Abstract
Otsuka Long-Evans Tokushima Fatty (OLETF) rats lack the CCK-1 receptor and are hyperphagic and obese. CCK-1 receptors play a role in prepulse inhibition (PPI) by modulating mesolimbic dopamine transmission, a modulator of sensorimotor gating. Therefore, the present study assessed the effects of brief, daily sucrose access on PPI and acoustic startle response (ASR) in OLETF rat and age-matched non-mutant Long-Evans Tokushima Otsuka (LETO) rats. The results revealed that OLETF rats with sucrose access showed an increased ASR [F(1,16) = 6.84; P < 0.01)], relative to sucrose receiving LETO rats. No significant sucrose effect (P = 0.283) on PPI was noted in OLETF rats, whereas sucrose receiving LETO rats had a significantly lower (P < 0.05) PPI percentage than non-sucrose controls. In contrast, sucrose-receiving OLETF rats expressed significantly higher PPI percentage than LETO rats with identical sucrose presentation (P < 0.01). Taken together, these results suggest that sucrose access alters PPI and ASR in general, and the CCK-1 receptors play a modulatory role in facilitating or inhibiting these responses, respectively. A similar effect may be contributory to the hyperphagic behavioral phenotype of obese animal models with altered central dopamine regulation.
Keywords: CCK, Dopamine, CCK-A, Preference, Sugar, Food
1. Introduction
Cholecystokinin (CCK) is one of the most widely studied endogenous gut peptides controlling satiation signaling and feeding behavior in rodents (see [25] for review). It is well established that of the two known CCK receptors, CCK-1 and CCK-2, the inhibitory feeding effects of CCK are mediated mainly via CCK-1 receptors [24]. In addition to its role in peripheral satiation signaling, CCK found within specific brain regions can play a modulatory role in the activity of several neurotransmitters, including dopamine (DA). Specifically, CCK stimulates DA release by acting on CCK-1 receptors [4], while activation of the CCK-2 receptor confers an inhibitory effect on DA release [22]. In contrast to the ubiquitous presence of CCK-2 receptors throughout the brain, populations of central CCK-1 receptors are only known to exist within limited regions, such as the dorsomedial hypothalamus [23,30] and striatum [10], with specifically high intensity in the caudo-medial shell of the nucleus accumbens (NAcc) [17,20]. In the caudal NAcc, DA and CCK are co-released in vivo after administration of drugs that increase DA neuronal firing rate [4,19]. A similar co-release of DA and CCK in the somatodendritic area of the mesoaccumbens DA system, in the ventral tegmental area (VTA), has also been shown to affect DA cell firing rate [16].
Natural and transgenic CCK receptor mutant animal models offer a unique opportunity for studying interactions between DA and CCK systems. The Otsuka Long-Evans Tokushima Fatty (OLETF) rat is an outbred strain of Long-Evans Tokushima (LETO) rat established as a model for dietary induced obesity and non-insulin dependent diabetes mellitus (NIDDM) [18]. These animals lack expression of the CCK-1 receptor due to a recently discovered spontaneous 6.8 kb deletion in the CCK-1 receptor gene [29]. Therefore, the OLETF rat represents a potentially important model to assess mesolimbic DA transmission in vivo where effects of CCK-1 receptor activation are absent. Indeed, recent data from Feifel and colleagues have demonstrated that OLETF rats exhibit enhanced amphetamine (AMPH) and cocaine-induced increases in NAcc DA release relative to LETO controls [9].
Prepulse inhibition (PPI) of acoustic startle reflex (ASR), where a brief loud burst of noise is immediately preceded (50–500 ms) by a softer non-startling sound, has been previously recognized as an operational measure of sensori-motor gating [28]. The mesolimbic DA pathway is one of the crucial systems in the brain that contributes to PPI response [27]. Specifically, DA agonists such as AMPH can disrupt PPI by inhibiting the normal reduction of startle response due to the prepulse [21]. Participation of CCK-1 receptors in mediating PPI responses has also been documented. For example, cerulein, a CCK agonist with preferential affinity for CCK-1 receptors partially reverses AMPH-induced reductions in PPI [7]. The CCK-1 receptor antagonist, devazepide, but not the CCK-2 antagonist, L-365,260, blocked cerulein’s effect on PPI, suggesting CCK-1 receptor mediation of CCK effects on PPI [7]. Furthermore, CCK-1 receptor deficient OLETF rats are unresponsive to AMPH-induced decreases in PPI compared to LETO rats, which show a disruption of PPI consistent with AMPH treatment in non-mutant animals [8].
Our laboratory has recently demonstrated that OLETF rats have an increased preference for both real and sham feeding of sucrose solutions [5]. The effects of sucrose on dopaminergic transmission are well recognized. Sham feeding sucrose in rats triggers NAcc DA release as a function of sucrose concentration [15]. When given intermittent access to sugar solutions, non-mutant rats exhibit increased binding of D1 DA receptors in the NAcc [3]. Despite the emerging indication of enhanced DA signaling through sucrose consumption, only the effects of sucrose on ASR have been addressed [6], where high sucrose feeders exhibit altered ASR relative to low sucrose feeders in response to acoustic stimuli. The impact of sucrose treatment on PPI remains unknown. Furthermore, the role of CCK-1 receptors in mediating ASR and PPI responses following repeated sucrose consumption has not been examined.
Therefore, the goal of the present study was twofold: (1) to test the effects of repeated brief sucrose access on ASR and PPI responses in general; and (2) to determine the specific involvement of CCK-1 receptors in this modulation by assessing strain differences in startle responses in OLETF and LETO rats after sucrose consumption.
2. Materials and methods
2.1. Subjects
Fourteen-week old male OLETF and LETO rats were obtained as a generous gift of the Tokushima Research Institute, Otsuka Pharmaceutical, Tokushima, Japan. All animals were individually housed in mesh-floored, stainless-steel hanging cages in a temperature-controlled vivarium while maintained on a constant 12:12-h light–dark cycle (lights on at 0600). Rats were handled daily for a minimum of 1 week prior to the onset of experimental procedures. Tap water and pelleted rat chow (Purina 5001) were available ad libitum throughout experiments. All protocols used were approved by The Pennsylvania State University Institutional Animal Care and Use Committee.
2.2. Procedures and experiments
2.2.1. Habituation to startle chamber
Twenty rats (10 OLETF and 10 LETO) with average body weights of 452.6 ± 10.84 g and 367.5 ± 2.3 g, respectively, were divided in two groups (n = 5 per strain) matched for body weight within each strain. All rats were acclimated to an SR-LAB (San Diego Instruments, San Diego, CA) acoustic startle chamber (ASC) three times prior to experimental onset. Rats within the same stimulus group were run on the same day, i.e., sucrose (SUC) rats (n = 5) alternated daily with control, non-sucrose (NON), rats (n = 5).
2.2.2. Sucrose training
For sucrose regimen, we have adapted a protocol from our previous studies that demonstrated potency of brief, daily 20-min sucrose access on altering feeding behavior as well as cause functional and histological alterations in dopamine markers as early as after 6 days [12]. Following habituation to the startle chamber, SUC rats within each strain were trained to drink 10.0 ml of 0.3 M (10.3% w/v) sucrose during a brief 20 min access period, while the NON group never received sucrose access at any time. Three additional days of sucrose training occurred once each SUC rat consumed all 10 ml within the 20 min allotted period. Sucrose was presented at either 0900, 1000, or 1100 h during these three training days in order to dissociate any specific time cues of expectation associated with sucrose access. Starting at 0800 h on training day 4, all rats were tested in startle chambers. For SUC rats, this occurred immediately following sucrose access, while NON rats were tested at simultaneous intervals without sucrose access. Each rat was presented with 10 ml of 0.3 M sucrose. Immediately following consumption, the rat was placed in the ASC for testing. Rats within treatment groups and strain were randomly selected for time of testing.
2.2.3. Acoustic startle protocol
For testing ASRs, the rats were placed in the startle chamber for a 5 min acclimation period with a 65 dB background noise. Each rat was tested according to the same predetermined pseudo-randomized sequence of startle trials. The trial series consisted of: (1) a 120 dB, 40 ms noise burst presented alone, or (2) the same 120 dB, 40 ms noise burst preceded 100 ms by one of three prepulses (20 ms noise burst) that were 4, 8, or 12 dB above background. Each test session consisted of 7 presentations of the 4 experimental trials as described above, presented in a pseudo-randomized order. Variable inter-trial intervals averaged 8 s (range 4–12 s). The mean value of all 7 trials within each pulse-pairing was used as the rat’s value for each pulse-pairing. If the number of usable trials within a pulse-pairing was less than 4, then that pulse-pair was excluded from subsequent analyses.
2.2.4. Statistical analysis
The startle response computer output file contains two main values of interest: Vmax and Tmax. Vmax is the maximum amplitude spike that occurs during each trial, while Tmax is the time at which this value is recorded. In our protocol, Tmax occurs at ~20 ms (for pulse alone) or ~120 ms (for all prepulse-pulse pairings) after trial recording begins, which corresponds to the timeframe immediately following the 120 dB acoustic stimuli at 20 ms after recording (pulse alone trial) or the 120 ms mark after recording (for all prepulse paired trials). In a few cases, Tmax values occurred prior to the initiation of any acoustic stimuli, indicating that the displacement measured was not due to the acoustic stimuli, and thus was not a measure of startle response. In all, less than 10 such cases were recorded for individual trials, appearing randomly among the four experimental groups. These data were excluded from subsequent analyses.
PPI is defined as the percent reduction in startle amplitude in the presence of the prepulse stimulus compared to the amplitude in the absence of the prepulse stimulus [100 − (100 × amplitude on prepulse trial/amplitude on 120 dB pulse trial alone)]. ASR and PPI were calculated by two-way repeated measures analysis of variance (rmANOVA) with strain and sucrose treatment as main factors. ANOVA results were subsequently analyzed by Tukey’s honestly significant difference (HSD) test post-hoc tests when applicable. All data are expressed as means ± SEM. Differences were considered statistically significant if P < 0.05. All statistical analyses were carried out with PC-SAS (version 8.02, SAS Institute, Carey, NC).
3. Results
A significant interaction [F(3,16) = 4.84; P < 0.05] of strain × sucrose access on acoustic startle response was observed. As shown in Fig. 1, there were no significant difference in baseline (i.e., with no sucrose) ASR between OLETF and LETO rats (137.5 ± 26.1 mV and 224.3 ± 37.0 mV for LETO and OLETF rats, respectively), although there was a trend toward higher ASR in OLETFs (P = 0.0867). In contrast, in animals receiving brief access to sucrose, a significant genotype main effect [F(1,16) = 6.84; P < 0.01)] was observed as OLETF rats receiving sucrose had significantly higher (P < 0.01) ASR than LETO rats receiving sucrose (174.6 ± 21.8 mV and 364.7 ± 53.0 mV for LETO and OLETF rats, respectively).
Fig. 1.
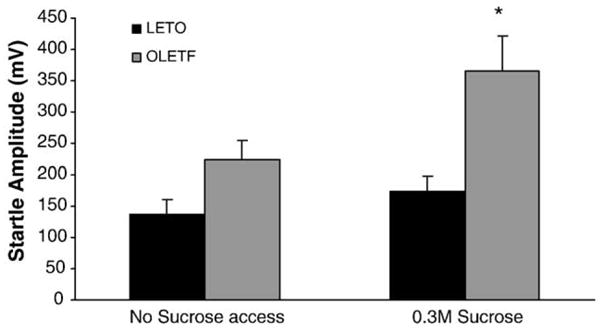
OLETF rats receiving sucrose had a higher ASR magnitude than LETO rats receiving sucrose. In contrast, there were no differences in baseline ASR between OLETF and LETO rats. (*P < 0.05 between strains).
This experiment also yielded a significant genotype × sucrose treatment interaction on prepulse inhibition [F(3,16) = 7.93; P < 0.02]. Fig. 2A shows that while non-sucrose receiving controls (NON) exhibited statistically identical prepulse inhibition percentages (45.5 ± 5.8% and 47.7 ± 2.3% for LETO and OLETF rats, respectively), LETO rats receiving sucrose treatment had a significantly lower PPI percentage (P < 0.01) than OLETF rats receiving the same treatment (32.4 ± 2.3% and 57.7 ± 3.1% for LETO and OLETF rats, respectively). Additionally, sucrose receiving LETO rats had a significantly lower (P < 0.05) PPI percentage than non-sucrose controls (32.4 ± 2.3% and 45.5 ± 5.8% for sucrose and non-sucrose treatments, respectively). No significant sucrose effects on overall PPI (P = 0.283) were noted in OLETF rats (57.7 ± 3.1% and 47.7 ± 2.3% for sucrose and non-sucrose treatments, respectively). Fig. 2B illustrates the results of post-hoc analyses which revealed that LETO rats exhibited significantly reduced PPI across all three levels of prepulse intensity [P < 0.05, for PPI-4 and PPI-8 trials; P < 0.01, for PPI-12 trials], while OLETF rats showed no response [all Ps > 0.05].
Fig. 2.
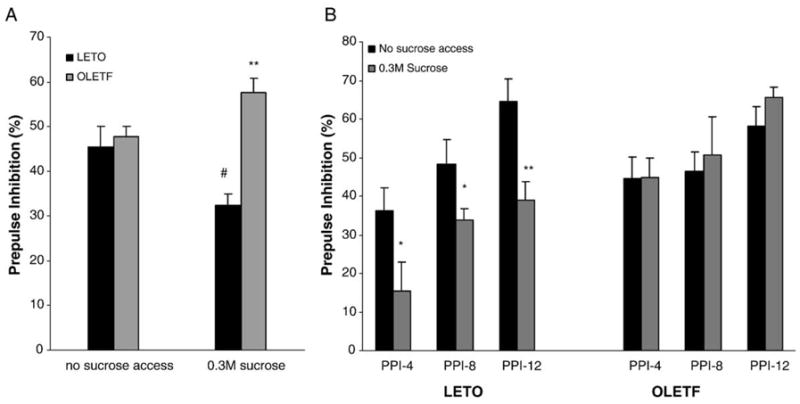
(A) LETO rats receiving sucrose treatment had a lower overall PPI percentage than OLETF rats, while non-sucrose receiving controls exhibited similar PPI percentages. Additionally, sucrose receiving LETO rats had lower PPI percentage than non-sucrose LETO rats. (**P < 0.01, between strains; #P < 0.05 within strain). (B) When examining specific effects of prepulse intensity on PPI of baseline and sucrose-induced changes in PPI, LETO rats show decreased PPI at all levels of prepulse intensity, while OLETF rats are unresponsive. (**P < 0.01, *P < 0.05; between strains).
4. Discussion
These results show that short, repeated access to a palatable sucrose solution can disrupt PPI in non-mutant rodents. Furthermore, we demonstrated that using a sucrose access model, rats lacking CCK-1 receptors are insensitive to the PPI effects of sucrose across three levels of prepulse intensity compared with their non-mutant LETO controls. Additionally, the present data demonstrate that brief access to sucrose resulted in higher ASR in OLETF rats compared to LETO rats.
The specific mechanisms through which sucrose affects PPI are unknown, although several lines of evidence point to the activation of the mesolimbic DA system. For example, sucrose consumption produces a rise in NAcc DA [11]. Furthermore, bilateral lesions in the pontine parabrachial nucleus (PBN), the second major relay in the gustatory pathway, attenuate sucrose-related DA responses in the NAcc, while similar lesions to the gustatory thalamus do not [13]. This finding demonstrates a functional connection between gustatory information processing and accumbens DA, while also suggesting that these actions occur within the brainstem and/or limbic DA systems, rather than the thalamo-corticostriatal circuitry. Thus, it is likely that sucrose consumption could alter PPI via increased mesoaccumbens DA release. Evidence supporting this notion comes from studies using AMPH or cocaine challenge [9]. For example, OLETF rats show enhanced DA release and do not respond to drug-induced disruptions of PPI from AMPH or the NMDA receptor antagonist, dizocilpine [8]. Overall, these data indicate that OLETF rats may have abnormalities secondary to the inborn absence of CCK-1 receptors that may impair DA autoregulation. Therefore, it is possible that impaired sucrose-induced DA signaling due to the lack of CCK-1 receptor in OLETF rats could account for the blocked sucrose PPI response in these animals. However, this hypothesis remains to be directly tested.
There are also data suggesting cross-sensitization effects of sucrose and AMPH. Rats previously sensitized to AMPH showed increased locomotor activity after brief sucrose access than rats that were prior treated with saline [1]. A similar cross-sensitization was also observed where rats receiving intermittent sucrose access exhibited higher subsequent locomotor activity by AMPH challenge, compared to rats that did not receive AMPH injections [2]. Additionally, rats having higher baseline consumption of sucrose show increased AMPH-induced dopamine overflow in the posterior nucleus accumbens relative to low sucrose feeders [26]. This is consistent with our recent results demonstrating a greater sensitization to AMPH in OLETF compared to LETO rats after access to sucrose [14] and may explain our findings of an increased preference for sucrose in OLETF rats [5].
We also showed that sucrose access resulted in increased ASR in OLETF, but not LETO rats. To our knowledge, this is the first observation of increased ASR using brief access to sucrose as a stimulus. The results are in overall agreement with previous data showing a higher magnitude of dopamine agonist-induced ASR in OLETF relative to LETO rats [8]. Our finding of a trend toward increased baseline ASR in OLETF compared to LETO rats parallels those by Feifel and colleagues, although we did not achieve statistical significance. However, it is important to emphasize that the lack of statistical difference between the groups cannot be interpreted as evidence of a lack of effect of sucrose. It is possible that a larger sample size would have born out an effect.
It is well known that ASR is a commonly used model of anxiety, where increased ASR signifies greater anxiogenic effect. Yamamoto and colleagues recently observed that OLETF rats exhibit higher anxious behaviors in an elevated plus-maze test relative to LETO controls [31]. Thus, is it possible the higher anxiety may explain increased ASR in the OLETF rat. How this behavior would interact with sucrose access to promote a genotype-dependent increase in ASR is not known; however, it is possible that altered sensitivity to the rewarding properties of sucrose may differentially affect the basal anxiety state in OLETF and LETO rats. In these studies, although baseline ASR in OLETF rats was somewhat higher than in LETO, baseline PPI was identical between strains, while sucrose treated OLETF rats showed both higher ASR and PPI relative to LETO rats. The different directional change in ASR and PPI measures in the LETO rats is not surprising since previous data also show that ASR and PPI can change independently. Some caution in the interpretation of these results is warranted, in particular due to the modest sample size. However, the magnitude of the effect size was sufficient to detect statistical differences between main effects. A larger sample size, combined with a detailed careful study of the time course of the reported effects across different sucrose concentrations, is necessary for the interpretation of the reported findings. Despite this, the present findings do support a role of CCK-1 receptors in modulating sucrose-induced alterations on PPI and ASR responses.
Taken together, this study has identified repeated brief sucrose access as a stimulus model able to disrupt prepulse inhibition. While the exact mechanism for this effect is not yet known, it is likely that the mesolimbic dopamine system, that also mediates affective characteristics of palatable sucrose, is compromised in disruption of PPI by sucrose ingestion. In the sweet-preferring OLETF rats, sucrose had no effect, that is PPI was unaltered after sucrose access in these animals. Therefore, it is possible that developmental defects due to the congenital lack of CCK-1 receptor leads to alterations in DA signaling that becomes manifest by stimulation from sucrose ingestion which, in turn, leads to altered startle responsiveness. These changes, together with reported deficits in satiation signals of OLETF rats, may be responsible for the hyperphagia and obesity that characterize this model.
Acknowledgments
The authors wish to thank Otsuka Pharmaceutical Co. (Tokushima, Japan) for the generous donation of the OLETF and LETO animals used in this research. This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK065709.
Abbreviations
- ASR
Acoustic Startle Response
- PPI
Percent Pre-Pulse inhibition
References
- 1.Avena NM, Hoebel BG. Amphetamine-sensitized rats show sugar-induced hyperactivity (cross-sensitization) and sugar hyperphagia. Pharmacol Biochem Behav. 2003;74:635–639. doi: 10.1016/s0091-3057(02)01050-x. [DOI] [PubMed] [Google Scholar]
- 2.Avena NM, Hoebel BG. A diet promoting sugar dependency causes behavioral cross-sensitization to a low dose of amphetamine. Neuroscience. 2003;122:17–20. doi: 10.1016/s0306-4522(03)00502-5. [DOI] [PubMed] [Google Scholar]
- 3.Colantuoni C, Schwenker J, McCarthy J, Rada P, Ladenheim B, Cadet JL, Schwartz GJ, Moran TH, Hoebel BG. Excessive sugar intake alters binding to dopamine and mu-opioid receptors in the brain. NeuroReport. 2001;12:3549–3552. doi: 10.1097/00001756-200111160-00035. [DOI] [PubMed] [Google Scholar]
- 4.Crawley JN. Cholecystokinin-dopamine interactions. Trends Pharmacol Sci. 1991;12:232–236. doi: 10.1016/0165-6147(91)90558-a. [DOI] [PubMed] [Google Scholar]
- 5.De Jonghe BC, Hajnal A, Covasa M. Increased oral and decreased intestinal sensitivity to sucrose in obese, prediabetic CCK-A receptor-deficient OLETF rats. Am J Physiol: Regul, Integr Comp Physiol. 2005;288:R292–R300. doi: 10.1152/ajpregu.00481.2004. [DOI] [PubMed] [Google Scholar]
- 6.Desousa NJ, Wunderlich GR, De Cabo C, Vaccarino FJ. Individual differences in sucrose intake predict behavioral reactivity in rodent models of anxiety. Pharmacol Biochem Behav. 1998;60:841–846. doi: 10.1016/s0091-3057(98)00069-0. [DOI] [PubMed] [Google Scholar]
- 7.Feifel D, Reza T, Robeck S. Antipsychotic potential of CCK-based treatments: an assessment using the prepulse inhibition model of psychosis. Neuropsychopharmacology. 1999;20:141–149. doi: 10.1016/S0893-133X(98)00041-4. [DOI] [PubMed] [Google Scholar]
- 8.Feifel D, Priebe K, Shilling PD. Startle and sensorimotor gating in rats lacking CCK-A receptors. Neuropsychopharmacology. 2001;24:663–670. doi: 10.1016/S0893-133X(00)00235-9. [DOI] [PubMed] [Google Scholar]
- 9.Feifel D, Shilling PD, Kuczenski R, Segal DS. Altered extracellular dopamine concentration in the brains of cholecystokinin-A receptor deficient rats. Neurosci Lett. 2003;348:147–150. doi: 10.1016/s0304-3940(03)00767-5. [DOI] [PubMed] [Google Scholar]
- 10.Graham WC, Hill DR, Woodruff GN, Sambrook MA, Crossman AR. Reduction of [125I]Bolton Hunter CCK8 and [3H]MK-329 (devazepide) binding to CCK receptors in the substantia nigra/VTA complex and its forebrain projection areas following MPTP-induced hemi-parkinsonism in the monkey. Neurosci Lett. 1991;131:129–134. doi: 10.1016/0304-3940(91)90353-u. [DOI] [PubMed] [Google Scholar]
- 11.Hajnal A, Norgren R. Accumbens dopamine mechanisms in sucrose intake. Brain Res. 2001;904:76–84. doi: 10.1016/s0006-8993(01)02451-9. [DOI] [PubMed] [Google Scholar]
- 12.Hajnal A, Norgren R. Repeated access to sucrose augments dopamine turnover in the nucleus accumbens. NeuroReport. 2002;13:2213–2216. doi: 10.1097/00001756-200212030-00010. [DOI] [PubMed] [Google Scholar]
- 13.Hajnal A, Norgren R. Taste pathways that mediate accumbens dopamine release by sapid sucrose. Physiol Behav. 2005;84:363–369. doi: 10.1016/j.physbeh.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 14.Hajnal A, Covasa M, Acharya N, Bello NT. Altered dopamine functions in obese CCK-A receptor deficient (OLETF) rats: reduced motor activity and responsiveness to amphetamine and chronic sucrose feeding, lower dopamine transporter binding. Abstr - Soc Neurosci. 2004 Abstract. [Google Scholar]
- 15.Hajnal A, Smith GP, Norgren R. Oral sucrose stimulation increases accumbens dopamine in the rat. Am J Physiol: Regul, Integr Comp Physiol. 2004;286:R31–R37. doi: 10.1152/ajpregu.00282.2003. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton ME, Freeman AS. Effects of administration of cholecystokinin into the VTA on DA overflow in nucleus accumbens and amygdala of freely moving rats. Brain Res. 1995;688:134–142. doi: 10.1016/0006-8993(95)00518-u. [DOI] [PubMed] [Google Scholar]
- 17.Heidbreder C, Gewiss M, De Potter W, De Witte P. Regional amines levels in the rat brain following intra-accumbens cholecystokinin and intraperitoneal amphetamine pre-treatment. Arch Int Physiol, Biochim Biophys. 1992;100:267–273. doi: 10.3109/13813459208998113. [DOI] [PubMed] [Google Scholar]
- 18.Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes. 1992;41:1422–1428. doi: 10.2337/diab.41.11.1422. [DOI] [PubMed] [Google Scholar]
- 19.Ladurelle N, Durieux C, Roques BP, Dauge V. Different modifications of the dopamine metabolism in the core and shell parts of the nucleus accumbens following CCK-A receptor stimulation in the shell region. Neurosci Lett. 1994;178:5–10. doi: 10.1016/0304-3940(94)90276-3. [DOI] [PubMed] [Google Scholar]
- 20.Lanca AJ, De Cabo C, Arifuzzaman AI, Vaccarino FJ. Cholecystokinergic innervation of nucleus accumbens subregions. Peptides. 1998;19:859–868. doi: 10.1016/s0196-9781(98)00032-1. [DOI] [PubMed] [Google Scholar]
- 21.Mansbach RS, Geyer MA, Braff DL. Dopaminergic stimulation disrupts sensorimotor gating in the rat. Psychopharmacology (Berlin) 1988;94:507–514. doi: 10.1007/BF00212846. [DOI] [PubMed] [Google Scholar]
- 22.Marshall FH, Barnes S, Hughes J, Woodruff GN, Hunter JC. Cholecystokinin modulates the release of dopamine from the anterior and posterior nucleus accumbens by two different mechanisms. J Neurochem. 1991;56:917–922. doi: 10.1111/j.1471-4159.1991.tb02009.x. [DOI] [PubMed] [Google Scholar]
- 23.Moran TH, Robinson PH, Goldrich MS, McHugh PR. Two brain cholecystokinin receptors: implications for behavioral actions. Brain Res. 1986;362:175–179. doi: 10.1016/0006-8993(86)91413-7. [DOI] [PubMed] [Google Scholar]
- 24.Moran TH, Ameglio PJ, Schwartz GJ, McHugh PR. Blockade of type A, not type B, CCK receptors attenuates satiety actions of exogenous and endogenous CCK. Am J Physiol. 1992;262:R46–R50. doi: 10.1152/ajpregu.1992.262.1.R46. [DOI] [PubMed] [Google Scholar]
- 25.Ritter RC. Gastrointestinal mechanisms of satiation for food. Physiol Behav. 2004;81:249–273. doi: 10.1016/j.physbeh.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Sills TL, Vaccarino FJ. Individual differences in sugar consumption following systemic or intraaccumbens administration of low doses of amphetamine in nondeprived rats. Pharmacol Biochem Behav. 1996;54:665–670. doi: 10.1016/0091-3057(96)00024-x. [DOI] [PubMed] [Google Scholar]
- 27.Swerdlow NR, Caine SB, Geyer MA. Regionally selective effects of intracerebral dopamine infusion on sensorimotor gating of the startle reflex in rats. Psychopharmacology (Berlin) 1992;108:189–195. doi: 10.1007/BF02245306. [DOI] [PubMed] [Google Scholar]
- 28.Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology (Berlin) 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- 29.Takiguchi S, Takata Y, Funakoshi A, Miyasaka K, Kataoka K, Fujimura Y, Goto T, Kono A. Disrupted cholecystokinin type-A receptor (CCKAR) gene in OLETF rats. Gene. 1997;197:169–175. doi: 10.1016/s0378-1119(97)00259-x. [DOI] [PubMed] [Google Scholar]
- 30.Woodruff GN, Hill DR, Boden P, Pinnock R, Singh L, Hughes J. Functional role of brain CCK receptors. Neuropeptides. 1991;19(Suppl):45–56. doi: 10.1016/0143-4179(91)90082-t. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto Y, Akiyoshi J, Kiyota A, Katsuragi S, Tsutsumi T, Isogawa K, Nagayama H. Increased anxiety behavior in OLETF rats without cholecystokinin-A receptor. Brain Res Bull. 2000;53:789–792. doi: 10.1016/s0361-9230(00)00407-x. [DOI] [PubMed] [Google Scholar]


