Abstract
An unbiased conditioned place preference paradigm was used to evaluate the effect of dextro-morphine on the morphine-produced reward in male CD rats. Morphine sulfate (1-10 mg/kg) given intraperitoneally dose-dependently produced the conditioned place preference. Pretreatment with dextro-morphine at a dose from 0.1 to 3 μg/kg given subcutaneously dose-dependently attenuated the morphine-produced conditioned place preference. However, dextro-morphine at a higher dose 100 μg/kg did not affect the morphine-produced conditioned place preference. Thus, dextro-morphine pretreatment induces an U-shaped dose-response curve for attenuating the morphine-produced conditioned place preference. The attenuation of the morphine-produced conditioned place preference was reversed by the pretreatment with the sigma1 receptor antagonist BD1047 (N-[2-(3,4-Dichlorophenyl)ethyl]-N-methyl-2-(dimethylamino)ethylamine dihydrobromide. dextro-Morphine or BD1047 given alone did not affect the baseline place conditioning. It is concluded that dextro-morphine attenuated the morphine-produced conditioned place preference via the sigma1 receptor activation.
Keywords: sigma receptors, addiction, opioid, morphine, rat
1. Introduction
The naturally occurring morphine alkaloid, which is isolated from the juice of the opium poppy, papaver somniferum, is stereochemically identified as a levorotatory form. Morphine interacts with μ-opioid receptors to produce potent analgesic, addictive and other pharmacological effects. The dextrorotatory dextro-morphine, which is synthesized from sinomenine (Iijima et al., 1978), does not have any affinity for μ-opioid receptors and therefore does not produce any μ-opioid receptor mediated pharmacological effects (Jacquet et al., 1977). However, we have previously demonstrated that dextro-morphine pretreatment attenuates the analgesia produced by morphine in mice (Wu et al., 2005). The interesting finding inspired us to determine if dextro-morphine can also exert the anti-addictive effect. The effect of dextro-morphine on the conditioned place preference produced by morphine was then studied. We now report that dextro-morphine given systemically attenuates the systemic morphine-produced conditioned place preference.
The most likely receptor for dextro-morphine to act for producing the antianalgesic and anti-addictive effects is the sigma1 receptor. Other dextro-opiates such as (+)-pentazocine and (+)-N-ally-normetazocine interact with sigma1receptors, which are distinct from classical opioid receptors. We have found that the attenuation of the morphine-produced tail-flick inhibition induced by dextro-morphine is reversed by the sigma1 receptor antagonist BD1047, indicating that the attenuation of the morphine analgesia is mediated by the sigma1 receptor activation (Wu and Tseng, 2007). Sigma1 receptors have been reported to play an important role in the modulation of analgesia produced by μ-, δ- or κ-opioid receptor agonists (Mei and Pasternak, 2002; Marrazzo et al., 200653226; Chien and Pasternak, 1994) and the conditioned place preference produced by nicotine, cocaine, amphetamine or alcohol (Romieu et al., 2000, 2002; Matsumoto et al., 2001, 2002; Horan et al., 2001; Miyatake et al., 2004; Maurice et al., 2003; Liu et al., 2005; Nguyen et al., 2005). The experiment was then undertaken to determine if the attenuation of the morphine-produced conditioned place preference induced by dextro-morphine is mediated by the sigma1 receptor activation. We now report that BD1047 pretreatment reverses the attenuation of the morphine-produced conditioned place preference induced by dextro-morphine pretreatment. The finding provides the evidence that activation of sigma1 receptors is involved in the attenuation of the morphine-produced conditioned place preference induced by dextro-morphine.
2. Materials and Methods
2.1. Animals
Male CD rats weighing 300-350 g (Charles River Breeding Laboratory, Wilmington, MA) were used. Animals were housed two per cage in a room maintained at 22 ± 0.5°C with an alternating 12-h light-dark cycle. Food and water were available ad libitum. Each animal was used only once. All experiments were approved by and conformed to the guidelines of the Animal Care Committee of the Medical College of Wisconsin.
2.2. Conditioned place preference
The place conditioning experiment consisted of pre-conditioning, conditioning and post-conditioning phases (Bals-Kubik et al., 1993; Terashvili et al., 2004).Injections of vehicle or drug were only done during the conditioning phase. A two-compartment box (60 × 29.2 × 29.2 cm) with a transparent Plexiglas front separated by a gray cylinder platform (10.3 cm in diameter and 12 cm in height) was used. One compartment was white with a textured floor and the other was black with a smooth floor. For preconditioning, rats were initially placed on the neutral cylinder gray platform and allowed to step down off of the platform to either the white or black compartment. A sliding wall was then put down on the platform and the rat was free to access either compartment through two openings (9.5 × 12 cm) on each side of the platform. The amount of time spent in the black or white compartment was manually measured for 15 min. For preconditioning control, rat was tested once in the morning for their preference one day before conditioning sessions; only those rats that did not exhibit a significant preference between black and white compartments (350-550/900 s) were used for experiments. Rats, which spent less than 5 min 50 s in either the white or black compartment, were considered not to be neutral in preference for either side and were excluded from further study (less than 5% of rats).
The place conditioning session was carried out on days 2 to 4. The box was divided into two equal-sized compartments by putting down a sliding wall after removal of the gray cylinder platform. Conditioning session was conducted twice daily, morning and afternoon, and repeated for 3 days. Rats were placed in either the black or white compartment immediately following the injection and left in that compartment for 40 min. Forty min was in agreement with previous studies (Bals-Kubik et al., 1993; Shippenberg and Heidbreder, 1995). Rats were confined to either the black or white compartment after injection of drugs tested in the morning session of each day, and were confined to the opposite compartment after the injection of vehicle for the afternoon session and vice versa. Animals receiving vehicle in both sessions served as controls. Drug treatment consisted of dextro-morphine or BD1047 ( N-[2-(3,4-Dichlorophenyl)ethyl]-N-methyl-2-(dimethylamino)ethylamine dihydrobromide) given intraperitoneally or morphine sulfate given subcutaneously. The morning session was carried out from 8-11 a.m. and the afternoon session was carried out from 2-5 p.m. of the day.
The post-conditioning session was carried out on day 5 and was exactly the same as the pre-conditioning. The scores for the drug-paired place were then calculated by subtracting the pre-conditioning score from post-conditioning score. A positive score represented conditioning place preference, while a negative score represented conditioning place aversion.
2.3. Drugs and drug-administration
Morphine sulfate and dextro-morphine base were obtained from the National Institute on Drug Abuse (Baltimore, MD). N-[2-(3,4-Dichlorophenyl)ethyl]-N-methyl-2-(dimethylamino)ethylamine dihydrobromide (BD1047) was purchased from Tocris (Ellisville, MO). Morphine and BD1047 were dissolved in 0.9% saline. The dextro-morphine was initially dissolved in 10 N hydrochloric acid and then titrated with 1 N sodium hydroxide to pH 7.0, which was then diluted to the intended concentrations in 0.9% saline for injection. Drugs were injected subcutaneously or intraperitoneally in an injection volume of 0.1 ml per 100 g body weight with a 26-gauge needle.
2.4. Statistical analysis
Conditioning scores were expressed as mean ± S.E.M. The Student paired t-test was used to analyze the differences of the score between pre- and post-conditioning of each group of rats. One-way analysis of variance (ANOVA) followed by Dunnett’s post-test was used to compare the difference between drug treated groups and the vehicle treated group. In all experiments, P < 0.05 was considered a significant difference. The Prism statistical software was used to perform the statistics (version 4.1; GraphPad Software, Inc., San Diego, CA).
3. Results
3.1. Effect of morphine sulfate given subcutaneously on the production of the conditioned place preference
Groups of rats were injected subcutaneously with different doses of morphine sulfate (1, 5 or 10 mg/kg) or saline vehicle and placed in the conditioned place preference box immediately for place conditioning. Morphine sulfate at a dose, 1 and 5 mg/kg, dose-dependently increased the conditioned place preference. However, morphine sulfate at a higher dose, 10 mg/kg, produced no more increase of the conditioned place preference. Subcutaneous injection of the vehicle did not affect the baseline place conditioning response (Fig. 1). Five mg/kg of morphine sulfate was then chosen for the following experiments.
Fig. 1.
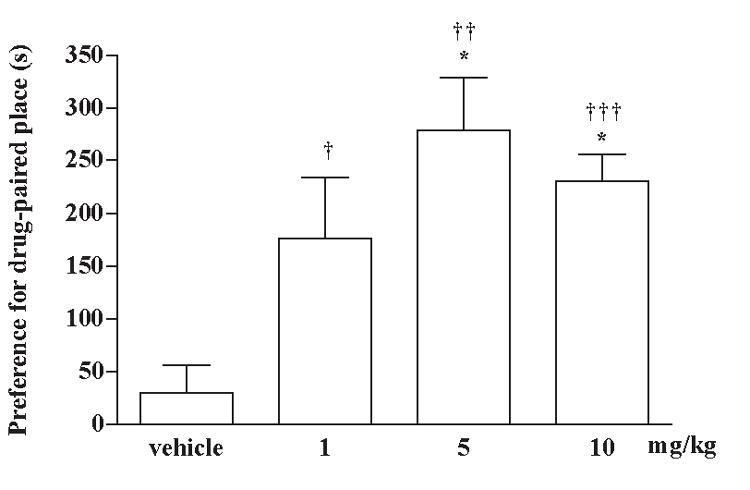
Conditioned place preference produced by morphine sulfate given subcutaneously in rats. Groups of rats were injected subcutanesously with a various dose of morphine sulfate (1-10 mg/kg) alternatively with saline vehicle and placed in the conditioning box for place conditioning. Each column represents the mean and the vertical bar represents the S.E.M. with 8 rats in each group; † P < 0.05, †† P < 0.01, ††† P < 0.001 compared with the pre-conditioning score of the individual group; * P < 0.01 compared with the vehicle-injected group.
3.2. Effects of dextro-morphine given intraperitoneally on the conditioned place preference produced by morphine sulfate given subcutaneously
Groups of rats were pretreated intraperitoneally in the home cage with different doses (0.1-100 μg/kg) of dextro-morphine or saline vehicle for 1 h before subcutaneous injection of morphine sulfate (5 mg/kg) and were placed in the conditioned place preference box for place conditioning. Pretreatment with dextro-morphine at a dose from 0.1 to 3 μg/kg dose-dependently attenuated the morphine-produced increase of the conditioned place preference. However, dextro-morphine at a higher dose100 μg/kg did not attenuate the increase of the conditioned place preference produced by morphine (Fig. 2). Thus, dextro-morphine at a dose range from 0.1 to 100 μg/kg produced a U-shaped dose-response curve with a maximal inhibition at 3 μg/kg for attenuating the morphine-produced conditioned place preference. However, dextro-morphine at 3 μg/kg, which produced a maximal inhibition, still did not completely block the increase of the conditioned place preference produced by morphine. Pretreatment with dextro-morphine (3 μg/kg) given intraperitoneally alone did not affect the baseline place conditioning in rats treated subcutaneously with saline vehicle (Fig. 2).
Fig. 2.
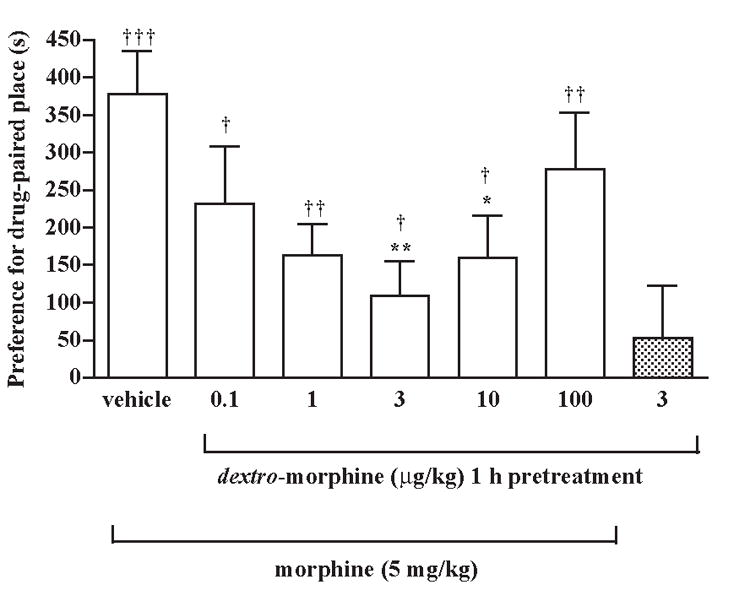
Pretreatment with dextro-morphine attenuates the morphine-produced conditioned place preference in rats. Groups of rats were pretreated intraperitoneally with a various doses of dextro-morphine in the home cage for 1 h before subcutaneous injection of morphine sulfate (5 mg/kg) and were placed in the conditioning box for place conditioning. Each column represents the mean and the vertical bar represents the S.E.M. with 8-12 rats in each group; † P < 0.05, †† P < 0.01, ††† P < 0.001 compared with the pre-conditioning score of the individual group; * P < 0.05, ** P < 0.01 compared with the vehicle injected group. The dextro-morphine (3 μg/kg) given intraperitoneally alone (the first column from the right) produced neither conditioning place preference or conditioned place aversion.
3.2. Effects of sigma1 receptor antagonist BD1047given intraperitoneally on the attenuation of morphine-produced conditioned place preference induced by dextro-morphine
Since most (+)-opiates such as (+)-pentazocine and (+)-N-allyl-normetazocine interact stereospecifically with the sigma1 receptors, the possibility that the sigma1 receptors are involved in attenuating the increase of the morphine-produced conditioned place preference induced by dextro-morphine was then explored. The sigma1 receptor antagonist BD1047 was used to determine if the effect of dextro-morphine is mediated by the sigma1 receptor activation. Groups of rats were pretreated intraperitonally in the home cage with a dose 1, 3 or 6 mg/kg of BD1047 and 3 μg/kg of dextro-morphine for 1 h before subcutaneous injection of morphine sulfate (5 mg/kg) and were placed in the conditioned place preference box for place conditioning. Pretreatment with dextro-morphine given alone attenuated the increase of the conditioned place preference produced by morphine. The attenuation of the morphine-produced conditioned place preference was reversed dose-dependently by the BD1047 pretreatment. Pretreatment with BD1047 (3 mg/kg) given alone did not affect the morphine-produced conditioned place preference, nor did it affect the baseline place preference in rats treated with saline vehicle (Fig. 3).
Fig. 3.
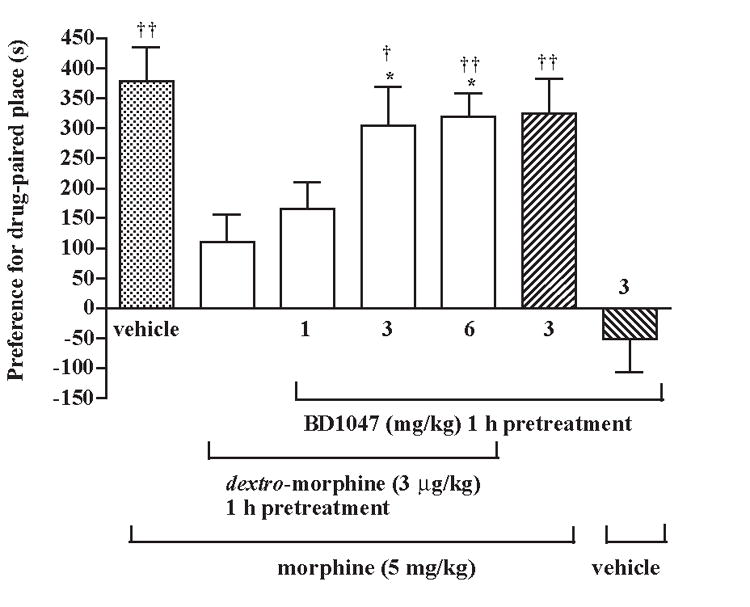
Pretreatment with the sigma1 receptor antagonist BD1047 reverses the attenuation of the morphine-produced conditioned place preference induced by dextro-morphine in rats. Groups of rats were pretreated intraperitoneally with a various dose of BD1047 (1, 3 or 6 mg/kg) and 3 μg/kg of dextro-morphine in the home cage for 1 h before subcutaneous injection of morphine sulfate (5 mg/kg) or vehicle and were placed in the conditioning box for place conditioning. Each column represents the mean and the vertical bar represents the S.E.M. with 8-12 rats in each group; † P < 0.01, †† P < 0.001 compared with the pre-conditioning score of the individual group; * P < 0.05, ** P < 0.01 compared with the vehicle injected group. The dextro-morphine (3 μg/kg) given intraperitoneally alone (the first column from the right) produced neither conditioning place preference or conditioned place aversion.
4. Discussion
4.1. Morphine, but not dextro-morphine, produces conditioned place preference given subcutaneously
The place conditioning, a technique that measures the secondary reinforcing effects of drugs, was used to assess the effect of dextro-morphine on the morphine-produced motivational effects. In this procedure, the association that develops between the presentation of a drug and a previously neutral stimulus, e.g. different colored compartment of a shuttle box is evaluated. The results obtained with this paradigm for rewarding drugs done by others are largely identical to those obtained with the self-administration paradigm technique (Spyraki, 1988; Carr et al., 1989).
Previous studies have demonstrated that μ-opioid agonists such as morphine, D-Ala2-N-MePhe4-Gly5-ol-enkephalin or endomorphin-1 given systemically or intracerebroventricularly in mice or rats produce conditioned place preference (Neisewander et al., 1990; Piepponen et al., 1997; Suzuki et al., 1991; Terashvili et al., 2004; Wu et al., 2004). The results of our present studies are consistent with previous findings that morphine given subcutaneously dose-dependently produced conditioned place preference. The conditioned place preference produced by morphine is blocked by μ-opioid receptor antagonist naltrexone or naloxonazine, indicating that the effect is mediated by the stimulation of μ-opioid receptors (Olmstead and Burns, 2005; Piepponen et al., 1997).
Unlike optically levorotatory morphine alkaloid, which produces analgesia and other μ-opioid receptor-mediated pharmacological effects, the dextrorotatory dextro-morphine does not have any affinity and efficacy for μ-opioid receptors and therefore does not produce analgesia and other μ-opioid receptor mediated effects (Jacquet et al., 1977). We found in the present studies that dextro-morphine at a dose 3 μg/kg given systemically did not produce conditioned place preference or nor did it produce the conditioned place aversion. Mucha and Herz (1986) have previously reported that dextro-morphine even at a much higher dose 4 mg/kg given systemically does not produce any conditioned place preference or conditioned place aversion. Morphine at the same dose, on the other hand, produces conditioned place preference. These findings are consistent with the view that dextro-morphine does not interact with the μ-opioid receptors and does not produce conditioned place preference or conditioned place aversion (Jacquet et al., 1977; Mucha and Herz, 1986). Thus, the conditioned place preference produced by morphine is stereospecific; it is only produced by the opioid receptor active isomers, such as levorotatory morphine, but not dextrorotatory dextro-morphine.
4.2. dextro-Morphine at a dose range 0.1 –100 μg/kg given systemically produce a U-shaped dose-response curve in attenuating the morphine-produced conditioned place preference
We found in the present study that dextro-morphine at a dose range of 0.1-3 μg/kg dose-dependently attenuated the morphine-produced conditioned place preference. Paradoxically, dextro-morphine at a higher dose 100 μg/kg was ineffective in attenuating the morphine-produced conditioned place preference. Thus, dextro-morphine at a dose range from 0.1 to 100 μg/kg induces a U-shaped dose-response curve with a maximal attenuation at a dose of 3 μg/kg for attenuating the morphine-produced conditioned place preference. The therapeutic window of the dose of dextro-morphine for the anti-addiction therapy should be in the μg/kg dose range. Higher doses of dextro-morphine would not be effective in providing the therapeutic effect. dextro-Morphine can potentially be developed for the treatment of opiate addiction.
The U-shaped dose-response curve is also known as hormesis (Calabrese and Baldwin, 2003). A well-documented example of a U-shaped dose-response relationship concerns the actions of corticosteroid hormones in the CA1 area of the hippocampus (Diamond et al., 1992; Joels and de Kloet, 1994; Joels, 2006), a brain region that is important for learning and memory formation. The neurosteroids have been proposed to be the endogenous ligand for sigma1 receptors in the central nervous system (Maurice, 2004; Maurice et al., 2001; Monnet and Maurice, 2006). In a modified passive-avoidance learning task in mice, pre-training or post-training administration of neurosteroids, pregnenolone sulfate or dehydroepiandrosterone sulfate enhances memory retention of passive-avoidance training. In both treatments, an inverted U-shaped dose-response curve is obtained covering 2- to 5-fold dose range in a manner typical for memory-enhancing substance. The neurosteroid-induced facilitation of memory retention may involve central sigma receptors, because the effect of neurosteroids is completely antagonized by sigma receptor antagonist haloperidol (Reddy and Kulkarni, 1998). It is postulated that dextro-morphine may mimic the effect of endogenous neurosteroids to stimulate the sigma1 receptors for producing the anti-addictive effect (see details in the next section below). The U-shaped dose-response curve for dextro-morphine to attenuate the morphine conditioned place preference is consistent with this hypothesis.
4.3. The attenuation of the morphine-produced conditioned place preference by dextro-morphine is mediated by sigma1 receptor activation
Most of dextrorotatory opiates such as (+)-pentazocine and (+)-N-allyl-normetazocine ((+)-SKF10047) exhibit the sigma1 receptor binding activity (Walker et al., 1992; Martin et al., 1984; de Costa et al., 1989). We therefore speculate that dextro-morphine acts on sigma1 receptors to attenuate the increase of the conditioned place preference produced by morphine. BD1047, a selective sigma1 receptor antagonist (McCracken et al., 1999) was then used to determine if the effect of dextro-morphine is mediated by the sigma1 receptor activation. We found in the present study that pretreatment with BD1047 dose-dependently reversed the attenuation of the morphine-produced conditioned place preference induced by dextro-morphine, indicating that sigma1 receptors are involved in dextro-morphine-induced anti-addiction. The sigma1 receptor stimulatory property of dextro-morphine can also be found in our other studies of the antianalgesic effect of dextro-morphine. Pretreatment with dextro-morphine attenuates the morphine-produced analgesia (Wu et al., 2005). The attenuation of the morphine-produced analgesia was also blocked by the pretreatment of the BD1047 (unpublished observation).
A role for sigma1 receptors in antinociception has been demonstrated from the studies that show a relationship between sigma1 receptor system and opioid analgesia (Chien and Pasternak, 1993, 1994, 1995; Guitart, 2004). Activation of sigma1 receptors by the selective sigma1 receptor agonist (+)-pentazocine given supraspinally or systemically attenuate the antinociception produced by morphine. (+)-Pentazocine given supraspinally also attenuates the antinociception produced by δ- and κ-opioid agonists as well. On the other hand, the blockade of sigma1 receptors with the sigma1 antagonist haloperidol potentiates the morphine-produced analgesia (Chien and Pasternak, 1995; Mei and Pasternak, 2002). Pretreatment with antisense oligodeoxynucleotide to down regulate the sigma1 receptors markedly enhances the antinociception produced by morphine (Mei and Pasternak, 2002). These observations indicate that sigma1 receptors also play an important role in modulating the opioid analgesia.
The recent demonstration of Romieu et al. (2003) that neurosteroids, endogenous ligands for sigma1 receptors, are able to modulate the acquisition of the cocaine-induced conditioned place preference suggests their influence on acquisition of drug addiction. Neurosteroids pregnenolone and dehydroxypregnolone potentiates the acquisition of the cocaine-induced conditioned place preference, as do selective sigma1 receptor agonists, like igmesine or PRE-084, and the effect is blocked by the sigma1 receptor antagonist BD1047 (Romieu et al., 2002). Selective sigma1 receptor antagonist, like BD1047 or NE100, or antisense oligodeoxynucleotide probe targeting the sigma1 receptor also blocks acquisition or expression of the cocaine-induced conditioned place preference in mice (Romieu et al., 2000, 2002; Maurice et al., 2002). Similar observations could be presented for abuse of amphetamine and alcohol, which also involve sigma receptor activation (Nguyen et al., 2005;Stefanski et al., 2004; Maurice et al, 2003). The activation of sigma1 receptors by sigma1 receptor agonist SA4503 (1-(3,4-dimethoxyphenethyl)-4-(phenylpropyl)pipperazine) attenuates the acquisition of the conditioned place preference produced by nicotine (Horan et al., 2001). These observations clearly indicate that sigma1 receptors are involved in the conditioned place preference produced by cocaine, amphetamine, alcohol and nicotine as well.
It is concluded that dextro-morphine at a μg/kg dose range given systemically attenuates the morphine-produced conditioned place preference. The attenuation of the morphine-produced conditioned place preference induced by dextro-morphine was reversed by the pretreatment with the sigma1 receptor antagonist BD1047, indicating that the effect of dextro-morphine is mediated by sigma1 receptor activation. It is speculated that dextro-morphine can potentially be developed for the treatment of opiate addiction.
Acknowledgments
This work was supported by grant DA12588 from the National Institute of Health, National Institute on Drug Abuse (PI: Leon F.Tseng) and Research Affair Committee, Medical College of Wisconsin (PI: Hsiang-En Wu).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication.As a service to our customers we are providing this early version of the manuscript.The manuscript will undergo copyediting, typesetting, and review of the resulting proofbefore it is published in its final citable form. Please note that during the productionprocess errorsmaybe discovered which could affect the content, and all legal disclaimersthat apply to the journal pertain.
References
- Bals-Kubik R, Ableitner A, Shippenberg TB. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther. 1993;264:489–495. [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Hormesis: The dose-response revolution. Annu Rev Pharmacol Toxicol. 2003;43:175–197. doi: 10.1146/annurev.pharmtox.43.100901.140223. [DOI] [PubMed] [Google Scholar]
- Carr CD, Fibinger HC, Phillips AG. Conditioning place preference as a measure of drug reward. In: Liebman JM, cooper SJ, editors. The Neuropharmacological Basis of Reward. Oxford: Sci Publ Press; 1989. pp. 264–319. [Google Scholar]
- Chien CC, Pasternak GW. Functional antagonism of morphine analgesia by (+)-pentazocine: evidence for an anti-opioid sigma1 system. Eur J Pharmacol. 1993;250:R7–R8. doi: 10.1016/0014-2999(93)90650-7. [DOI] [PubMed] [Google Scholar]
- Chien CC, Pasternak GW. Selective antagonism of opioid analgesia by a sigma system. J Pharmacol Exp Ther. 1994;271:1583–1884. [PubMed] [Google Scholar]
- Chien CC, Pasternak GW. Sigma antagonists potentiate opioid analgesia in rats. Neurosci Lett. 1995;190:137–139. doi: 10.1016/0304-3940(95)11504-p. [DOI] [PubMed] [Google Scholar]
- De Costa BR, Bowen WD, Hellewell SB, Walker JM, Thurkauf A, Jacobson AE, Rice KC. Synthesis and evaluation of optically pure [3H](+)-pentazocine, a highly potent and selective radioligand for sigma receptors. FEBS Lett. 1989;251:53–58. doi: 10.1016/0014-5793(89)81427-9. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Bennett MC, Fleshner M, Rose GM. Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus. 1992;2:421–430. doi: 10.1002/hipo.450020409. [DOI] [PubMed] [Google Scholar]
- Guitart X, Codony X, Monroy X. Sigma receptors; biology and therapeutic potential. Psychopharmacology. 2004;174:301–319. doi: 10.1007/s00213-004-1920-9. [DOI] [PubMed] [Google Scholar]
- Horan B, Gardner EL, Dewey SL, Brodie JD, Ashby CR., Jr The selective sigma 1 receptor agonist, 1-(3,4-dimethoxyphenethyl)-4-(phenylpropyl)piperazine (SA4503), blocks the acquisition of the conditioned place preference response to (-)-nicotine in rats. Eur J Pharamcol. 2001;426:R1–R2. doi: 10.1016/s0014-2999(01)01229-8. [DOI] [PubMed] [Google Scholar]
- Iijima I, Minamikawa JI, Jacobsen AE, Brossi A, Rice KE. Studies in the (+)-morphinan series-4. A markedly improved synthesis of (+)-morphine. J Org Chem. 1978;43:1462–1463. [Google Scholar]
- Jacquet YF, Klee WA, Rice KC, Iijima I, Minamikaw J. Stereospecfic and nonstereospecific effects of (+)- and (-)-morphine: evidence for a new class of receptors? Science. 1977;198:842–845. doi: 10.1126/science.199942. [DOI] [PubMed] [Google Scholar]
- Joëls M. Corticosteroid effects in the brain: U-shape it. Trends Pharmacol Sci. 2006;27:244–250. doi: 10.1016/j.tips.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Joëls M, de Kloet ER. Mineralocorticoid and glucocorticoid receptors in the brain. Implication for ion permeability and transmitter systems. Prog Neurobiol. 1994;43:1–36. doi: 10.1016/0301-0082(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Liu Y, Chen GD, Lerner MR, Brackett DJ, Matsumoto RR. Cocaine up-regulates fra-2 and σ-1 receptor gene and protein expression in brain regions involved in addiction and reward. J Pharmacol Exp Ther. 2005;314:770–779. doi: 10.1124/jpet.105.084525. [DOI] [PubMed] [Google Scholar]
- Marrazzo A, Parenti C, Scavo V, Ronsisvalle S, Scoto GM, Ronsisvalle G. In vivo evaluation of (+)-MR200 as a selective sigma ligand modulating MOP, DOP and KOP supraspinal analgesia. Life Sci. 2006;78:2449–2553. doi: 10.1016/j.lfs.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Martin BR, Katzen JS, Woods JA, Tripathi HL, Harris LS, May EL. Stereoisomers of [3H]-N-allylnormetazocine bind to different sites in mouse brain. J Pharmacol Exp Ther. 1984;231:539–544. [PubMed] [Google Scholar]
- Matsumoto RR, McCracken KA, Friedman MJ, Pouw B, De Costa BR, Bowen WD. Conformationally restricted analogs of BD1008 and an antisense oligodeoxynucleotide targeting sigma1 receptors produce anti-cocaine effects in mice. Eur J Pharmacol. 2001;419:163–174. doi: 10.1016/s0014-2999(01)00968-2. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, McCracken KA, Pouw B, Zhang Y, Bowen WD. Involvement of sigma receptors in the behavioral effects of cocaine: evidence from novel ligands and antisense oligodeoxynucleotides. Neuropharmacology. 2002;42:1042–1055. doi: 10.1016/s0028-3908(02)00056-4. [DOI] [PubMed] [Google Scholar]
- Maurice T. Neurosteroids and sigma1 receptor, biochemical and behavioral relevance. Pharmacopsychiatry. 2004;(37 Suppl):S171–182. doi: 10.1055/s-2004-832675. [DOI] [PubMed] [Google Scholar]
- Maurice T, Casalino M, Lacroix M, Romieu P. Involvement of the sigma1 receptor in the motivational effects of ethanol in mice. Pharmacol Biochem Behav. 2003;74:869–876. doi: 10.1016/s0091-3057(03)00002-9. [DOI] [PubMed] [Google Scholar]
- Maurice T, Martin-Fardon R, Romieu P, Matsumoto RR. Sigma1 receptor antagonists represent a new strategy against cocaine addiction and toxicity. Neurosci Biobehav Rev. 2002;26:499–527. doi: 10.1016/s0149-7634(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Maurice T, Urani A, Phan VL, Romieu P. The interaction between neuroactive steroids and the σ1 receptor function: behavioral consequences and therapeutic opportunities. Brain Res Rev. 2001;37:116–132. doi: 10.1016/s0165-0173(01)00112-6. [DOI] [PubMed] [Google Scholar]
- McCracken KA, Bowen WD, de Costa BR, Matsumoto RR. Two novel σ receptor ligands, BD1047 and LR172, attenuate cocaine-induced toxicity and locomotor activity. Eur J Pharmacol. 1999;370:225–232. doi: 10.1016/s0014-2999(99)00113-2. [DOI] [PubMed] [Google Scholar]
- Mei J, Pasternak GW. σ1 Receptor modulation of opioid analgesia in the mouse. J Pharmacol Exp Ther. 2002;300:1070–1074. doi: 10.1124/jpet.300.3.1070. [DOI] [PubMed] [Google Scholar]
- Miyatake R, Furukawa A, Matsuchita S, Higuchi S, Suwaki H. Functional polymorphisms in the sigma1 receptor gene associated with alcoholism. Biol Psychiatry. 2004;55:85–90. doi: 10.1016/j.biopsych.2003.07.008. [DOI] [PubMed] [Google Scholar]
- Monnet FP, Maurice T. The sigma-1 protein as a target for the non-genomic effects of neuro(active)steroid: molecular, physiological and behavioral aspects. J Pharmacol Sci. 2006;100:93–118. doi: 10.1254/jphs.cr0050032. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Herz A. Preference conditioning produced by opioid active and inactive isomers of levophanol and morphine in rat. Life Sci. 1986;38:241–249. doi: 10.1016/0024-3205(86)90309-7. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Pierce RC, Bardo MT. Naloxone enhances the expression of morphine-induced conditioned place preference. Psychopharmacology. 1990;100:201–205. doi: 10.1007/BF02244406. [DOI] [PubMed] [Google Scholar]
- Nguyen EC, McCracken KA, Liu Y, Pouw B, Matsumoto RR. Involvement of sigma receptor in the acute actions of methamphetamine: receptor binding and behavioral studies. Neuropharmacology. 2005;49:638–645. doi: 10.1016/j.neuropharm.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Olmstead MC, Burns LH. Ultra-low-dose naltrexone suppresses rewarding effects of opiates and aversive effects of opiate withdrawal in rats. Psychopharmacology. 2005;12:1–6. doi: 10.1007/s00213-005-0022-7. [DOI] [PubMed] [Google Scholar]
- Piepponen TP, Kivastik T, Katajamaki J, Zharkovsky A, Ahtee L. Involvement of opioid mu 1 receptors in morphine-induced conditioned place preference in rats. Pharamcol Biochem Behav. 1997;58:275–279. doi: 10.1016/s0091-3057(96)00567-9. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Kulkarni SK. The effects of neurosteroids on acquisition and retention of a modified passive-avoidance learning task in mice. Brain Res. 1998;791:108–116. doi: 10.1016/s0006-8993(98)00085-7. [DOI] [PubMed] [Google Scholar]
- Romieu P, Martin-Fardon R, Maurice T. Involvement of the σ1 receptor in the cocaine-induced conditioned place preference. NeuroReport. 2000;11:2885–2888. doi: 10.1097/00001756-200009110-00011. [DOI] [PubMed] [Google Scholar]
- Romieu P, Martin-Fardon R, Bowen WD, Maurice T. Sigma1 (σ1) receptor-related neuroactive steroids modulate cocaine-induced reward. J Neurosci. 2003;23:3572–3576. doi: 10.1523/JNEUROSCI.23-09-03572.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romieu P, Phan VL, Martin-Fardon R, Maurice T. Involvement of the sigma 1 receptor in cocaine-induced conditioned place preference: possible dependence on dopamine uptake blockade. Neuropsychopharmacology. 2002;26:444–455. doi: 10.1016/S0893-133X(01)00391-8. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Heidbreder C. The delta-opioid antagonist naltrindol prevents sensitization to the conditioned rewarding effects of cocaine. Eur J Pharmacol. 1995;280:55–61. doi: 10.1016/0014-2999(95)00185-n. [DOI] [PubMed] [Google Scholar]
- Spyraki C. Drug reward studied by use of place conditioning in rats. In: lades M, editor. The Psychopharmacology of Addiction. Oxford: Oxford Univ Press; 1988. pp. 97–114. [Google Scholar]
- Strefanski R, Justinova Z, Hayashi T, Takebayashi M, Goldberg SR, Su TP. Sigma1 receptor upregulation after chronic methamphetamine self-administration in rats: a study with yoked controls. Psychopharmacology. 2004;175:68–75. doi: 10.1007/s00213-004-1779-9. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Funada M, Narita M, Misawa M, Nagase H. Pertussis toxin abolishes mu- and delta-opioid agonist-induced place preference. Eur J Pharmacol. 1991;205:85–88. doi: 10.1016/0014-2999(91)90774-k. [DOI] [PubMed] [Google Scholar]
- Terashvili M, Wu H, Leitermann RJ, Hung K, Clithero AD, Schwasinger ET, Tseng LF. Differential conditioned place preference responses to endomorphin-1 and endomorphin-2 microinjected into posterior nucleus accumbens shell and ventral tegmental area in the rat. J Pharmacol Exp Ther. 2004;309:816–824. doi: 10.1124/jpet.103.059287. [DOI] [PubMed] [Google Scholar]
- Walker JM, Bowen WD, Goldstein SR, Roberts AH, Patrick SL, Hohmann AG, deCosta B. Autoradiographic distribution of [3H](+)-pentazocine and [3H]1,3-di-o-tolylguanidine (DTG) binding sites in guinea pig brain: a comparative study. Brain Res. 1992;581:33–38. doi: 10.1016/0006-8993(92)90340-f. [DOI] [PubMed] [Google Scholar]
- Wu H, MacDougall RS, Clithe AD, Terashvili M, Tseng LF. Opposite conditioned place preference responses to endomorphin-1 and endomorphin-2 in the mouse. Nuerosci Lett. 2004;356:157–161. doi: 10.1016/j.neulet.2004.03.093. [DOI] [PubMed] [Google Scholar]
- Wu H, Thompson J, Sun H, Terashvili M, Tseng LF. Antianalgesia: stereo- selective action of dextro-morphine over levo-morphine on glia in the mouse spinal cord. J Pharmacol Exp Ther. 2005;314:1101–1108. doi: 10.1124/jpet.105.087130. [DOI] [PubMed] [Google Scholar]


