Abstract
Inosine 5′-monophosphate dehydrogenase (IMPDH) is the critical, rate-limiting enzyme in the de novo biosynthesis pathway for guanine nucleotides. Two separate isoenzymes, designated IMPDH types I and II, contribute to IMPDH activity. An additional pathway salvages guanine through the activity of hypoxanthine-guanine phosphoribosyltransferase (HPRT) to supply the cell with guanine nucleotides. In order to better understand the relative contributions of IMPDH types I and II and HPRT to normal biological function, a mouse deficient in IMPDH type I was generated by standard gene-targeting techniques and bred to mice deficient in HPRT or heterozygous for IMPDH type II. T-cell activation in response to anti-CD3 plus anti-CD28 antibodies was significantly impaired in both single- and double-knockout mice, whereas a more general inhibition of proliferation in response to other T- and B-cell mitogens was observed only in mice deficient in both enzymes. In addition, IMPDH type I−/− HPRT−/0 splenocytes showed reduced interleukin-4 production and impaired cytolytic activity after antibody activation, indicating an important role for guanine salvage in supplementing the de novo synthesis of guanine nucleotides. We conclude that both IMPDH and HPRT activities contribute to normal T-lymphocyte activation and function.
Guanine nucleotides are crucial prerequisites for many cellular functions including transmembrane and intracellular signaling, DNA replication, and RNA and protein synthesis. Two separate biosynthetic pathways contribute to intracellular guanine nucleotide pools. The rate-limiting enzyme in the de novo pathway is inosine 5′-monophosphate dehydrogenase (IMPDH), which catalyzes the conversion of IMP to GMP. The salvage enzyme hypoxanthine-guanine phosphoribosyltransferase (HPRT) catalyzes the conversion of guanine to GMP. The relative roles of these two enzymes in cellular guanine nucleotide biosynthesis are unknown.
The enzymatic activity of IMPDH is composed of two distinct but nearly identical isoforms designated type I and type II. The two isoforms are 84% identical at the amino acid level (4, 24) and have substrate affinities, catalytic activities, and Ki values that are virtually indistinguishable (1, 12). IMPDH activity has been shown to increase during cell proliferation and transformation (3, 14, 23), and inhibitors of this enzymatic activity are effective immunosuppressive drugs (6, 7, 13, 22). Depletion of guanine nucleotides in peripheral blood T lymphocytes elicits cell cycle arrest in late G1 and is associated with a variety of perturbations in cell cycle-related protein synthesis (20, 33). Differences in the regulation and expression of the two isoforms have been well documented. In most tissues, IMPDH type II mRNA is expressed at higher levels than type I mRNA, which has been believed to be constitutive (30). However, both type I and type II mRNA levels increase with T-cell activation (5, 35). Studies of the promoter region of human IMPDH type I have revealed three separate transcription initiation sites, which suggests a possible tissue- and/or developmentally specific expression (10), whereas the type II gene gives rise to a single transcript (36).
Loss of both IMPDH type II alleles as a consequence of targeted homologous recombination in the mouse results in very early embryonic lethality despite normal HPRT and IMPDH type I activity (11). Mice with loss of a single IMPDH type II allele are phenotypically normal, with normal lymphocyte subsets and normal responses to mitogenic stimuli. However, lymphocytes from mice with an IMPDH type II+/− HPRT−/0 genotype have a 30% mean reduction in GTP levels and a diminished proliferative response when activated with anti-CD3 plus anti-CD28 antibodies, which are T-cell mitogens. Activation in response to other T-cell mitogens and to phorbol-12-myristate-13-acetate (PMA) and ionomycin is also decreased, as is cytolytic T-cell activity against allogeneic target cells. These results indicate that murine T-lymphocyte activation and function are significantly impaired when guanine nucleotide synthesis is reduced.
In order to better understand the relative contributions of the de novo and salvage pathways, as well as the relative roles of the IMPDH isoforms, for guanine nucleotide biosynthesis, lymphocyte biology, and murine development, we have successfully targeted the IMPDH type I gene in mice.
MATERIALS AND METHODS
Cloning of the murine IMPDH type I gene.
Murine type I IMPDH cDNA was used to probe a bacterial artificial chromosome (BAC) library at Genome Systems. A single genomic clone (BACM-238-A11) was identified with this probe. A HindIII subclone spanning exon 2 through exon 13 was cloned from the genomic BAC clone into the HindIII site of the pBluescipt vector (see Fig. 1). Exon 1 and the remaining part of intron 1 were cloned by PCR using oligonucleotide primers derived from exon 1 and exon 2. The amplified DNA was then used to identify another genomic HindIII subclone containing ∼5 kb upstream of exon 1, exon 1, and part of intron 1. The region spanning exon 13 to exon 14 was also amplified by using PCR and subcloned into a blunted EcoRV site of the pT7-Blue vector (Novagen, Madison, Wis.).
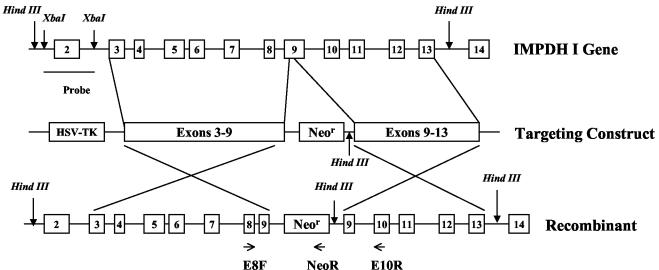
FIG. 1.
Targeted disruption of the IMPDH type I gene. Shown are a schematic diagram of the IMPDH type I gene with exons 2 to 14 (open boxes), the targeting construct with exons 3 to 9 and exons 9 to 13 cloned adjacent to the neo gene, and the expected result of homologous recombination. The 2.3-kb XbaI fragment used as a probe for Southern analysis and oligonucleotides (E8F, NeoR, and E10R) used for PCR are indicated.
Targeting the IMPDH type I gene and ES cell selection.
The IMPDH type I gene was inactivated by using a knockout construct consisting of IMPDH type I exons 3 to 13 with an insertion of the 1.2-kb PGK-Neo cassette accompanied by a 9-amino-acid (9-aa) (aa 323 to 331) deletion at the end of exon 9 (see Fig. 1). It has been demonstrated that amino acids G326 and C331 line the IMP binding pocket of the IMPDH, and mutation at either of these sites leads to near-complete loss of IMPDH activity (8). Mouse embryonic stem (ES) cells of strain 129/R1 were transfected with this construct by electroporation and were selected positively and negatively in the presence of 200 μg of G418/ml and 0.5 μg of ganciclovir/ml, respectively. Genomic DNA from selected ES cell clones was digested with HindIII, separated on 0.7% agarose gels, transferred to Zeta-probe nylon membranes (Bio-Rad Laboratories Inc., Hercules, Calif.), and hybridized with an [α-32P]dCTP-labeled 2.3-kb DNA probe (3,000 Ci/mmol; Amersham Pharmacia Biotech, Piscataway, N.J.) that included the entire exon 2 (Fig. 1). Labeling was carried out using the Random Primer Labeling kit (Promega Corp., Madison, Wis.). Southern hybridization was performed in a High Efficiency Hybridization system (Molecular Research Center, Inc., Cincinnati, Ohio) at 65°C for 16 to 24 h. The membrane was then washed twice in 1× SSC (0.15 M NaCl plus 0.015 M sodium citrate)-0.2% sodium dodecyl sulfate (SDS) at room temperature for 10 min, followed by a wash in 0.5× SSC-0.1% SDS at 60°C for 30 min.
To confirm the genotype, PCR was performed using the three-primer strategy as indicated in Fig. 1: a forward primer (E8F) from exon 8 of the type I gene, 5′-GGAACTCAGTGTATCAGATCGCC-3′; a reverse primer (NeoR) from the neo gene, 5′-CTATCAGGACATAGCGTTGGCTACC-3′; and a second reverse primer (E10R) from exon 10 of the type I gene, 5′-TACTCGGCCACCTTGTAGACAGC-3′. PCR was performed in a total volume of 50 μl consisting of approximately 300 ng of DNA, 200 μM deoxynucleoside triphosphates, 5% dimethyl sulfoxide, 100 ng of each of the reverse primers and 200 ng of the forward primer, and 1 U of Taq polymerase in 1× PCR buffer (Boehringer Mannheim, GmbH, Mannheim, Germany). Samples were amplified for 30 cycles, each consisting of denaturation at 94°C for 1 min, annealing at 55°C for 1 min, and elongation at 72°C for 2.5 min, and products were visualized on 1% agarose gels. ES cell clones heterozygous for targeted homologous recombination at the IMPDH type I gene locus were microinjected into blastocysts of the mouse strain C57BL/6 (B6) and then injected into the uteri of pseudopregnant B6 females (The Jackson Laboratory, Bar Harbor, Maine) in order to produce chimeric offspring.
Breeding and genotyping for single- and double-knockout mice.
Male mice with 60 to 95% chimerism based on coat color were bred to B6 females with successful germ line transmission. Confirmed heterozygous mice were further interbred to generate homozygous mice. Genotyping was performed on tail DNA of 3- to 4-week-old offspring by a combination of Southern hybridization and PCR analysis as described above. To generate IMPDH type I-plus-type II double-knockout mice, type I homozygous mice were bred with type II heterozygous mice. Offspring with heterozygosity for both type I and type II were further interbred to generate mice deficient in both type I (−/−) and type II (+/−). Genotyping for IMPDH type II was performed by Southern hybridization as described previously (11). To generate IMPDH type I-plus-HPRT double-knockout mice, type I homozygous null male mice were bred with HPRT homozygous female mice (C57BL/6J Hprtb-m3; The Jackson Laboratory). Mice heterozygous for both type I and HPRT were further interbred, and male offspring were selected for double-knockout mice (see Fig. 3). To assess loss of the HPRT allele, genomic DNA was digested with BamHI and Southern hybridization was performed using a 180-bp probe corresponding to exon 3 of the HPRT gene, obtained by reverse transcription-PCR from a normal mouse brain.
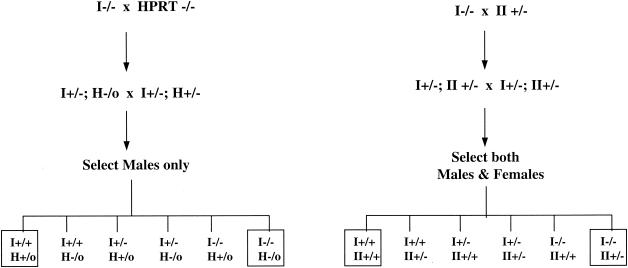
FIG. 3.
Breeding strategy to generate double-knockout mice. Boxed genotypes indicate animals used for experiments.
Northern hybridization.
Isolation of total RNA and Northern blot analysis were performed as previously described (11). Briefly, tissue or cellular RNA was extracted using Tri-Reagent (Molecular Research Center, Inc.). Total RNA (10 to 20 μg) was separated on a formaldehyde-agarose gel, transferred to Nytran membranes (Schleicher and Schuell, Keene, N.H.), and hybridized with [α-32P]dCTP-labeled IMPDH type I or type II or HPRT cDNA probes as previously described.
Immunoblotting.
Tissues were homogenized in extraction buffer containing 200 mM Tris-HCl (pH 7.5), 200 mM NaCl, 2 mM dithiothreitol, 5 mM MgCl2, 1 mM EGTA, 2 μg of leupeptin/ml, 0.1 mM phenylmethylsulfonyl fluoride, and 2 μg of pepstatin/ml. Cells were lysed by three cycles of freezing and thawing followed by centrifugation at 4°C for 15 min. Ten micrograms of total protein was resolved on an SDS-15% polyacrylamide gel and transferred onto Immobilon-P polyvinylidene difluoride membranes (Millipore, Bedford, Mass.). IMPDH proteins were detected by using a polyclonal antibody generated against the C-terminal 12 aa of type I protein. This region is highly conserved in type II (10 of 12 aa are identical), and the antibody (a gift from Vertex Pharmaceuticals Inc., Cambridge, Mass.) recognizes both type I and type II proteins.
Determination of intracellular GTP levels.
Splenocytes were stimulated with immobilized anti-CD3 (2 μg/ml) plus CD28 (5 μg/ml) for 48 h and washed once with phosphate-buffered saline (PBS). Cell pellets were then extracted with 5% cold perchloric acid and incubated on ice for 20 min. After centrifugation at 15,000 × g for 10 min, supernatants were adjusted to pH 6 by using 3 M KOH-3 M KHCO3. Samples were incubated on ice for an additional 10 min and centrifuged for 10 min at 4°C. Supernatants were stored at −80°C until use. GTP levels were measured by high-performance liquid chromatography (HPLC) (Partisil 5 SAX, RAC 11 column; VWR, Suwanee, Ga.). The Student one-tailed t test was performed to evaluate significance.
Enzyme assays.
To measure IMPDH enzyme activity, total tissue lysates were assayed in a 50-μl reaction volume containing 40 μM [8-14C]IMP (55 mCi/mmol; Moravek Biochemicals, Brea, Calif.), 100 mM Tris-HCl (pH 8.0), 100 mM KCl, 0.25 mM NAD, and 3 mM EDTA. For HPRT enzyme activity, 20 μg of protein extracted from tissues was assayed in a 50-μl reaction volume containing 125 μM [8-14C]hypoxanthine (48 mCi/mmol; ICN Biomedicals, Inc., Irvine, Calif.), 50 mM Tris-HCl (pH 7.4), 5 mM MgCl2, and 5 mM phosphoribosylpyrophosphate. Reactions were carried out at 37°C for 60 min, and 20 μl of the reaction product was spotted onto thin-layer chromatography plates (cellulose polyethyleneimine-F; J. T. Baker Inc., Phillipsburg, N.J.) and separated in 0.25 M sodium formate (pH 4.3) buffer.
Splenocyte activation and proliferation.
Mouse splenocyte culture and activation of T and B cells were performed as previously described (11). Briefly, mouse splenocytes were either stimulated with concanavalin A (ConA) (5 μg/ml), lipopolysaccharide (LPS) (20 μg/ml), or PMA (10 ng/ml) plus ionomycin (250 ng/ml) or added to a plate precoated with anti-CD3 (2 μg/ml) plus anti-CD28 (5 μg/ml) antibodies. Splenocytes were plated at a density of 5 × 105/ml and incubated at 37°C for 48 h, and 1 μCi of [methyl-3H]thymidine (80 Ci/mmol; Amersham Pharmacia Biotech) was added for the final 4 to 6 h of culture. Cells were harvested onto glass fiber filters (Packard Instrument Co., Meriden, Conn.), and radioactivity was measured in a Microscint 20 (Packard) by a Top Counter (Packard).
Cell cycle analysis.
Approximately 2 × 106 to 3 × 106 cells were fixed in 80% cold ethanol and stored at 4°C. For flow cytometry analysis, cells were spun down and washed once with PBS containing 0.2% bovine serum albumin. Cell pellets were then resuspended in 500 μl of PBS-0.2% bovine serum albumin containing 200 μg of RNase A/ml and 100 μg of propidium iodide/ml and were incubated at 37°C for 30 min. DNA fluorescence was measured by flow cytometry using a FACScan Flow (Becton Dickinson Immunocytometry, Mountain View, Calif.), and the percentage of cells in each phase of the cell cycle was determined by the ModFit Cell-Cycle Analysis program (Verity Software, Topsham, Maine). The Student one-tailed t test was used to determine statistical significance.
Cytokine determinations.
To measure cytokine secretion resulting from antibody stimulation, 24-well plates were coated with 2 μg of anti-CD3/ml and 5 μg of anti-CD28/ml at 4°C overnight. Plates were washed twice with PBS before seeding of cells. Splenocytes were plated at 2 × 106/ml in each well, and supernatants were collected at the indicated times. To determine levels of interleukin-2 (IL-2) and IL-4, the DuoSet ELISA Development kits were used according to the manufacturer's instructions (R&D Systems, Inc. Minneapolis, Minn.). Assays were performed in duplicate. Amounts of IL-2 and IL-4 in the supernatant were determined by using standard curves, which were performed for each experiment.
51Cr release assay.
Generation of allogeneic-specific cytotoxic T lymphocytes and the 51Cr release assay were performed as previously described (11). Briefly, wild-type or mutant splenocytes (from C57BL/6 [H-2b] mice) were mixed with irradiated (30 Gy) splenocytes from DBA/2J (H-2d) mice for 5 days (mixed lymphocyte reaction). Target cells (P815 cell line [H-2d]) were labeled with 150 μCi of sodium [51Cr]chromate (250 to 500 mCi/mg of Cr; Amersham Pharmacia Biotech). The function of cytotoxic T lymphocytes generated from the mixed lymphocyte reaction was measured by mixing them with labeled target cells at indicated effector-to-target cell ratios (E:T ratios) and incubating at 37°C for 4 h. Wells containing only target cells and medium were used as spontaneous release controls, and wells containing target cells with 5% Triton X-100 were used as maximum release controls. Results are expressed as percentages of specific lysis and were calculated by the following formula: (release with effector cells − spontaneous release)/(maximum release − spontaneous release). A one-tailed Student t test was performed at each E:T ratio.
RESULTS
Generation of IMPDH type I-deficient mice.
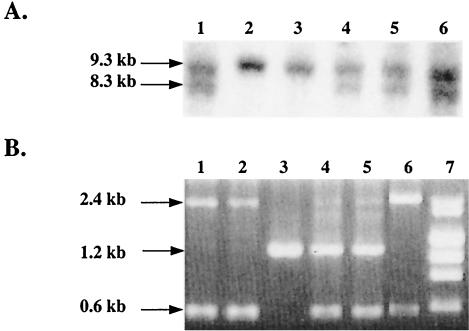
The IMPDH type I gene was disrupted by homologous recombination (Fig. 1). The targeting vector deleted 25 bp from exon 9 of the IMPDH type I gene, resulting in the loss of 9 aa (aa 323 to 331) that are essential for enzyme folding and catalytic function (8). A neo gene was inserted into exon 9 under the regulation of the phosphoglycerate kinase (PGK) promoter, conferring resistance to G418. A targeted recombination event with this vector would introduce a HindIII site at the 3′ end of the neo gene, giving rise to a distinct 8.3-kb HindIII band in the knockout allele as opposed to the 9.3-kb HindIII genomic fragment detected by a probe homologous to exon 2 (Fig. 2A). The genotype was also confirmed by using the 3-oligonucleotide PCR method as described in Materials and Methods. A wild-type allele will produce a 1.2-kb band only (Fig. 2B), representing amplification between exons 8 and 10, whereas a knockout allele will produce both a 0.6-kb band generated by amplification from exon 8 to the neo gene and a 2.4-kb band resulting from amplification between exons 8 and 10 including the insertion of the 1.2-kb neo gene in exon 9. ES cells from mice of strain 129 (substrain R1) were electroporated with linearized targeting vector and selected in the presence of G418 and ganciclovir. Genotyping revealed that approximately 11% of the clones analyzed underwent targeted homologous recombination.
FIG. 2.
Analysis of homologous recombination. (A) A representative Southern hybridization demonstrating the 9.3-kb (wild-type allele) and 8.3-kb (knockout allele) HindIII fragments. (B) PCR analysis demonstrating a 1.2-kb fragment (wild-type allele) or 0.6- and 2.4-kb fragments (knockout allele). Lane 3, wild type; lanes 1, 2, and 6, homozygous; lanes 4 and 5, heterozygous; lane 7, molecular weight markers consisting of lambda/HindIII and φX174/HeaIII digests.
Two ES cell clones heterozygous for the disrupted IMPDH type I gene were microinjected into B6 blastocysts in order to generate chimeric mice. Chimeric male mice were bred with B6 females, and one mouse exhibited successful germ line transmission. Tail DNA was isolated from all agouti offspring at the age of 3 or 4 weeks for genotyping by both Southern hybridization and PCR methods. Mice heterozygous for IMPDH type I gene disruption were further interbred to generate homozygous mutant mice. As summarized in Table 1, homozygous, heterozygous, and wild-type mice were born in the expected 1:2:1 ratio, with male and female progeny born in the expected 1:1 ratio. IMPDH type I homozygous mice did not show any obvious growth or behavioral abnormalities up to the age of 8 months and were fertile.
TABLE 1.
IMPDH type I heterozygous breeding
| Mice | No. analyzed | No. (%) wild type | No. (%) heterozygous | No. (%) homozygous |
|---|---|---|---|---|
| Total born | 131 | 30 (22.9) | 74 (56.5) | 27 (20.6) |
| Males | 63 | 15 (50) | 36 (48.6) | 12 (44.4) |
| Females | 68 | 15 (50) | 38 (51.4) | 15 (55.6) |
Generation of double-knockout mice.
We had previously attempted targeted disruption of the IMPDH type II gene. Mice homozygous for disruption of the IMPDH type II gene die during early embryogenesis. Mice heterozygous for the type II gene showed a significant impairment in T-lymphocyte activation when crossed with HPRT-deficient mice (11). In order to magnify the defect in the guanine nucleotide synthesis pathway and to compare phenotypes with those of IMPDH type I-deficient mice, we crossed IMPDH type I-deficient mice with IMPDH type II-heterozygous mice to generate mice deficient in both type I (−/−) and type II (+/−) genes. We also crossed IMPDH type I-deficient mice with HPRT-deficient mice to generate mice deficient in both type I (−/−) and HPRT (−/0) genes. The breeding strategy is outlined in Fig. 3. Since HPRT is an X-chromosome-linked gene, only male offspring were selected for genotyping in order to minimize the breeding complexity. Only mice that were genotyped as wild type or deficient at both loci (Fig. 3) were used for further experiments.
Expression of IMPDH type I, IMPDH type II, and HPRT in mutant mice.
In order to determine whether the expression of IMPDH type I, IMPDH type II, and HPRT was coordinately regulated, total cellular mRNA was isolated from various tissues of wild-type or double-knockout mice. Northern hybridizations were performed using IMPDH type I, type II, and HPRT probes (Fig. 4A). In wild-type mice, expression of IMPDH type I was relatively higher in the thymus, lung, and brain, indicating that type I may be important for function in these tissues, whereas it was barely detected in the liver and testis. Tissues from both types of double-knockout mice (type I−/− type II+/− and type I−/− HPRT−/0) had no detectable IMPDH type I mRNA. Expression of IMPDH type II mRNA was approximately 50% lower in tissues of IMPDH type I−/− type II+/− mice than in those from wild-type mice. IMPDH type II expression in IMPDH type I−/− HPRT−/0 mice was similar to that observed in wild-type mice. These results indicate that expression of IMPDH type II is not increased in response to the absence of type I or HPRT activity. Similarly, expression of HPRT was not elevated in response to a deficiency of IMPDH type I and partial loss of IMPDH type II. It has previously been demonstrated that expression of IMPDH type I is not increased when type II activity is reduced (11).
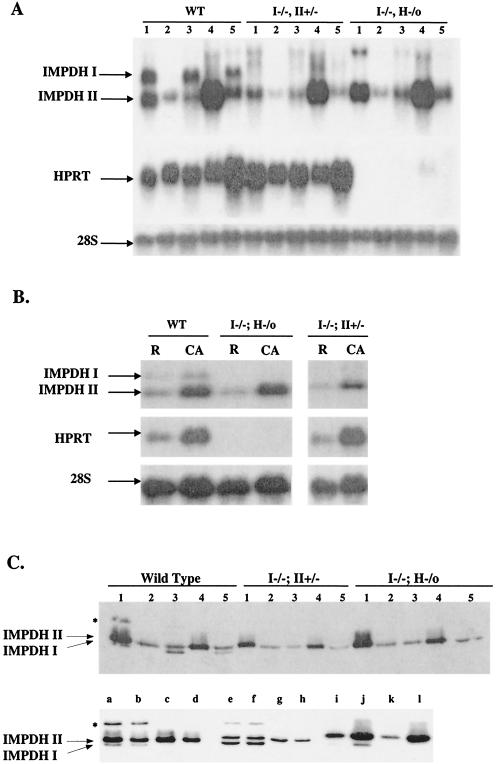
FIG. 4.
Expression of IMPDH type I, IMPDH type II, and HPRT mRNAs and IMPDH proteins in wild-type and double-knockout mice. (A) Northern hybridizations were performed on 20 μg of total RNA from tissues of wild-type (WT) and double-knockout mice. Lanes: 1, thymus; 2, liver; 3, lung; 4, testis; 5, brain. (B) Total RNA (10 μg) from resting (R) or ConA-activated (CA) splenocytes from wild-type or double-knockout mice. Both blots were probed with IMPDH type I, type II, or HPRT cDNA probes, as indicated. Levels of 28S RNA were used for quantitation. (C) (Upper panel) Expression of IMPDH type I and type II proteins in tissues from wild-type and double-knockout mice. Lanes containing tissue types are as in panel A. (Lower panel) Expression of IMPDH type I and type II proteins in thymuses (lanes a through d) of wild-type (lanes a and b) and type I−/− HPRT−/0 (lanes c and d) mice, lungs (lanes e through h) of wild type (lanes e and f) and type I−/− (lanes g and h) mice, and resting (lanes i and k) and ConA-activated (lanes j and l) lymphocytes from wild-type (lanes i and j) and type I−/− (lanes k and l) mice. Lower arrow, type I proteins; upper arrow, type II proteins. Asterisk indicates a high-molecular-weight variant of IMPDH I in the thymuses and lungs of wild-type mice. Total-cell lysates (10 μg) were separated on SDS-polyacrylamide gel electrophoresis gels and probed with an anti-IMPDH specific antibody.
Northern blot analysis was also performed on resting and ConA-activated splenocytes (Fig. 4B), since any compensatory increase in expression would be more likely to be manifested during proliferation after mitogenic stimulation. Type I, type II, and HPRT mRNA levels were increased 2.8-, 2.7-, and 3.4-fold, respectively, upon ConA activation of wild-type splenocytes. The increase in type II mRNA levels in activated mutant (type I−/− type II+/−) splenocytes was approximately half that in wild-type splenocytes, whereas type II mRNA levels in activated type I−/− HPRT−/0 splenocytes did not differ from those in wild-type splenocytes. These results further demonstrate the absence of a compensatory mechanism regulating expression of the type I and type II genes. Similarly, expression of HPRT mRNA in activated type I−/− type II+/− splenocytes was equal to or only slightly greater than that in wild-type cells (Fig. 4B).
IMPDH type I and type II proteins from tissues of wild-type and mutant mice were also analyzed (Fig. 4C). As was observed for mRNA expression, type I protein levels were relatively higher in the thymus, lung, and brain in wild-type mice (Fig. 4C, upper panel). Expression of the type II protein was not increased by IMPDH type I deficiency. A higher-molecular-weight protein band detected by an IMPDH-specific antibody was observed in the wild-type thymus (lane 1) and lung (lane 3) and was absent in the IMPDH type I−/− thymus and lung, indicating an IMPDH type I protein variant. To confirm the complete absence of type I protein in mutant mice, we performed Western blot analysis of thymuses, lungs, and resting and activated lymphocytes from five pairs of wild-type and type I−/− mice. As shown in the lower panel of Fig. 4C, type I protein is completely absent in tissues from type I−/− mice. The high-molecular-weight band consistently observed in thymus and lung samples from wild-type mice is also absent in mutant mice, confirming that this band represents an IMPDH I variant. In order to exclude the possibility that any truncated N-terminal protein was produced in type I−/− mice, we also performed Western blot analysis using a type I-specific antibody raised against aa 167 to 177 (a gift from Vertex Pharmaceuticals Inc.). We did not detect any additional N-terminal protein fragments in these animals (data not shown).
Intracellular GTP levels in mutant mice.
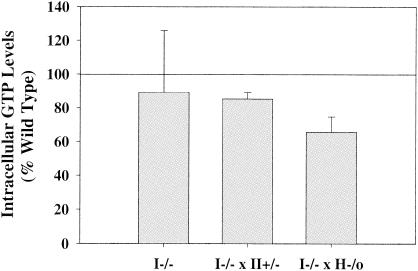
It has been shown previously that upon stimulation with anti-CD3 plus anti-CD28, the GTP levels in splenocytes of IMPDH type II+/− HPRT−/0 mice were 30% lower than those in wild-type splenocytes (11). GTP levels in antibody-activated IMPDH type I−/− splenocytes were not significantly different from those in wild-type cells (Fig. 5). However, splenocytes from type I−/− type II+/− and type I−/− HPRT−/0 mice both had significantly lower GTP levels than those from wild-type mice after antibody activation (85% ± 3% [P = 0.0108] and 65% ± 6% [P = 0.0024] of wild-type levels, respectively).
FIG. 5.
Intracellular GTP levels. Splenocytes from wild-type, IMPDH type I−/−, and double-mutant (type I−/− type II+/− or type I−/− HPRT−/0) mice were stimulated with anti-CD3 plus anti-CD28 antibodies for 48 h. GTP levels in resting and antibody-activated wild-type splenocytes were 0.2 ± 0.05 and 1.8 ± 0.3 nmol per 107 cells, respectively (n = 11). Values from activated splenocytes (IMPDH type I−/− [n = 4], type I−/− type II+/− [n = 3], and type I−/− HPRT−/0 [n = 4]) were plotted as percentages of wild-type levels (means ± standard deviations).
IMPDH and HPRT enzyme activities in mutant mice.
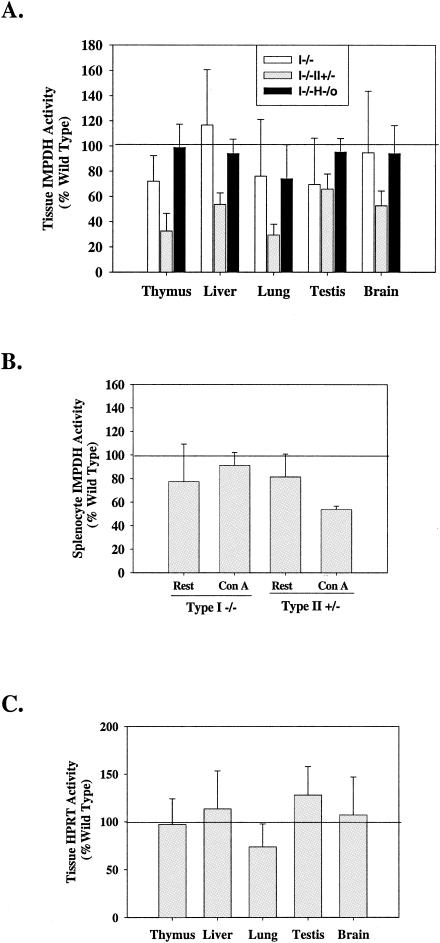
IMPDH enzyme activities were measured in various tissues from wild-type and mutant mice. As shown in Fig. 6A, IMPDH activity in tissues from mice deficient in IMPDH type I alone or deficient in both IMPDH type I and HPRT was not significantly different from that of wild-type mice. There was, however, a significant decrease in IMPDH activity in all these tissues from type I−/− type II+/− mice, indicating that expression of type II contributes in a major way to total IMPDH activity. Since expression of IMPDH type I mRNA was increased nearly threefold upon ConA stimulation in wild-type mice, we determined whether loss of type I would affect total IMPDH enzyme activity in lymphocytes. In contrast to the IMPDH type II+/− splenocytes, where the increase in IMPDH activity upon ConA activation was reduced by 40%, there was no significant difference in total IMPDH activity in activated splenocytes between IMPDH type I−/− and wild-type mice (Fig. 6B). There was also no significant difference in HPRT activity between mutant mice and wild-type mice (Fig. 6C), consistent with the inability of HPRT to compensate for partial loss of IMPDH activity at the mRNA level.
FIG. 6.
IMPDH and HPRT enzymatic activities in mutant mice. (A) IMPDH enzyme activity was measured in tissues of single- and double-knockout mice. Total-cell lysates of 20 μg (thymus), 50 μg (liver, brain, and testis), and 60 μg (lung) were used for each assay. Activities from mutant mice (IMPDH type I−/− [n = 4], type I−/− type II+/− [n = 5], and type I−/− HPRT−/0 [n = 5]) were plotted as percentages of activities obtained from wild-type tissues (n = 14). (B) IMPDH enzyme activities were measured on 10 μg of splenocyte lysates from mice with an IMPDH type I−/− or type II+/− genotype before and after ConA activation and plotted as percentages of wild-type activities. (C) HPRT activities were measured from type I−/− type II+/− double-knockout mice (n = 4) and plotted as percentages of wild-type activity. Values are means ± standard deviations, with each determination performed in duplicate.
Lymphocyte development and distribution in mutant mice.
Thymocytes and bone marrow cells were assessed for T- and B-cell distribution by flow cytometry (17). Splenocytes were stained with antibody Thy1.2 for T cells and antibody B220 for B cells. There were no significant differences in the size of the thymus or spleen or in T- and B-cell maturation between wild-type and mutant mice. There was a small but highly significant (P < 0.003) decrease in the proportion of Thy1.2-positive T cells, to 73% ± 15% of that in the control, in spleens from IMPDH type I−/− HPRT[−]/0 mice. This decrease was not observed for IMPDH type II+/− HPRT−/0 mice.
Effects of mutations on lymphocyte proliferation and activation.
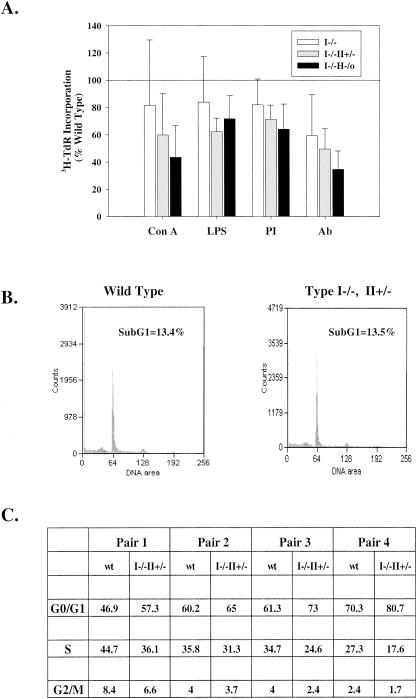
It has previously been demonstrated that lymphocytes from mice with an IMPDH type II+/− HPRT−/0 genotype have a significantly impaired response to T-cell-receptor stimulation (11). In order to acquire a better understanding of how guanine nucleotide biosynthesis is regulated by the de novo and salvage pathways and of the relative biological importance of the two IMPDH isoforms and HPRT in lymphocyte activation and function, we examined the proliferation of lymphocytes from mice with the IMPDH type I−/− genotype or a double knockout (the type I−/− type II+/− or type I−/− HPRT−/0 genotype) following activation by various stimuli (Fig. 7A). We observed a significant decrease in lymphocyte proliferation in response to anti-CD3 plus anti-CD28 in both single- and double-knockout mice. A more profound impairment of splenocyte proliferation in response to other T- and B-cell mitogens was demonstrated only in double-knockout mice. To assess whether the decrease in lymphocyte proliferation might be due to increased apoptosis, we analyzed cells by flow cytometry. There was no significant increase in the sub-G1 apoptotic region in IMPDH type I−/− type II+/− splenocytes (Fig. 7B). Cell cycle profiles indicated a small but significant increase in the proportion of cells in G0/G1 (116% ± 6% [P = 0.006]) and a decrease in S phase (76% ± 10% [P = 0.008]) in IMPDH type I−/− type II+/− splenocytes compared with wild-type levels after antibody activation (Fig. 7C).
FIG. 7.
Effects of loss of IMPDH type I, IMPDH type II, and HPRT activities on splenocyte proliferation. (A) Splenocytes from wild-type (100%), IMPDH type I-deficient, or double-knockout (type I−/− type II+/− or type I−/− HPRT−/0) mice were stimulated with ConA, LPS, PMA plus ionomycin (PI), or anti-CD3 plus anti-CD28 antibodies (Ab) as indicated for 48 h. Proliferation was measured by [3H]thymidine incorporation. Data are means ± standard deviations from three experiments performed in triplicate. (B) Splenocytes from wild-type and type I−/− type II+/− mice were stimulated with antibodies for 48 h, and cell cycle analysis was performed after PI labeling. The sub-G1 population was quantitated. Results of an experiment representative of three determinations are shown. (C) Cell cycle analysis of activated splenocytes from paired wild-type and type I−/− type II+/− littermates. Cells were stimulated with anti-CD3 plus anti-CD28 antibodies for 48 h. Data are percentages of cells in each stage of the cell cycle.
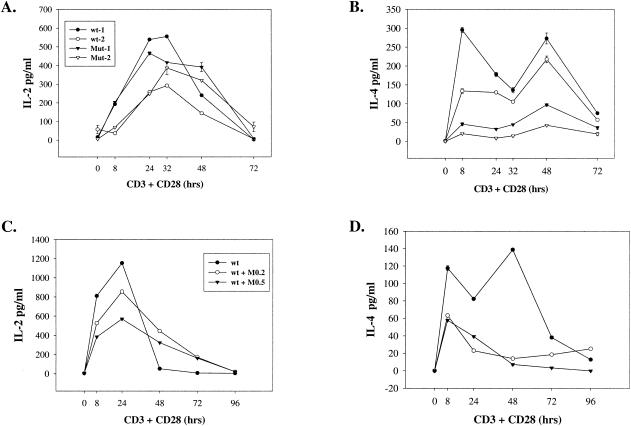
To determine whether cytokine secretion is affected in mutant mice, we cultured splenocytes in the presence of anti-CD3 plus anti-CD28 for various times. IL-2 is normally secreted early in activation with a peak around 24 h, whereas IL-4 is secreted in two phases with peaks around 8 and 48 h. As shown in Fig. 8, splenocytes of double-knockout mice (IMPDH type I−/− HPRT−/0) secreted similar amounts of IL-2 but significantly less IL-4 (Fig. 8A and B) than those from wild-type mice. This decrease in IL-4 secretion was also seen in about half of the mice with the type I−/− type II+/− genotype but was not seen in type I−/− animals. We have also measured gamma interferon (IFN-γ) levels from wild-type and double-knockout (type I−/− type II+/−) mice and observed no significant difference following antibody activation (data not shown). To confirm that the decrease in IL-4 secretion is due to a decrease in GTP levels, we used the IMPDH-specific inhibitor mycophenolic acid (MPA) to treat wild-type splenocytes. IL-2 secretion was somewhat impaired at early time points by treatment with MPA at 0.2 and 0.5 μM concentrations but tended to be more sustained (Fig. 8C). In contrast to the minimal effect on IL-2 secretion, MPA treatment markedly and significantly decreased IL-4 secretion at all time points tested (Fig. 8D).
FIG. 8.
(A and B) Levels of IL-2 and IL-4 secreted by splenocytes of wild-type and type I−/− HPRT −/0 mice. Splenocytes from two wild-type (wt-1 and wt-2) and two IMPDH type I−/− HPRT−/0 (Mut-1 and Mut-2) animals were stimulated with anti-CD3 plus anti-CD28 antibodies. Culture supernatants were collected at the indicated times and measured for the presence of IL-2 (A) and IL-4 (B). Enzyme-linked immunosorbent assays were performed in duplicate. (C and D) Splenocytes from wild-type (wt) mice were activated by antibodies in the absence (solid circles) or presence of 0.2 μM MPA (M0.2) (open circles) or 0.5 μM MPA (M0.5) (solid inverted triangles), and levels of IL-2 (C) and IL-4 (D) secretion were determined in duplicate.
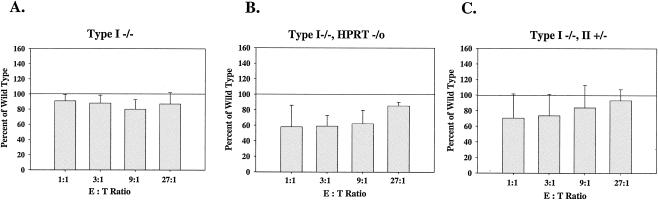
The cytolytic function of T cells was also analyzed using allogeneic T lymphocytes generated from the mixed lymphocyte reaction. It has been shown previously that T cells from IMPDH type II+/− HPRT−/0 mice have impaired cytolytic activity (11). IMPDH type I−/− T cells have no significant defect in cytolytic activity (Fig. 9A), nor do T cells from type II+/− mice (data not shown). However, cytotoxic T cells from IMPDH type I−/− HPRT−/0 mice showed a significant decrease in killing activity against allogeneic target cells (P < 0.05 for all E:T ratios) (Fig. 9B). Lymphocytes from mice with the IMPDH type I−/− type II+/− genotype also demonstrated a decrease in killing activity that achieved marginal significance at E:T ratios of 1:1 and 3:1 but was not statistically significant at E:T ratios of 9:1 and 27:1 (Fig. 9C). These results clearly implicate HPRT activity as an important component of cytolytic T-cell function.
FIG. 9.
Effects of loss of IMPDH and HPRT activities on the cytolytic activities of cytotoxic T lymphocytes. The functions of cytotoxic T lymphocytes from wild-type (100%), IMPDH type I−/− (n = 3) (A), type I−/− HPRT−/0 (n = 5) (B), or type I−/− type II+/− (n = 6) (C) mice were analyzed in 51Cr release assays. Data are means ± standard deviations. Assays were performed in triplicate.
DISCUSSION
The relative contributions of IMPDH type I, IMPDH type II, and HPRT to guanine nucleotide biosynthesis have not been elucidated to date, despite strong evidence that these enzymes are important for the normal activation of peripheral blood lymphocytes. IMPDH inhibitors impair the activation of both T and B lymphocytes in vitro (7, 15, 33) and are in clinical use as immunosuppressive drugs in preserving organ transplants and in treating graft-versus-host disease and autoimmune diseases (13, 16, 22). These IMPDH inhibitors have not been selective for either isoenzyme (15, 31). The specificity of these drugs for the immune system has been attributed to an overall dependence of peripheral blood lymphocyte activation on the de novo rather than the salvage pathway of guanine nucleotide biosynthesis, and the assumption based on relative levels of gene expression has been that IMPDH type II is the more important enzyme in this process. However, the role of IMPDH type I and its ability to compensate for loss of IMPDH type II have never been examined. In view of the widespread expression of the type I enzyme, its upregulation during lymphocyte activation, and the complex promoter region of the human gene, we elected to explore the role of this isoenzyme in tissue development and lymphocyte function in a murine model.
Our results demonstrate that IMPDH type I enzymatic activity is not essential for normal mouse development or fertility. However, the expression of IMPDH type I is clearly tissue specific; levels of mRNA in the thymus, lung, and brain are essentially equivalent to those of the type II gene. In contrast, the liver and testis had no detectable type I mRNA or protein. A higher-molecular-weight form of type I protein, as confirmed by its absence in the knockout animals, was routinely found in the thymus and the lung. This form of the protein is presumably the product of an alternatively spliced mRNA species.
No compensatory increase in either IMPDH type II or HPRT was observed in response to the loss of IMPDH type I at either the mRNA or the protein level. This finding is of interest in relation to the clear demonstration of regulation of IMPDH type II mRNA, possibly at the posttranscriptional level, by alterations in intracellular levels of guanine nucleotides (9); human promyelocytic or melanoma cells treated with the IMPDH inhibitor MPA had elevated levels of type II IMPDH mRNA, while cells exposed to guanosine had reduced levels. It is possible that in our IMPDH type I−/− or type II+/− mice the GTP pools did not decrease sufficiently to trigger this compensatory increase. Alternatively, this may be a cell type-specific phenomenon or an acute rather than a chronic response to a reduction in guanine nucleotide pools.
Since the major objective of this study was to determine the relative contributions of IMPDH type I to lymphocyte activation and function, we determined the effects of the loss of this enzyme, either alone or in combination with the loss of one allele of the type II gene or the loss of HPRT, on splenocyte activation in response to various mitogenic stimuli. Loss of IMPDH type I alone in the absence of other mutations did not significantly reduce proliferation in response to ConA, LPS, or PMA plus ionomycin, whereas double-knockout mice demonstrated a more general inhibition of proliferation. However, both single- and double-knockout mice showed significant reductions in lymphocyte activation in response to anti-CD3 plus anti-CD28 antibodies. These results strongly suggest a more selective inhibitory effect on activation through the T-cell-receptor signaling pathway.
Upon T-cell activation, T helper (Th) cells can be differentiated into at least two subsets: Th1 cells, producing IL-2, IFN-γ, and lymphotoxin, and Th2 cells, producing IL-4, IL-5, and IL-13 (25). Previous studies had indicated that treatment of T cells with an IMPDH inhibitor did not affect IL-2 or IL-2 receptor mRNA expression levels after stimulation with PMA and ionomycin (33). However, other studies have demonstrated that IMPDH inhibitors can decrease IFN-γ production after allogeneic stimulation (21) or reduce both Th1 and Th2 cytokine gene expression in animal models of allergic encephalomyelitis (32). We have demonstrated that mice deficient in both IMPDH type I and HPRT have both a lower level of IL-4 secretion after antibody stimulation and a 35% reduction in GTP levels, whereas mice with the IMPDH type I−/− type II+/− genotype have a less consistent decrease in IL-4 secretion. To validate the relationship between the drop in guanine nucleotide synthesis and IL-4 secretion, we showed that MPA treatment of wild-type splenocytes resulted in a marked reduction in IL-4 production, with only a slight effect on IL-2. Production of IL-5 from activated wild-type splenocytes was totally abolished by MPA treatment (data not shown). Since the production of IL-4 during early activation is important for further development of Th2 cells, our results suggest that, with lower levels of guanine nucleotides, Th2 cell development following anti-CD3 plus anti-CD28 stimulation might be impaired. Th1 cell differentiation appears to be less sensitive to GTP levels, since we did not detect a decrease in IL-2 levels in our double-mutant (type I−/− HPRT−/0 or type I−/− type II+/−) mice. It has been demonstrated that both T-cell-receptor-mediated activation of the Ras-mitogen-activated protein kinase pathway (18, 34) and the costimulatory CD28 signaling pathway (19, 28) are very important for Th2 cell differentiation and Th2 cytokine production. It is possible that the decrease in GTP levels, in addition to inhibiting T-cell proliferation, may also impair Th2 cell development and result in a reduction in Th2 cytokine production.
Finally, lymphocytes from IMPDH type I−/− mice crossed with HPRT-deficient mice showed a significant decrease in cytotoxic T-lymphocyte function, whereas IMPDH type I−/− and type I−/− type II+/− splenocytes did not. The consistent effect of the loss of HPRT in enhancing the effects of the loss of IMPDH type I or type II on proliferation as well as cytolytic function, in conjunction with the striking increase in HPRT mRNA levels with T-cell activation, strongly supports a role for this enzyme in lymphocyte activation. We conclude from these studies that there are overlapping contributions from IMPDH type I, IMPDH type II, and HPRT to the provision of the increased levels of guanine nucleotides required for normal lymphocyte activation in response to mitogens and for cytokine production and normal cytolytic T-cell function. The availability of genetic models of deficiencies of these enzymes should aid in the dissection of the specific signaling pathway sensing mechanisms, such as Rho family GTPases (27), phosphatidylinositol 3-kinase (2, 26), and ZAP-70 (29), that display reduced function under conditions of guanine nucleotide reduction.
Acknowledgments
We thank Carol Beach at the Protein Sequencing Analysis and Macromolecular Structure Analysis Facility, University of Kentucky, for analyzing GTP levels by HPLC.
This work was supported by NIH grant RO1-CA64192 to B. S. Mitchell.
REFERENCES
- 1.Carr, S. F., E. Papp, J. C. Wu, and Y. Natsumeda. 1993. Characterization of human type I and type II IMP dehydrogenases. J. Biol. Chem. 268:27286-27290. [PubMed] [Google Scholar]
- 2.Carrera, A. C., L. Rodriguez-Borlado, C. Martinez-Alonso, and I. Merida. 1994. T cell receptor-associated α-phosphatidylinositol 3-kinase becomes activated by T cell receptor cross-linking and requires pp56lck. J. Biol. Chem. 269:19435-19440. [PubMed] [Google Scholar]
- 3.Collart, F. R., C. B. Chubb, B. L. Mirkin, and E. Huberman. 1992. Increased inosine-5′-phosphate dehydrogenase gene expression in solid tumor tissues and tumor cell lines. Cancer Res. 52:5826-5828. [DOI] [PubMed] [Google Scholar]
- 4.Collart, F. R., and E. Huberman. 1988. Cloning and sequence analysis of the human and Chinese hamster inosine-5′-monophosphate dehydrogenase cDNAs. J. Biol. Chem. 263:15769-15772. [PubMed] [Google Scholar]
- 5.Dayton, J. S., T. Lindsten, C. B. Thompson, and B. S. Mitchell. 1994. Effects of human T lymphocyte activation on inosine monophosphate dehydrogenase expression. J. Immunol. 152:984-991. [PubMed] [Google Scholar]
- 6.Dayton, J. S., L. A. Turka, C. B. Thompson, and B. S. Mitchell. 1992. Comparison of the effects of mizoribine with those of azathioprine, 6-mercaptopurine, and mycophenolic acid on T lymphocyte proliferation and purine ribonucleotide metabolism. Mol. Pharmacol. 41:671-676. [PubMed] [Google Scholar]
- 7.Eugui, E. M., S. J. Almquist, C. D. Muller, and A. C. Allison. 1991. Lymphocyte-selective cytostatic and immunosuppressive effects of mycophenolic acid in vitro: role of deoxyguanosine nucleotide depletion. Scand. J. Immunol. 33:161-173. [DOI] [PubMed] [Google Scholar]
- 8.Futer, O., M. D. Sintchak, P. R. Caron, E. Nimmesgern, M. T. DeCenzo, D. J. Livingston, and S. A. Raybuck. 2002. A mutational analysis of the active site of human type II inosine 5′-monophosphate dehydrogenase. Biochim. Biophys. Acta 1594:27-39. [DOI] [PubMed] [Google Scholar]
- 9.Glesne, D. A., F. R. Collart, and E. Huberman. 1991. Regulation of IMP dehydrogenase gene expression by its end products, guanine nucleotides. Mol. Cell. Biol. 11:5417-5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu, J. J., J. Spychala, and B. S. Mitchell. 1997. Regulation of the human inosine monophosphate dehydrogenase type I gene. Utilization of alternative promoters. J. Biol. Chem. 272:4458-4466. [DOI] [PubMed] [Google Scholar]
- 11.Gu, J. J., S. Stegmann, K. Gathy, R. Murray, J. Laliberte, L. Ayscue, and B. S. Mitchell. 2000. Inhibition of T lymphocyte activation in mice heterozygous for loss of the IMPDH II gene. J. Clin. Investig. 106:599-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hager, P. W., F. R. Collart, E. Huberman, and B. S. Mitchell. 1995. Recombinant human inosine monophosphate dehydrogenase type I and type II proteins. Purification and characterization of inhibitor binding. Biochem. Pharmacol. 49:1323-1329. [DOI] [PubMed] [Google Scholar]
- 13.Hauser, I. A., and R. B. Sterzel. 1999. Mycophenolate mofetil: therapeutic applications in kidney transplantation and immune-mediated renal disease. Curr. Opin. Nephrol. Hypertens. 8:1-6. [DOI] [PubMed] [Google Scholar]
- 14.Jackson, R. C., G. Weber, and H. P. Morris. 1975. IMP dehydrogenase, an enzyme linked with proliferation and malignancy. Nature 256:331-333. [DOI] [PubMed] [Google Scholar]
- 15.Jain, J., S. J. Almquist, D. Shlyakhter, and M. W. Harding. 2001. VX-497: a novel, selective IMPDH inhibitor and immunosuppressive agent. J. Pharm. Sci. 90:625-637. [DOI] [PubMed] [Google Scholar]
- 16.Kokado, Y., M. Ishibashi, H. Jiang, S. Takahara, and T. Sonoda. 1989. A new triple-drug induction therapy with low dose cyclosporine, mizoribine and prednisolone in renal transplantation. Transplant. Proc. 21:1575-1578. [PubMed] [Google Scholar]
- 17.Kontgen, F., R. J. Grumont, A. Strasser, D. Metcalf, R. Li, D. Tarlinton, and S. Gerondakis. 1995. Mice lacking the c-rel proto-oncogene exhibit defects in lymphocyte proliferation, humoral immunity, and interleukin-2 expression. Genes Dev. 9:1965-1977. [DOI] [PubMed] [Google Scholar]
- 18.Koprak, S., M. J. Staruch, and F. J. Dumont. 1999. A specific inhibitor of the p38 mitogen activated protein kinase affects differentially the production of various cytokines by activated human T cells: dependence on CD28 signaling and preferential inhibition of IL-10 production. Cell. Immunol. 192:87-95. [DOI] [PubMed] [Google Scholar]
- 19.Kubo, M., M. Yamashita, R. Abe, T. Tada, K. Okumura, J. T. Ransom, and T. Nakayama. 1999. CD28 costimulation accelerates IL-4 receptor sensitivity and IL-4-mediated Th2 differentiation. J. Immunol. 163:2432-2442. [PubMed] [Google Scholar]
- 20.Laliberte, J., A. Yee, Y. Xiong, and B. S. Mitchell. 1998. Effects of guanine nucleotide depletion on cell cycle progression in human T lymphocytes. Blood 91:2896-2904. [PubMed] [Google Scholar]
- 21.Lui, S. L., V. Ramassar, J. Urmson, and P. F. Halloran. 1998. Mycophenolate mofetil reduces production of interferon-dependent major histocompatibility complex induction during allograft rejection, probably by limiting clonal expansion. Transplant. Immunol. 6:23-32. [DOI] [PubMed] [Google Scholar]
- 22.Mita, K., N. Akiyama, T. Nagao, H. Sugimoto, S. Inoue, T. Osakabe, Y. Nakayama, K. Yokota, K. Sato, and H. Uchida. 1990. Advantages of mizoribine over azathioprine in combination therapy with cyclosporine for renal transplantation. Transplant. Proc. 22:1679-1681. [PubMed] [Google Scholar]
- 23.Natsumeda, Y., T. Ikegami, K. Murayama, and G. Weber. 1988. De novo guanylate synthesis in the commitment to replication in hepatoma 3924A cells. Cancer Res. 48:507-511. [PubMed] [Google Scholar]
- 24.Natsumeda, Y., S. Ohno, H. Kawasaki, Y. Konno, G. Weber, and K. Suzuki. 1990. Two distinct cDNAs for human IMP dehydrogenase. J. Biol. Chem. 265:5292-5295. [PubMed] [Google Scholar]
- 25.O'Garra, A., and N. Arai. 2000. The molecular basis of T helper 1 and T helper 2 cell differentiation. Trends Cell Biol. 10:542-550. [DOI] [PubMed] [Google Scholar]
- 26.Pages, F., M. Ragueneau, R. Rottapel, A. Truneh, J. Nunes, J. Imbert, and D. Olive. 1994. Binding of phosphatidylinositol-3-OH kinase to CD28 is required for T-cell signalling. Nature 369:327-329. (Erratum, 370:157.) [DOI] [PubMed]
- 27.Reif, K., and D. A. Cantrell. 1998. Networking Rho family GTPases in lymphocytes. Immunity 8:395-401. [DOI] [PubMed] [Google Scholar]
- 28.Rulifson, I. C., A. I. Sperling, P. E. Fields, F. W. Fitch, and J. A. Bluestone. 1997. CD28 costimulation promotes the production of Th2 cytokines. J. Immunol. 158:658-665. [PubMed] [Google Scholar]
- 29.Salojin, K. V., J. Zhang, and T. L. Delovitch. 1999. TCR and CD28 are coupled via ZAP-70 to the activation of the Vav/Rac-1-/PAK-1/p38 MAPK signaling pathway. J. Immunol. 163:844-853. [PubMed] [Google Scholar]
- 30.Senda, M., and Y. Natsumeda. 1994. Tissue-differential expression of two distinct genes for human IMP dehydrogenase (E.C. 1.1.1.205). Life Sci. 54:1917-1926. [DOI] [PubMed] [Google Scholar]
- 31.Sintchak, M. D., M. A. Fleming, O. Futer, S. A. Raybuck, S. P. Chambers, P. R. Caron, M. A. Murcko, and K. P. Wilson. 1996. Structure and mechanism of inosine monophosphate dehydrogenase in complex with the immunosuppressant mycophenolic acid. Cell 85:921-930. [DOI] [PubMed] [Google Scholar]
- 32.Tran, G. T., N. Carter, and S. J. Hodgkinson. 2001. Mycophenolate mofetil treatment accelerates recovery from experimental allergic encephalomyelitis. Int. Immunopharmacol. 1:1709-1723. [DOI] [PubMed] [Google Scholar]
- 33.Turka, L. A., J. Dayton, G. Sinclair, C. B. Thompson, and B. S. Mitchell. 1991. Guanine ribonucleotide depletion inhibits T cell activation. Mechanism of action of the immunosuppressive drug mizoribine. J. Clin. Investig. 87:940-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamashita, M., M. Kimura, M. Kubo, C. Shimizu, T. Tada, R. M. Perlmutter, and T. Nakayama. 1999. T cell antigen receptor-mediated activation of the Ras/mitogen-activated protein kinase pathway controls interleukin 4 receptor function and type-2 helper T cell differentiation. Proc. Natl. Acad. Sci. USA 96:1024-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zimmermann, A. G., J. J. Gu, J. Laliberte, and B. S. Mitchell. 1998. Inosine-5′-monophosphate dehydrogenase: regulation of expression and role in cellular proliferation and T lymphocyte activation. Prog. Nucleic Acid Res. Mol. Biol. 61:181-209. [DOI] [PubMed] [Google Scholar]
- 36.Zimmermann, A. G., J. Spychala, and B. S. Mitchell. 1995. Characterization of the human inosine-5′-monophosphate dehydrogenase type II gene. J. Biol. Chem. 270:6808-6814. [DOI] [PubMed] [Google Scholar]