Abstract
The silencer of death domains (SODD) has been proposed to prevent constitutive signaling of tumor necrosis factor receptor 1 (TNFR1) in the absence of ligand. Besides TNFR1, death receptor 3 (DR3), Hsp70/Hsc70, and Bcl-2 have been characterized as binding partners of SODD. In order to investigate the in vivo role of SODD, we generated mice congenitally deficient in expression of the sodd gene. No spontaneous inflammatory infiltrations were observed in any organ of these mice. Consistent with this finding, in the absence of SODD no alteration in the activation patterns of nuclear factor κB (NF-κB), stress kinases, or ERK1 or -2 was observed after stimulation with tumor necrosis factor (TNF). Activation of NF-κB by DR3 was also unchanged. The extents of DR3- and TNF-induced apoptosis were comparable in gene-deficient and wild-type cells. Protection of cells against heat shock as mediated by the Hsp70 system and against staurosporine-induced apoptosis was independent of SODD. Furthermore, resistance to high-dose lipopolysaccharide (LPS) injections, LPS-d-GalN injections, and infection with listeriae was similar in wild-type and gene-deficient mice. In conclusion, our data do not support the concept of a unique, nonredundant role of SODD for the functions of TNFR1, Hsp70, and DR3.
In a yeast-two-hybrid screen, the silencer of death domains (SODD) was isolated as the binding partner of death receptor 3 (DR3), a member of the tumor necrosis factor receptor (TNFR) family which has been described as playing a role in negative selection during thymocyte development (20, 47). The cytoplasmic death domain of DR3 was identified as the domain interacting with SODD. The death domain of DR3 shares a high degree of homology with its counterpart in TNFR1, and not surprisingly, SODD was also found to bind to the death domain of TNFR1, although it did not bind to the death domains of other death receptor family members such as Fas, DR4, and DR5. Likewise, SODD was unable to interact with TNFR2 (20). SODD is a 457-amino-acid (aa) cytosolic protein that lacks death domains. Overexpression of SODD and RNA antisense experiments suggested a role for SODD in preventing ligand-independent TNFR1 signaling (20). According to the proposed model, SODD associates with TNFR1 in the absence of tumor necrosis factor (TNF) and thereby keeps the receptor molecules in a conformation that inhibits spontaneous signaling. In the presence of TNF, SODD dissociates, and the receptor-triggered signaling cascades can be initiated by binding of TRADD and other downstream signaling proteins, leading to activation of NF-κB and stress kinases or to apoptosis. In vivo, uncontrolled signaling of TNFR1 can lead to hyperinflammation or to cell death by apoptosis (23, 24). Recently, it has been shown that (i) TNFR1 is present in aggregates in ATP-depleted cells, (ii) SODD is able to disassemble TNFR1 aggregates only in the presence of ATP, and (iii) TNFR1 has the potential to function as an ATPase (27). From these data the authors deduced a model in which a nucleotide-dependent conformational change in TNFR1 has a key role in regulating TNF signaling.
Structurally, SODD belongs to the BAG (bcl-2-associated athanogene) protein family (42), and therefore it is also named BAG-4. Members of the BAG protein family are defined by the presence of a conserved C-terminal domain (about 45 to 70 aa) referred to as the BAG domain. The prototype and best-characterized member of the BAG protein family is BAG-1. BAG-1 inhibits the chaperone activity of the Hsp/Hsc70 system (4, 38, 40) and, when overexpressed, confers enhanced resistance to apoptosis (9, 37, 41). The latter effect may depend on the capacity of BAG-1 to bind to the Hsp/Hsc70 system and/or to the antiapoptotic protein Bcl-2 (41). SODD/BAG-4 also has the potential to interact directly, by virtue of the C-terminal BAG domain, with Hsp70 and Bcl-2, both proteins that have functions in apoptosis regulation (1, 27). Bcl-2 inhibits the activation of the downstream apoptosis system by preventing the release of cytochrome c from the mitochondria into the cytosol (22, 48). Hsp70 can hinder the recruitment of procaspase-9 to the “apoptosome,” a signaling complex containing released cytochrome c and Apaf-1 (3, 36). Hsp70 has been shown to block apoptosis induced by many different agents including TNF (45). It has been speculated that SODD serves as a cellular adaptor recruiting Hsp/Hsc70 to the TNFR1 complex and thereby inducing conformational changes that prevent receptor signaling in the absence of ligand (43). In view of the fact that overexpression of SODD prevents TNF-induced apoptosis (20), it seems conceivable that apoptosis in human pancreatic cancer cells is impaired by SODD, since high expression of SODD was selectively found in such tumors (31).
BAG-3, SODD/BAG-4, and BAG-5 contain closely related BAG domains (6). BAG-3 is known to bind Bcl-2 and Hsp/Hsc70 and to function as an apoptosis inhibitor when overexpressed (1). Although it was initially surmised that the BAG domain is not responsible for binding to TNFR1 (43), it has recently been demonstrated that SODD can bind through its BAG domain to TNFR1 (27). This raises the possibility that other BAG domain-containing proteins may also associate with TNFR1. To date it is unclear whether BAG proteins other than SODD/BAG-4 can actually bind to TNFR1.
Despite the extensive knowledge about binding partners of SODD such as TNFR1, DR3, Hsp/Hsc70, and Bcl-2, it is not known whether in vivo SODD is necessary or dispensable for the function of these proteins. To determine the in vivo role of SODD, we generated mice that were deficient in the expression of SODD. Unexpectedly, no signs of uncontrolled TNFR1 signaling such as hyperinflammation were identified in these mice. Moreover, in sodd-deficient cells, NF-κB and stress kinases were not constitutively active, and after TNF stimulation, they were activated and again deactivated in the same way as in wild-type cells. DR3- and TNF-induced apoptosis and NF-κB activation by DR3 were also unaltered in the absence of SODD. In agreement with these findings, no difference was observed between wild-type and gene-deficient mice when they were challenged with listeriae, lipopolysaccharide (LPS), or LPS-d-galactosamine hydrochloride (LPS-d-GalN). Survival of these challenges greatly depends on the functional mode of TNFR1 (12, 24, 34, 35), and thus one would expect to see differences if TNFR1 signaling was significantly altered. Moreover, Hsp70-mediated protection against lethal heat shock and Bcl-2-influenced, staurosporine-induced apoptosis were independent of SODD. In summary, our data argue against a major, nonredundant role of SODD in the function of TNFR1, DR3, Hsp70, or Bcl-2.
MATERIALS AND METHODS
Generation of sodd−/− mice.
Full-length murine SODD cDNA was cloned by reverse transcription-PCR on a splenic cDNA library (Invitrogen). For this purpose, sequences from a public expressed sequence tag (EST) database (National Center for Biotechnology Information DNA database) were used to design primers which were positioned upstream of the start codon and downstream of the stop codon. A mouse genomic bacterial artificial chromosome (BAC) library (Genome Systems Inc.) was screened by using a probe whose sequence was derived from the first coding exon of sodd. One BAC clone containing all protein-coding exons was identified. The sodd locus was mapped by restriction enzyme digestions and sequencing of all exons and exon-intron boundaries. A conventional targeting construct was cloned in order to replace the first coding exon shortly after the start codon by insertion of a neomycin resistance cassette in the opposite orientation. The targeting vector was linearized with SalI and electroporated into E14.1 embryonic stem (ES) cells as described previously (34). G418- and ganciclovir-resistant ES cell colonies were picked and subsequently screened for homologous recombination by PCR (5′-TGTCGATGTCCTCGATGAGGC-3′ and 5′-ACGAGTTCTTCTGAGGGGATCG-3′; expected size of PCR product, 715 bp) and Southern blotting after digestion of ES cell DNA with EcoRI and hybridization with a flanking probe (a 0.5-kb fragment located directly 5′ of the targeting vector in the genomic locus). Single integration of the targeting vector was verified by Southern probing with the neomycin resistance cassette. Chimeric mice were produced by microinjection of targeted ES cells into C57BL/6 blastocysts according to standard methods (34) and were mated with wild-type C57BL/6 mice to produce heterozygous mice. Littermates from the mating of heterozygous mice were then analyzed. Mice were housed in an animal facility with barrier conditions. Routine genotyping was performed by PCR analysis of genomic tail DNA (for the knockout allele, primers 5′-TTCCGCGCTAGGCGTACAAGG-3′ and 5′-ACGAGTTCTTCTGAGGGGATCG-3′ were used; for the wild-type allele, primers were 5′-GCCCCGGCCGAGACCACCTG-3′ and 5′-TCGCATCTCTGGTCCTCGTC-3′).
Cells, antibodies, reagents, and Western blotting.
In a given experiment, gene-deficient and wild-type murine embryonic fibroblasts (MEFs) derived from the same litter were used. Experiments were repeated with MEFs (+/+ and −/−) from a second litter, in all cases confirming the earlier results. A total of (1.2 to 1.5) × 105 MEFs were grown in 6-well-plates in Dulbecco's modified Eagle medium (GIBCO BRL) supplemented with 5% heat-inactivated fetal calf serum (Seromed), 2 mM l-glutamine (Seromed), 50 μM 2-mercaptoethanol (GIBCO BRL), 50 μg of streptomycin (Seromed)/ml, and 100 U of penicillin/ml and were treated as indicated in the figure legends. After treatment, cells were lysed in the wells in lysis buffer containing 50 mM HEPES (pH 7.5), 150 mM NaCl, 1 mM EDTA, 10% glycerol, 1% Triton X-100, 5 mM para-nitrophenylphosphate, 20 mM β-glycerophosphate, 10 μg of leupeptin/ml, 2 mM orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 10 mM sodium fluoride, and 10 μg of aprotinin/ml. Lysates were cleared by centrifugation at 10,000 × g for 10 min, boiled in sodium dodecyl sulfate (SDS) sample buffer, resolved on a 10% acrylamide-SDS gel, and blotted onto nitrocellulose membranes. Membranes were probed with the indicated antibodies and visualized using enhanced chemiluminescence (ECL; Amersham) for detection. Tissue lysates were prepared by homogenization in a buffer containing 30 mM NaHCO3, 1 mM dithiothreitol, and 1:25-diluted proteinase inhibitor mix (Roche). The homogenates were centrifuged at 1,000 × g for 5 min at 4°C. The resultant supernatants were spun at 25,000 × g for 90 min at 4°C. The supernatants from the latter step were taken for determination of protein concentrations. After adjustment of the total protein concentration to 7.5 mg/ml, supernatants were boiled in SDS sample buffer and processed for immunoblotting as described above. The following primary antibodies were used: polyclonal rabbit anti-C-terminal SODD (Imgenex), polyclonal rabbit anti-N-terminal SODD (ProSci), monoclonal mouse anti-Hsp70 (StressGen), polyclonal rabbit anti-phospho-p38 mitogen-activated protein kinase (Thr180/Tyr182) (New England Biolabs), polyclonal rabbit anti-p38 mitogen-activated protein kinase (New England Biolabs), polyclonal rabbit anti-phospho-SAPK/JNK (Thr183/Tyr185) (New England Biolabs), polyclonal rabbit anti-SAPK/JNK (New England Biolabs), polyclonal rabbit anti-phospho-ERK1/2 (Thr202/204) (New England Biolabs), polyclonal rabbit anti-ERK1/2 (Upstate Biotechnology), and polyclonal rabbit anti-IκBα (Upstate Biotechnology). For stimulation of cells, murine TNF (R&D Systems) and human TNF (Genzyme) were used at the indicated concentrations. Staurosporine and cycloheximide were purchased from Sigma.
Luciferase assays.
To investigate TNF- and DR3-induced NF-κB transcriptional activity, we transfected MEFs with 5 μg of the NF-κB-dependent immunoglobulin(κ) [Ig(κ)]-luciferase reporter vector and 0.1 μg of a constitutive β-galactosidase expression vector. For measurement of DR3-induced NF-κB activity, 10 μg of a DR3-expression vector (pTRAMP; a kind gift of J. Tschopp) was cotransfected. The overall amount of plasmid DNA was held at 20 μg per electroporation by addition of the appropriate empty expression vector. Electroporation of 1.5 × 106 cells was performed in a 400-μl final volume (RPMI-25% fetal calf serum) at 250 V and 960 μF in a Bio-Rad gene pulser. After electroporation, cells were washed and split onto 12-well-plates (2.5 × 105 cells per well). At 24 h posttransfection, cells were treated as indicated in the figure legends and tables. Cell extracts were prepared, and luciferase assays were performed, according to the manufacturer's instructions (Promega).
MTT assays.
MEFs (1.5 × 104/well) were grown in 6-well-plates and treated as indicated in the figure legends. For the relative quantification of cell survival, cells were once washed in phosphate-buffered saline (PBS) and cultivated for 5 h at 37°C in 1 ml of Dulbecco's modified Eagle medium (supplemented as described above) containing 0.5 mg of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma), allowing living cells to convert the MTT compound. The reaction was stopped by addition of 1.5 ml of hydrochloric acid (0.04 N) dissolved in isopropanol. After 1 h the absorbance at 570 nm was measured.
Apoptosis induction by heat shock.
MEFs (1.5 × 104/well) were grown in 6-well-plates at 37°C under 8% carbon dioxide. For heat shock treatment, plates were sealed in plastic bags and submerged for the indicated time in a 45°C water bath. After this, the culture plates were unpacked and again incubated at 37°C under 8% carbon dioxide as described in the figure legends. The relative amount of surviving cells was determined by an MTT-assay.
High-dose LPS injections, LPS-d-GalN injections, and listeria infections.
The indicated amounts of LPS (Sigma; catalog no. L-2880) dissolved in PBS or LPS together with 20 mg of d-GalN (Roth) dissolved in PBS were intraperitoneally (i.p.) injected into mice in a volume of 200 μl. The survival of mice was monitored for 1 week. For listeria infections, overnight cultures of Listeria monocytogenes (ATCC 43251) were grown in brain-heart infusion (Difco), adjusted to an optical density of 0.7, and serially 10-fold diluted in medium. A 350-μl volume of the second (5 × 106 bacteria) to fourth (5 × 104 bacteria) dilution was injected i.p. into mice. The dose of bacteria was checked by plating 10-μl duplicate aliquots of the serial dilutions on Columbia blood agar plates and counting the CFU after overnight incubation at 37°C. The survival of mice was observed for 20 days.
RESULTS
Generation of sodd−/− mice.
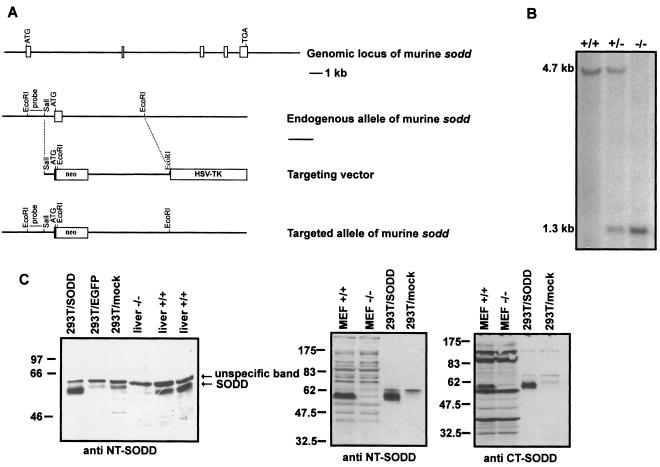
The strategy used to inactivate the sodd gene in ES cells is shown in Fig. 1A. We chose to disrupt the first coding exon by replacing most of its sequence downstream of the start codon with a neomycin resistance cassette inserted in the antisense direction. By leaving the start codon intact, transcription starting from the endogenous sodd promoter of the manipulated locus would lead to multiple in-frame translational termination sites within the neomycin resistance cassette. Homologous recombination into the sodd locus was visualized by reduction of a 4.7-kb wild-type EcoRI fragment to a 1.3-kb fragment on Southern blots hybridized with a flanking probe taken from a region upstream of the targeting vector's short arm (data not shown). Single-site integration could be proven by demonstrating a single band of the expected size (3.6 kb) on Southern blots by using a neo probe and HindIII-digested ES cell DNA (data not shown). The homologous recombination frequency was 2.6%. After ES cell injection into blastocysts and mating of the resulting chimeras with C57BL/6 mice, mice heterozygous for the targeted allele were identified by screening for 5′ recombination in genomic Southern blots of EcoRI-digested tail DNA (Fig. 1B). Breeding of heterozygous (sodd+/−) mice generated homozygous sodd-null (sodd−/−) mice in normal Mendelian and male/female ratios (data not shown). The absence of SODD was confirmed in protein extracts of sodd−/− livers and sodd−/− MEFs by Western blotting using polyclonal antibodies directed against the N- and C-terminal ends of SODD, respectively (Fig. 1C). The polyclonal antibodies used were cross-reactive to human and murine SODD. For specificity control, murine SODD was overexpressed in 293T human embryonic kidney cells, leading to detection of a strong (double) band with an apparent molecular size of 62 kDa, representing murine SODD (Fig. 1C). In MEFs and livers from wild-type littermate controls, expression of SODD could be visualized easily (Fig. 1C).
FIG. 1.
Genetic inactivation of murine sodd. (A) Targeting strategy used for inactivation of the murine sodd gene. (B) Southern blot analysis of genomic tail DNA digested with the restriction enzyme EcoRI. As predicted, a single 4.7-kb wild-type band and a single 1.3-kb recombinant band were seen. The position of the flanking probe used is shown in panel A. (C) Immunoblots using antibodies directed against the N-terminal (NT) or C-terminal (CT) end of SODD. The absence of SODD protein in protein extracts from livers and MEFs derived from sodd-deficient animals was demonstrated. For control of specificity, murine SODD was overexpressed in 293T human embryonic kidney cells.
Absence of inflammation and normal development of major organs, peripheral lymphoid organs, and lymphocyte subsets in sodd−/− mice.
Gene-deficient mice showed no signs of illness during an observation period ranging from birth to the age of 1 year. Histological analysis of all major organs including the brain, heart, large and small intestines, kidneys, lungs, spleen, and joints revealed no abnormalities (data not shown). The concept of SODD negatively regulating TNFR1 signaling predicted spontaneous inflammatory responses in organs as a sign of constitutive TNFR1 signaling (20). However, no such signs of inflammation were observed in any organ. Peripheral lymphoid organs such as the thymus, spleen, lymph nodes, and Peyer's patches were all normally developed. A regular splenic microarchitecture including primary and secondary B-cell follicles and follicular dendritic cell networks was demonstrated by immunohistochemical labeling of the respective cell populations (data not shown) (for antibodies used, see Endres et al. [11]). In contrast to our findings for sodd−/− mice, Peyer's patches, B-cell follicles, and follicular dendritic cells are absent in tnfr1−/− mice (30, 32, 33). The percentages of splenic CD19+ B-cell and CD3+ T-cell populations were not significantly different in wild-type versus sodd−/− mice (data not shown). There were also no significant differences in the weights and sizes of spleens and thymuses (data not shown).
Comparable TNF- and DR3-induced NF-κB activation in sodd−/− and wild-type mice.
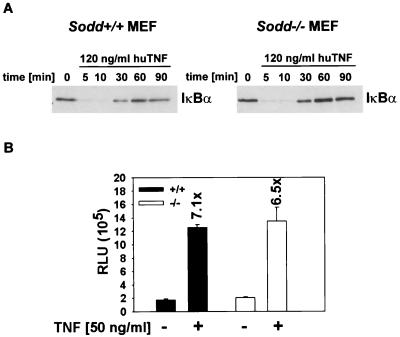
Overexpression of SODD in 293 HEK cells had been shown to prevent NF-κB activation, and in accordance, the expression of antisense SODD RNA had resulted in enhanced NF-κB activation after TNF stimulation (20). We expected to detect a similar overactivation in sodd−/− cells. However, inhibitor of κB alpha (IκBα) was not constitutively degraded in unstimulated sodd−/− MEFs (Fig. 2A), and after TNF stimulation, the time kinetics of the degradation and reaccumulation of IκBα protein were comparable for sodd−/− and wild-type MEFs (Fig. 2A). In agreement with these findings, the transcriptional activity of NF-κB complexes was not altered by the absence of SODD as measured in luciferase assays. For these experiments an NF-κB-dependent promoter was used to drive luciferase expression in sodd−/− or wild-type cells before and after stimulation with human TNF (Fig. 2B). Because SODD is also known to bind to the death domain of DR3, SODD may have an impact on DR3-induced NF-κB activation. Overexpression of DR3 in wild-type MEFs led to a fourfold induction of luciferase activity compared to activity in mock-transfected cells (Table 1). Yet a similar induction of luciferase activity was seen in sodd−/− MEFs (Table 1). Thus, our data strongly argue against a dominant role for SODD in regulating the TNFR1 and DR3 signaling pathways that lead to NF-κB activation.
FIG. 2.
Lack of difference in TNF-induced NF-κB activation between wild-type and gene-deficient cells. (A) Immunoblots showing the kinetics of IκBα degradation after stimulation of MEFs with human TNF (120 ng/ml). For the loading control of the immunoblot, see Fig. 3C, where similar amounts of unphosphorylated ERK1 and -2 per lane are detectable. (B) Luciferase assay using an NF-κB-dependent promoter sequence derived from the Ig(κ) gene. MEFs were transfected with the reporter construct and a vector leading to the constitutive expression of β-galactosidase. After 24 h, cells were either stimulated with human TNF (50 ng/ml) for 8 h or left untreated. Luciferase activity was measured and normalized for β-galactosidase expression. RLU, relative light units. Data are means ± standard deviations for two replicate samples.
TABLE 1.
Lack of effect of SODD on DR3-induced NF-κB-activationa
| MEF genotype | NF-κB activationb
|
Fold activation | |
|---|---|---|---|
| Without DR3 | With DR3 | ||
| sodd+/+ | 1.0 ± 0.07 | 4.36 ± 0.39 | 4.4 |
| sodd−/− | 1.14 ± 0.01 | 4.93 ± 0.29 | 4.3 |
MEFs were transfected by electroporation with 15 μg of the empty vector pEF (without DR3) or with 5 μg of the DR3 expression vector pTRAMP and 10 μg of pEF. All cells were also transfected with 5 μg of an NF-κB-dependent Ig(κ)-luciferase reporter gene and 0.1 μg of the β-galactosidase expression vector pEF-βGal. After 24 h, luciferase activity was measured and normalized for β-galactosidase expression.
Expressed as relative luciferase activity. Data are means ± standard deviations of two replicate samples.
Unaltered activation and deactivation of stress kinases and ERK1 and -2.
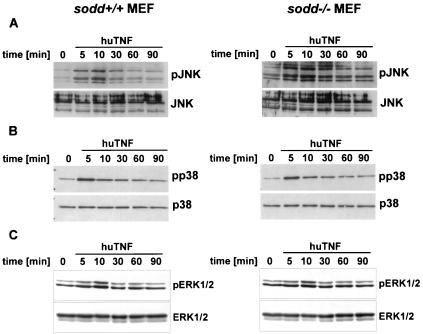
After TNF stimulation, recruitment of TRAF2 to the TNFR1 signaling complex precedes the activation of stress kinases (49). In the absence of TNF, SODD was reported to block the interaction between TRADD, TRAF2, and TNFR1 and thereby to prevent further downstream signaling events leading to the activation of stress kinases such as Jun N-terminal kinase (JNK) and p38 (20). In order to analyze the activation of stress kinases in sodd−/− cells, we triggered the TNFR1 signaling pathways by addition of either human TNF or murine TNF (40 ng/ml) to MEFs. In the mouse, human TNF selectively binds to TNFR1 whereas murine TNF shows specificity for both TNFR1 and TNFR2 (25). In sodd+/+ and sodd−/− cells, the kinetics of phosphorylation and dephosphorylation of JNK and p38 were identical after addition of human TNF (Fig. 3 A and B) or murine TNF (data not shown). Moreover, phosphorylated forms of ERK1 and -2 appeared and disappeared at similar times and quantities after stimulation of MEFs with either human (Fig. 3C) or murine (data not shown) TNF. Collectively, these data indicate that the absence of SODD has no apparent effect on the activation and subsequent deactivation of stress kinases and ERK1 and -2 by TNFR1.
FIG. 3.
Stress kinases and ERK1 and -2 are phosphorylated and dephosphorylated with similar kinetics in wild-type and sodd-deficient cells after TNF stimulation. Immunoblots were performed on protein extracts from MEFs by using antibodies specific for the phosphorylated forms (upper panels) of JNK (A), p38 (B), and ERK1 and -2 (C) as well as antibodies reactive with the total proteins (lower panels) of JNK (A), p38 (B), and ERK1 and -2 (C).
SODD has no critical role in apoptosis triggered by TNF or DR3.
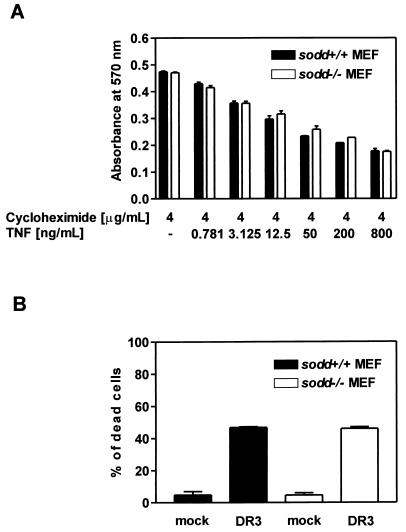
Signals mediated by TNFR1 or DR3 can initiate programmed cell death (5, 8, 15). Overexpression of SODD protected cells against TNF-induced apoptosis, and the presence of SODD antisense RNA increased the rate of apoptosis after TNF stimulation (20). However, an increased rate of apoptosis could not be observed when sodd−/− MEFs were treated with titrated amounts of TNF in the presence of a constant concentration of cycloheximide (Fig. 4A). For analysis of DR3-initiated apoptosis in sodd−/− cells, DR3 was overexpressed in sodd+/+ and sodd−/− MEFs. Transfected apoptotic cells and transfected viable cells were identified by cell morphology as described previously (28). The proportions of apoptotic cells in wild-type versus sodd−/− cells did not differ significantly (Fig. 4B). Thus, our data do not support the notion that SODD has a crucial antiapoptotic function in the TNFR1 and DR3 signal pathways.
FIG. 4.
The rates of TNF- and DR3-induced apoptosis are comparable in wild-type and sodd-deficient MEFs. (A) TNF was titrated on MEFs (150 × 103 cells/well) in the presence of cycloheximide (4 μg/ml). After a 24-h incubation time, the relative number of surviving cells was determined by an MTT assay (see Materials and Methods). The means for two replicate samples are shown. (B) A total of 3 × 106 cells were cotransfected by electroporation either with 15 μg of the empty vector pEF and 5 μg of a β-galactosidase expression vector or with 15 μg of the DR3 expression vector pTRAMP and 5 μg of a β-galactosidase expression vector. Twenty-four hours later, a substrate for visualization of β-galactosidase activity was added. After two more days, transfected viable cells and transfected dead cells were identified by blue staining and cell morphology as described previously (28). The percentage of transfected dead cells in relation to all transfected cells is shown.
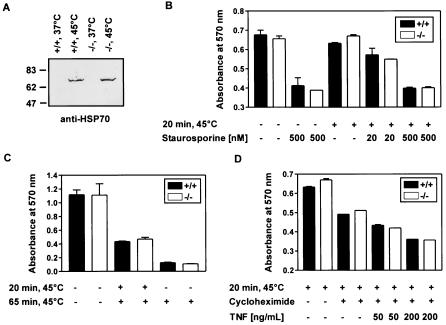
Protection of cells against heat shock- or staurosporine-induced apoptosis is not affected by the absence of SODD.
Hsp70 protects cells against heat-induced death (21) by hindering recruitment of procaspase-9 to the apoptosome (3, 36). In addition, in certain cell types Hsp70 has been reported to impair programmed cell death caused by a variety of different stimuli including TNF (17, 29, 45). Another inhibitor of apoptosis that has been described to great detail is the cellular protein Bcl-2. Bcl-2 acts upstream or at the level of mitochondria and prevents the release of cytochrome c, which occurs as a major signaling step in many cases of apoptosis (22, 48). SODD binds to Bcl-2 (1) and to the ATPase domain of Hsp70 (27) and thus might influence the functions of Bcl-2 and Hsp70, respectively. To test this hypothesis, we induced strong expression of Hsp70 by incubating MEFs at 45°C for 20 min, followed by a 6-h incubation at 37°C (Fig. 5A). SODD was not significantly induced by such treatment (data not shown). High expression of Hsp70 did not alter the susceptibility of MEFs to staurosporine treatment (Fig. 5B), whereas Bcl-2 is known to be a potent inhibitor of staurosporine-induced apoptosis (19, 41). However, the rates of staurosporine-mediated apoptosis in sodd+/+ and sodd−/− cells did not differ significantly (Fig. 5B). MEFs containing high levels of Hsp70 showed enhanced resistance to a subsequent prolonged heat shock (Fig. 5C). Again, however, no difference was observed between wild-type and sodd−/− cells (Fig. 5C). A short heat pretreatment did also not lead to apparent differences in TNF-mediated apoptosis between sodd+/+ and sodd−/− cells (Fig. 5D). Taken together, these data make it appear unlikely that SODD plays an indispensable role in the antiapoptotic functions of Hsp70 or Bcl-2.
FIG. 5.
In the presence of Hsp70 expressed at high levels, sodd-deficient cells do not differ from wild-type cells in their responses to (i) staurosporine, (ii) prolonged heat shock, and (iii) TNF-cycloheximide treatment. (A) Wild-type and sodd-deficient MEFs were incubated for 20 min at 45°C or left at 37°C. After an additional 6 h of incubation at 37°C, an immunoblot using a primary antibody directed against Hsp70 revealed comparable induction of Hsp70 in short-term heat-shocked wild-type and sodd-deficient cells. (B, C, and D) MEFs either were not preincubated or were preincubated for 20 min at 45°C. After a subsequent 6-h incubation at 37°C, cells were treated (or not) with the indicated amounts of staurosporine for 12 h (B), with a prolonged (65-min) heat shock at 45°C followed by a 12-h incubation at 37°C (C), or with the indicated amounts of TNF-cycloheximide for 12 h (D). The relative amount of viable cells was determined by an MTT assay (see Materials and Methods). Data are means ± standard deviations from two or three replicate samples.
Comparable susceptibilities of sodd-deficient and wild-type mice to titrated doses of LPS, LPS-d-GalN, and listeriae.
Overcoming acute listeriosis and sensitivity to LPS-d-GalN injection depends on functional TNFR1 signaling (12, 34). Uncurbed TNFR1 signaling, on the other hand, results in hypersensitivity to LPS (24). To test a potential impact of SODD on TNFR1 signaling in vivo, sodd−/− and wild-type control mice were injected with titrated amounts of LPS (Table 2), LPS-d-GalN (Table 3), and listeriae (Table 4), and survival was monitored. No genotype-dependent differences were observed in survival rates, arguing against a significant in vivo role of SODD in TNFR1 signaling.
TABLE 2.
Survival after high-dose LPS injection
| Mouse genotype | No. of mice surviving/no. of mice challenged with the following dose of LPS (per g of body weight):
|
||
|---|---|---|---|
| 17.5 μg | 35 μg | 52.5 μg | |
| sodd+/+ | 4/6 | 2/6 | 0/7 |
| sodd−/− | 6/7 | 3/7 | 1/7 |
TABLE 3.
Survival after LPS-d-GalN injection
| Mouse genotype | No. of mice surviving/no. of mice challenged with the following dose of LPS (per 20 g of body weight) plus 20 mg of d-GalN:
|
||
|---|---|---|---|
| 0.01 μg | 0.1 μg | 1 μg | |
| sodd+/+ | 6/16 | 1/6 | 0/5 |
| sodd+/− | 7/17 | 0/8 | 0/8 |
| sodd−/− | 5/15 | 2/8 | 1/8 |
TABLE 4.
Survival after listeria infection
| Mouse genotype | No. of mice surviving/no. challenged i.p. with the following no. of listeriae:
|
||
|---|---|---|---|
| 5 × 104 | 5 × 105 | 5 × 106 | |
| sodd+/+ | 8/11 | 4/8 | 1/5 |
| sodd+/− | 7/12 | 4/8 | 1/5 |
| sodd−/− | 7/12 | 3/8 | 0/5 |
DISCUSSION
In TNFR1-expressing cells, binding of TNF is principally capable of triggering activation and nuclear translocation of NF-κB, activation of stress kinases, and activation of caspases. A tight negative regulation of TNFR1 signaling is therefore pivotal for the survival of single cells as well as for the normal physiology of the whole mammalian organism. Artificial overexpression of TNFR1 causes cell death in a ligand-independent manner due to spontaneous self-aggregation and conformational change of death domains. This phenomenon occurs at a higher frequency when these proteins are expressed above physiological levels. In vivo, uncurbed TNFR1 signaling can lead to hyperinflammation and premature death, as seen in mice deficient in the A20 gene (24) and in mice expressing greater than physiological amounts of TNF (23). Several mechanisms for negative regulation of TNFR1 signaling have been identified; internalization of ligand-bound receptors and down-regulation of surface receptors are believed to limit the intensity of TNFR1-mediated signals (14, 16, 44, 46). Expression of the cytoplasmic zinc finger protein A20 is rapidly increased by TNF treatment of cells and leads to a reduced kinase activity of the IκB kinase (IKK) complex and thereby to reduced phosphorylation and degradation of IκBα (24). Phosphorylation of C-terminal serine residues of IKK2 negatively regulates the kinase activity of the IKK complex (10). Furthermore, de novo synthesis of IκBα protein is induced by TNFR1 signaling (2, 39). Increased levels of IκBα can sequester NF-κB complexes and thereby shut off the transcriptional activity of this proinflammatory transcription factor. These mechanisms appear to be necessary in order to turn off TNFR1 signaling once it has been initiated.
SODD has been proposed to prevent constitutive TNFR1 signaling in the absence of TNF by inhibiting intrinsic self-aggregation properties of the death domains and maintaining TNFR1 in an inactive, monomeric state (20). The conception of TNFR1 molecules switching from a monomeric to a trimeric or oligomeric state after binding of ligand has been questioned by a study of Chan et al. showing that in the absence of ligand, TNFR1 molecules preassociate as oligomers on the cell surface (7). According to their model, preassociation depends on the presence of an extracellular domain called the pre-ligand binding assembly domain (PLAD), which is distinct from the ligand binding domain. Although these data appear to be at odds with the earlier model (i.e., that TNF brings monomer receptor chains into apposition in threefold complexes that recruit cytoplasmic signal transduction proteins), a negative regulatory role for SODD in TNFR1 signaling would still appear conceivable. In the preassociated TNFR1 complexes SODD could be considered necessary for maintaining the intracellular death domains in a nonsignaling conformation in the absence of ligand. However, our data make this assumption unlikely.
Signal transduction pathways emanating from TNFR1 have been found to use the same set of interaction partners in all cell types examined. Therefore, we focused our study on the analysis of TNFR1 signaling in one cell type (i.e., MEFs) derived from sodd−/− and wild-type control mice. In the presence and absence of TNF, the functional modes of all major signaling pathways of TNFR1 were not altered to any detectable degree in the absence of SODD. The results were not confounded by TNFR2 signaling, because not only murine TNF but also human TNF was used for stimulation of cells in different sets of experiments. Human TNF specifically binds murine TNFR1 but not murine TNFR2 (25). In agreement with the in vitro findings, in vivo the degrees of resistance against LPS, LPS-d-GalN, and listeriae were not different for sodd−/− and wild-type mice, although survival after injection of these toxic agents and microorganisms is known to depend on the functional mode of TNFR1 signaling (12, 24, 34, 35). Moreover, no signs of (hyper)inflammation were observed in sodd−/− mice. It is hard to reconcile these findings with the conception of a dominant role for SODD in TNFR1 signaling. Likewise, no indication of a major influence of SODD on DR3-induced apoptosis and NF-κB activation was found. Thus, either there are proteins other than SODD that regulate TNFR1 and/or DR3 signaling in a similar way (which would mean that SODD is “functionally redundant”) or SODD is not involved in TNFR1 and/or DR3 signaling at all.
The solution structure of the BAG domain from SODD/BAG-4 was determined by multidimensional nuclear magnetic resonance methods and compared to those of the corresponding domains in other BAG proteins. This structural comparison revealed two subfamilies of BAG proteins, one consisting of BAG-3, SODD/BAG-4, and BAG-5 and the other represented by BAG-1 (6). BAG-1, BAG-3, and SODD/BAG-4 efficiently bind to the ATPase domain of Hsp70 by virtue of their BAG domains (1, 27). In addition, like BAG-1, BAG-3 and SODD/BAG-4 are able to bind to the Bcl-2 oncoprotein (1). The antiapoptotic members of the Bcl-2 protein family such as Bcl-2 and Bcl-XL operate at the level of the mitochondria by preventing the release of cytochrome c during apoptosis (22, 48). Proteins of the Hsp70/Hsc70 system counteract apoptosis at a point downstream of cytochrome c release (3, 18, 26, 36). BAG-1 showed anti-cell death activity after exposure to a variety of apoptotic stimuli (9, 41). BAG-3 overexpression modestly inhibited apoptosis resulting from cytokine deprivation of interleukin-3-dependent 32D cells (1). Expression of full-length SODD, but not SODD with the BAG domain deleted, protected Madin-Darby canine kidney cells from apoptosis after cell matrix detachment (13). The interaction of BAG-3 and SODD with proteins from two families of apoptosis regulators is intriguing, leading to questions about the in vivo significance of these proteins in apoptosis induction. However, our data make a dominant impact of SODD/BAG-4 on Hsp70-mediated protection against heat-induced apoptosis unlikely. Staurosporine-induced apoptosis, a process known to be strongly influenced by the antiapoptotic protein Bcl-2 (19, 41), was also unaltered in sodd−/− cells. These findings raise the possibility of redundancy within the BAG protein family. For example, BAG-3 may functionally replace SODD/BAG-4 in sodd−/− cells. To decide whether such redundancy actually exists, further gene-deficient mouse strains (e.g., bag-3 or bag-5 deficient) have to be generated and crossed to sodd−/− mice. The phenotype of such double- or triple-gene-deficient mice might provide novel insights into the complex functions of this class of proteins in apoptosis and host defense.
Acknowledgments
The continuous and generous support of H. Wagner is greatly appreciated. We thank J. Tschopp for providing the pTRAMP vector and J. Meinicke and J. Vier for excellent technical assistance.
This work was supported by the Deutsche Forschungsgemeinschaft (Sonderforschungsbereich 391 and 576, Schwerpunktprogramm “Angeborene Immunität” Pf 259/3-1, Sachbeihilfe Pf 259/2-5/6).
REFERENCES
- 1.Antoku, K., R. S. Maser, W. J. J. Scully, S. M. Delach, and D. E. Johnson. 2001. Isolation of Bcl-2 binding proteins that exhibit homology with BAG-1 and suppressor of death domains protein. Biochem. Biophys. Res. Commun. 286:1003-1010. [DOI] [PubMed] [Google Scholar]
- 2.Arenzana-Seisdedos, F., J. Thompson, M. S. Rodriguez, F. Bachelerie, D. Thomas, and R. T. Hay. 1995. Inducible nuclear expression of newly synthesized IκBα negatively regulates DNA-binding and transcriptional activities of NF-κB. Mol. Cell. Biol. 15:2689-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beere, H. M., B. B. Wolf, K. Cain, D. D. Mosser, A. Mahboubi, T. Kuwana, P. Tailor, R. I. Morimoto, G. M. Cohen, and D. R. Green. 2000. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat. Cell Biol. 2:469-475. [DOI] [PubMed] [Google Scholar]
- 4.Bimston, D., J. Song, D. Winchester, S. Takayama, J. C. Reed, and R. I. Morimoto. 1998. BAG-1, a negative regulator of Hsp70 chaperone activity, uncouples nucleotide hydrolysis from substrate release. EMBO J. 17:6871-6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodmer, J. L., K. Burns, P. Schneider, K. Hofmann, V. Steiner, M. Thome, T. Bornand, M. Hahne, M. Schroter, K. Becker, A. Wilson, L. E. French, J. L. Browning, H. R. MacDonald, and J. Tschopp. 1997. TRAMP, a novel apoptosis-mediating receptor with sequence homology to tumor necrosis factor receptor 1 and Fas(Apo-1/CD95). Immunity 6:79-88. [DOI] [PubMed] [Google Scholar]
- 6.Briknarova, K., S. Takayama, S. Homma, K. Baker, E. Cabezas, D. W. Hoyt, Z. Li, A. C. Satterthwait, and K. R. Ely. 2002. BAG4/SODD protein contains a short BAG domain. J. Biol. Chem. 277:31172-31178. [DOI] [PubMed] [Google Scholar]
- 7.Chan, F. K., H. J. Chun, L. Zheng, R. M. Siegel, K. L. Bui, and M. J. Lenardo. 2000. A domain in TNF receptors that mediates ligand-independent receptor assembly and signaling. Science 288:2351-2354. [DOI] [PubMed] [Google Scholar]
- 8.Chinnaiyan, A. M., K. O'Rourke, G. L. Yu, R. H. Lyons, M. Garg, D. R. Duan, L. Xing, R. Gentz, J. Ni, and V. M. Dixit. 1996. Signal transduction by DR3, a death domain-containing receptor related to TNFR-1 and CD95. Science. 274:990-992. [DOI] [PubMed] [Google Scholar]
- 9.Clevenger, C. V., K. Thickman, W. Ngo, W. P. Chang, S. Takayama, and J. C. Reed. 1997. Role of Bag-1 in the survival and proliferation of the cytokine-dependent lymphocyte lines, Ba/F3 and Nb2. Mol. Endocrinol. 11:608-618. [DOI] [PubMed] [Google Scholar]
- 10.Delhase, M., M. Hayakawa, Y. Chen, and M. Karin. 1999. Positive and negative regulation of IκB kinase activity through IKKβ subunit phosphorylation. Science. 284:309-313. [DOI] [PubMed] [Google Scholar]
- 11.Endres, R., M. B. Alimzhanov, T. Plitz, A. Futterer, M. H. Kosco-Vilbois, S. A. Nedospasov, K. Rajewsky, and K. Pfeffer. 1999. Mature follicular dendritic cell networks depend on expression of lymphotoxin beta receptor by radioresistant stromal cells and of lymphotoxin beta and tumor necrosis factor by B cells. J. Exp. Med. 189:159-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Endres, R., A. Luz, H. Schulze, H. Neubauer, A. Futterer, S. M. Holland, H. Wagner, and K. Pfeffer. 1997. Listeriosis in p47(phox−/−) and TRp55−/− mice: protection despite absence of ROI and susceptibility despite presence of RNI. Immunity 7:419-432. [DOI] [PubMed] [Google Scholar]
- 13.Frisch, S. M. 1999. Evidence for a function of death-receptor-related, death-domain-containing proteins in anoikis. Curr. Biol. 9:1047-1049. [DOI] [PubMed] [Google Scholar]
- 14.Higuchi, M., and B. B. Aggarwal. 1994. TNF induces internalization of the p60 receptor and shedding of the p80 receptor. J. Immunol. 152:3550-3558. [PubMed] [Google Scholar]
- 15.Hsu, H., H. B. Shu, M. G. Pan, and D. V. Goeddel. 1996. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell 84:299-308. [DOI] [PubMed] [Google Scholar]
- 16.Imamura, K., D. Spriggs, and D. Kufe. 1987. Expression of tumor necrosis factor receptors on human monocytes and internalization of receptor bound ligand. J. Immunol. 139:2989-2992. [PubMed] [Google Scholar]
- 17.Jaattela, M., D. Wissing, P. A. Bauer, and G. C. Li. 1992. Major heat shock protein hsp70 protects tumor cells from tumor necrosis factor cytotoxicity. EMBO J. 11:3507-3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaattela, M., D. Wissing, K. Kokholm, T. Kallunki, and M. Egeblad. 1998. Hsp70 exerts its anti-apoptotic function downstream of caspase-3-like proteases. EMBO J. 17:6124-6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobsen, M. D., M. Weil, and M. C. Raff. 1996. Role of Ced-3/ICE-family proteases in staurosporine-induced programmed cell death. J. Cell Biol. 133:1041-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang, Y., J. D. Woronicz, W. Liu, and D. V. Goeddel. 1999. Prevention of constitutive TNF receptor 1 signaling by silencer of death domains. Science 283:543-546. [DOI] [PubMed] [Google Scholar]
- 21.Johnston, R. N., and B. L. Kucey. 1988. Competitive inhibition of hsp70 gene expression causes thermosensitivity. Science 242:1551-1554. [DOI] [PubMed] [Google Scholar]
- 22.Kluck, R. M., E. Bossy-Wetzel, D. R. Green, and D. D. Newmeyer. 1997. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 275:1132-1136. [DOI] [PubMed] [Google Scholar]
- 23.Kontoyiannis, D., M. Pasparakis, T. T. Pizarro, F. Cominelli, and G. Kollias. 1999. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity 10:387-398. [DOI] [PubMed] [Google Scholar]
- 24.Lee, E. G., D. L. Boone, S. Chai, S. L. Libby, M. Chien, J. P. Lodolce, and A. Ma. 2000. Failure to regulate TNF-induced NF-κB and cell death responses in A20-deficient mice. Science 289:2350-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis, M., L. A. Tartaglia, A. Lee, G. L. Bennett, G. C. Rice, G. H. Wong, E. Y. Chen, and D. V. Goeddel. 1991. Cloning and expression of cDNAs for two distinct murine tumor necrosis factor receptors demonstrate one receptor is species specific. Proc. Natl. Acad. Sci. USA 88:2830-2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li, C. Y., J. S. Lee, Y. G. Ko, J. I. Kim, and J. S. Seo. 2000. Heat shock protein 70 inhibits apoptosis downstream of cytochrome c release and upstream of caspase-3 activation. J. Biol. Chem. 275:25665-25671. [DOI] [PubMed] [Google Scholar]
- 27.Miki, K., and E. M. Eddy. 2002. Tumor necrosis factor receptor 1 is an ATPase regulated by silencer of death domain. Mol. Cell. Biol. 22:2536-2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miura, M., H. Zhu, R. Rotello, E. A. Hartwieg, and J. Yuan. 1993. Induction of apoptosis in fibroblasts by IL-1β-converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell 75:653-660. [DOI] [PubMed] [Google Scholar]
- 29.Mosser, D. D., A. W. Caron, L. Bourget, C. Denis-Larose, and B. Massie. 1997. Role of the human heat shock protein hsp70 in protection against stress-induced apoptosis. Mol. Cell. Biol. 17:5317-5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neumann, B., A. Luz, K. Pfeffer, and B. Holzmann. 1996. Defective Peyer's patch organogenesis in mice lacking the 55-kD receptor for tumor necrosis factor. J. Exp. Med. 184:259-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ozawa, F., H. Friess, A. Zimmermann, J. Kleeff, and M. W. Buchler. 2000. Enhanced expression of Silencer of death domains (SODD/BAG-4) in pancreatic cancer. Biochem. Biophys. Res. Commun. 271:409-413. [DOI] [PubMed] [Google Scholar]
- 32.Pasparakis, M., L. Alexopoulou, V. Episkopou, and G. Kollias. 1996. Immune and inflammatory responses in TNF-α-deficient mice: a critical requirement for TNF-α in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J. Exp. Med. 184:1397-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pasparakis, M., L. Alexopoulou, M. Grell, K. Pfizenmaier, H. Bluethmann, and G. Kollias. 1997. Peyer's patch organogenesis is intact yet formation of B lymphocyte follicles is defective in peripheral lymphoid organs of mice deficient for tumor necrosis factor and its 55-kDa receptor. Proc. Natl. Acad. Sci. USA 94:6319-6323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfeffer, K., T. Matsuyama, T. M. Kundig, A. Wakeham, K. Kishihara, A. Shahinian, K. Wiegmann, P. S. Ohashi, M. Kronke, and T. W. Mak. 1993. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell 73:457-467. [DOI] [PubMed] [Google Scholar]
- 35.Rothe, J., W. Lesslauer, H. Lotscher, Y. Lang, P. Koebel, F. Kontgen, A. Althage, R. Zinkernagel, M. Steinmetz, and H. Bluethmann. 1993. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature 364:798-802. [DOI] [PubMed] [Google Scholar]
- 36.Saleh, A., S. M. Srinivasula, L. Balkir, P. D. Robbins, and E. S. Alnemri. 2000. Negative regulation of the Apaf-1 apoptosome by Hsp70. Nat. Cell Biol. 2:476-483. [DOI] [PubMed] [Google Scholar]
- 37.Sekiya, M., M. Adachi, S. Takayama, J. C. Reed, and K. Imai. 1997. IFN-γ upregulates anti-apoptotic gene expression and inhibits apoptosis in IL-3-dependent hematopoietic cells. Biochem. Biophys. Res. Commun. 239:401-406. [DOI] [PubMed] [Google Scholar]
- 38.Stuart, J. K., D. G. Myszka, L. Joss, R. S. Mitchell, S. M. McDonald, Z. Xie, S. Takayama, J. C. Reed, and K. R. Ely. 1998. Characterization of interactions between the anti-apoptotic protein BAG-1 and Hsc70 molecular chaperones. J. Biol. Chem. 273:22506-22514. [DOI] [PubMed] [Google Scholar]
- 39.Sun, S. C., P. A. Ganchi, D. W. Ballard, and W. C. Greene. 1993. NF-κB controls expression of inhibitor IκBα: evidence for an inducible autoregulatory pathway. Science 259:1912-1915. [DOI] [PubMed] [Google Scholar]
- 40.Takayama, S., D. N. Bimston, S. Matsuzawa, B. C. Freeman, C. Aime-Sempe, Z. Xie, R. I. Morimoto, and J. C. Reed. 1997. BAG-1 modulates the chaperone activity of Hsp70/Hsc70. EMBO J. 16:4887-4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takayama, S., T. Sato, S. Krajewski, K. Kochel, S. Irie, J. A. Millan, and J. C. Reed. 1995. Cloning and functional analysis of BAG-1: a novel Bcl-2-binding protein with anti-cell death activity. Cell 80:279-284. [DOI] [PubMed] [Google Scholar]
- 42.Takayama, S., Z. Xie, and J. C. Reed. 1999. An evolutionarily conserved family of Hsp70/Hsc70 molecular chaperone regulators. J. Biol. Chem. 274:781-786. [DOI] [PubMed] [Google Scholar]
- 43.Tschopp, J., F. Martinon, and K. Hofmann. 1999. Apoptosis: silencing the death receptors. Curr. Biol. 9:R381-R384. [DOI] [PubMed] [Google Scholar]
- 44.van der Poll, T., S. M. Coyle, A. Kumar, K. Barbosa, J. M. Agosti, and S. F. Lowry. 1997. Down-regulation of surface receptors for TNF and IL-1 on circulating monocytes and granulocytes during human endotoxemia: effect of neutralization of endotoxin-induced TNF activity by infusion of a recombinant dimeric TNF receptor. J. Immunol. 158:1490-1497. [PubMed] [Google Scholar]
- 45.Van Molle, W., B. Wielockx, T. Mahieu, M. Takada, T. Taniguchi, K. Sekikawa, and C. Libert. 2002. HSP70 protects against TNF-induced lethal inflammatory shock. Immunity 16:685-695. [DOI] [PubMed] [Google Scholar]
- 46.Vuk-Pavlovic, S., and J. S. Kovach. 1989. Recycling of tumor necrosis factor-alpha receptor in MCF-7 cells. FASEB J. 3:2633-2640. [DOI] [PubMed] [Google Scholar]
- 47.Wang, E. C., A. Thern, A. Denzel, J. Kitson, S. N. Farrow, and M. J. Owen. 2001. DR3 regulates negative selection during thymocyte development. Mol. Cell. Biol. 21:3451-3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang, J., X. Liu, K. Bhalla, C. N. Kim, A. M. Ibrado, J. Cai, T. I. Peng, D. P. Jones, and X. Wang. 1997. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 275:1129-1132. [DOI] [PubMed] [Google Scholar]
- 49.Yeh, W. C., A. Shahinian, D. Speiser, J. Kraunus, F. Billia, A. Wakeham, J. L. de la Pompa, D. Ferrick, B. Hum, N. Iscove, P. Ohashi, M. Rothe, D. V. Goeddel, and T. W. Mak. 1997. Early lethality, functional NF-κB activation, and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity 7:715-725. [DOI] [PubMed] [Google Scholar]