Abstract
Chloride secretion is important because it is the driving force for fluid movement into the intestianl lumen. Flow of accummulated fluid flushes out invading micro-orgnaisms in defense of the host. Chloride secretion is regulated by neurons in the submucosal plexus of the enteric nervous system. Mechanosensitive enterochromaffin cells that release 5-hydroxytryptamine (5-HT)activate intrinsic afferent neurons in the submucosal plexus and initiate chloride secretion. Mechanical stimulation by distention may also triggers reflexes by a direct action on intrinsic afferent neurons. Dysregulation of 5-HT release or altered activity of intrinsic afferents is likely to occur in states of inflammation and other disorders.
Keywords: Intestinal secretion, chloride, 5-hydroxytryptamine, enteric nerves
Introduction
This chapter will focus on Cl− secretion and its regulation by the enteric nervous system. Cl− secretion is a major determinant of net movement of fluid across the mucosal surface into the intestinal lumen. The extremes in secretion reflect clinical symptoms of constipation or diarrhea. Who are the players? The players that determine secretion are 1) the crypt epithelial cell as the effector, 2) the enterochromaffin cell or afferent neuron that is a sensor and 3) the neurons in the neural secretory reflex. The secretory reflex consists of intrinsic afferents which deliver signals from the mucosa to the secretomotor neurons that innervate the epithelial cells to drive fluid secretion.
The Crypt Epithelial Cell
The intestinal mucosa is comprised mainly of epithelial villous and columnar absorptive cells; however, it is the crypt cell that maintains levels of Cl− secretion (Figure 1A). Cl− accumulates in the cell by a Na+/2Cl−/K+ transporter (NKCC1) on the basolateral membrane and exits via apical Cl− channels (Figure 1A). The Cl− channel most well studied is the cystic fibrosis transmembrane regulator (CFTR) Cl− channel that is activated by cAMP or cGMP (Barrett and Keely, 2006). However, evidence is accumulating to support the presence of other functional Cl− channels that provide an important, alternative route for Cl− secretion other than CFTR channels. These new Cl− channels include the Ca+2 activated CLCA Cl− channels and, the still some what controversial, cCAMP mediated ClC-2 channels on the apical membrane of crypt secreting cells (Cuppoletti and Malinowska 2006). K+ channels (IK-1, KvlQT1) on the basolateral side open and contribute to Cl−secretion by providing a favorable electrochemical gradient when K+ is lost. The basolateral Na+/K+ ATPase recycles K+ into the cell and maintains low intracellular concentrations of cytosolic Na+ (Figure 1A).
Figure 1.
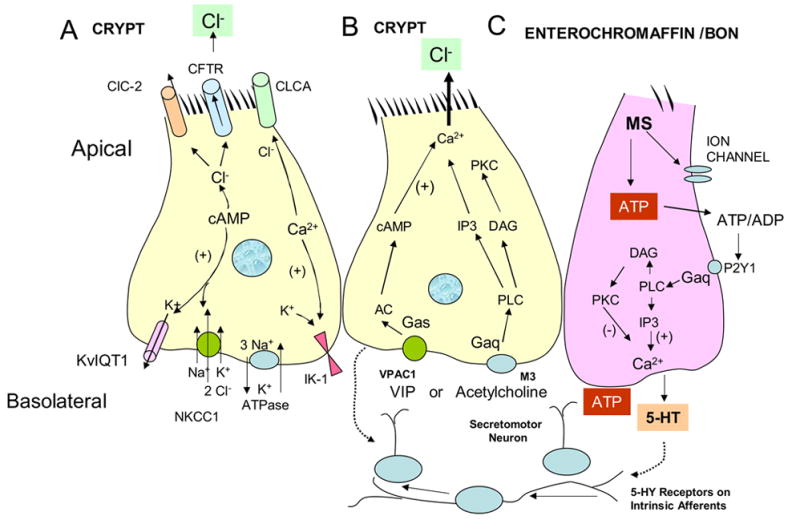
A. Crypt cell. Cl− secretion through cystic fibrosis transmembrane regulator Cl− channels (CFTR) or other Cl− channels (CIC-2 and CLCA). Cl- secretion through ion channels is dependent on the electrochemical driving forces. (See test page 2). B. Crypt cell with intracellular Gαq signaling for mobilization of Ca+2 when acetylcholine binds to M3 receptors, and with Gαs signaling pathways for elevation of cAMP by VIP binding to VPAC1 receptors. C. Enterochromaffin cell model (human BON cell). Mechanical stimulation by mucosal stroking or rotational shaking (MS), releases adenosine triphosphate, (ATP). It exists t he cell and is converted to adenosine di-phosphate (ADP). ADP activates P2Y1 receptors coupled to Gaq signaling and 5-HT release. 5-HT activates 5-HT receptors on intrinsic afferents and neurotransmission through secretomotor neurons release VIP or acetylcholine. Inostitol triphosphate (IP3), phospholipase C (PLC), diacylglycerol (DAG), protein kinase C and intracellular mobilization of intracellular Ca+2, adelylyl cyclase (AC), protein kinase A (PKA).
Cyclic AMP-mediated Cl− secretion has slow kinetics taking about 10–15 minutes to reach a peak response (Barrett and Keely, 2006; Cuppoletti and Malinowska, 2006). Secretogogues of interest in stimulating cAMP in crypt cells are vasoactive intestinal peptide (VIP), a neurotransmitter released by enteric neurons at epithelial junctions, and prostaglandins a paracrine inflammatory messenger that contributes to maintaining a resting level of Cl− secretion. These messengers act by binding to receptors on the crypt cell (Figure 1B).
Ca+2 mediated Cl− secretion has a fast onset and is transient (Barrett and Keely, 2006; Cuppoletti and Malinowska, 2006). The neurotransmitter of interest that utilizes a Ca+2 pathway is acetylcholine. It activates muscarinic M3 receptors on epithelial cells to mediate transient Ca+2 activated Cl- secretion (Figure 1B). The transient peak secretory response to Ca+2 is suppressed by anti-secretory signals that are not well defined (Barrett and Keely, 2006). They may include recruitment of phosphotidylinositol 3-kinase and various metabolites (inositol phosphate-3,4,5,6-)P4. cAMP and Ca+2 augment each others effects on secretion, possibly through G-protein crosstalk, although the specifics are not clear.
Enteric Nervous System
Specialized layers make up the gastrointestinal tract: The longitudinal muscle, myenteric plexus, circular muscle, submucosal plexus in the submucosa and epithelium. There are two ganglionated plexuses, the myenteric plexus lying between the longitudinal and circular muscle, and the submucosal ganglia lying in the submucosa, between the circular muscle and the epithelium. The myenteric plexus and the submucosal plexus are interconnected for efficient inter-communication to coordinate secretion with mixing, and propulsion of the intestinal contents, and with delivery of oxygen and nutrients to nourish the cells (Grider and Jin, 1994). To understand this complex regulatory mechanism requires knowledge of neural reflexes that are activated by appropriate stimuli. The secretory reflex depends on a sensor, an intrinsic afferent neuron, a secretomotor neuron, and an epithelial cell.
Sensors: Enterochromaffin Cells
Enterochromaffin cells, a type of enteroendocrine cell, are known to synthesize, store and release 5-hydroxytryptamine (5-HT). They contain more than 90% of the 5-HT stores in the body, the rest of 5-HT being stored in platelets, in mast cells in some species or in enteric neurons (Bertrand, 1997; 2003; Crowell, 2004; Galligan, 2004; Gershon, 2004; Kim et al., 2001). The intestinal track decodes (mechanical and chemical) stimuli such as stretch, touch, shear forces, compression, acid pH and nutrients that are involved in digestive function. Sensors detect mechanical signals that are generated by the mixing and propulsive waves occurring during contraction and stretch of smooth muscle cells (Jeffrey, et al. 2003). Gastric chyme emptying onto the duodenum contains acid that triggers 5-HT release from enterochromaffin cells. How these mechanical and chemical stimuli are sensed is unknown. Understanding how mechanical forces and chemicals impact on 5-HT release is important for understanding the physiological regulation of gut secretory and motility reflexes (Bertrand, 1997; 2003; Kellum 1999).
Alterations in 5-HT release, its content or its reuptake mechanisms by epithelial cells occur in a variety of diseases and syndromes. For example, irritable bowel syndrome, chronic constipation, carcinoid syndrome, diarrhea due to radiotherapy, chemotherapy, enterocolitis and inflammatory bowel disease may have different degrees of involvement of 5-HT (Farthing, 2002; Furness, 2006; Furness et al., 1999; Gershon, 2004; Linden et al., 2003; Mawe, et al., 1989; Wood, 2006A, 2006B). Understanding how 5-HT release is regulated at the cellular and molecular level is a necessary for understanding the basis of these disorders (Beubler et al., 1989; Crowell, 2004; Galligan, 2004;Gershon, 2006).
Sensory transduction mechanisms fall into several categories (Hamill and Martinac, 2001). 1). A force or chemical or mechanical stimulus is detected by a sensory cell that responds by triggering release of a mediator such as 5-HT. The mediator then activates an afferent neuron to initiate the reflex. An example of this is the reflex generated by stroking the surface of the intestinal mucosa with a brush or by shaking cells. 2) The second category of sensor is an afferent neuron that is activated directly by a stimulus causing a biological response such as opening of ion channels. This leads to depolarization, firing of action potentials and initiation of the reflex. An example of this is stretch activation of a subset of intrinsic and extrinsic enteric neurons.
Enterochromaffin cells transduce luminal stimuli such as acid, nutrients and applied mechanical forces. They defend the host from invading micro-organisms by releasing 5-HT that activates a neural reflex pathway to stimulate Cl- secretion and water movement into the intestinal lumen (Figure 1C). Together with motility responses, the accumulated fluid sweeps and flushes out the intestinal contents. To carry out this action, the enterochromaffin cells utilize an intricate signaling system that includes generation of intracellular messengers, extracellular messengers and cell surface receptors that participate in the release of 5-HT (Kim, et al. 2001A; 2001B; Raybould, 2004,) Defining these signaling pathways has been made difficult because of the sparse distribution of enterochromaffin cells and the impediments to isolating a pure population of enterochromaffin cells. In the absence of good models for studying 5-HT release, the human carcinoid BON cell line has emerged as a suitable model (Evers et al, 1991).
Adenosine triphosphate (ATP) has emerged as a signaling molecule released during mechanical stimulation in a variety of cells including urinary bladder, human colon, and nasal epithelium (Chien et al, 1998; Cooke, et al., 2003; Wynn, et al 2004). One of the pathways for 5-HT release is mechanosensitive and involves early release of ATP (Figure 1C). Rotational shaking of BON cells (60–100 revolutions/minute), and mucosal stroking of the intestinal lining with a brush can stimulate enterochromaffin cells and thus be converted to a biological event such as opening of a mechanosensitive ion channel (Kim, et al. 2001A). ATP then exits the sensory enterochromaffin cell by unknown mechanisms that could include exocytosis of secretory granules or efflux through ion channels (Wynn, et al., 2004). Once ATP leaves the cell and reaches the extracellular compartment, it can act as an autocrine messenger to stimulate other endochromaffin cells directly or as a paracrine messenger to activate P2Y receptors or P2X receptors that may be present on intrinsic afferent neurons (Figure 1B, 1C, Figure 2).
Figure 2.
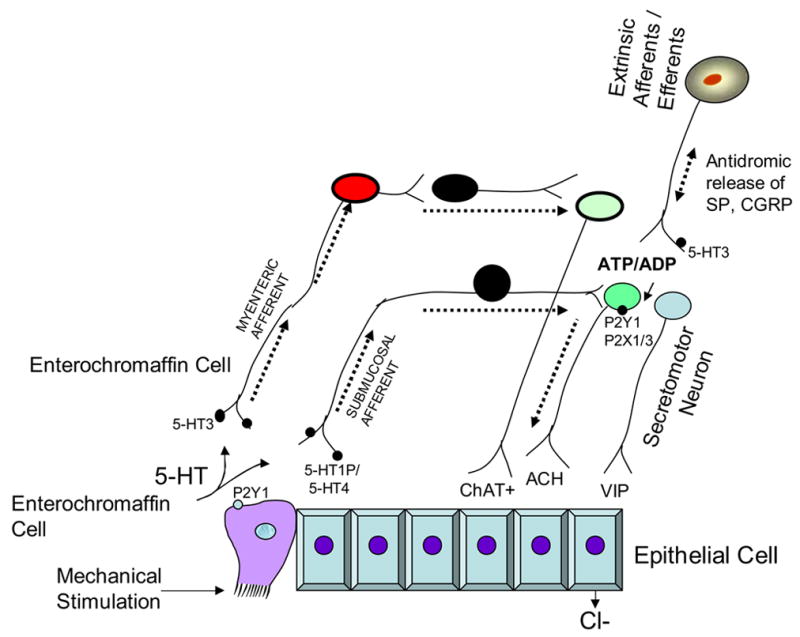
Reflex activity stimulated by mucosal stroking the intestinal lining or rotational shaking of cultured enterochromaffin derived cell line, BON. The mechanotransduction pathway is initiated by 5-HT released from enterochromaffin cells which activates a reflex arc within the submucosal plexus. P2Y1 receptors are on enterochromaffin cells. P2Y1 receptors are down stream in neural pathways and found on cholinergic secretomotor neurons in the guinea pig colon and VIP secretomotor neurons in the ileum. Thus the mechanosensitive pathway triggered by mucosal stroking involves release of 5-HT and activation of intrinsic afferents that do not express P2Y1 receptors. This may alter the excitability of secretomotor neurons. In contrast, stretch is expected to activate submucosal afferents directly without the need for 5-HT. Secretory reflexes are shown to go through the submucosal plexus. Mechanically evoked 5-HT can also activate myenteric neurons via 5-HT3 receptors to initiate long reflexes.
ATP is metabolized to ADP, AMP and adenosine by ecto-ATPases and ectonucleotidases and the latter reaches levels of 50–80 nmole/liter at rest (Christofi et al., 2004; Kukulski and Komoszynski, 2003). Adenosine that comes from the breakdown of nucleotides or other sources is an inhibitor of baseline or resting Cl− secretion and can mask some of the secretory effects of excitatory mediators that are released spontaneously (Christofi et al 2004). The ATP metabolite, ADP, then acts on a cognate P2Y1 receptor on the enterochromaffin cells (Figure 1B, 1C). The activation of receptor/Gαq by binding of ATP/ADP to P2Y1 receptors causes phospholipase C-mediated hydrolysis of phospholipids, and yields inositol-triphosphate (IP3) that binds to IP3 receptor on the endoplasmic recticulum of the enterochromaffin cell. Mobilization of Ca+2 from intracellular stores increases 5-HT release (Kim et al, 2001A) (Figure 1). This is the main pathway for release of 5-HT; however, 5-HT release from enterochromaffin cells can be modified by formation of diacylglycerol and protein kinase C. This description of the events completes the story for release of 5-HT. 5-HT now must play a paracrine role to activate intrinsic afferents to initiate the neural reflex (Figure 1C).
Sensors: Afferents
Enteric intrinsic afferent neurons are characterized by large smooth cell bodies and multiple axonal processes with few or no dendrites. Studies in rat, pig, human, mouse and guinea pig demonstrate that sensory afferents directly respond to stretch or to touch (Bornstein, 2006). These neurons have projections to the mucosa and to submucosal neurons or to myenteric neurons. Their projections suggest that they regulate the spread of neural activity around the circumference of the small intestine in the guinea pig.
Intrinsic afferents are cholinergic with immunoreactivity for choline acetyltransferase signifying the presence of acetylcholine. In general, about 80% of cholinergic neurons contain substance P. A subset of substance P intrinsic afferents also expresses glutamate. Calcitonin gene-related peptide (CGRP) has also been reported to be present in submucosal afferents (Kirchgessner, 2001; Pan, 2000). This chemical coding suggests that acetylcholine, substance P, glutamate and CGRP are putative neurotransmitters in that mediate synaptic transmission to other afferents or to secretomotor neurons.
Intrinsic afferent neurons are distinguished by a high resting membrane potential, discharge of one or a few spikes during a depolarizing current injection, and prolonged hyperpolarizing after-potentials (Wood, 2006A; 2006B). These neurons respond to touch by bursts of action potentials (Bornstein, 2006). Thus most of the neurons with after-hyperpolarizing currents in the myenteric plexus are stretch activated and respond to touch (Bornstein, 2006).
One population of submcosal afferents responds to gadolinium, a stretch activated inhibitor of ion channels. Gadolinium also causes a reduction in secretion (Frieling et al, 1992, Weber et al, 2002001). There are two components to secretion: one due to intrinsic mechanisms and the other due to extrinsic mechanisms. Distention evoked secretion due to activation of the submucosal afferents is revealed when capsaicin is added to block release of substance P from extrinsic afferents (Frieling et al, 1992; Weber et al., 2001). The capsaicin-insensitive Cl− secretion was inhibited by NK1 and NK3 antagonists showing that stretch activation of ion channels in the submucosal plexus involves tachykinin transmission. In the guinea pig ileum a subset of substance P afferents are chemosensitive and gluco-responsive, being activated by 5 mM D-glucose. Whether they are the same subset that recognizes stretch is unclear. These gluco-responsive neurons and the enterochromaffin cell model, BON cells, appear to utilize different signaling pathways for detection of D-glucose because the threshold for evoking a response in the neurons was 5 mM and was 5 fold higher for the BON cells (Kim et al, 2001A; 2001B; Liu, et al., 1999).
An important and unique feature of intrinsic afferents not shared by other sensory neurons is the presence of many neurotransmitter receptors on the cell bodies, suggesting that they receive synaptic inputs from other neurons (Furness, 2006). They respond to physiological stimuli and are identified as sensory neurons. Slow excitatory synaptic potentials drive other afferents around the circumference of the intestine. This arrangement suggests that these neurons also act as interneurons that simultaneously regulate circumferential and distal spread of neural activity (Bornstein, 2006). These characteristics suggest that afferents also play a dual role as interneurons that transmit information to the secretomotor neurons.
In the guinea pig colon, submucosal afferents have receptors for tachykinins (NK1, NK3), serotonin (5-HT1P, 5-HT3, 5-HT4), purines (P2X, P2Y), somatostatin, adenosine (A1) and receptors for inflammatory mediators (bradykinin B2, histamine H2) (Bertrand, 2006; Cooke and Christofi, 2006; Wynn, et al., 2004). The large number of receptors on these intrinsic afferent neurons suggest that they are active participants in the neural microcircuits and not simply relay stations from the mucosa. The presence of receptors for immune mediators on intrinsic afferents indicates that intrinsic afferents are targets for excitatory inputs during inflammation (Bornstein, 2006).
In summary, there appear to be at least two types of sensors regulating secretion in response to mechanical stimulation: Enterochromaffin cells detect force and release ATP and 5-HT and submucosal intrinsic afferents respond to stretch by opening of ion channels (Bornstein, 2006; Frieling et al, 1992). Studies using enterochromaffin cell models demonstrate that chemicals or nutrients such as D-glucose also stimulate the release of 5-HT. The mechanisms by which they affect release of 5-HT are still unknown (Kim et al, 2001A. 2001B)
Secretomotor neurons
Secretomotor or vasomotor neurons have uniaxonal morphology, low resting membrane potentials, high input resistance, repetitive spike discharge and no prolonged after hyperpolarizing responses. In the guinea pig ileum about half of submucosal neurons are cholinergic and half are VIP neurons (Brookes and Costa, 2006). One subset of cholinergic neurons expresses calretinin and is vasomotor. The remainder of submucosal cholinergic neurons contain neuropeptide Y as a marker, and another subset has no peptide markers. VIP does not co-localize with acetylcholine in secretomotor neurons in the guinea pig. VIP secretomotor neurons can be subdivided into those neurons that signal via cAMP those that do not. Some of the cAMP-dependent neurons also express adenosine A2A receptors. However, none of the cholinergic neurons with neuropeptide Y had cAMP-dependent responses or A2A receptors (Cooke and Christofi, 2006). The physiological significance of this pattern is unknown; however, there may be discrete subsets of cholinergic and VIP secretomotor neurons that are activated by different inputs. Further evidence of the diversity of functional subsets of secretomotor neurons comes from the finding that distribution of the cholinergic and VIP secretomotor neurons are polarized. The mucosa is innervated by a small fraction of ascending cholinergic (13% of ascending neurons), secretomotor neurons, and by a population of descending VIP secretomotor neurons. There are two small populations (1% each) of myenteric secretomotor neurons that are cholinergic or VIP containing (Costa et al, 2000). A small population of VIP interneurons project from the submucosal ganglia to the myenteric ganglia and may be important in coordinating secretion and motility (Figure 2).
Neural Secretory Reflex
Input from intrinsic afferents converge onto the same secretomotor neurons which clearly are separated into several categories. (Figure 2). Short reflexes occur over distances of a few millimeters in response to mucosal stroking that releases 5-HT (Figure 2). 5-HT has a great impact on secretion due to its activation of several different receptors on intrinsic afferents. 5-HT causes fast excitatory responses by activation of 5-HT3 receptors/ion channels and slow excitatory responses that are mediated by 5-HT4 receptors. Presynaptic inhibition of neurotransmitter release suppresses the neural synapses, but the receptor identity is unknown. 5-HT also excites intrinsic afferent neurons in the submucosal (5-HT1P, 5-HT4) and myenteric ganglia (5-HT3) and in extrinsic afferents as well (5-HT3) (Galligan, 2004, Gershon, 2004; Kellum et al., 1999; Wood, 2001), (Figure 2). This response can be inhibited with blockers of NK1, M3, VPAC1, P2Y1 but not P2X1/3 receptors (Cooke et al, 2004). The implication is that submucosal tachykinin-expressing intrinsic afferents are involved and transmit to both cholinergic and VIP secretomotor neurons and release acetylcholine and VIP at neuro-epithelial junctions (Figure 2). Chloride secretion is mediated by VIP acting on VPAC1 receptors, and acetylcholine via muscarinic receptors to elevate intracellular cAMP and Ca2+. While the majority of neurotransmission occurs through the submucosal plexus, there are some instances when long reflexes drive secretion via activation of myenteric afferent neurons that synapse with myenteric secretomotor neurons (Reed and Vanner, 2003; Kojima, et al., 2004). This is the pathway that cholera toxin may take to amplify cAMP responses already elevated in epithelial crypt cells when exposed to the toxin. The cystic fibrosis transmembrane regulator (CFTR) chloride channel provides a conduit for exit of chloride into the lumen (Barrett and Keely, 2006).
Role of Purinergic Receptors in Mechanically Evoked Mucosal Reflexes
One of the challenges is to elucidate the role for purinergic receptors in mechanically evoked mucosal reflexes. During mechanical stimulation, If P2X and P2Y receptors are in proximity to the site of ATP release by mechanical stimulation, ATP or a metabolite may bind and activate the receptor. The success in activating any P2 receptors at the target site will be dependent on diffusion distances, the presence and density of the receptors on the target tissues and presence of degrading enzymes. Because P2Y (P2Y1, 2, 4, 6, 8, 11, 12, 13) receptors are found in most cells, identifying a role for these receptors in secretory reflexes is difficult even with the use of new gene silencing techniques. For example, altering one component of receptor-mediated Cl− secretion by knocking down the P2Y receptor gene of interest can affect the balance among the different receptor-mediated components of secretion and perhaps mask the effect of gene knock-out of the receptor in question. An alternative approach to identify roles for these receptors is to over-express the cloned receptor in other cell systems to reveal its role in mechanosensory transduction. Furthermore, as in the case of mouse knockout models, some cell lines do not express or minimally express purinergic receptors and these could be used to determine the function of a receptor.
The role of P2Y1 in mechanosensory transduction of 5-HT release is another area in need of investigation. In some cell systems, P2Y1 receptors have been suggested to be a missing link in the mechanosensory signaling pathway. We have already shown that P2Y1 receptors are important for 5-HT release from an enterochromaffin cell model; yet, P2Y1 receptors are down stream of the release of ATP and therefore may not be the sensor per se (Kim et al, 2001A). This is an area certainly in need of further investigation.
Some reports suggest that P2Y1 receptors are necessary to convey mechanosensitive properties to cells that do not otherwise respond to mechanical stimulation. In Xenopous oocytes injected with P2Y1 receptor cRNA, touch elicited sensory potentials, whereas those without the cRNA did not respond (Nakamara and Strittmatter, 1996). This implies a need for the P2Y1 receptor in mechanosensory transduction and the need for further investigation of each of the steps in the 5-HT signaling of enterochromaffin cells (Ishida et al., 1997).
Several of the P2Y receptors signal through mobilization of intracellular Ca+2 that leads to 5-HT release. Many of the components of the signaling pathway including, PLC, IP3, PKC, Gαq and others are reported to be found in caveolin-1 associated membrane domains, called caveolae, that are rich in cholesterol and sphingolipids (Galbiati et al 1998; Kaiser, et al., 2002). Although caveolae have been suggested to play a role in mechanosensory transmission, caveolae may provide a scaffold for docking of signaling molecules in the resting state. They are present in intestinal epithelial cells, endothelial cells, enterochromaffin cell/BON cells, lung alveolar type 1 cells, fibroblasts and others (Qaddoumi et al 2003). Recent studies show that Gαq is sequestered in caveolin-1 associated domains in resting conditions in BON cells, but dissociates from caveolin-1 associated domains during mechanical stimulation of BON cells (Kim et al 2007; Qaddoumi et al 2003; Razani and Lisanti, 2001). Sequestration of P2Y1 receptors in cholesterol dependent signaling domains allows for agonist specific responses to elevation of Ca+2. This suggests that cells can respond disparately to various agonists by activation of the receptors coupled through a common Ca+2 release pathway. Cholesterol depletion with cyclodextrins causes BON cells to lose their ability to release 5-HT during mechanical stimulation by rotational shaking. (Qaddoumi et al., 2003). Further studies are necessary to define a role in 5-HT signaling. (Razani and Lisanti, 2001).
Extrinsic Afferents
The intestinal tract has a rich innervation of extrinsic afferent neurons that are characterized as vagal or spinal depending on their presence in cell bodies in nodose ganglia (NDG) and in the dorsal root ganglia (DRG), respectively. In general, vagal afferents convey the normal physiological state, whereas spinal afferents are concerned with nociception. The afferents run predominantly in the vagal tracts and spinal cord to convey information from the gut to the brain (afferent function); however, transmission may be bidirectional. In some instances, when an action potential propagates through axon collaterals, neurotransmitters are released retrogradely and act at neural targets close by.
Extrinsic afferents are polymodal. These respond to balloon distention, saline infusion, muscle contraction, nutrients, protons, activation of ligand-gated ion channels, 5-HT3, P2X and P2Y, ASICs,TRPV1 (Beyak et al., 2006; Clerc and Furness, 2004).
Extrinsic afferents directly innervate submucosal ganglia and alter function of the downstream targets, namely epithetical cells, arterioles and immune cells (Raybould et al, 2004, Wood 2006A, 2006B). Capsaicin treatment releases and then depletes extrinsic afferents of substance P and consequently has been used to eliminate the contribution of extrinsic afferents to secretion. Capsaicin acts through a vanilloid 1 receptor (TRPV1) receptor, a member of the TRP family of ion channels. Jejunal distention is attenuated in TRPV1 knock-out mouse models (Rong et al., 2004). TRPV1 Is gated by pH < 6, heat, capsicum, vanilloid compounds, and blocked by capsaicin (Beyek, 2006; Vanner and MacNaughton, 1995). Different latencies and threshold for the responses to different stimuli suggest there may be more than one sensor.
There are a number of sensors that play a role in gut function mediated by extrinsic afferents. Electrical stimulation of the nerve trunks containing extrinsic afferents in the intestinal mesentery of guinea pigs releases substance P, possibly from axon collaterals associated with the epithelium, immune cells or other cell types. Distention of rat or guinea pig intestine also causes Cl− secretion which is reduced by acute capsaicin treatment and tetrodotoxin (Eutamene et al., 1997; Weber et al., 2001). The ability of capsaicin to reduce the secreting response by about 50% suggests that extrinsic afferents play a significant role in secretion. Because NK1 and NK3 antagonists abolished the capsaicin-sensitive Cl− current the response was due to release of substance P and activation of NK1 and NK3 receptors on cholinergic and VIP-expressing secretomotor neurons (Weber et al., 2001). Thus, both intrinsic and extrinsic pathways converge into common secretomotor neurons in the distention response.
The intestinal mucosa serves as a barrier to invasion of microbes and bacteria. Several lines of defense include release of mediators from mast cells and macrophages and this is accomplished in part by activation of extrinsic afferents serving an efferent function, i.e. bi-directional communication. Substance P and CGRP released by stimulation of these nerves binds to their respective receptors on mast cells to release histamine and possibly epithelial cells to release other mediators (Wang et al, 2006 AGA abstract). Histamine then causes a blanket-like excitatory response in enteric neurons (Wang, et al., 2006). Alteration in sensory neurons during inflammation may contribute to alterations of secretory and motility patterns. Associated with inflammation and changes in pH other mechanically evoked reflexes may interact to cause sensitization of afferents
Chasing a role for acid in neural reflexes
Do acid-sensing ion channels have a potential role in gut function (Kellum, 1999)? Capsaicin-sensitive extrinsic afferents express acid-sensing ion channels (ASICs) found in many cell types. Although pH changes can affect a number of different ion channels, ASICs are unique, because they are directly activated by protons (Krishtal 2003). ASICs are known to play a role in visceral nociception and mechanotransduction. Four ASIC genes (ASIC1-4) encode at least 6 individual ASIC subunits which associate into homomeric or heteromeric channels with distinct properties (Kellenberger and Schild 2002). ASIC1 knockouts have increased sensitivity in all colonic mechanoreceptor subtypes (Page, Brierley et al. 2004; 2005). Mice lacking ASIC2 and ASIC3 channels have altered sensitivity in neurons that detect light touch (Page, Brierley et al. 2004; 2005). In addition, ASICs play a role in sensitization of mechanosensory afferents following inflammation. ASIC current density increases following acid ulceration of the stomach (Sugiura, Dang et al. 2005). In humans, ASIC3 protein levels increase in DRG following inflammation of the intestine (Yiangou, Facer et al. 2001). Furthermore, ASIC3 is required for sensitization of mechanosensitive afferents to acid-inflammatory “soup” (Jones, Xu et al. 2005). How ASICs impact mechanotransduction is unknown. It is thought that ASICs are modulators since mechanosensation has not been reported to activate ASICs directly. ASICs may also play a role in the release of serotonin from enterochromaffin cells in response to acid. However, the exact distribution of ASICs in the GI tract is unclear and a definitive role of ASICs in gut secretory function remains to be determined.
Sympathetic and Parasympathetic Divisions
Both efferent parasympathetic and sympathetic outflow includes release of transmitters such as acetylcholine or norepinephrine. The release is directed to the myenteric and submucosal ganglia to modulate the ongoing neural programs that govern the rates of blood flow, secretion and motility. Cranial parasympathetic outflow occurs via cranial nerves III, VII, IX and X (vagus) and via sacral parasympathetic outflow to the viscera, bladder and genitalia. Signals are sent via craniosacral parasympathetic pathways to activate many of the effectors including lacrimal, submandibular salivary, parotid, bronchial and intestinal crypt glands causing secretion across mucosal epithelial surfaces (Beyak et al, 2006). Thoraco-lumbar sympathetic outflow emerges with spinal T1-L3 or L4 and passes from sympathetic ganglia to destination organs. Intestinal crypt glands receive input from the sympathetic fibers and modulate secretion across mucosal epithelial surfaces (Beyak et al, 2006). The same effectors stimulated by parasympathetic fibers are targets for sympathetic input except lacrimal and bronchial glands which have no input from the sympathetic.
The effect of parasympathetic and sympathetic outflows to the intestine involve release of transmitters such as acetylcholine and norepinephrine, respectively. Neurotransmission from efferent fibers is directed to the myenteric and submucosal ganglia to modulate enteric neural pathways that govern the rates of blood flow, secretion and motility. Sympathetic fibers may be in proximity to epithelial cells as well, and therefore stimulation of sympathetics shuts down run-away secretion by a combined pre-synaptic inhibitory action of norepinephrine to reduce neurotransmitter release of some neurons and by acting at α2-adrenergic receptors on epithelial cells and diminishing secretion
In summary, the regulation of secretion across mucosal surfaces is a complex interaction of enterochromaffin cells, afferent neurons of intrinsic and extrinsic origin, secretomotor neurons and epithelial cells. In the guinea pig model, mucosal stroking of the intestinal epithelial mucosa stimulates release of 5-HT from the enterochromaffin cell to act on submucosal afferents. It is likely that stretch of submucosal afferents, like their myenteric counterparts, respond directly to stretch as a mechanical stimulus Muscarinic M3 and VPAC1 receptors on epithelial cells bind acetylcholine and VIP to stimulate secretion. Cholinergic neuronal inputs diverge to VIP secretomotor neurons to synchronize their activity in the same ganglion. This basic reflex is modified by intrinsic and extrinsic inputs and altered by sympathetic and parasympathetic activities. Understanding how the autonomic nervous system regulates intestinal fluid and electrolytes secretion is challenging due to the multitude of receptors and their interactions or different cells involved in mucosal reflex activity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jiajing Xue, Department of Neuroscience, 333 West 10th Avenue, The Ohio State University, Columbus, OH 43210.
Candice Askwith, Department of Neuroscience, 333 West 10th Avenue, The Ohio State University, Columbus, OH 43210.
Najma H. Javed, Department of Physiology and Health Science, 2000 University Avenue, Ball State University, Muncie, IN 47306
Helen J. Cooke, Department of Neuroscience, 333 West 10th Avenue, The Ohio State University, Columbus, OH 43210
References
- 1.Barrett KB, Keely SJ. Integrative physiology and pathophysiology of intestinal electrolyte transport. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. Academic press; 2006. pp. 1931–1951. [Google Scholar]
- 2.Bertrand PP, Kunze WA, Bornstein JC, Furness JB, Smith ML. Analysis of the responses of myenteric neurons in the small intestine to chemical stimulation of the mucosa. Am J Physiol. 1997;273(2 Pt 1):G422–35. doi: 10.1152/ajpgi.1997.273.2.G422. [DOI] [PubMed] [Google Scholar]
- 3.Bertrand PP. ATP and sensory transduction in the enteric nervous system. Neuroscientist Rev. 2003;9(4):243–60. doi: 10.1177/1073858403253768. [DOI] [PubMed] [Google Scholar]
- 4.Beubler E, Kollar G, Saria A, Bukhave K, Rask-Madsen J. Involvement of 5-hydroxytryptamine, prostaglandin E2, and cyclic adenosine monophosphate in cholera toxin-induced fluid secretion in the small intestine of the rat in vivo. Gastroenterology. 1989;96(2 Pt 1):368–76. doi: 10.1016/0016-5085(89)91560-6. [DOI] [PubMed] [Google Scholar]
- 5.Beyak MJ, Bulmer DCE, Jiang W, Keating C, Rong W, Grundy D. Extrinsic sensory afferent nerves innervating the gastrointestinal tract. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. Academic press; 2006. pp. 685–725. [Google Scholar]
- 6.Bornstein JC. Intrinsic sensory neurons of mouse gut--toward a detailed knowledge of enteric neural circuitry across species. Focus on “characterization of myenteric sensory neurons in the mouse small intestine”. J Neurophysiol. 2006 Sep;96(3):973–4. doi: 10.1152/jn.00511.2006. [DOI] [PubMed] [Google Scholar]
- 7.Brookes SJH, Costa M. Functional histonantomy of the enteric nervous system. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. Academic press; 2006. pp. 577–602. [Google Scholar]
- 8.Chien S, Li S, Shy YJ. Effects of mechanical forces on signal transduction and gene expression in endothelial cells. Hypertension. 1998;31(1 Pt 2):162–9. doi: 10.1161/01.hyp.31.1.162. [DOI] [PubMed] [Google Scholar]
- 9.Christofi FL, Kim M, Wunderlich JE, Xue J, Suntres Z, Cardounel A, Javed NH, Yu JG, Grants I, Cooke HJ. Endogenous adenosine differentially modulates 5-Hydroxytryptamine release from a human enterochromaffin cell model. Gastroenterology. 2004;127(1):188–202. doi: 10.1053/j.gastro.2004.04.070. [DOI] [PubMed] [Google Scholar]
- 10.Clerc N, Furness JB. Intrinsic primary afferent neurons of the digestive tract. Neurogastroenterol Motil. 2004;16(Suppl 1):24–7. doi: 10.1111/j.1743-3150.2004.00470.x. [DOI] [PubMed] [Google Scholar]
- 11.Cooke HJ, Wunderlich J, Christofi FL. “The Force Be With You” - ATP in gut mechanosensory transduction. News in Physiological Sciences. 2003;18:43–49. doi: 10.1152/nips.01411.2002. [DOI] [PubMed] [Google Scholar]
- 12.Cooke HJ, Xue J, Yu J-G, Wunderlich J, Wang Y-Z, Guzman J, Javed J, Christofi FL. Mechanical Stimulation Releases Nucleotides that Activate P2Y1 Receptors to Trigger Neural Reflex Chloride Secretion in Guinea Pig Distal Colon. J Comp Neurology. 2004;469:1–15. doi: 10.1002/cne.10960. [DOI] [PubMed] [Google Scholar]
- 13.Cooke HJ, Christofi FL. Enteric neural regulation of mucosal secretion. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. Academic press; 2006. pp. 737–762. [Google Scholar]
- 14.Costa M, Brookes SJ, Hennig GW. Anatomy and physiology of the enteric nervous system. Gut. 2000;47(Suppl 4):iv15–9. doi: 10.1136/gut.47.suppl_4.iv15. discussion iv26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crowell MD. Role of serotonin in the pathophysiology of the irritable bowel syndrome. Br J Pharmacol. 2004;141:1285–93. doi: 10.1038/sj.bjp.0705762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuppoletti J, Malinowska DH. Ion channels of the epithelia of the gastrointestinal tract. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. Academic press; 2006. pp. 1917–1929. [Google Scholar]
- 17.Eutamene H, Theodorou V, Fioramonti J, Bueno L. Rectal distention-induced colonic net water secretion in rats involves tachykinins, capsaicin sensory, and vagus nerves. Gastroenterology. 1997;112(5):1595–602. doi: 10.1016/s0016-5085(97)70041-6. [DOI] [PubMed] [Google Scholar]
- 18.Evers BM, Townsend CM, Sr, UPP JR, et al. Establishment and characterization of a human carcinoid in nude mice and effect of various agents on tumor growth. Gastroenterology. 1991;101:301–311. doi: 10.1016/0016-5085(91)90004-5. [DOI] [PubMed] [Google Scholar]
- 19.Farthing MJ. Novel targets for the control of secretory diarrhoea. Gut. 2002;50(Suppl 3):III15–8. doi: 10.1136/gut.50.suppl_3.iii15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frieling T, Wood JD, Cooke HJ. Submucosal reflexes: distension-evoked ion transport in the guinea pig distal colon. Am J Physiol. 1992;263(1 Pt 1):G91–6. doi: 10.1152/ajpgi.1992.263.1.G91. [DOI] [PubMed] [Google Scholar]
- 21.Furness JB, Kunze WA, Clerc N. Nutrient tasting and signaling mechanisms in the gut. II. The intestine as a sensory organ: neural, endocrine, and immune responses. Am J Physiol. 1999;277(5 Pt 1):G922–8. doi: 10.1152/ajpgi.1999.277.5.G922. [DOI] [PubMed] [Google Scholar]
- 22.Furness JB. Novel gut afferents: Intrinsic afferent neurons and intestinofugal neurons. Auton Neurosci. 125. 2006;(1–2):81–85. doi: 10.1016/j.autneu.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Galbiati F, Volonte D, Engelman JA, Watanabe G, Burk R, Pestell RG, Lisanti MP. Targeted downregulation of caveolin-1 is sufficient to drive cell transformation and hyperactivate the p42/44 MAP kinase cascade. EMBO J. 1998;16;17(22):6633–48. doi: 10.1093/emboj/17.22.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galligan JJ. 5-hydroxytryptamine, ulcerative colitis, and irritable bowel syndrome: molecular connections. Gastroenterology. 2004;26(7):1897–9. doi: 10.1053/j.gastro.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 25.Gershon MD. Serotonin receptors and transporters -- roles in normal and abnormal gastrointestinal motility. Aliment Pharmacol Ther, Rev. 2004;20(Suppl 7):3–14. doi: 10.1111/j.1365-2036.2004.02180.x. [DOI] [PubMed] [Google Scholar]
- 26.Grider JR, Jin JG. Distinct populations of sensory neurons mediate the peristaltic reflex elicited by muscle stretch and mucosal stimulation. J Neurosci. 1994;14(5 Pt 1):2854–60. doi: 10.1523/JNEUROSCI.14-05-02854.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamill OP, Martinac B. Molecular basis of mechanotransduction in living cells. Physiol Rev. 2001;81(2):685–740. doi: 10.1152/physrev.2001.81.2.685. [DOI] [PubMed] [Google Scholar]
- 28.Ishida T, Takahashi M, Corson MA, Berk BC. Fluid shear stress-mediated signal transduction: how do endothelial cells transduce mechanical force into biological responses? Ann N Y Acad Sci. 1997;15:811, 12–23. doi: 10.1111/j.1749-6632.1997.tb51984.x. discussion 23–4. [DOI] [PubMed] [Google Scholar]
- 29.Jeffrey B, Udaykumar HS, Schulze KS. Flow fields generated by peristaltic reflex in isolated guinea pig ileum: impact of contraction depth and shoulders. Am J Physiol Gastrointest Liver Physiol. 2003;285(5):G907–18. doi: 10.1152/ajpgi.00062.2003. [DOI] [PubMed] [Google Scholar]
- 30.Jones RC, 3rd, Xu L, et al. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vaniloid 1 and acid-sensing ion channel 3. J Neurosci. 2005;25(47):10981–9. doi: 10.1523/JNEUROSCI.0703-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaiser RA, Oxhorn BC, Andrews G, Buxton IL. Functional compartmentation of endothelial P2Y receptor signaling. Cir Rev. 2002;91(4):292–9. doi: 10.1161/01.res.0000030711.21521.ac. [DOI] [PubMed] [Google Scholar]
- 32.Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev. 2002;82(3):735–67. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- 33.Kellum JM, Albuquerque FC, Stoner MC, Harris RP. Stroking human jejunal mucosa induces 5-HT release and Cl- secretion via afferent neurons and 5-HT4 receptors. Am J Physiol. 1999;277(3 Pt 1):G515–20. doi: 10.1152/ajpgi.1999.277.3.G515. [DOI] [PubMed] [Google Scholar]
- 34.Kim M, Javed NH, Yu JG, Christofi F, Cooke HJ. Mechanical stimulation activates G alphaq signaling pathways and 5-hydroxytryptamine release from human carcinoid BON cells. J Clin Invest. 2001A;108(7):1051–9. doi: 10.1172/JCI12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim M, Cooke HJ, Javed NH, Carey HV, Christofi F, Raybould HE. D-glucose releases 5-hydroxytryptamine from human BON cells as a model of enterochromaffin cells. Gastroenterology. 2001B;121(6):1400–6. doi: 10.1053/gast.2001.29567. [DOI] [PubMed] [Google Scholar]
- 36.Kim M, Christofi F, Xue J, Robinson JM, Cooke HJ. Mechanically-evoked 5- hydroxytriptamine release is mediated by caveolin-associated cholesterol rich membrane domains. Neurogastroenterology Motility. 2007 doi: 10.1111/j.1365-2982.2007.00912.x. In Press. [DOI] [PubMed] [Google Scholar]
- 37.Kojima S, Ueda S, Ikeda M, Kamikawa Y. Calcitonin gene-related peptide facilitates serotonin release from guinea-pig colonic mucosa via myenteric neurons and tachykinin NK2/NK3 receptors. Br J Pharmacol. 2004;141(3):385–90. doi: 10.1038/sj.bjp.0705624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krishtal O. The ASICs: signaling molecules? Modulators? Trends Neurosci. 2003;26(9):477–83. doi: 10.1016/S0166-2236(03)00210-8. [DOI] [PubMed] [Google Scholar]
- 39.Kirchgessner AL. Glutamate in the enteric nervous system. Curr Opin Pharmacol. 2001 Dec;1(6):591–6. doi: 10.1016/s1471-4892(01)00101-1. Review. [DOI] [PubMed] [Google Scholar]
- 40.Kukulski F, Komoszynski M. Purification and characterization of NTPDase 1 (ecto-apyrase) and NTPDase2 (ecto-ATPase) from porcine brain cortex synaptosomes. Eur J Biochem. 2003;270:3447–3454. doi: 10.1046/j.1432-1033.2003.03734.x. [DOI] [PubMed] [Google Scholar]
- 41.Linden DR, Chen JX, Gershon MD, Sharkey KA, Mawe GM. Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2003;285:G207–16. doi: 10.1152/ajpgi.00488.2002. [DOI] [PubMed] [Google Scholar]
- 42.Liu M, Seino S, Kirchgessner AL. Identification and characterization of gluco-responsive neurons in the enteric nervous system. J Neurosci. 1999;19(23):10305–17. doi: 10.1523/JNEUROSCI.19-23-10305.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mawe GM, Branchek TA, Gershon MD. Blockade of 5-HT-mediated enteric slow EPSPs by BRL 24924 gastrokinetic effects. Am J Physiol. 1989;257(3 Pt 1):G386–96. doi: 10.1152/ajpgi.1989.257.3.G386. [DOI] [PubMed] [Google Scholar]
- 44.Nakamura F, Strittmatter SM. P2Y1 purinergic receptors in sensory neurons: contribution to touch-induced impulse generation. Proc Natl Acad Sci USA. 1996;93(19):10465–70. doi: 10.1073/pnas.93.19.10465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Page AJ, Brierley SM, et al. The ion channel ASIC1 contributes to visceral but not cutaneous mechanoreceptor function. Gastroenterology. 2004;127(6):1739–47. doi: 10.1053/j.gastro.2004.08.061. [DOI] [PubMed] [Google Scholar]
- 46.Page AJ, Brierley SM, et al. Different contributions of ASIC channels 1a, 2, and 3 in gastrointestinal mechanosensory function. Gut. 2005;54(10):1408–15. doi: 10.1136/gut.2005.071084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pan H, Gershon MD. Activation of intrinsic afferent pathways in submucosal ganglia of the guinea pig small intestine. J Neurosci. 2000;20(9):3295–309. doi: 10.1523/JNEUROSCI.20-09-03295.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qaddoumi MG, Gukasyan HJ, Davda J, Labhasetwar V, Kim KJ, Lee VH. Clathrin and caveolin-1 expression in primary pigmented rabbit conjunctival epithelial cells: role in PLGA nanoparticle endocytosis. Mol Vis. 2003 Oct 15;9:559–68. [PubMed] [Google Scholar]
- 49.Raybould HE, Cooke HJ, Christofi FL. Sensory mechanisms: transmitters, modulators and reflexes. Neurogastroenterol Motil 16 Suppl. 2004;1:60–3. doi: 10.1111/j.1743-3150.2004.00477.x. [DOI] [PubMed] [Google Scholar]
- 50.Razani B, Lisanti MP. Caveolins and caveolae: molecular and functional relationships. Exp Cell Res, Rev. 2001;15271(1):36–44. doi: 10.1006/excr.2001.5372. [DOI] [PubMed] [Google Scholar]
- 51.Reed DE, Vanner SJ. Long vasodilator reflexes projecting through the myenteric plexus in guinea-pig ileum. J Physiol. 2003;553(Pt 3):911–24. doi: 10.1113/jphysiol.2003.053140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rong WK, Hillsley JB, Davis GA, Hicks WJ, Winchester DG. Jejunely afferent nerve sensitivity in wild type and TRPV1 knock-out mice. J Physiol. 2004;560:867–881. doi: 10.1113/jphysiol.2004.071746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sugiura T, Dang K, et al. Acid-sensing properties in rat gastric sensory neurons from normal and ulcerated stomach. J Neurosci. 2005;25(10):2617–27. doi: 10.1523/JNEUROSCI.2894-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vanner S, MacNaughton WK. Capsaicin-sensitive afferent nerves activate submucosal secretomotor neurons in guinea pig ileum. Am J Physiol. 1995;269:G203–209. doi: 10.1152/ajpgi.1995.269.2.G203. [DOI] [PubMed] [Google Scholar]
- 55.Wang GD, Wang XY, Liu S, Fei GJ, Hu HZ, Xia Y, Wood JD. Extrinsic primary afferent neurons stimulate enteric mast cells to release histamine as a paracrine modulator in the enteric nervous system in the guinea-pig intestine. Gastroenterology. 2006;130:A-30. [Google Scholar]
- 56.Weber E, Neunlist M, Schemann M, Frieling T. Neural components of distension-evoked secretory responses in the guinea-pig distal colon. J Physiol. 2001;536(pt3):741–751. doi: 10.1111/j.1469-7793.2001.00741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wood JD. Enteric nervous system, serotonin, and the irritable bowel syndrome. Curr Opin Gastroenterol. 2001;17(1):91–7. doi: 10.1097/00001574-200101000-00017. [DOI] [PubMed] [Google Scholar]
- 58.Wood JD. Integrative functions of enteric nervous system. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. Academic press; 2006A. pp. 665–683. [Google Scholar]
- 59.Wood JD. Pathophysiology underlying the irritable bowel syndrome. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. Academic press; 2006B. pp. 1009–1031. [Google Scholar]
- 60.Wynn G, Ma B, Ruan HZ, Brunstock G. Purinergic component of mechanosensory transduction in a rat model. Am J Physiol Gastrointest Liver Physiol. 2004;287:G647–657. doi: 10.1152/ajpgi.00020.2004. [DOI] [PubMed] [Google Scholar]
- 61.Yiangou Y, Facer P, et al. Increased acid-sensing ion channel ASIC-3 in inflamed human intestine. Eur J Gastroenterol Hepatol. 2001;13(8):891–6. doi: 10.1097/00042737-200108000-00003. [DOI] [PubMed] [Google Scholar]


