Abstract
The Werner syndrome protein (WRN) is a caretaker of the human genome, and the Abl kinase is a regulator of the DNA damage response. Aberrant DNA repair has been linked to the development of cancer. Here, we have identified a direct binding between WRN and c-Abl in vitro via the N-terminal and central regions of WRN and the Src homology domain 3 of c-Abl. After bleomycin treatment in culture, WRN and c-Abl are dissociated and followed by an Abl kinase-dependent WRN relocalization to the nucleoplasm. WRN is a substrate of c-Abl in vitro and in vivo. WRN is tyrosine phosphorylated either transiently by treatment of HeLa cells with bleomycin or constitutively in cells from chronic myeloid leukemia (CML) patients, and these phosphorylations are prevented by treatment with the Abl kinase inhibitor STI-571. Tyrosine phosphorylation of WRN results in inhibition of both WRN exonuclease and helicase activities. Furthermore, anti-WRN immunoprecipitates from CML cells treated with STI-571 show increased 3′→5′ exonuclease activity. These findings suggest a novel signaling pathway by which c-Abl mediates WRN nuclear localization and catalytic activities in response to DNA damage.
The human WRN gene encodes a multifunctional nuclear protein, WRN, which belongs to the RecQ family of DNA helicases (54). WRN possesses 3′→5′ helicase, 3′→5′ exonuclease, and DNA-dependent ATPase activities (17, 18). The human syndromes defective in the RecQ helicase genes, including Werner, Bloom, and Rothmund-Thomson syndromes, are autosomal recessive disorders that share a common feature of genomic instability associated with segmental progeroid and cancer predisposition (27). However, Werner syndrome (WS) displays more symptoms of normal aging than others and is considered a model system for segmental progeroid (26). WS cells are hypersensitive to certain DNA cross-linking therapeutic drugs and, to a lesser extent, ionizing radiation (33, 34, 52). WS cells also show defects in resolving recombinational intermediates and in maintaining the stability of broken DNA ends (31, 35, 37). It has been shown that WRN relocalizes from the nucleolus to the nucleoplasm in response to DNA damage (16, 38); however, the mechanism of this WRN trafficking is not understood. A number of protein partners of WRN have been identified. WRN interacts physically and functionally with replication protein A, Ku70, Ku80, the catalytic subunit of DNA-PK (DNA-PKCS), p53, DNA polymerase δ, Bloom syndrome protein (BLM), and FEN-1 (reviewed in reference 5). The known functions of these proteins suggest that WRN is likely involved in pathways of DNA replication, recombination, and DNA repair.
The nuclear form of the ubiquitously expressed c-Abl tyrosine kinase is activated by genotoxic stress, including DNA double strand breaks and cross-links (23). In response to ionizing radiation, the ataxia telangiectasia mutated (ATM) kinase activates nuclear c-Abl (3, 43). Another pathway of c-Abl activation is mediated via interaction with and phosphorylation by DNA-PK, a complex containing DNA, Ku70, Ku80, and DNA-PKCS (20, 22). Activation of the nuclear c-Abl by DNA damage contributes to apoptosis by mechanisms that partly depend on p53, p73, and Rad9 (1, 15, 53, 55, 57). In addition, cellular responses to DNA damage involve interaction of c-Abl with DNA repair proteins, including Rad51, Rad52, BRCA1, and a UV-damaged DNA binding protein (9, 14, 24, 56). Tyrosine phosphorylation by c-Abl plays important regulatory roles in the DNA damage response. Phosphorylation of Rad51 by c-Abl inhibits its strand exchange activity (56), and phosphorylation of DNA-PKCS by c-Abl dissociates the Ku heterodimer from DNA-PK (20, 22). Recently, an ATM-dependent BRCA1 phosphorylation by c-Abl has been identified (14). Thus, c-Abl appears to function in mediating pathways involved in homologous recombination and/or nonhomologous end-joining (NHEJ).
Almost all patients with chronic myeloid leukemia (CML) and a subset of those with acute lymphocytic leukemia carry the constitutively activated BCR-ABL tyrosine kinase, a fusion product of a reciprocal chromosome translocation (40). Structurally, c-Abl tyrosine kinase activity is self-suppressed by its N-terminal sequence (32). For BCR-ABL, this inhibitory sequence is removed, resulting in an uncontrolled, constitutive activation of its tyrosine kinase activity that contributes to CML. BCR-ABL is antiapoptotic when it resides in the cytoplasm but becomes proapoptotic when it binds to the compound STI-571 (Gleevec or Imatinib) and, in turn, promotes the BCR-ABL relocalization to the nucleus (48). The 2-phenylaminopyrimidine derivative STI-571 binds to the kinase domain of the Abl kinase with an exceptionally high affinity, resulting in an inhibition of its tyrosine kinase activity (42). Cells expressing BCR-ABL show increased chromosomal aberrations and decreased levels of DNA-PKCS (12). In contrast, expression of BCR-ABL in mouse myeloid cells stimulates Rad51 expression and DNA recombinational repair (46). Therefore, understanding the molecular mechanisms of DNA metabolism in CML may provide insight into strategies for therapeutic intervention.
While WRN can be serine/threonine phosphorylated by DNA-PK (21, 52), there has been no report of tyrosine phosphorylation of WRN. Recent observations show that, in K562 lymphoblasts from CML patients, WRN distributes throughout the nucleus instead of the nucleolus, where WRN primarily resides in normal cells (38). Therefore, Abl tyrosine kinase may play a specific role in modulating the biological function of WRN. Increasing evidence suggests that WRN plays a role in recombinational repair (34, 37). In addition, nuclear c-Abl is also likely to be involved in this process because it acts as a negative regulator of Rad51, Rad52, and BRCA1, three proteins that promote recombinational repair (14, 24, 56). Both WS and CML cells show increased chromosomal aberrations (5, 12). However, in contrast to WS cells, CML cells are resistant to the DNA cross-linker mitomycin C and to gamma irradiation (46). We thus hypothesized that WRN may interact with the Abl tyrosine kinase in the DNA damage response. Here, we provide evidence for a functional and physical interaction between WRN and c-Abl, including WRN relocalization in response to DNA damage, suggesting that this protein-protein interaction participates in a shared pathway of genome surveillance.
MATERIALS AND METHODS
Cell cultures and proteins.
Human HeLa, WS (AG11395), and 293T cells and mouse wild-type and c-Abl−/− embryonic fibroblasts (MEFs) were cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum, 50 U of penicillin/ml, and 50 μg of streptomycin/ml. K562 and Ramos lymphoblasts were cultured in RPMI medium 1640 containing 10% fetal bovine serum and antibiotics. The human cells were from the American Type Culture Collection (Manassas, Va.) or Coriell Cell Repositories (Camden, N.J.). The bacterially expressed glutathione S-transferase (GST)-WRN fragments (6, 50) and GST-c-Abl-Src homology domain 2 (SH2) and SH3 fragments (53) and the purified histidine-tagged WRN (30) and c-Abl (53) from a baculovirus insect expression system were prepared as previously described. The GST-Crk (amino acids [aa] 120 to 225) and GST-Crk (aa 120 to 212, lacking a c-Abl consensus sequence) fragments were used as controls for kinase assays (36). The c-Abl fragment (45 kDa, containing the SH2 and kinase domains) (Fig. 1D) and the leukocyte antigen-related tyrosine phosphatase (LAR) were purchased from New England BioLabs (Beverly, Mass.). In some experiments, HeLa cells were incubated with bleomycin (Sigma, St. Louis, Mo.) in serum-free media as indicated.
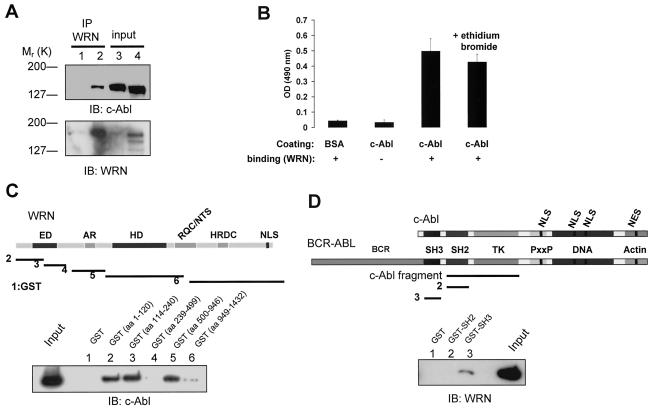
FIG. 1.
WRN interacts with c-Abl. (A) Lysates from WS (lanes 1 and 3) and control (lanes 2 and 4) fibroblasts were immunoprecipitated (IP) with polyclonal anti-WRN antibodies (lanes 1 and 2). The immunoprecipitates were analyzed by immunoblotting (IB) with anti-Abl and anti-WRN antibodies. Lysates containing 10% of the amount for immunoprecipitation were loaded as the input controls (lanes 3 and 4). (B) Direct interaction between WRN and c-Abl by ELISA. The purified full-length c-Abl was coated onto ELISA plates and followed by incubation with purified WRN. Rabbit antibodies against WRN (Ab200; Novus) were used to detect the bound WRN. Values are means ± standard errors (n = 3). OD, optical density; +, present; −, absent. (C) Known structural motifs and domains of WRN. ED, exonuclease domain; AR, acidic regions; HD, helicase domain; RQC/NTS, RecQ C-terminal/nucleolar targeting sequence; HRDC, helicase-related domain; NLS, nuclear localization signal. Bacterially expressed GST and GST-WRN fragments were bound to glutathione beads and incubated with 100 ng of purified c-Abl in the presence of ethidium bromide. After extensive washing, proteins were eluted, separated, and analyzed by IB with anti-Abl antibody. The input lane was loaded with 10 ng of c-Abl protein. (D) Schematic diagram of c-Abl domains in c-Abl and BCR-ABL proteins. TK, tyrosine kinase; PxxP, PXXP motif; DNA and actin, DNA- and actin-binding domains; NLS, nuclear localization signal; NES, nuclear export signal. The c-Abl fragment used for the in vitro kinase and WRN catalytic activity assays contains SH2 and TK domains. The binding assays were conducted as described except that 100 ng of WRN protein was used and followed by IB with anti-WRN antibody.
Immunoprecipitation and immunoblot analysis.
Cells were washed in ice-cold phosphate-buffered saline and lysed in a lysis buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1% NP-40, 5 mM EDTA) containing leupeptin and aprotinin at 10 μg/ml, 1 mM phenylmethylsulfonyl fluoride, 10 mM NaF, and tyrosine phosphatase inhibitors (cocktail II, 1:100; Sigma) for 30 min on ice. The suspension was centrifuged at 20,000 × g for 20 min at 4°C. The supernatants (soluble fraction) were separated from the pellets, which were resuspended (1/3 volume of the soluble fraction) and sonicated in a sonication buffer (50 mM Tris-HCl [pH 8], 450 mM NaCl, 1% Tween-20, 5 mM EDTA, 15 mM MgCl2, and proteinase and phosphatase inhibitors). After centrifugation, the supernatant was transferred and 2 volumes of the sonication buffer without NaCl were added to generate the insoluble fraction. Equal amounts of soluble and insoluble fraction were combined to generate the whole-cell extract. The precleared lysates were immunoprecipitated with rabbit anti-WRN (H-300; Santa Cruz, Santa Cruz, Calif.) or mouse monoclonal anti-Abl (Ab-3; Oncogene Science, San Diego, Calif.) antibodies. The protein G Sepharose-precipitated proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinylidene difluoride membranes, and analyzed by immunoblotting with anti-WRN (clone 30; BD Biosciences, Lexington, Ky.), anti-Abl (8E9; BD Biosciences), or anti-phosphotyrosine (4G10; Cell Signaling Technology, Beverly, Mass.) monoclonal antibodies followed by the chemiluminescent method for detection with SuperSignal substrates (Pierce, Rockford, Ill.). Membranes were stripped by using the Restore Western blot stripping buffer (Pierce) when necessary.
Immunofluorescence.
Exponentially growing HeLa cells on glass coverslips were treated with bleomycin (40 μg/ml) for 0 to 30 min. Some cells were pretreated with STI-571 (1 μM) for 24 h as indicated. Cells were washed with phosphate-buffered saline, fixed with ice-cold 3.7% paraformaldehyde for 10 min, permeabilized with 0.3% Triton X-100 at room temperature for 10 min, and incubated with rabbit antiserum against WRN (ab200; Novus, Littleton, Colo.) and mouse anti-nucleolin antibody (MS-3; Santa Cruz). 4′,6′-Diamidino-2-phenylindole (DAPI) was used to stain the nuclei. The immunostaining was visualized by a fluorescence microscope (Zeiss Axiovert 200 M), and images were processed by using deconvolution with the software AxioVision, version 3.1.
GST pull-down experiments and ELISA.
The GST-WRN and GST-c-Abl fragments and GST were incubated with purified c-Abl and WRN, respectively, and the adsorbents were analyzed by SDS-PAGE as described previously (6). The membrane was analyzed by immunoblotting with the monoclonal anti-Abl or anti-WRN antibody, followed by amido black staining. Enzyme-linked immunosorbent assays (ELISA) were performed as previously described (50). Briefly, either bovine serum albumin (BSA) or full-length c-Abl (0.6 pmol) was coated onto 96-well plates and incubated with full-length WRN (1.2 pmol) in the presence or absence of ethidium bromide (20 ng/μl) for 2 h at 37°C. After extensive washing, the bound WRN was detected with anti-WRN antibodies (Novus) and followed by horseradish peroxidase-conjugated secondary antibody detection.
In vitro kinase assay and in vivo phosphorylation.
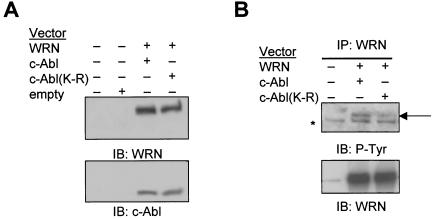
For in vitro kinase assays, the affinity-purified WRN and GST-Crk (aa 120 to 225 or 120 to 212) were incubated with the c-Abl fragment in the presence of [γ-32P]ATP in a kinase buffer (50 mM Tris-HCl, 10 mM MgCl2, 1 mM EGTA, 2 mM dithiothreitol) for 15 min at 28°C. Either the tyrosine phosphatase LAR or the c-Abl kinase inhibitor STI-571 (Novartis Pharma AG) (13) was used as a control. For immunocomplex kinase assays, the anti-c-Abl immunoprecipitates from MEFs were added with WRN and incubated in the presence of the phosphatidylinositol-3 kinase inhibitor wortmannin (100 nM) and [γ-32P]ATP in a kinase buffer (53). Alternatively, the wortmannin (100 nM)-treated anti-WRN immunoprecipitates were incubated with [γ-32P]ATP in the kinase buffer. Phosphorylated proteins were separated by SDS-PAGE and analyzed by autoradiography and immunoblotting. For in vivo phosphorylation, 293T cells (2.4 × 106 cells/10-cm-diameter culture dish) were cotransfected with 1.5 μg of WRN (16) and 6 μg of wild-type c-Abl or the dominant-negative c-Abl (K-R) mutant vectors (41) for 30 h with the PolyFect reagent (Qiagen, Valencia, Calif.). The cells were lysed in the lysis buffer and followed by SDS-PAGE and immunoblotting to detect phosphotyrosine, c-Abl, and WRN.
WRN activities.
Helicase and exonuclease activity assays were conducted as previously described (29). For assays with purified proteins, the c-Abl fragment was preincubated with WRN in a reaction buffer (40 mM Tris-HCl [pH 8.0], 4 mM MgCl2, 5 mM dithiothreitol, 2 mM ATP, 0.1 mg of BSA/ml) for 5 min at 28°C prior to the addition of the 22- or 34-bp forked duplex (5′ end-labeled with 32P) as a substrate for measuring WRN helicase or exonuclease, respectively. For assays with immunocomplexes, the anti-immunoglobulin G (IgG) or anti-WRN (Santa Cruz) immunoprecipitates (200 μg of protein) were washed three times in lysis buffer and two times in the WRN activity reaction buffer, followed by the addition of the exonuclease substrate. Helicase and exonuclease products were run on 12% native and 14% denaturing polyacrylamide gels, respectively, visualized with a PhosphorImager, and quantitated by using ImageQuant 5.2 software. For exonuclease assays, the percent digestion was calculated based on the following formula: (intensity of the digested bands/intensity of the digested plus undigested bands) × 100%. Statistics were performed by using the t test.
RESULTS
Physical interaction between WRN and c-Abl.
We first investigated whether WRN interacts with c-Abl by coimmunoprecipitation experiments. Analyses of anti-WRN immunoprecipitates by immunoblotting with an anti-Abl antibody demonstrated an association between WRN and c-Abl in normal, but not in WS, fibroblasts (Fig. 1A, lanes 1 to 2). The level of c-Abl protein was similar in these two cell lines (Fig. 1A, lanes 3 to 4). We also detected an association between WRN and c-Abl in primary normal human fibroblasts, AG11076 (data not shown). Thus, WRN and c-Abl are coimmunoprecipitated from cell lysates, suggesting that they are associated in the same complex in vivo.
To determine whether WRN physically associates with c-Abl, we performed ELISA with His-tagged purified full-length proteins (Fig. 1B). The results demonstrated that WRN bound c-Abl directly. Because the interaction was unaffected by the presence of ethidium bromide, this binding was not dependent on DNA. As a control, WRN did not bind to BSA. Therefore, WRN binds to c-Abl directly in vitro.
To map this direct binding, we incubated purified c-Abl with bacterially expressed WRN fragments fused to GST. Analyses of the adsorbates demonstrated that c-Abl bound to the N-terminal GST-WRN (aa 1 to 120 and 114 to 240) and the central GST-WRN (aa 500 to 946) fragments but not to the acidic GST-WRN (aa 239 to 499), the C-terminal GST-WRN (aa 949 to 1432) fragment, or GST alone (Fig. 1C). To map the region of c-Abl that binds to WRN, we incubated purified WRN with two c-Abl signaling domains, SH2 and SH3, fused to GST. Analyses of the adsorbates demonstrated that WRN bound to the GST-c-Abl-SH3 domain but not to the GST-c-Abl-SH2 domain or GST alone (Fig. 1D). Equal amounts of GST and the GST fragments for incubation were confirmed by amido black staining (data not shown). Taken together, our data indicate that c-Abl binds to two regions of WRN containing the exonuclease and helicase domains, and WRN binds to the SH3 domain of c-Abl.
WRN is tyrosine phosphorylated and dissociates from c-Abl after bleomycin treatment followed by WRN relocalization.
Next, we were interested in exploring whether the cellular association between WRN and c-Abl differs during the process of a cellular DNA damage response pathway. The radiomimetic therapeutic agent bleomycin leads to DNA strand breaks, WRN serine/threonine phosphorylation, and WRN relocalization (21, 22, 38). We therefore tested whether the cellular association between WRN and c-Abl is affected by this compound. Treatment of HeLa cells with bleomycin at 10 μg/ml resulted in a time-dependent decrease of the amount of WRN in the anti-c-Abl immunoprecipitates (Fig. 2A, left panels). When HeLa cells were treated with a higher dose of bleomycin (40 μg/ml), we found a complete disruption of the association between WRN and c-Abl 10 min after the treatment (Fig. 2A, right panels). c-Abl protein levels in the immunoprecipitates remained unchanged, except for those from cells at 60 min after the high-dose bleomycin treatment (Fig. 2A). Thus, bleomycin treatment disrupts the in vivo association between WRN and c-Abl. In comparison with the input control, it is estimated that about 10% of WRN is c-Abl associated in the unstressed cells.
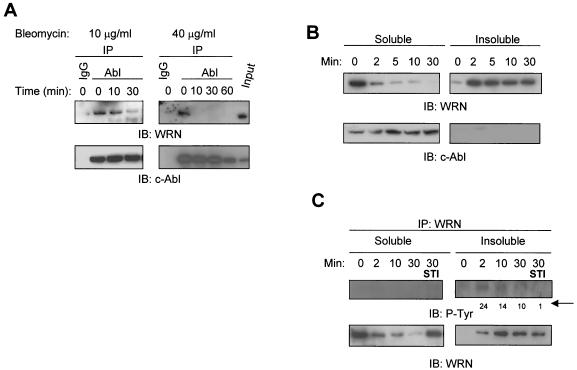
FIG.2.
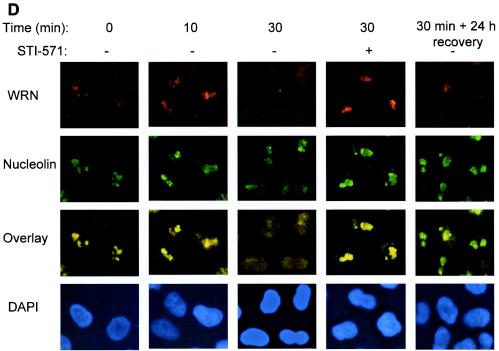
Bleomycin-induced WRN tyrosine phosphorylation and dissociation from c-Abl and c-Abl tyrosine kinase-dependent WRN relocalization. (A) HeLa cells were incubated with bleomycin (10 or 40 μg/ml for 0 to 60 min). Soluble cell lysates were immunoprecipitated with anti-immunoglobulin G (IgG) or anti-Abl antibodies. The anti-Abl immunoprecipitates (IP) or input (10% of the amount for IP) were analyzed by immunoblotting (IB) with anti-WRN antibodies and anti-Abl antibodies. (B and C) HeLa cells were incubated with bleomycin (40 μg/ml) with or without STI-571 pretreatment (1 μM, 24 h), and the soluble and insoluble lysates were IB with anti-WRN or anti-Abl antibodies. Alternatively, the anti-WRN IPs were analyzed by IB by using anti-phosphotyrosine (P-Tyr) antibodies followed by anti-WRN antibodies. The numbers indicated by an arrow are relative intensities of the bands. (D) HeLa cells were pretreated in the absence or presence of STI-571 (1 μM, 24 h) and then incubated with bleomycin (40 μg/ml) for 0 to 30 min. Some cells were replaced with normal medium 30 min after the bleomycin treatment and were stained 24 h thereafter. The cells were costained with rabbit anti-WRN (red) (Novus) and mouse anti-nucleolin (green) (MS-3; Santa Cruz) antibody and were visualized by a fluorescent microscope. The nuclei were stained with DAPI. +, present; −, absent.
WRN relocalizes from the nucleolus to the nucleoplasm in response to cellular exposure of various DNA-damaging agents (38). To link the events between WRN relocalization and the bleomycin-induced dissociation of WRN and c-Abl, we tested for distribution of WRN by immunoblotting in a time course study. We found that almost all of the WRN protein was in the soluble fraction in the untreated cells (Fig. 2B). However, most of WRN was found in the insoluble fraction as early as 2 min following the bleomycin treatment (40 μg/ml). In contrast, c-Abl was found only in the soluble fraction both in the absence and presence of bleomycin. To further extend this observation, we assayed for WRN tyrosine phosphorylation in the soluble and insoluble fractions of cells before and after bleomycin treatment (40 μg/ml). In the soluble fraction, no tyrosine-phosphorylated WRN was detected at the 2-min time point (Fig. 2C). In the insoluble fraction, tyrosine-phosphorylated WRN was found at 2 min and the intensity was decreased thereafter. Remarkably, pretreatment of cells with STI-571 inhibited WRN tyrosine phosphorylation. In the anti-WRN immunoprecipitates, WRN was mainly found in the soluble fraction from untreated cells or in the insoluble fraction from bleomycin-treated cells.
Next, we tested for the location of the endogenous WRN by immunostaining in a time course study. While nucleolin remains in the nucleolus regardless of the bleomycin treatment (40 μg/ml), a proportion of WRN was found in the nucleoplasm at 10 min, followed by a significant WRN translocation at 30 min (Fig. 2D). Twenty-four hours after removing bleomycin, a significant proportion of the nucleoplasmic WRN returned to the nucleolus (Fig. 2D), and there was no detectable WRN tyrosine phosphorylation in either the soluble or the insoluble fraction (data not shown). Strikingly, pretreatment of cells with STI-571 (1 μM, 24 h) attenuated the bleomycin-induced WRN relocalization, suggesting a functional link between the DNA damage-induced nuclear c-Abl kinase activity and WRN relocalization (Fig. 2D). We observed that, at 10 min after bleomycin treatment, the majority of WRN was found in the insoluble fraction and in the nucleolus by immunoblotting and immunofluorescence methods, respectively. It is likely that the tyrosine-phosphorylated WRN is released during the process of soluble protein extraction. Collectively, these results suggest the following sequence of events in response to DNA damage: (i) WRN is tyrosine phosphorylated, (ii) it dissociates from c-Abl, and (iii) it relocalizes from the nucleolus to the nucleoplasm.
WRN is tyrosine phosphorylated by c-Abl in vitro and in vivo.
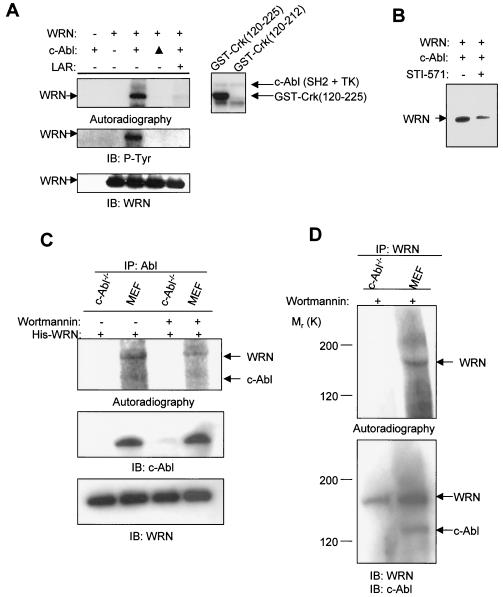
To determine whether WRN is a substrate for c-Abl, we incubated purified WRN with a c-Abl fragment containing the SH2 and tyrosine kinase domains (Fig. 1D, map) in the presence of [γ-32P]ATP. Analysis of the products by autoradiography showed that c-Abl tyrosine kinase phosphorylated WRN in vitro, whereas there was no detectable phosphorylation of WRN when the c-Abl was heat inactivated or when the phosphorylation reactions were reversed by incubation with LAR (Fig. 3A, upper left panel). Immunoblotting with the same membrane demonstrated that WRN was tyrosine phosphorylated and that total WRN protein levels were not affected by the treatment (Fig. 3A, middle and bottom panels). The c-Abl fragment was autophosphorylated and was resistant to the tyrosine phosphatase treatment (data not shown). As positive and negative controls, the c-Abl fragment phosphorylated GST-Crk (aa 120 to 225) but not the GST-Crk (aa 120 to 212), which does not contain tyrosine phosphorylation sites (Fig. 3A, right panel). The WRN phosphorylation by c-Abl was also attenuated in the presence of STI-571 (Fig. 3B).
FIG. 3.
c-Abl phosphorylates WRN in vitro. (A) Kinase assays were conducted by incubating purified WRN protein (left panels) or GST-Crk (aa 120 to 225 or 120 to 212) (right panel) with the c-Abl fragment (Fig. 1D) as described in Materials and Methods. The phosphorylated proteins were separated, transferred to a polyvinylidene difluoride membrane, and followed by autoradiography. After satisfactory exposures, the membrane was used for immunoblotting (IB) to detect phosphotyrosine and WRN. ▴, heat-inactivated c-Abl; LAR, a tyrosine phosphatase; +, present; −, absent. (B) Kinase assays were conducted with purified WRN and the c-Abl kinase in the presence of STI-571 (50 nM). (C) Kinase assays were conducted with anti-Abl immunoprecipitates (IP) from c-Abl−/− and wild-type MEFs, with the addition of the purified WRN protein, in the absence or presence of wortmannin (100 nM). Autoradiography and immunoblotting were performed by using the respective antibodies against individual proteins. (D) Kinase assays were conducted by using anti-WRN immunoprecipitates from c-Abl−/− and wild-type MEFs in the presence of wortmannin (100 nM) followed by autoradiography and immunoblotting with the respective antibodies.
To confirm that WRN is a substrate of full-length c-Abl and to determine whether WRN is tyrosine-phosphorylated in a c-Abl-dependent manner in cell extracts, we conducted immunocomplex kinase assays with cell lysates from wild-type and c-Abl−/− MEFs. Because both c-Abl and WRN are associated with DNA-PK (21, 22, 52), we excluded the contribution of DNA-PK to the WRN phosphorylation by adding wortmannin to the kinase reactions. Analysis of the products by autoradiography showed that the anti-c-Abl immunoprecipitates from wild-type MEFs, but not from c-Abl−/− MEFs, were able to phosphorylate purified WRN protein (Fig. 3C). Adding wortmannin attenuated the WRN phosphorylation in the immunoprecipitates from wild-type MEFs, suggesting that DNA-PK and possibly other members of the phosphatidylinositol-3 kinase family were in the complex containing c-Abl and WRN. To confirm the c-Abl-dependent WRN phosphorylation, kinase assays were conducted by using anti-WRN immunoprecipitates from both MEFs in the presence of wortmannin. WRN was phosphorylated in the anti-WRN immunoprecipitates from the wild-type MEFs but not from the c-Abl−/− MEFs (Fig. 3D). These results demonstrate a c-Abl-dependent WRN phosphorylation with anti-c-Abl or anti-WRN immunocomplexes from cell extracts.
To test whether WRN is phosphorylated by c-Abl in vivo, we cotransfected 293T cells with expression vectors for WRN and wild-type c-Abl or the kinase-inactive c-Abl(K-R) mutant. Results from immunoblotting showed similar amounts of c-Abl protein and of WRN protein in the different cotransfected cells (Fig. 4A). Analysis of anti-WRN immunoprecipitates with an anti-phosphotyrosine antibody demonstrated WRN tyrosine phosphorylation by c-Abl, but this phosphorylation was dramatically reduced in cells expressing the kinase-inactive c-Abl (K-R) (Fig. 4B). Some bands are nonspecific, as they appear at comparable amounts in all three lanes. Therefore, WRN is an in vivo substrate of c-Abl.
FIG. 4.
c-Abl phosphorylates WRN in vivo. (A) 293T cells were cotransfected with WRN and c-Abl or c-Abl(K-R) expression vectors. Cell lysates were immunoblotted (IB) with anti-WRN and anti-Abl antibodies. (B) The anti-WRN immunoprecipitates (IP) were subjected to immunoblotting with antibodies against phosphotyrosine and WRN. The arrow indicates the tyrosine-phosphorylated WRN, and the star indicates a cross-reacting nonspecific protein. +, present; −, absent.
WRN protein levels and tyrosine phosphorylation in CML.
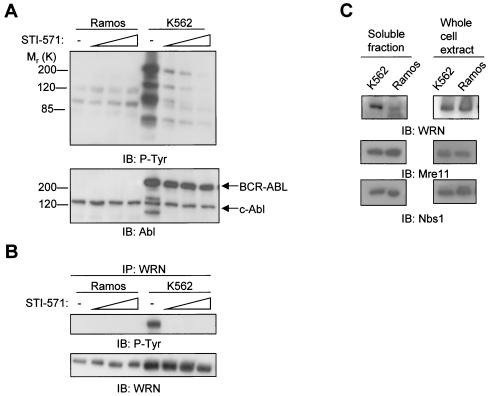
The Abl kinase-active K562 lymphoblasts from CML patients, which contain a BCR-involved chromosomal translocation, were compared with the control Ramos (Burkitt lymphoma) lymphoblasts, which contain a Myc-involved chromosomal translocation. Western analyses demonstrated STI-571 dose-dependent inhibition of protein tyrosine phosphorylation in K562, but not in Ramos lymphoblasts (Fig. 5A, upper panel), confirming the specificity of this drug for inhibiting BCR-ABL kinase-dependent tyrosine phosphorylation. BCR-ABL was expressed only in K562 cells, whereas c-Abl was expressed at a comparable level between these two cell lines (Fig. 5A, bottom panel). To determine whether WRN is tyrosine phosphorylated in the K562 cells and whether this event is reversible, lysates were prepared from the two cell lines with or without STI-571 pretreatment. Analyses of anti-WRN immunoprecipitates demonstrated that WRN was tyrosine phosphorylated only in K562 cells and that STI-571 treatment resulted in a disappearance of WRN tyrosine phosphorylation (Fig. 5B). WRN was not tyrosine phosphorylated in Ramos cells. The STI-571 treatment did not change the WRN protein level in either cell line (Fig. 5B). However, there was a much greater amount of WRN protein in the soluble fraction of K562 than in Ramos cells (Fig. 5C, left panels). Therefore, a threefold-greater amount of protein from Ramos cells was used for immunoprecipitation in an attempt to equalize the WRN protein level between the two cell lines. Neither the Mre11 exonuclease nor the nucleoplasmic Nbs1 protein levels differed between these two cells. However, Western analysis with whole-cell lysates showed comparable amounts of WRN, Mre11, and Nbs1 proteins between the two cell lines (Fig. 5C, right panels).
FIG. 5.
Levels of Abl and WRN proteins and the effects of STI-571 on total protein and WRN tyrosine phosphorylation in K562 and Ramos lymphoblasts. (A) Ramos and K562 lymphoblasts were treated with STI-571 (0, 0.6, 1.3, or 2.5 μM) (53) for 24 h. The lysates (50 μg of protein) were subjected to immunoblotting (IB) with antibodies against phosphotyrosine and Abl. (B) The lysates were immunoprecipitated (IP) with anti-WRN antibodies followed by immunoblotting with the respective antibodies. (C) Lysates from soluble fractions (left panels) and whole-cell extracts (right panels) of the untreated K562 and Ramos lymphoblasts were subject to immunoblotting with antibodies against WRN, Mre11, or Nbs1. −, absent.
Tyrosine phosphorylation of WRN inhibits its catalytic activities in vitro and in CML cells.
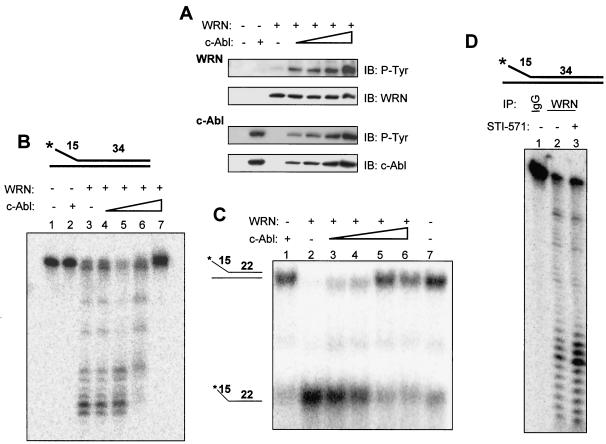
The c-Abl fragment (containing the SH2 and kinase domain) (Fig. 1D) phosphorylated WRN (Fig. 3A) without forming a stable association with it (data not shown). This c-Abl fragment was thus used in an experiment to determine whether tyrosine phosphorylation on WRN affects its catalytic activities. In this way, potential confounding effects of the constitutive interaction of WRN with full-length c-Abl would be eliminated. We preincubated the purified WRN protein with the c-Abl fragment prior to the addition of WRN substrates. Immunoblotting demonstrated that WRN was tyrosine phosphorylated under the buffer conditions used for the WRN exonuclease (Fig. 6A) or helicase activity (data not shown). There was a c-Abl dose-dependent increase of WRN tyrosine phosphorylation while the amounts of WRN protein were comparable. WRN exonuclease activity was examined by using a 34-bp forked duplex substrate (Fig. 6B), which is not completely unwound by the WRN helicase activity but is digested by the WRN exonuclease starting from the blunt end (29). WRN (13 nM) alone digested ∼80% of the substrate (Fig. 6B, lane 3), and incubation with increasing amounts of the c-Abl fragment resulted in a complete inhibition of the WRN exonuclease activity (Fig. 6B, lanes 4 to 7). c-Abl alone did not digest the WRN exonuclease substrate (Fig. 6B, lane 2). These results demonstrate that WRN phosphorylation by Abl kinase inhibits its exonuclease activity on the 34-bp forked duplex DNA. Next, we used a 22-bp forked duplex substrate (Fig. 6C) (29) to determine whether tyrosine phosphorylation of WRN affects its helicase activity. WRN (1.0 nM) alone almost completely unwound the substrate (Fig. 6C, lane 2), whereas c-Abl alone displayed no unwinding activity (Fig. 6C, lane 1). Preincubation of WRN with c-Abl resulted in a c-Abl dose-dependent inhibition (up to 87% inhibition) (Fig. 6, lane 5) of WRN helicase activity (Fig. 6C, lanes 3 to 6). It should be noted that, similar to BCR-ABL, lack of the N-terminal inhibitory sequence makes this c-Abl fragment highly active, so the amounts of the c-Abl fragment used are much smaller than those of WRN. Gel shift experiments demonstrated that the c-Abl fragment did not form a stable complex with either of the two substrates (data not shown), excluding the possibility that the inhibition of WRN activities was due to blockage of the substrate by c-Abl. Therefore, tyrosine phosphorylation of WRN by c-Abl inhibits both its exonuclease and helicase activities.
FIG. 6.
Tyrosine phosphorylation of WRN by c-Abl inhibits its exonuclease and helicase activities. (A) WRN was incubated with the c-Abl fragment by using the same protein concentrations and reaction conditions as those for the exonuclease assay. The proteins were analyzed by immunoblotting (IB) with anti-phosphotyrosine (P-Tyr) antibodies followed by anti-WRN or anti-Abl antibodies. (B) The structure of the exonuclease substrate is shown at the top. WRN (13 nM, lanes 3 to 7) was incubated in a 10-μl reaction volume with a 0, 0.02, 0.04, 0.09 (lanes 3 to 6), or 0.18 (lanes 2 and 7) nM concentration of the c-Abl fragment for 5 min at 28°C prior to the addition of the DNA substrate (0.5 nM, final concentration) under the conditions described in Materials and Methods, and products were analyzed on 14% denaturing polyacrylamide gels. (C) The structure of the helicase substrate is shown on the left. Purified WRN (1.0 nM, lanes 2 to 6) was incubated with the c-Abl fragment at a 0, 0.02, 0.04, 0.09 (lanes 2 to 5), or 0.18 (lanes 1 and 6) nM concentration in a 20-μl reaction volume for 5 min at 28°C prior to the addition of the DNA substrate (0.5 nM, final concentration) under the conditions described in Materials and Methods. Products were analyzed on 12% native polyacrylamide gels. Lane 7, no enzyme control. (D) Lysates from K562 cells with (0.6 μM, lane 3) or without (lanes 1 and 2) STI-571 treatment were immunoprecipitated with antibodies against immunoglobulin G (IgG) (lane 1) or WRN (lanes 2 and 3). The washed immunocomplexes were incubated with the exonuclease substrate (top, 0.5 nM, final concentration) as described. A representative was shown (n = 2). +, present; −, absent.
To gain insight into the potential roles for WRN in CML, we measured exonuclease activity in anti-WRN immunocomplexes from K562 cells with or without STI-571 treatment (0.6 μM). The washed anti-IgG or anti-WRN immunoprecipitates were incubated with the exonuclease substrate. Figure 6D shows that the anti-WRN immunoprecipitates digested the 34-bp forked duplex substrate in the 3′→5′ direction, and the digestion was enhanced (80% increase, P < 0.05) when the cells were pretreated with STI-571. Similar results were obtained by using a 32-bp, 3′-recessed substrate with one blunt end (10) (data not shown), indicating that this effect is not substrate specific. It should be emphasized that, although the majority of proteins in the immunocomplexes are WRN, we cannot exclude the possibility that other proteins with similar roles in the WRN complex also act on the substrate. These results suggest that treatment of the K562 CML cells with STI-571 increases the exonuclease activity in the WRN complex in vivo.
DISCUSSION
We have found a novel physical and functional interaction between WRN and the Abl kinase. The association is constitutive; however, treating cells with a DNA strand break inducer disrupts this interaction. This dissociation precedes WRN relocalization and is significantly prevented by an Abl kinase inhibitor. c-Abl phosphorylates WRN in vivo, and the tyrosine-phosphorylated form of WRN is found constitutively in CML lymphoblasts or after bleomycin treatment in HeLa fibroblasts. Although WRN can be serine/threonine phosphorylated by DNA-PK (21, 52), we have now provided the first evidence for WRN tyrosine phosphorylation by c-Abl in vivo and in vitro. Tyrosine phosphorylation of WRN by c-Abl inhibits WRN exonuclease and helicase activities. Our findings suggest that WRN and c-Abl may participate in a common DNA metabolic pathway and provide insight into the mechanism by which DNA damage plays a role in the pathogenesis of CML.
De novo synthesized WRN is destined to the nucleolus by its nucleolar targeting sequence (49). Some DNA damage agents induce WRN relocalization to foci in the nucleoplasm via a poorly understood mechanism (16, 38). DNA strand breaks induce an ATM- or DNA-PK-dependent c-Abl activation (3, 22), which is likely to tyrosine-phosphorylate WRN in the nucleolus and to release it into the nucleoplasm. Consistent with this notion, in CML cells where Abl kinase is constitutively activated, WRN is distributed throughout the nucleus (38). This observation is in line with the association between DNA damage-induced WRN acetylation and its relocalization (4), as c-Abl is required for some DNA damage-induced acetylation events (11). As both c-Abl and BLM interact with WRN and are part of the BRCA1-associated genome surveillance complex (BASC) (50, 51), WRN may dissociate from BASC after DNA damage, as does c-Abl (14). It is likely that WRN exists in a complex with c-Abl in the nucleolus together with other BASC components prior to DNA damage (2). It is unclear why WRN relocalization induced by 4-nitroquinoline-1-oxide or serum depletion can be prevented by a tyrosine phosphatase inhibitor, Na3VO4, in COS cells (16). This c-Abl/WRN pathway may depend on the stage of cell cycle because DNA damage does not activate c-Abl kinase until cells are committed to enter the S phase (25), and WRN does not localize to the nucleolus in S-phase-arrested cells (16). Collectively, we propose that there is an Abl kinase-mediated cellular response to DNA damage that phosphorylates and translocates the nucleolar WRN to the sites of DNA damage.
What is the biological significance of the inhibition of WRN catalytic activities by c-Abl? Similar to DNA-PK (21, 52), c-Abl also inhibits WRN exonuclease and helicase activities. The ATM kinase is considered an early responder to DNA damage, especially of DNA strand breaks. The bleomycin-induced WRN serine/threonine phosphorylation by DNA-PK appears to be independent of ATM (21, 52). In contrast, ionizing radiation-induced c-Abl activation requires the ATM kinase (3), suggesting that WRN phosphorylation by c-Abl is a separate pathway. NHEJ and homologous recombination are the two major pathways that repair DNA double strand breaks. As DNA-PK is required for NHEJ, the inhibition of WRN catalytic activities due to its phosphorylation by DNA-PK may prevent cells from shuttling damage to this error-prone pathway. In contrast, WRN phosphorylation by activated Abl kinase may facilitate WRN export from the nucleoli while temporarily holding WRN in a catalytically inactive state during the process of relocalization. Dissociation of WRN from c-Abl, and possibly dephosphorylation of WRN by the c-Abl-mediated tyrosine phosphatase (8), may attenuate the tyrosine phosphorylation of WRN such that the inhibition of WRN catalytic activities is reversed at repair foci. As the c-Abl fragment does not bind WRN stably but is sufficient to phosphorylate WRN, the inhibition of WRN catalytic activities appears to be independent of c-Abl binding to WRN. However, there may exist another pathway of WRN in ribosomal DNA metabolism (44), as a small portion of WRN is usually retained in the nucleolus after DNA damage. Therefore, WRN may act as a central coordinating protein of different DNA repair pathways, and its role may be regulated by phosphorylation and other forms of posttranscriptional modification.
In untreated cells, c-Abl localizes to both the nucleus and the cytoplasm while BCR-ABL is exclusively cytoplasmic. Inhibition of tyrosine kinase activity and nuclear export by using STI-571 and leptomycin B, respectively, cause a portion (20 to 25%) of BCR-ABL to reside in the nucleus (48). This raises intriguing questions. Which Abl protein phosphorylates the nuclear WRN, and how does this phosphorylation respond to STI-571 treatment in CML cells? Evidence suggests that cellular localization of c-Abl and BCR-ABL is quite dynamic between the nucleus and the cytoplasm (19, 48), partially due to the coexistence of the nuclear localization and export signals in their C termini (Fig. 1D). It is conceivable that the cytoplasmic BCR-ABL phosphorylates and activates c-Abl when it is in the cytoplasm. After cycling back to the nucleus, c-Abl may phosphorylate WRN. Alternatively, the newly synthesized WRN could be tyrosine phosphorylated prior to translocation to the nucleus. A search for the consensus sequence of the c-Abl phosphorylation motif revealed two putative WRN phosphorylation sites: Tyr640 (VYVTP) and Tyr839 (VIHYGAP). Future experiments should be conducted to verify tyrosine phosphorylation sites on WRN.
As a caretaker of the human genome (28), inactivation of WRN catalytic activities may contribute to increased genomic instability in CML cells. BCR-ABL-expressing cells accumulate more DNA damage than normal cells, and they exhibit an induction of homologous recombination and a reduction of NHEJ (12, 46). Rad51 plays a central role in homologous recombination (47). Prince et al. and Saintigny et al. (35, 37) have demonstrated a coordinated regulation of WRN and Rad51 in the homologous recombination pathway. It has been shown that Rad51 colocalizes with WRN in the nuclear foci in response to DNA damage (38). Interestingly, this Rad51-WRN colocalization is observed in the K562 CML cells where the Abl kinase activity is constitutively activated. Whether this interaction holds true in non-CML cells remains to be determined; however, Abl kinase is likely to mediate this repair pathway (7, 56). As multiple mutations in the kinase domain of BCR-ABL are found in patients who develop Imatinib resistance (39), new therapeutic strategies should also aim at optimizing the DNA repair response in CML.
Importantly, c-Abl tyrosine kinase activity is increased in cells from individuals with BRCA1 mutations (14). As WRN and BRCA1 follow similar kinetics of their interaction with c-Abl, this suggests that an increase in oncogenic tyrosine kinase activity may be associated with the increased cancer predisposition found in WS and other genome instability syndromes. Indeed, genomic instability is common in both normal aging and many types of cancers characterized by activated tyrosine kinase (45). By cDNA microarray analysis, we have recently found that genes transcriptionally affected by normal aging or WRN mutations are very similar (K. J. Kyng and V. A. Bohr, unpublished data). Since WS reflects segmental progeroid in many ways, this type of uncontrolled tyrosine phosphorylation may contribute to the mechanisms of aging and its associated increase in cancer incidence.
Acknowledgments
We thank J. Oshima for human WRN vectors, J. Harrigan and M. Seidman for reagents and critical reading of the manuscript, L. Dawut for technical assistance, and P. Gearhart and K. Hashiguchi for suggestions.
This work was supported in part by grant CA-29431 (to D.K.).
REFERENCES
- 1.Agami, R., G. Blandino, M. Oren, and Y. Shaul. 1999. Interaction of c-Abl and p73α and their collaboration to induce apoptosis. Nature 399:809-813. [DOI] [PubMed] [Google Scholar]
- 2.Andersen, J. S., C. E. Lyon, A. H. Fox, A. K. Leung, Y. W. Lam, H. Steen, M. Mann, and A. I. Lamond. 2002. Directed proteomic analysis of the human nucleolus. Curr. Biol. 12:1-11. [DOI] [PubMed] [Google Scholar]
- 3.Baskaran, R., L. D. Wood, L. L. Whitaker, C. E. Canman, S. E. Morgan, Y. Xu, C. Barlow, D. Baltimore, A. Wynshaw-Boris, M. B. Kastan, and J. Y. Wang. 1997. Ataxia telangiectasia mutant protein activates c-Abl tyrosine kinase in response to ionizing radiation. Nature 387:516-519. [DOI] [PubMed] [Google Scholar]
- 4.Blander, G., N. Zalle, Y. Daniely, J. Taplick, M. D. Gray, and M. Oren. 2002. DNA damage-induced translocation of the Werner helicase is regulated by acetylation. J. Biol. Chem. 277:50934-50940. [DOI] [PubMed] [Google Scholar]
- 5.Brosh, R. M., Jr., and V. A. Bohr. 2002. Roles of the Werner syndrome protein in pathways required for maintenance of genome stability. Exp. Gerontol. 37:491-506. [DOI] [PubMed] [Google Scholar]
- 6.Brosh, R. M., Jr., C. von Kobbe, J. A. Sommers, P. Karmakar, P. L. Opresko, J. Piotrowski, I. Dianova, G. L. Dianov, and V. A. Bohr. 2001. Werner syndrome protein interacts with human flap endonuclease 1 and stimulates its cleavage activity. EMBO J. 20:5791-5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, G., S. S. Yuan, W. Liu, Y. Xu, K. Trujillo, B. Song, F. Cong, S. P. Goff, Y. Wu, R. Arlinghaus, D. Baltimore, P. J. Gasser, M. S. Park, P. Sung, and E. Y. Lee. 1999. Radiation-induced assembly of Rad51 and Rad52 recombination complex requires ATM and c-Abl. J. Biol. Chem. 274:12748-12752. [DOI] [PubMed] [Google Scholar]
- 8.Cong, F., S. Spencer, J. F. Cote, Y. Wu, M. L. Tremblay, L. A. Lasky, and S. P. Goff. 2000. Cytoskeletal protein PSTPIP1 directs the PEST-type protein tyrosine phosphatase to the c-Abl kinase to mediate Abl dephosphorylation. Mol. Cell 6:1413-1423. [DOI] [PubMed] [Google Scholar]
- 9.Cong, F., J. Tang, B. J. Hwang, B. Q. Vuong, G. Chu, and S. P. Goff. 2002. Interaction between UV-DDB proteins and the c-Abl tyrosine kinase. J. Biol. Chem. 277:34870-34878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper, M. P., A. Machwe, D. K. Orren, R. M. Brosh, D. Ramsden, and V. A. Bohr. 2000. Ku complex interacts with and stimulates the Werner protein. Genes Dev. 14:907-912. [PMC free article] [PubMed] [Google Scholar]
- 11.Costanzo, A., P. Merlo, N. Pediconi, M. Fulco, V. Sartorelli, P. A. Cole, G. Fontemaggi, M. Fanciulli, L. Schiltz, G. Blandino, C. Balsano, and M. Levrero. 2002. DNA damage-dependent acetylation of p73 dictates the selective activation of apoptotic target genes. Mol. Cell 9:175-186. [DOI] [PubMed] [Google Scholar]
- 12.Deutsch, E., A. Dugray, B. AbdulKarim, E. Marangoni, L. Maggiorella, S. Vaganay, R. M'Kacher, S. D. Rasy, F. Eschwege, W. Vainchenker, A. G. Turhan, and J. Bourhis. 2001. BCR-ABL down-regulates the DNA repair protein DNA-PKcs. Blood 97:2084-2090. [DOI] [PubMed] [Google Scholar]
- 13.Druker, B. J., S. Tamura, E. Buchdunger, S. Ohno, G. M. Segal, S. Fanning, J. Zimmermann, and N. B. Lydon. 1996. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat. Med. 2:561-566. [DOI] [PubMed] [Google Scholar]
- 14.Foray, N., D. Marot, V. Randrianarison, N. D. Venezia, D. Picard, M. Perricaudet, V. Favaudon, and P. Jeggo. 2002. Constitutive association of BRCA1 and c-Abl and its ATM-dependent disruption after irradiation. Mol. Cell. Biol. 22:4020-4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gong, J. G., A. Costanzo, H. Q. Yang, G. Melino, W. G. Kaelin, Jr., M. Levrero, and J. Y. Wang. 1999. The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature 399:806-809. [DOI] [PubMed] [Google Scholar]
- 16.Gray, M. D., L. Wang, H. Youssoufian, G. M. Martin, and J. Oshima. 1998. Werner helicase is localized to transcriptionally active nucleoli of cycling cells. Exp. Cell Res. 242:487-494. [DOI] [PubMed] [Google Scholar]
- 17.Gray, M. D., J. C. Shen, A. S. Kamath-Loeb, A. Blank, B. L. Sopher, G. M. Martin, J. Oshima, and L. A. Loeb. 1997. The Werner syndrome protein is a DNA helicase. Nat. Genet. 17:100-103. [DOI] [PubMed] [Google Scholar]
- 18.Huang, S., B. Li, M. D. Gray, J. Oshima, I. S. Mian, and J. Campisi. 1998. The premature ageing syndrome protein, WRN, is a 3′→5′ exonuclease. Nat. Genet. 20:114-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito, Y., P. Pandey, N. Mishra, S. Kumar, N. Narula, S. Kharbanda, S. Saxena, and D. Kufe. 2001. Targeting of the c-Abl tyrosine kinase to mitochondria in endoplasmic reticulum stress-induced apoptosis. Mol. Cell. Biol. 21:6233-6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin, S., S. Kharbanda, B. Mayer, D. Kufe, and D. T. Weaver. 1997. Binding of Ku and c-Abl at the kinase homology region of DNA-dependent protein kinase catalytic subunit. J. Biol. Chem. 272:24763-24766. [DOI] [PubMed] [Google Scholar]
- 21.Karmakar, P., J. Piotrowski, R. M. Brosh, Jr., J. A. Sommers, S. P. Miller, W. H. Cheng, C. M. Snowden, D. A. Ramsden, and V. A. Bohr. 2002. Werner protein is a target of DNA-dependent protein kinase in vivo and in vitro, and its catalytic activities are regulated by phosphorylation. J. Biol. Chem. 277:18291-18302. [DOI] [PubMed] [Google Scholar]
- 22.Kharbanda, S., P. Pandey, S. Jin, S. Inoue, A. Bharti, Z. M. Yuan, R. Weichselbaum, D. Weaver, and D. Kufe. 1997. Functional interaction between DNA-PK and c-Abl in response to DNA damage. Nature 386:732-735. [DOI] [PubMed] [Google Scholar]
- 23.Kharbanda, S., R. Ren, P. Pandey, T. D. Shafman, S. M. Feller, R. R. Weichselbaum, and D. W. Kufe. 1995. Activation of the c-Abl tyrosine kinase in the stress response to DNA-damaging agents. Nature 376:785-788. [DOI] [PubMed] [Google Scholar]
- 24.Kitao, H., and Z. M. Yuan. 2002. Regulation of ionizing radiation-induced Rad52 nuclear foci formation by c-Abl-mediated phosphorylation. J. Biol. Chem. 277:48944-48948. [DOI] [PubMed] [Google Scholar]
- 25.Liu, Z. G., R. Baskaran, E. T. Lea-Chou, L. D. Wood, Y. Chen, M. Karin, and J. Y. Wang. 1996. Three distinct signalling responses by murine fibroblasts to genotoxic stress. Nature 384:273-276. [DOI] [PubMed] [Google Scholar]
- 26.Martin, G. M., S. N. Austad, and T. E. Johnson. 1996. Genetic analysis of aging: role of oxidative damage and environmental stresses. Nat. Genet. 13:25-34. [DOI] [PubMed] [Google Scholar]
- 27.Mohaghegh, P., and I. D. Hickson. 2001. DNA helicase deficiencies associated with cancer predisposition and premature ageing disorders. Hum. Mol. Genet. 10:741-746. [DOI] [PubMed] [Google Scholar]
- 28.Nakayama, H. 2002. RecQ family helicases: roles as tumor suppressor proteins. Oncogene 21:9008-9021. [DOI] [PubMed] [Google Scholar]
- 29.Opresko, P. L., J. P. Laine, R. M. Brosh, Jr., M. M. Seidman, and V. A. Bohr. 2001. Coordinate action of the helicase and 3′ to 5′ exonuclease of Werner syndrome protein. J. Biol. Chem. 276:44677-44687. [DOI] [PubMed] [Google Scholar]
- 30.Orren, D. K., R. M. Brosh, Jr., J. O. Nehlin, A. Machwe, M. D. Gray, and V. A. Bohr. 1999. Enzymatic and DNA binding properties of purified WRN protein: high affinity binding to single-stranded DNA but not to DNA damage induced by 4NQO. Nucleic Acids Res. 27:3557-3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oshima, J., S. Huang, C. Pae, J. Campisi, and R. H. Schiestl. 2002. Lack of WRN results in extensive deletion at nonhomologous joining ends. Cancer Res. 62:547-551. [PubMed] [Google Scholar]
- 32.Pluk, H., K. Dorey, and G. Superti-Furga. 2002. Autoinhibition of c-Abl. Cell 108:247-259. [DOI] [PubMed] [Google Scholar]
- 33.Poot, M., K. A. Gollahon, M. J. Emond, J. R. Silber, and P. S. Rabinovitch. 2002. Werner syndrome diploid fibroblasts are sensitive to 4-nitroquinoline-N-oxide and 8-methoxypsoralen: implications for the disease phenotype. FASEB J. 16:757-758. [DOI] [PubMed] [Google Scholar]
- 34.Poot, M., J. S. Yom, S. H. Whang, J. T. Kato, K. A. Gollahon, and P. S. Rabinovitch. 2001. Werner syndrome cells are sensitive to DNA cross-linking drugs. FASEB J. 15:1224-1226. [DOI] [PubMed] [Google Scholar]
- 35.Prince, P. R., M. J. Emond, and R. J. Monnat, Jr. 2001. Loss of Werner syndrome protein function promotes aberrant mitotic recombination. Genes Dev. 15:933-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ren, R., Z. S. Ye, and D. Baltimore. 1994. Abl protein-tyrosine kinase selects the Crk adapter as a substrate using SH3-binding sites. Genes Dev. 8:783-795. [DOI] [PubMed] [Google Scholar]
- 37.Saintigny, Y., K. Makienko, C. Swanson, M. J. Emond, and R. J. Monnat, Jr. 2002. Homologous recombination resolution defect in werner syndrome. Mol. Cell. Biol. 22:6971-6978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakamoto, S., K. Nishikawa, S. J. Heo, M. Goto, Y. Furuichi, and A. Shimamoto. 2001. Werner helicase relocates into nuclear foci in response to DNA damaging agents and co-localizes with RPA and Rad51. Genes Cells 6:421-430. [DOI] [PubMed] [Google Scholar]
- 39.Sawyers, C. L. 1999. Chronic myeloid leukemia. N. Engl. J. Med. 340:1330-1340. [DOI] [PubMed] [Google Scholar]
- 40.Sawyers, C. L. 2002. Disabling Abl-perspectives on Abl kinase regulation and cancer therapeutics. Cancer Cell 1:13-15. [DOI] [PubMed] [Google Scholar]
- 41.Sawyers, C. L., J. McLaughlin, A. Goga, M. Havlik, and O. Witte. 1994. The nuclear tyrosine kinase c-Abl negatively regulates cell growth. Cell 77:121-131. [DOI] [PubMed] [Google Scholar]
- 42.Schindler, T., W. Bornmann, P. Pellicena, W. T. Miller, B. Clarkson, and J. Kuriyan. 2000. Structural mechanism for STI-571 inhibition of abelson tyrosine kinase. Science 289:1938-1942. [DOI] [PubMed] [Google Scholar]
- 43.Shafman, T., K. K. Khanna, P. Kedar, K. Spring, S. Kozlov, T. Yen, K. Hobson, M. Gatei, N. Zhang, D. Watters, M. Egerton, Y. Shiloh, S. Kharbanda, D. Kufe, and M. F. Lavin. 1997. Interaction between ATM protein and c-Abl in response to DNA damage. Nature 387:520-523. [DOI] [PubMed] [Google Scholar]
- 44.Shiratori, M., T. Suzuki, C. Itoh, M. Goto, Y. Furuichi, and T. Matsumoto. 2002. WRN helicase accelerates the transcription of ribosomal RNA as a component of an RNA polymerase I-associated complex. Oncogene 21:2447-2454. [DOI] [PubMed] [Google Scholar]
- 45.Skorski, T. 2002. Oncogenic tyrosine kinases and the DNA-damage response. Nat. Rev. Cancer 2:351-360. [DOI] [PubMed] [Google Scholar]
- 46.Slupianek, A., C. Schmutte, G. Tombline, M. Nieborowska-Skorska, G. Hoser, M. O. Nowicki, A. J. Pierce, R. Fishel, and T. Skorski. 2001. BCR/ABL regulates mammalian RecA homologs, resulting in drug resistance. Mol. Cell 8:795-806. [DOI] [PubMed] [Google Scholar]
- 47.Sung, P. 1997. Function of yeast Rad52 protein as a mediator between replication protein A and the Rad51 recombinase. J. Biol. Chem. 272:28194-28197. [DOI] [PubMed] [Google Scholar]
- 48.Vigneri, P., and J. Y. Wang. 2001. Induction of apoptosis in chronic myelogenous leukemia cells through nuclear entrapment of BCR-ABL tyrosine kinase. Nat. Med. 7:228-234. [DOI] [PubMed] [Google Scholar]
- 49.von Kobbe, C., and V. A. Bohr. 2002. A nucleolar targeting sequence in the Werner syndrome protein resides within residues 949-1092. J. Cell Sci. 115:3901-3907. [DOI] [PubMed] [Google Scholar]
- 50.von Kobbe, C., P. Karmakar, L. Dawut, P. Opresko, X. Zeng, R. M. Brosh, Jr., I. D. Hickson, and V. A. Bohr. 2002. Colocalization, physical and functional interaction between Werner and Bloom syndrome proteins. J. Biol. Chem. 277:22035-22044. [DOI] [PubMed] [Google Scholar]
- 51.Wang, Y., D. Cortez, P. Yazdi, N. Neff, S. J. Elledge, and J. Qin. 2000. BASC, a super complex of BRCA1-associated proteins involved in the recognition and repair of aberrant DNA structures. Genes Dev. 14:927-939. [PMC free article] [PubMed] [Google Scholar]
- 52.Yannone, S. M., S. Roy, D. W. Chan, M. B. Murphy, S. Huang, J. Campisi, and D. J. Chen. 2001. Werner syndrome protein is regulated and phosphorylated by DNA-dependent protein kinase. J. Biol. Chem. 276:38242-38248. [DOI] [PubMed] [Google Scholar]
- 53.Yoshida, K., K. Komatsu, H. G. Wang, and D. Kufe. 2002. c-Abl tyrosine kinase regulates the human Rad9 checkpoint protein in response to DNA damage. Mol. Cell. Biol. 22:3292-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu, C. E., J. Oshima, Y. H. Fu, E. M. Wijsman, F. Hisama, R. Alisch, S. Matthews, J. Nakura, T. Miki, S. Ouais, G. M. Martin, J. Mulligan, and G. D. Schellenberg. 1996. Positional cloning of the Werner's syndrome gene. Science 272:258-262. [DOI] [PubMed] [Google Scholar]
- 55.Yuan, Z. M., Y. Huang, M. M. Fan, C. Sawyers, S. Kharbanda, and D. Kufe. 1996. Genotoxic drugs induce interaction of the c-Abl tyrosine kinase and the tumor suppressor protein p53. J. Biol. Chem. 271:26457-26460. [DOI] [PubMed] [Google Scholar]
- 56.Yuan, Z. M., Y. Huang, T. Ishiko, S. Nakada, T. Utsugisawa, S. Kharbanda, R. Wang, P. Sung, A. Shinohara, R. Weichselbaum, and D. Kufe. 1998. Regulation of Rad51 function by c-Abl in response to DNA damage. J. Biol. Chem. 273:3799-3802. [DOI] [PubMed] [Google Scholar]
- 57.Yuan, Z. M., H. Shioya, T. Ishiko, X. Sun, J. Gu, Y. Y. Huang, H. Lu, S. Kharbanda, R. Weichselbaum, and D. Kufe. 1999. p73 is regulated by tyrosine kinase c-Abl in the apoptotic response to DNA damage. Nature 399:814-817. [DOI] [PubMed] [Google Scholar]