior appears to be highly context dependent, in that it is greatly influenced by the absence or presence of pRB and the levels and/or activities of the remaining E2F species. The simplest explanation for this finding is that the individual E2Fs have both positive and negative functions that are integrated to determine the rate of cell proliferation and/or survival. In this manner, the changes in the individual E2Fs could either promote or suppress tumor formation depending on the balance of positive and negative activities in individual tissues.
Acknowledgments
We thank Alicia Caron for excellent technical support in the generation of histological sections and stainings. We are also extremely grateful for the gift of key reagents from Tyler Jacks (Rb mutant mice), David MacPherson (γ-irradiated fibroblasts), and Frances Connor (p53−/− tumor specimen). We thank members of the Lees lab for comments on the manuscript.
This work was supported by stipends to U.Z. from the DFG zi/98 and MERCK/MIT and by a grant to J.A.L. from the NIH (PO1-CA42063).
REFERENCES
- 1.Clarke, A. R., E. R. Maandag, M. van Roon, N. M. van der Lugt, M. van der Valk, M. L. Hooper, A. Berns, and H. te Riele. 1992. Requirement for a functional Rb-1 gene in murine development. Nature 359:328-330. [DOI] [PubMed] [Google Scholar]
- 2.Cloud, J. E., C. Rogers, T. L. Reza, U. Ziebold, J. R. Stone, M. H. Picard, A. M. Caron, R. T. Bronson, and J. A. Lees. 2002. Mutant mouse models reveal the relative roles of E2F1 and E2F3 in vivo. Mol. Cell. Biol. 22:2663-2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cook, W. D., and B. J. McCaw. 2000. Accommodating haploinsufficient tumor suppressor genes in Knudson's model. Oncogene 19:3434-3438. [DOI] [PubMed] [Google Scholar]
- 4.DeGregori, J., G. Leone, A. Miron, L. Jakoi, and J. R. Nevins. 1997. Distinct roles for E2F proteins in cell growth control and apoptosis. Proc. Natl. Acad. Sci. USA 94:7245-7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dyson, N. 1998. The regulation of E2F by pRB-family proteins. Genes Dev. 12:2245-2262. [DOI] [PubMed] [Google Scholar]
- 6.Friend, S. H., R. Bernards, S. Rogelj, R. A. Weinberg, J. M. Rapaport, D. M. Albert, and T. P. Dryja. 1986. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature 323:643-646. [DOI] [PubMed] [Google Scholar]
- 7.Fung, Y. K., A. L. Murphree, A. T'Ang, J. Qian, S. H. Hinrichs, and W. F. Benedict. 1987. Structural evidence for the authenticity of the human retinoblastoma gene. Science 236:1657-1661. [DOI] [PubMed] [Google Scholar]
- 8.Garcia, I., M. Murga, A. Vicario, S. J. Field, and A. M. Zubiaga. 2000. A role for E2F1 in the induction of apoptosis during thymic negative selection. Cell Growth Differ. 11:91-98. [PubMed] [Google Scholar]
- 9.Hanahan, D., and R. A. Weinberg. 2000. The hallmarks of cancer. Cell 100:57-70. [DOI] [PubMed] [Google Scholar]
- 10.Harrison, D. J., M. L. Hooper, J. F. Armstrong, and A. R. Clarke. 1995. Effects of heterozygosity for the Rb-1t19neo allele in the mouse. Oncogene 10:1615-1620. [PubMed] [Google Scholar]
- 11.Hsieh, J. K., S. Fredersdorf, T. Kouzarides, K. Martin, and X. Lu. 1997. E2F1-induced apoptosis requires DNA binding but not transactivation and is inhibited by the retinoblastoma protein through direct interaction. Genes Dev. 11:1840-1852. [DOI] [PubMed] [Google Scholar]
- 12.Hu, N., A. Gutsmann, D. C. Herbert, A. Bradley, W. H. Lee, and E. Y. Lee. 1994. Heterozygous Rb-1 delta 20/+ mice are predisposed to tumors of the pituitary gland with a nearly complete penetrance. Oncogene 9:1021-1027. [PubMed] [Google Scholar]
- 13.Humbert, P. O., C. Rogers, S. Ganiatsas, R. L. Landsberg, J. M. Trimarchi, S. Dandapani, C. Brugnara, S. Erdman, M. Schrenzel, R. T. Bronson, and J. A. Lees. 2000. E2F4 is essential for normal erythrocyte maturation and neonatal viability. Mol. Cell 6:281-291. [DOI] [PubMed] [Google Scholar]
- 14.Humbert, P. O., R. Verona, J. M. Trimarchi, C. Rogers, S. Dandapani, and J. A. Lees. 2000. E2f3 is critical for normal cellular proliferation. Genes Dev. 14:690-703. [PMC free article] [PubMed] [Google Scholar]
- 15.Jacks, T., A. Fazeli, E. M. Schmitt, R. T. Bronson, M. A. Goodell, and R. A. Weinberg. 1992. Effects of an Rb mutation in the mouse. Nature 359:295-300. [DOI] [PubMed] [Google Scholar]
- 16.Lee, E. Y., H. Cam, U. Ziebold, J. B. Rayman, J. A. Lees, and B. D. Dynlacht. 2002. E2F4 loss suppresses tumorigenesis in Rb mutant mice. Cancer Cell 2:463-472. [DOI] [PubMed] [Google Scholar]
- 17.Lee, E. Y., C. Y. Chang, N. Hu, Y. C. Wang, C. C. Lai, K. Herrup, W. H. Lee, and A. Bradley. 1992. Mice deficient for Rb are nonviable and show defects in neurogenesis and haematopoiesis. Nature 359:288-294. [DOI] [PubMed] [Google Scholar]
- 18.Lee, W. H., R. Bookstein, F. Hong, L. J. Young, J. Y. Shew, and E. Y. Lee. 1987. Human retinoblastoma susceptibility gene: cloning, identification, and sequence. Science 235:1394-1399. [DOI] [PubMed] [Google Scholar]
- 19.Lees, J. A., M. Saito, M. Vidal, M. Valentine, T. Look, E. Harlow, N. Dyson, and K. Helin. 1993. The retinoblastoma protein binds to a family of E2F transcription factors. Mol. Cell. Biol. 13:7813-7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin, W. C., F. T. Lin, and J. R. Nevins. 2001. Selective induction of E2F1 in response to DNA damage, mediated by ATM-dependent phosphorylation. Genes Dev. 15:1833-1844. [PMC free article] [PubMed] [Google Scholar]
- 21.Lukas, J., B. O. Petersen, K. Holm, J. Bartek, and K. Helin. 1996. Deregulated expression of E2F family members induces S-phase entry and overcomes p16INK4A-mediated growth suppression. Mol. Cell. Biol. 16:1047-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macleod, K. F., Y. Hu, and T. Jacks. 1996. Loss of Rb activates both p53-dependent and -independent cell death pathways in the developing mouse nervous system. EMBO J. 15:6178-6188. [PMC free article] [PubMed] [Google Scholar]
- 23.Maser, R. S., O. K. Mirzoeva, J. Wells, H. Olivares, B. R. Williams, R. A. Zinkel, P. J. Farnham, and J. H. Petrini. 2001. Mre11 complex and DNA replication: linkage to E2F and sites of DNA synthesis. Mol. Cell. Biol. 21:6006-6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meng, R. D., P. Phillips, and W. S. El-Deiry. 1999. p53-independent increase in E2F-1 expression enhances the cytotoxic effects of etoposide and of adriamycin. Int. J. Oncol. 14:5-14. [PubMed] [Google Scholar]
- 25.Morgenbesser, S. D., B. O. Williams, T. Jacks, and R. A. DePinho. 1994. p53-dependent apoptosis produced by Rb-deficiency in the developing mouse lens. Nature 371:72-74. [DOI] [PubMed] [Google Scholar]
- 26.Mulligan, G., and T. Jacks. 1998. The retinoblastoma gene family: cousins with overlapping interests. Trends Genet. 14:223-229. [DOI] [PubMed] [Google Scholar]
- 27.Murga, M., O. Fernandez-Capetillo, S. J. Field, B. Moreno, L. R. Borlado, Y. Fujiwara, D. Balomenos, A. Vicario, A. C. Carrera, S. H. Orkin, M. E. Greenberg, and A. M. Zubiaga. 2001. Mutation of E2F2 in mice causes enhanced T lymphocyte proliferation, leading to the development of autoimmunity. Immunity 15:959-970. [DOI] [PubMed] [Google Scholar]
- 28.Nikitin, A., and W. H. Lee. 1996. Early loss of the retinoblastoma gene is associated with impaired growth inhibitory innervation during melanotroph carcinogenesis in Rb+/− mice. Genes Dev. 10:1870-1879. [DOI] [PubMed] [Google Scholar]
- 29.Pan, H., C. Yin, N. J. Dyson, E. Harlow, L. Yamasaki, and T. Van Dyke. 1998. Key roles for E2F1 in signaling p53-dependent apoptosis and in cell division within developing tumors. Mol. Cell 2:283-292. [DOI] [PubMed] [Google Scholar]
- 30.Park, M. S., J. Rosai, H. T. Nguyen, P. Capodieci, C. Cordon-Cardo, and A. Koff. 1999. p27 and Rb are on overlapping pathways suppressing tumorigenesis in mice. Proc. Natl. Acad. Sci. USA 96:6382-6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phillips, A. C., S. Bates, K. M. Ryan, K. Helin, and K. H. Vousden. 1997. Induction of DNA synthesis and apoptosis are separable functions of E2F-1. Genes Dev. 11:1853-1863. [DOI] [PubMed] [Google Scholar]
- 32.Qin, X. Q., D. M. Livingston, W. G. Kaelin, Jr., and P. D. Adams. 1994. Deregulated transcription factor E2F-1 expression leads to S-phase entry and p53-mediated apoptosis. Proc. Natl. Acad. Sci. USA 91:10918-10922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quon, K. C., and A. Berns. 2001. Haplo-insufficiency? Let me count the ways. Genes Dev. 15:2917-2921. [DOI] [PubMed] [Google Scholar]
- 34.Rayman, J. B., Y. Takahashi, V. B. Indjeian, J. H. Dannenberg, S. Catchpole, R. J. Watson, H. te Riele, and B. D. Dynlacht. 2002. E2F mediates cell cycle-dependent transcriptional repression in vivo by recruitment of an HDAC1/mSin3B corepressor complex. Genes Dev. 16:933-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riley, D. J., C. C. Lai, C. Y. Chang, D. Jones, E. Y. Lee, and W. H. Lee. 1994. Susceptibility to tumors induced in mice by ethylnitrosourea is independent of retinoblastoma gene dosage. Cancer Res. 54:6097-6101. [PubMed] [Google Scholar]
- 36.Saavedra, H. I., B. Maiti, C. Timmers, R. Altura, Y. Tokuyama, K. Fukasawa, and G. Leone. 2003. Inactivation of E2F3 results in centrosome amplification. Cancer Cell 3:333-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saavedra, H. I., L. Wu, A. de Bruin, C. Timmers, T. J. Rosol, M. Weinstein, M. L. Robinson, and G. Leone. 2002. Specificity of E2F1, E2F2, and E2F3 in mediating phenotypes induced by loss of Rb. Cell Growth Differ. 13:215-225. [PubMed] [Google Scholar]
- 38.Shan, B., and W. H. Lee. 1994. Deregulated expression of E2F-1 induces S-phase entry and leads to apoptosis. Mol. Cell. Biol. 14:8166-8173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sherr, C. J. 1996. Cancer cell cycles. Science 274:1672-1677. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi, Y., J. B. Rayman, and B. D. Dynlacht. 2000. Analysis of promoter binding by the E2F and pRB families in vivo: distinct E2F proteins mediate activation and repression. Genes Dev. 14:804-816. [PMC free article] [PubMed] [Google Scholar]
- 41.Trimarchi, J. M., and J. A. Lees. 2002. Sibling rivalry in the E2F family. Nat. Rev. Mol. Cell. Biol. 3:11-20. [DOI] [PubMed] [Google Scholar]
- 42.Tsai, K. Y., Y. Hu, K. F. Macleod, D. Crowley, L. Yamasaki, and T. Jacks. 1998. Mutation of E2f-1 suppresses apoptosis and inappropriate S phase entry and extends survival of Rb-deficient mouse embryos. Mol. Cell 2:293-304. [DOI] [PubMed] [Google Scholar]
- 43.Tsai, K. Y., D. MacPherson, D. A. Rubinson, D. Crowley, and T. Jacks. 2002. ARF is not required for apoptosis in Rb mutant mouse embryos. Curr. Biol. 12:159-163. [DOI] [PubMed] [Google Scholar]
- 44.Vooijs, M., H. te Riele, M. van der Valk, and A. Berns. 2002. Tumor formation in mice with somatic inactivation of the retinoblastoma gene in interphotoreceptor retinol binding protein-expressing cells. Oncogene 21:4635-4645. [DOI] [PubMed] [Google Scholar]
- 45.Weinberg, R. A. 1992. The retinoblastoma gene and gene product. Cancer Surv. 12:43-57. [PubMed] [Google Scholar]
- 46.Williams, B. O., L. Remington, D. M. Albert, S. Mukai, R. T. Bronson, and T. Jacks. 1994. Cooperative tumorigenic effects of germline mutations in Rb and p53. Nat. Genet. 7:480-484. [DOI] [PubMed] [Google Scholar]
- 47.Wu, L., C. Timmers, B. Maiti, H. I. Saavedra, L. Sang, G. T. Chong, F. Nuckolls, P. Giangrande, F. A. Wright, S. J. Field, M. E. Greenberg, S. Orkin, J. R. Nevins, M. L. Robinson, and G. Leone. 2001. The E2F1-3 transcription factors are essential for cellular proliferation. Nature 414:457-462. [DOI] [PubMed] [Google Scholar]
- 48.Wu, X., and A. J. Levine. 1994. p53 and E2F-1 cooperate to mediate apoptosis. Proc. Natl. Acad. Sci. USA 91:3602-3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamasaki, L., R. Bronson, B. O. Williams, N. J. Dyson, E. Harlow, and T. Jacks. 1998. Loss of E2F-1 reduces tumorigenesis and extends the lifespan of Rb1+/− mice. Nat. Genet. 18:360-364. [DOI] [PubMed] [Google Scholar]
- 50.Yamasaki, L., T. Jacks, R. Bronson, E. Goillot, E. Harlow, and N. J. Dyson. 1996. Tumor induction and tissue atrophy in mice lacking E2F-1. Cell 85:537-548. [DOI] [PubMed] [Google Scholar]
- 51.Zhu, J. W., D. DeRyckere, F. X. Li, Y. Y. Wan, and J. DeGregori. 1999. A role for E2F1 in the induction of ARF, p53, and apoptosis during thymic negative selection. Cell Growth Differ. 10:829-838. [PubMed] [Google Scholar]
- 52.Zhu, J. W., S. J. Field, L. Gore, M. Thompson, H. Yang, Y. Fujiwara, R. D. Cardiff, M. Greenberg, S. H. Orkin, and J. DeGregori. 2001. E2F1 and E2F2 determine thresholds for antigen-induced T-cell proliferation and suppress tumorigenesis. Mol. Cell. Biol. 21:8547-8564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ziebold, U., T. Reza, A. Caron, and J. A. Lees. 2001. E2F3 contributes both to the inappropriate proliferation and to the apoptosis arising in Rb mutant embryos. Genes Dev. 15:386-391. [DOI] [PMC free article] [PubMed] [Google Scholar]