Abstract
Purpose
A new technique was developed to measure the flow of aqueous humor through the uveoscleral pathway in porcine eyes and to examine whether there is any outflow through the choroid into the vortex veins.
Methods
Enucleated porcine eyes were perfused in vitro under a constant pressure of 10 mm Hg. After total outflow was measured, the episcleral vessels were blocked with cyanoacrylate to eliminate outflow through the conventional pathway. The vortex veins were then blocked, to assess the amount of choroidal drainage.
Results
The average outflow in control eyes was found to be 2.8 ± 0.9 μL/min. After the exit sites of the conventional pathway were blocked, the average outflow decreased to 1.1 ± 0.5 μL/min. Blocking the vortex veins did not appear to alter uveoscleral outflow further (1.2 ± 0.8 μL/min).
Conclusions
The results suggest that choroidal drainage into the vortex veins is insignificant in the absence of blood perfusion. No significant washout effects in porcine eyes were observed.
Glaucoma is the leading cause of irreversible blindness in the United States. Blindness in those affected by the disease appears to be caused in most cases by compression of retinal ganglion cell axons in the optic nerve, due to elevated intraocular pressure (IOP). IOP increases over time because of impaired aqueous humor outflow. Many pharmaceuticals that lower IOP target aqueous secretion. Recently, prostaglandin and prostamide compounds have been introduced. They lower IOP primarily by increasing aqueous outflow through the unconventional, or uveoscleral, route. The precise molecular mechanisms by which these compounds lessen resistance in the outflow pathways to lower IOP are currently unknown.
Aqueous humor is actively secreted into the posterior chamber by the ciliary processes. The fluid then passes through the pupil and can be drained in two ways at the iridocorneoscleral angle. Aqueous humor exiting through the conventional pathway crosses the trabecular meshwork and flows through Schlemm’s canal into large collector channels that reach to the surface of the sclera, at which point they join episcleral vessels. Aqueous humor, following the unconventional or uveoscleral pathway, passes through the ciliary muscle bundles into the supraciliary and suprachoroidal spaces. The fluid then leaves through the sclera. Whether it can also be drained by the posterior choroid into the vortex veins remains a subject of controversy. Although, in tracer studies in the rhesus monkey, evidence of outflow through vortex veins has been found,1–3 no significant amount of tracer was detected in the posterior choroid in human and mouse eyes.4,5 It is possible that drainage through the choroid occurs in some species but not in others.
The uveoscleral pathway was first described by Bill,6 who reported that in the cynomolgus monkey, about half of the aqueous humor exits through the ciliary muscle. Bill and Phillips7 then observed this behavior in humans and found that the percentage of total aqueous outflow attributed to the uveoscleral pathway was approximately 10% to 20%. Later indirect calculations have produced values of approximately 35% in young adults and 3% in elderly persons over the age of 60.8,9 As reviewed by Nilsson,10 the relative importance of the unconventional pathway varies significantly with age and from species to species. In cats and rabbits, the uveoscleral outflow constitutes only 3% to 8% of the total drainage.
It has been suggested10 that the prostaglandin-induced increase in uveoscleral outflow is caused by two mechanisms: an initial relaxation of the ciliary muscle, and a subsequent decrease in the resistance of the pathway due to changes in the extracellular matrix. After multiple doses of prostaglandin F2α (PGF2α) the ciliary muscles bundles in cynomolgus monkey eyes are widened and a loss of type 1 and III collagen fibers from the connective tissues in these spaces is observed.11,12 Other studies have shown that prostaglandin F2α and latanoprost induce increased secretion of prometalloproteinases (MMPs) which results in a rearrangement of the extracellular matrix of the ciliary muscle with a reduction in the amount of collagen.13–15 The corresponding increase in spaces between the ciliary muscle fibers may greatly reduce the hydraulic resistance of the uveoscleral pathway.16
In this study, we developed a new technique to measure directly the uveoscleral outflow in porcine eyes and to examine whether there is any outflow through the choroid into the vortex veins. After having measured total outflow during constant-pressure perfusion, we blocked the episcleral vessels with cyanoacrylate to eliminate outflow through the conventional pathway. After isolation, we then blocked the vortex veins to assess the amount of choroidal drainage.
Methods
Fresh porcine eyes were obtained from a local abattoir. Enucleations were performed at the facility from which the eyes were obtained. Enucleated porcine eyes were carefully investigated under a dissecting microscope in ensure their integrity. Eyes were used within 30 hours of death.
In vitro single-level, constant-pressure perfusion studies were performed on porcine eyes, by using the hanging reservoir method of Bárány.17 Studies were performed in three phases for each pair of eyes. Polyethylene pressure line and Grant fittings were washed thoroughly with 70% ethyl alcohol and then deionized water before calibration. Two hanging reservoirs were loaded with Bárány solution and flushed to remove any bubbles in the line. The calibration speed for the flatbed recorder was set to 2 mm/s. With a gas-tight microliter syringe, 100 μL was pulled in 10-μL increments from the hanging bucket to calibrate the system by recording the distance traveled by the strip-chart pen for each increment. This step was repeated twice for each channel, there being one channel for each eye used during the experiment.
Excess tissue, including muscle and conjunctiva, was removed from the sclera of each eye using scissors and tweezers, keeping the eyes moist with saline. The eye was then wrapped with gauze moist with saline, and a trephination of the cornea was performed, followed by an iridectomy. A slit was cut radially in the iris, and the iris itself was left otherwise unaltered. Care was taken to avoid damage to any other ocular tissues. A Grant fitting was then inserted through the circular incision and tightened, and the eye was placed in plastic cups with adequate saline-moist gauze surrounding each eye. With the plastic cup attached to a ring stand, the height difference between hanging bucket and cornea of eye was adjusted so that the pressure difference remained constant at 10 mm Hg. Each hanging reservoir was connected to a Grant fitting using a polyethylene pressure line, and the system was flushed free of air bubbles.
The first step (phase I) of our study involved a baseline perfusion with whole eyes. Perfusion for all phases of this study were performed at a pressure of 10 mm Hg. The perfusion pressure was set by adjusting the height of the reservoir above the surface of the eye. Assuming an episcleral venous pressure of 8 to 10 mm Hg, this perfusion pressure would simulate an in vivo IOP of 18 to 22 mm Hg. Both experimental and control eyes underwent the establishment of a stable baseline outflow. The perfusion was ceased after 45 minutes of stability, as judged by constant outflow. Outflow data were collected throughout the experiment.
The second step (phase II) began with blockage of the episcleral vessels for the experimental eye while the control eye was kept under similar environmental conditions. The conventional pathway exit sites, or episcleral vessels, were found by a short perfusion of 2% sterile sodium fluorescein followed by observation under a cobalt blue light filter using low magnification. Each exit site was then dried with sponges (Weck Cel; Medtronic, Jacksonville, FL) and a minimal amount of ethyl cyanoacrylate was applied by needle to the surface of the sclera. This procedure was repeated for as many as 20 episcleral vessels. Once the sealant completely hardened, the exit sites were reexamined by perfusion of 2% sterile sodium fluorescein followed by observation under a blue-light filter with low magnification. This was repeated until no further vessels outlets were observed. A baseline perfusion was reestablished for 45 minutes and data were collected.
In the third step (phase III), the vortex veins were blocked for the experimental eye, whereas the control eye was kept under similar environmental conditions. The vortex veins were detectable with the naked eye. The surface of the sclera where the veins drained was dried with sponges (Weck Cel; Medtronics), and ethyl cyanoacrylate was applied through a needle. Once the sealant had completely hardened, the vortex vein exit sites were reexamined by perfusion of 2% sterile sodium fluorescein followed by observation under a blue-light filter, using low magnification to assure blockage. A final baseline perfusion was established for 45 minutes and data were collected.
The total perfusion time was therefore at least 135 minutes. Correcting for leaks and air bubbles in some cases increased the total perfusion time, which never exceeded 3 hours. The perfusion pressure remained constant throughout the entire experiment, as ensured by the constant height difference in height between the eye and the reservoir.
To ensure that the outflow across the conventional pathway was completely eliminated in the second step, we observed the blocked sites under cobalt blue light after perfusion with sodium fluorescein. Episcleral vessels just under the sclera surface may be followed for roughly 2 to 10 mL before they terminate at the surface. Ethyl cyanoacrylate was applied to the surface of the sclera where the vessel outlet could be observed. Minimal cyanoacrylate was applied to the surface of the sclera to avoid blocking potential uveoscleral outflow sites. It is possible that some exit sites for the conventional pathway were overlooked, because the episcleral vessels were observed for only a short time to avoid confusion with exit sites originating from unconventional outflow.
To make sure that drainage through the vortex veins was sealed off, similar steps were taken as in phase II. The sites were observed after application of cyanoacrylate with reapplication as needed. The outlet of the vortex veins and the veins themselves are much larger than the episcleral vessels. A relatively large amount of perfusate containing sodium fluorescein was observed on the surface of the sclera in the area of the vortex vein outlet, even with the naked eye. The preparations were observed under low magnification to assure complete blockage had been achieved. One limitation of this technique is that it might not differentiate between flow through and flow around the vortex veins, if the applied cyanoacrylate obstructed flow around the vessels as they pass through the sclera. Although we took care to avoid this effect, we cannot be certain that perivortex vein flow, if present, was not affected by this technique.
Ten pairs of eyes were examined. Three runs were discarded because of variation in outflow in the control eye between the three phases or variation between the control and the experimental eyes during the first phase.
The time between harvest and start of the experiment varied between 6 and 30 hours. We did not observe any correlation between the postmortem time and our outflow measurements.
The experimental eye-to-control eye outflow ratio was calculated for each step and averaged over the number of eyes studied. Statistical significance was tested using a two-tailed Student’s t-test for equal means.
Results
The objective of our study was to measure uveoscleral outflow and to assess the significance of drainage through the choroid with regard to this pathway. As shown in Table 1, we found that with IOP set to 10 mm Hg, the average outflow in control eyes was 2.8 ± 0.9 μL/min. After blocking the exit sites of the conventional pathway, the average outflow declined to 1.1 ± 0.5 μL/min. Blocking the vortex veins did not appear to further alter uveoscleral outflow (1.2 ± 0.8 μL/min). These results suggest that uveoscleral outflow contributes significantly to total aqueous humor outflow in porcine eyes and that in the enucleated porcine eye the choroid does not represent a significant exit route through the uveoscleral pathway, as illustrated in Figure 1.
Table 1.
Aqueous Humor Outflow Measurements in Porcine Eyes
| Phase I | Phase II | Phase III | |
|---|---|---|---|
| Control eye | 2.7 ± 0.8 | 2.8 ± 0.9 | 2.8 ± 0.9 |
| Experimental eye | 2.9 ± 1.1 | 1.1 ± 0.5* | 1.2 ± 0.8† |
| Experimental eye/control eye | 1.07 | 0.39 | 0.43 |
Phase I, baseline perfusion—that is, uncorrupted outflow; Phase II, blockage of episcleral vessels; Phase III, blockage of vortex veins.
Results are reported as the mean ± standard deviation (n = 7). Units are μL/min/mm Hg, except in the ratio row. All perfusions were performed at 10 mm Hg. Experimental eye results were not significantly different between phase II and phase III.
Statistically significant difference compared with control eye (P < 0.001).
Statistically significant difference compared with control eye (P < 0.005).
Figure 1.

Uveoscleral outflow in an experimental porcine eye during phase I (uncontrolled flow), phase II (after blockage of episcleral vessels), and phase III (after blockage of episcleral vessels and vortex veins). Three measurements were performed in each of the seven eyes.
Discussion
Our in vitro measurements of total aqueous humor outflow in porcine eyes appear to agree with other findings. As reviewed by Weinreb,18 uveoscleral outflow (Fu) has been found to occur in most species; however, its value varies significantly. Our results suggest that uveoscleral outflow in porcine eyes is in the upper range. Our estimate of 1.2 ± 0.8 μL/min is comparable to that reported in humans8,9 (i.e., 0.80 –1.04 μL/min), but is higher than that observed in other animals: 0.61 μL/min in the cynomolgus monkey,19 0.36 μL/min in the cat,20 and 0.26 μL/min in the rabbit.21 The fraction (f) of aqueous humor leaving through the uveoscleral route in porcine eyes, approximately 40% in the present study, also appears to be in the upper range. In cynomolgus monkeys, that percentage is normally in the range of 40% to 60%,10,19 whereas uveoscleral outflow only constitutes 3% to 8% of the total drainage in cats20 and rabbits.21 In humans, the uveoscleral outflow has been observed to decrease with age: f was measured as 36% in 24-year-old humans8 and as 3% in humans over 60.9 Because f and Fu may also depend on pressure, as discussed later, these comparisons should be made with caution.
The study in cynomolgus monkeys conducted by Bill22 suggested that the uveoscleral flow is pressure-independent as long as IOP is greater than 4 mm Hg. As summarized by Brubaker,23 recent experimental results suggest that prostanoids may cause the uveoscleral drainage pathway to become pressure sensitive. Accordingly, several investigators have proposed changes in the Goldmann equation, which relates the rate of aqueous humor formation to the IOP. Becker and Neufeld24 added a term comprising the facility of uveoscleral outflow, and Kaufman25 recommended including a pseudofacility term to account for the fact that the formation of aqueous humor is pressure dependent. Bill26 also suggested defining both a low and a high-pressure facility for the uveoscleral pathway. To avoid misinterpretation of aqueous dynamics data, other investigators believe it would be best to determine the collective hydraulic conductivity (or facility) of all pressure-dependent pathways out of the anterior chamber.23 We did not vary pressure in our experiments, given that perfusion time is limited for enucleated eyes, so that it was not possible to estimate any of these terms.
A washout effect has been observed by several investigators in different species. Bárány and Gassman27 observed that outflow facility varies between live and dead monkey eyes because of to a time-dependent, steady increase in outflow facility. Other studies have shown that this progressive increase in outflow facility, termed “washout,” does not happen in human eyes.28 To the best of our knowledge, there have been no published results regarding its occurrence in porcine eyes. In the present study, we did not report measurements in which outflow in the control eye varied by more than 15% between all three phases (Fig. 2). Taking into account all the experiments that we performed (n = 10; i.e., the results of three runs were discarded as described earlier), we observed that variations in control eye outflow between all three phases were always less than 25%. Our results thus show little evidence of a significant washout effect in porcine eyes.
Figure 2.
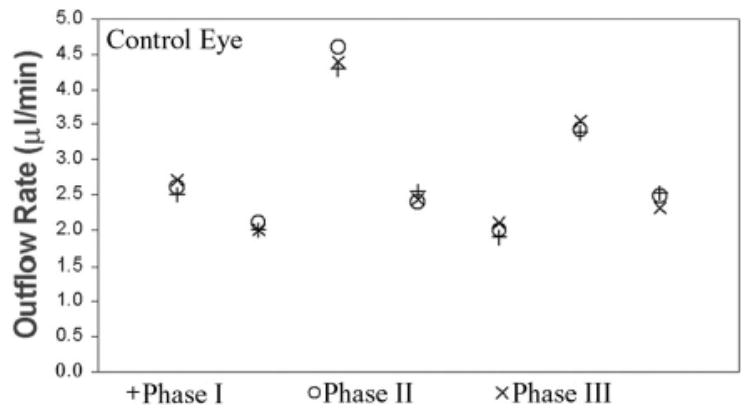
Uveoscleral outflow in control porcine eyes. Three measurements were performed in each of the seven eyes. Phases as in Figure 1.
Although we observed that uveoscleral outflow was not significantly affected by blocking vortex veins in vitro, it does not absolutely prove that the choroid does not constitute an important exit route in vivo, because the eye is normally perfused with blood. In the living eye, osmotic and oncotic pressures may draw fluid into the choroidal vasculature, providing an outlet for aqueous through the IOP independent pathway. Because enucleated eyes were studied, we were unable to evaluate this hypothesis in the present study. We were also unable to isolate the role of transscleral flow; this will require further investigation.
In summary, we have developed a new in vitro technique to measure aqueous humor outflow through the uveoscleral route, and the amount of fluid that is drained through the choroid. Our results indicate that flow of aqueous humor through the unconventional (or uveoscleral) pathway in porcine eyes is approximately 1.1 μL/min and that choroidal drainage into the vortex veins is insignificant in the absence of a functioning circulatory system. We also observed no significant washout effect in porcine eyes. This technique could be applied to human eyes to gain more understanding into the uveoscleral outflow pathway.
Acknowledgments
Supported in part by National Eye Institute Grants R01-EY13178 and P30-EY13078, by a grant from the Massachusetts Lions Eye Research Fund, and by Research to Prevent Blindness.
Footnotes
Disclosure: J.A. Wagner, None; A. Edwards, None; J.S. Schuman, None
References
- 1.Green K, Sherman SH, Laties AM, Pederson JE, Gaasterland DE, MacLellan HM. Fate of anterior chamber tracers in the living rhesus monkey eye with evidence for uveo-vortex outflow. Trans Ophthalmol Soc UK. 1977;97:731–739. [PubMed] [Google Scholar]
- 2.Pederson JE, Gaasterland DE, MacLellan HM. Uveoscleral aqueous outflow in the rhesus monkey: importance of uveal reabsorption. Invest Ophthalmol Vis Sci. 1977;16:1008 –1017. [PubMed] [Google Scholar]
- 3.Sherman SH, Green K, Laties AM. The fate of anterior chamber fluorescein in the monkey eye: the anterior chamber outflow pathways. Exp Eye Res. 1978;27:159 –173. doi: 10.1016/0014-4835(78)90086-6. [DOI] [PubMed] [Google Scholar]
- 4.Krohn J, Bertelsen T. Light microscopy of uveoscleral drainage routes after gelatine injections into the suprachoroidal space. Acta Ophthalmol Scan. 1998;76:521–527. doi: 10.1034/j.1600-0420.1998.760502.x. [DOI] [PubMed] [Google Scholar]
- 5.Lindsey JD, Weinreb RN. Identification of the mouse uveoscleral outflow pathway using fluorescent dextran. Invest Ophthalmol Vis Sci. 2002;43:2201–2205. [PubMed] [Google Scholar]
- 6.Bill A. The aqueous humor drainage mechanism in the cynomolgus monkey (Macaca irus) with evidence for unconventional routes. Invest Ophthalmol. 1965;4:911–919. [PubMed] [Google Scholar]
- 7.Bill A, Philipps CI. Uveoscleral drainage of aqueous humor in human eyes. Exp Eye Res. 1971;12:275–281. doi: 10.1016/0014-4835(71)90149-7. [DOI] [PubMed] [Google Scholar]
- 8.Townsend DJ, Brubaker RF. Immediate effects of epinephrine on aqueous formation in the normal human eye as measured by fluorophotometry. Invest Ophthalmol Vis Sci. 1980;19:256 –266. [PubMed] [Google Scholar]
- 9.Toris CB, Yablonski ME, Camras CB, Gleason ML. Uveoscleral outflow decreases with age in ocular normotensive humans. Invest Ophthalmol Vis Sci. 1996;37:407–412. [Google Scholar]
- 10.Nilsson SFE. The uveoscleral outflow routes. Eye. 1997;11:149 –154. doi: 10.1038/eye.1997.43. [DOI] [PubMed] [Google Scholar]
- 11.Lütjen-Drecoll E, Tamm E. Morphological study of the anterior segment of cynomolgus monkey eyes following treatment with prostaglandin F2α. Exp Eye Res. 1988;47:761–769. doi: 10.1016/0014-4835(88)90043-7. [DOI] [PubMed] [Google Scholar]
- 12.Tamm E, Lütjen-Drecoll E, Rohen JW. Age-related changed of the ciliary muscle in comparison with changes induced by treatment with prostaglandin F2α: an ultrastructural study in rhesus and cynomolgus monkeys. Mech Ageing Dev. 1990;51:101–120. doi: 10.1016/0047-6374(90)90093-u. [DOI] [PubMed] [Google Scholar]
- 13.Lindsey JD, Jashiwagi K, Boyle D, Kashiwagi F, Firestrin F, Weinreb RN. Prostaglandins increase proMMP-1 and proMMP-2 secretion by human ciliary smooth muscle cells. Curr Eye Res. 1996;15:869 –875. doi: 10.3109/02713689609017628. [DOI] [PubMed] [Google Scholar]
- 14.Ocklind A. Effect of latanoprost on the extracellular matrix of the ciliary muscle: a study on cultured cells and tissue sections. Exp Eye Res. 1998;67:179 –191. doi: 10.1006/exer.1998.0508. [DOI] [PubMed] [Google Scholar]
- 15.Sagara T, Gaton D, Lindsey JD, Gabelt BT, Kaufman PL, Weinreb RN. Topical prostaglandin F2α treatment reduces collagen types I, III, and IV in the monkey uveoscleral outflow pathway. Arch of Ophthalmo. 1999;117:794 –801. doi: 10.1001/archopht.117.6.794. [DOI] [PubMed] [Google Scholar]
- 16.Schachtschabel U, Lindsey JD, Weinreb RN. The mechanism of action of prostaglandins on uveoscleral outflow. Curr Opin Ophthalmol. 2000;11:112–116. doi: 10.1097/00055735-200004000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Bárány EH. Simultaneous measurement of changing intraocular pressure and outflow facility in the vervet monkey by constant pressure infusion. Invest Ophthalmol. 1964;3:135–143. [PubMed] [Google Scholar]
- 18.Weinreb RN. Uveoscleral outflow: the other outflow pathway. J Glaucoma. 2000;9:343–345. doi: 10.1097/00061198-200010000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Sperber GO, Bill A. A method for near-continuous determination of aqueous humour flow: effects of anaesthetics, temperature, and indomethacin. Exp Eye Res. 1984;39:435–453. doi: 10.1016/0014-4835(84)90044-7. [DOI] [PubMed] [Google Scholar]
- 20.Bill A. Formation and drainage of aqueous humor in cats. Exp Eye Res. 1966;5:185–190. [Google Scholar]
- 21.Poyer JF, Gabelt B, Kaufman PL. The effect of topical PGF2αon uveoscleral outflow and outflow facility in the rabbit eye. Exp Eye Res. 1992;54:277–283. doi: 10.1016/s0014-4835(05)90001-8. [DOI] [PubMed] [Google Scholar]
- 22.Bill A. Further studies on the influence of the intraocular pressure on aqueous humor dynamics in cynomolgus monkeys. Invest Ophthalmol. 1967;6:364 –372. [Google Scholar]
- 23.Brubaker RF. Goldmann’s equation and clinical measures of aqueous dynamics. Exp Eye Res. 2003 doi: 10.16/j.exer. 2003.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Becker B, Neufeld AH. Pressure dependence of uveoscleral outflow. J Glaucoma. 2002;11:464. doi: 10.1097/00061198-200210000-00017. Letter. [DOI] [PubMed] [Google Scholar]
- 25.Kaufman PL. Some thoughts on the pressure dependence of uveoscleral flow. J Glaucoma. 2003;12:89. doi: 10.1097/00061198-200302000-00018. Letter. [DOI] [PubMed] [Google Scholar]
- 26.Bill A. Some thoughts on the pressure dependence of uveoscleral flow. J Glaucoma. 2003;12:88 –89. doi: 10.1097/00061198-200302000-00017. [DOI] [PubMed] [Google Scholar]
- 27.Bárány EH, Gassman HB. The effect of death on outflow resistance in normal and sympathectomized rabbit eyes. Invest Ophthalmol. 1965;4:206 –210. [PubMed] [Google Scholar]
- 28.Erickson-Lamy K, Schroeder AM, Bassett-Chu S, Epstein DL. Absence of time-dependent facility increase (“washout”) in the perfused enucleated human eye. Invest Ophthalmol Vis Sci. 1990;31:2384 –2388. [PubMed] [Google Scholar]


