Abstract
Mre11, Rad50, and Nbs1 form a conserved heterotrimeric complex that is involved in recombination and DNA damage checkpoints. Mutations in this complex disrupt the S-phase DNA damage checkpoint, the checkpoint which slows replication in response to DNA damage, and cause chromosome instability and cancer in humans. However, how these proteins function and specifically where they act in the checkpoint signaling pathway remain crucial questions. We identified fission yeast Nbs1 by using a comparative genomic approach and showed that the genes for human Nbs1 and fission yeast Nbs1 and that for their budding yeast counterpart, Xrs2, are members of an evolutionarily related but rapidly diverging gene family. Fission yeast Nbs1, Rad32 (the homolog of Mre11), and Rad50 are involved in DNA damage repair, telomere regulation, and the S-phase DNA damage checkpoint. However, they are not required for G2 DNA damage checkpoint. Our results suggest that a complex of Rad32, Rad50, and Nbs1 acts specifically in the S-phase branch of the DNA damage checkpoint and is not involved in general DNA damage recognition or signaling.
DNA damage checkpoints are important regulatory programs that protect cells from the consequences of DNA damage (15, 46). These mechanisms maintain the integrity of the genome by ensuring that the cellular DNA is faithfully replicated and segregated in each cell cycle. In individuals lacking these critical quality control mechanisms, even low levels of spontaneous DNA damage result in mutations, chromosomal rearrangements, and aneuploidy, leading to a wide variety of cancers (22). DNA damage checkpoints consist of an evolutionarily conserved signaling pathway that regulates various targets throughout the cell cycle (15, 46). At the core of this signaling pathway is the ATM family of protein kinases (30). There are two such kinases in human cells: ATM, encoded by the gene mutated in the cancer-prone syndrome ataxia-telangiectasia (AT), and ATR (ATM-related) (3, 59). They are homologous to Rad3 in fission and to Mec1 in budding yeast (54). These ATM kinases, along with other conserved proteins, relay the DNA damage signal to various checkpoint targets (15, 57).
The checkpoint signaling pathways regulate the cell cycle at three major points: at the G1/S transition, during the S phase, and at the G2/M transition (15, 46). While the mechanisms of the G1 and G2 checkpoints are relatively well understood, the mechanism of the DNA damage checkpoint during the S phase is less well defined. Nevertheless, studies of human cancer-prone syndromes affecting the S-phase DNA damage checkpoint suggest that it plays a critical role in the cellular response to DNA damage (52). Three autosomal recessive diseases disrupt the S-phase DNA damage checkpoint: AT, AT-like disorder, and Nijmegen breakage syndrome (NBS) (1, 47, 63). AT is the best studied of the three disorders, and ATM, the gene mutated in AT, has been well characterized. Mutation of ATM disrupts the ability of all three major DNA damage checkpoints to respond to ionizing radiation (69). Not surprisingly, ATM−/− cells display chromosome instability, profound sensitivity to DNA damage, and predisposition to oncogenic transformation. Null mutations in Mre11 and Nbs1 (the genes mutated in AT-like disorder and NBS, respectively) are lethal in mammals, presumably due to an essential role for MRN in repairing spontaneous DNA damage during the S phase (9, 52, 64). However, hypomorphic mutations exist that primarily disrupt the S-phase checkpoint, leaving the G2 checkpoint intact and having no or an only partial effect on the G1 checkpoint (26, 29, 63, 70). Interestingly, these mutations have cellular and clinical phenotypes very similar to AT with respect to DNA damage sensitivity, chromosome instability, and predisposition to early-onset cancers (51). These observations suggest that loss of the S-phase DNA damage checkpoint significantly contributes to the phenotypes of AT. Likewise, in the budding yeast Saccharomyces cerevisiae, Mre11 and Xrs2 (the budding yeast homolog of Nbs1) are important for suppressing genomic rearrangements but are not required for the G2 DNA damage checkpoint (34, 41). Therefore, the S-phase DNA damage checkpoint seems to play a major role in maintaining genomic stability of normal cells—perhaps a greater role than the G1 or G2 DNA damage checkpoints.
Analysis of Mre11 and Nbs1 has provided some clues to their role in the S-phase DNA damage checkpoint. These two proteins associate with Rad50 to form a heterotrimeric complex known as MRN (8, 12, 20). In addition to its role in the S-phase DNA damage checkpoint, MRN is involved in DNA recombinational repair, telomere maintenance, and the formation of double-strand breaks in meiosis (12, 20). In vitro, Mre11 has a single-stranded DNA endonuclease activity and a 3′-5′ exonuclease activity that resects double-stranded DNA (49). Rad50 is a DNA-binding ATPase of the SMC (structural maintenance of chromosome) family that forms a long flexible coiled-coil (13, 24, 53). The two form a tight complex which is conserved from prokaryotes (in the form of the Escherichia coli SbcCD heterodimer) to humans (60). The Rad50 ATPase enhances the Mre11 nuclease activity, possibly by distorting double-stranded substrates (23, 49). Rad50 contains a zinc hook dimerization motif in which two cysteines from each of two zinc hooks cooperate to coordinate a zinc atom. This motif may allow MRN oligomers to hold together separate DNA molecules (13, 25). Although the precise in vivo biochemical role of the MRN complex in recombination is unclear, it is thought to be involved in bridging and processing DNA ends to generate productive recombination substrates (20, 64).
The third member of the complex, Nbs1, is believed to be a regulatory subunit (7, 50). Nbs1 contains N-terminal FHA and BRCT domains, both of which are found in various DNA damage response proteins and are thought to be involved in protein interactions and signal transduction. Interestingly, FHA domains are phosphopeptide-binding modules that recognize sequences similar to those phosphorylated by the ATM family of kinases, suggesting that the FHA domain of Nbs1 could directly link MRN to ATM signaling events (14, 32). Furthermore, ATM is believed to directly phosphorylate Nbs1 on serine 343, a phosphorylation that is required for the slowing of replication in response to DNA damage (35). Nbs1 has only weak similarity to its budding yeast counterpart, Xrs2, which has led to the suggestion that the two are unrelated proteins that have evolved in parallel to fill similar roles. Nonetheless, Xrs2 associates with Mre11 and Rad50 to form an MRX complex which is functionally analogous to mammalian MRN in its roles in homologous recombination and the S-phase DNA damage checkpoint (6, 11, 19, 64).
We have used a comparative genomic approach to identify Nbs1 in the fission yeast Schizosaccharomyces pombe and to demonstrate that budding yeast Xrs2 is a true evolutionary homolog of human Nbs1. We show that fission yeast Nbs1 is functionally conserved; in particular, it is required, along with Rad32 (the fission yeast homolog of Mre11) and Rad50, for the S-phase DNA damage checkpoint. However, these proteins are not required for the G2 DNA damage checkpoint. These results indicate that fission yeast MRN is not required for general DNA damage recognition or signaling and suggest that it has a specific function in the S-phase DNA damage checkpoint pathway, possibly as a downstream target.
MATERIALS AND METHODS
Identification of fission yeast Nbs1.
Proteins similar to S. cerevisiae Xrs2 (gi:465488) were identified among the predicted translation products of the genomes of Saccharomyces paradoxus, Saccharomyces mikatii, Saccharomyces bayanus, and Kluveromyces yarowii (27, 31). In each genome, a single putative Xrs2 homolog was found; their respective percentages of identity and similarity, as determined by pairwise BLASTP with S. cerevisiae Xrs2 (www.ncbi.nlm.nih.gov/gorf/bl2.html), are 71 and 81, 60 and 74, 52 and 69, and 23 and 41. The homology of S. cerevisiae Xrs2 to K. yarowii Xrs2 (gi:30350867) was confirmed by extensive synteny of the corresponding regions of the two genomes (M. Kellis, personal communication). BLASTP (www.sanger.ac.uk/Projects/S_pombe/blast_server.shtml) was used to identify a putative homolog of K. yarowii Xrs2 among the predicted translation products of the S. pombe genome. The most similar protein, SPBc3b1.09c (gi:29603355), is 25% identical and 44% similar over 307 carboxy-terminal amino acids. In addition, each protein has an amino-terminal FHA domain that is recognized by CDD (www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml), although the domains are not sufficiently similar to be aligned by BLASTP.
We compared the sequence of the fission yeast protein to that of human Nbs1 (gi:7513206) and found that the two were similar in their carboxy termini. Manual inspection of the genomic sequence upstream of the originally annotated SPBc3b1.09c ORF revealed four small potential exons that could code for an FHA domain and a sequence with similarity to the Nbs1 BRCT domain. When this coding potential is included in SPBc3b1.09c, its percentages of identity and similarity to human Nbs1 are 28 and 47, respectively, over 188 amino-terminal residues and 29 and 49, respectively, over 175 carboxy-terminal residues. Since the fission yeast protein is most similar to human Nbs1 and since the K. yarowii protein is most similar to S. cerevisiae Xrs2, we propose that the former be called Nbs1 and the latter be called Xrs2. When aligned pairwise with BLASTP, the respective P values for fission yeast Nbs1 with human Nbs1, K. yarowii Xrs2, and S. cerevisiae Xrs2 are 0.0012, 0.0019, and 0.79. A four-way alignment was generated with ClustalX (28) and plotted with njplot (pbil.univ-lyon1.fr/software/njplot.html) using S. cerevisiae Xrs2 as the outgroup.
Genetic, molecular, and biochemical methods.
Fission yeast were grown and manipulated as described previously (40). Unless otherwise stated, all strains were grown in YES (yeast extract-glucose with supplements) medium at 30°C. The strains that were used are described in (Table 1).
TABLE 1.
Strains used in this study
| Strain | Genotype | Source or reference |
|---|---|---|
| PR109 | h− ura4-D18 leu1-32 | Lab stock |
| CC3223 | h− leu1-32 ura4-D18 nbs1::kanMX | This study |
| CC3227 | h− leu1-32 ura4-D18 nbs1::kanMX rad32::ura4 | This study |
| CC3228 | h− leu1-32 ura4-D18 nbs1::kanMX rad50::kanMX | This study |
| CC3229 | h− leu1-32 ura4-D18 nbs1::kanMX rad50::kanMX rad32::ura4 | This study |
| CC3235 | h− leu1-32 ura4-D18 nbs1-TAP:kanMX rad32-13Myc:kanMX | This study |
| CC3230 | h? leu1-32 ura4-D18 his3? rad3::ura4 nbs1::kanMX | This study |
| CC3231 | h− leu1-32 ura4-D18 nbs1::kanMX tel1-D2::LEU2 | This study |
| CC3233 | h? leu1-32 ura4-D18 ade6-M216 his3-D1 rad3::ura4 rad50::kanMX | This study |
| EN2682 | h− leu1-32 ura4-D18 ade6-M210 cdc10-M17ts | Lab stock |
| NR1826 | h− leu1-32 ura4-D18 ade6-704 rad3::ura4 | 3 |
| NR2840 | h+ leu1-32 ura4-D18 ade6-M210 his3-D1 rad50::kanMX | This study |
| NR2841 | h− leu1-32 ura4-D18 ade6-M216 his3-D1 rad50::kanMX | This study |
| TMN2665 | h− leu1-32 ura4-D18 ade6-M210 his3-D1 | 45 |
| TMN2669 | h− leu1-32 ura4-D18 ade6-M210 his3-D1 trt1-D2::his3 | 45 |
| TMN2799 | h− leu1-32 ura4-D18 his3-D1 ade6-M210 rad32-D1::kanMX | 45 |
| TMN2937 | h− leu1-32 ura4-D18 ade6-M216 his3-D1 rad3::ura4 | 45 |
| TMN2967 | h− leu1-32 ura4-D18 ade6-M210 his3-D1 tel1-D1::kanMX | 45 |
| TMN2994 | h? leu1-32 ura4-D18 ade6-? his3-D1 rad3::ura4 rad32-D1::kanMX | 45 |
| TMN3052 | h? leu1-32 ura4-D18 ade6-M210 his3-D1 tel1-D1::kanMX rad3::ura4 | 45 |
| TMN3224 | h− leu1-32 ura4-D18 ade6-M216 his3-D1 nbs1::kanMX | This study |
| yFS260 | h+ leu1-32 ura4-D18 cdc10-M17ts rad3::ura4 | This study |
| yFS263 | h− leu1-32 ura4-D18 ade6-M210 his3-D1 cdc10-M17ts rad32-D1::kanMX | This study |
| yFS265 | h− leu1-32 ura4-D18 ade6-M210 his3-D1 cdc10-M17ts rad50::kanMX | This study |
| yFS267 | h− leu1-32 ura4-D18 cdc10-M17ts nbs1::kanMX | This study |
Genes were deleted and tagged by PCR cassette mutagenesis as previously described using the following oligonucleotides (2): for nbs1::kanMX, CCR0802-5 (GCGACCTATGCATCCTCAGTTGTGAAAACAGCATTCTGGTTTCTGAATTTTTTCAATAAAAAGAATTTTATGTTGAAAAACGGATCCCCGGGTTAATTAA) and CCR0802-6 (TAGGAGTTGAAAAATAAAATATTTGGATTTCATTCTGAATACGATATCTCAGGTAAGTAGTCAATAATAGTACAATATTAGAATTCGAGCTCGTTTAAAC); for nbs1-TAP::kanMX, CCR0802-6 (TAGGAGTTGAAAAATAAAATATTTGGATTTCATTCTGAATACGATATCTCAGGTAAGTAGTCAATAATAGTACAATATTAGAATTCGAGCTCGTTTAAAC) and CCR0802-7 (CGAGTAATTCATTTAAAGAACTATCTCCTAAAACCAATAATGACGAAGACGATGAATTTAATGATCTCAAGTTTCACTTTCGGATCCCCGGGTTAATTAA); for rad50::kanMX, NR123 (AAATAAAGTTACCTCGGTCATGTTAAAAACTACTATATAGTAAAGATAGACTACAAAGAAATAGAGAATCCAAGTCAATTGAATTCGAGCTCGTTTAAAC) and NR124 (GTAGCTATTATTATAGGGTGAAACGGGACACGAATCAGAAGAGCATGGTAAGGTTCGCTAACACAGTTATAGTACTAACTGAATTCGAGCTCGTTTAAAC). Other alleles have been described, namely, rad3::ura4 (3), rad32::ura4 (66), rad32-D1::kanMX (45), rad32-13Myc:kanMX (45), tel1-D1::kanMX (45), tel1-D2::LEU2 (45), and trt1-2::his3 (44).
To determine gamma radiation sensitivity, logarithmically growing cells were irradiated with gamma radiation at 3.2 Gy/min from a 137cesium source and immediately plated in duplicate on YES plates. For survival in methyl methanesulfonate (MMS), cells were plated in duplicate on freshly prepared YES plates containing the desired concentration of MMS. Colonies were counted after 4 days of growth at 30°C. Percent survival values for each strain were obtained after normalizing the number of colonies growing under a damage condition against the number of colonies appearing under untreated conditions. The assays were performed twice, and the average values were used to plot the survival curves.
nbs1 cDNA was amplified from an S. pombe cDNA library (Clontech) by using a high fidelity polymerase (KOD XL, Novagen) and the primers CCR0802-11 (5′-GGAATTCCATATGTGGATAATTGAGGCTGAGGGTG) and CCR0802-14 (5′-CGGGATCCCAAAGTGAAACTTGAGATCATTAAATTC). Two clones were sequenced.
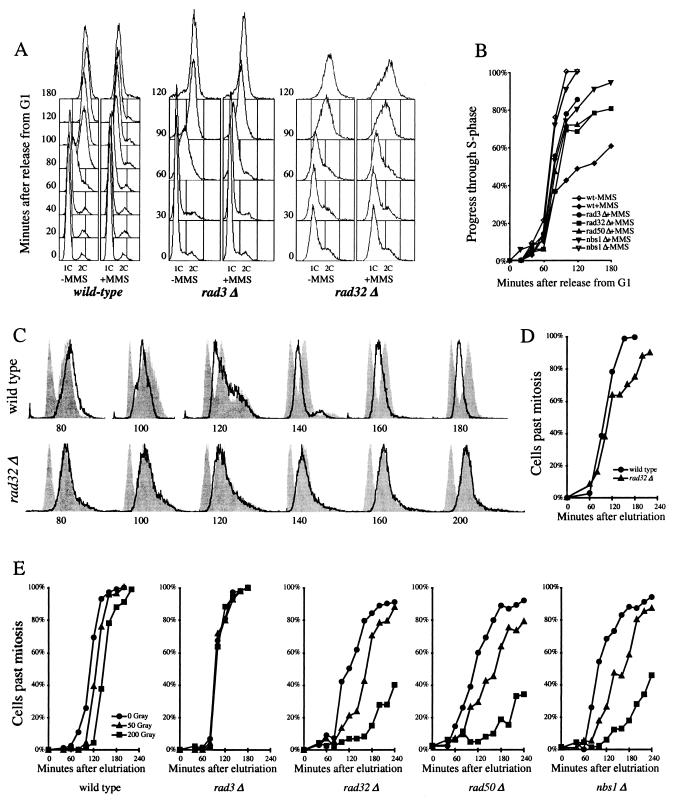
Cell cycle experiments were performed as previously described with the following exceptions (55, 58). In order to avoid a prolonged G1 arrest in the cdc10-M17ts synchronization experiments depicted in Fig. 5A, we arrested the cultures for 30 min at 35°C and then selected the smallest cells from the culture by centrifugal elutriation (55). These small cells are those that had just divided and thus had just encountered the G1 block. The S-phase DNA damage checkpoint was then activated with 0.03% MMS added 5 min after release from G1. For the experiments in Fig. 5C, asynchronous logarithmically growing cells were elutriated to select a population of synchronous G2 cells. After 60 min, the culture was divided in three; one part was left untreated, one part was treated with 0.01% MMS, and one part was treated with 10 mM hydroxyurea. For the G2 DNA damage checkpoint experiments in Fig. 5E, cells were irradiated at 10 Gy/min with a Faxitron 43855D X-ray source, ending 30 min after elutriation.
FIG. 5.
The MRN proteins are required for the S-phase DNA damage checkpoint but not the G2 DNA damage checkpoint. (A) Fluorescence-activated cell sorter (FACS) analysis of S-phase DNA damage checkpoint in cdc10-M17ts (EN2682), rad3Δ cdc10-M17ts (yFS260), and rad32Δ cdc10-M17ts (yFS263) cells. Synchronous G1 cultures were released from a G1 block in the presence or absence of 0.03% MMS. Progression through the S phase was determined by FACS. (B) Quantitation of the data in shown in panel A and data from rad50Δ cdc10-M17ts (yFS265) and nbs1Δ cdc10-M17ts (yFS267) cells. The percentage of progress through the S phase was calculated as the position of the mean of the FACS signal between the means of the 1C and 2C controls. (C) FACS analysis of S-phase DNA damage checkpoint in wild-type (PR109) and rad32Δ(TMN2799) cells. Elutriation-synchronized G2 cultures were treated with 0.01% MMS, a dose that does not activate the G2 DNA damage checkpoint but does activate the S-phase DNA damage checkpoint, and followed through mitosis and the S phase by FACS. For each time point, a separate set of FACS results is shown: the bold line represents the DNA content of the MMS-treated cells, the right-hand gray peak is a control consisting of the same time point of a parallel untreated culture, and the left-hand gray peak is a 1C control consisting of the 140-min sample of a parallel hydroxyurea-treated culture. (D) Division kinetics of the cells shown in panel C as determined by microscopically monitoring cell septation. (E) The response of wild-type (PR109), rad3Δ (NR1826), rad32Δ (TMN2799), rad50Δ (NR2841), and nbs1Δ (CC3223) cells to ionizing radiation during G2. Elutriation-synchronized G2 cultures were X-ray irradiated, and cells completing mitosis were identified by microscopically monitoring cell septation.
Telomere analysis, immunoprecipitations, and Western blots were performed as previously described (4, 45).
RESULTS
Identification of fission yeast Nbs1.
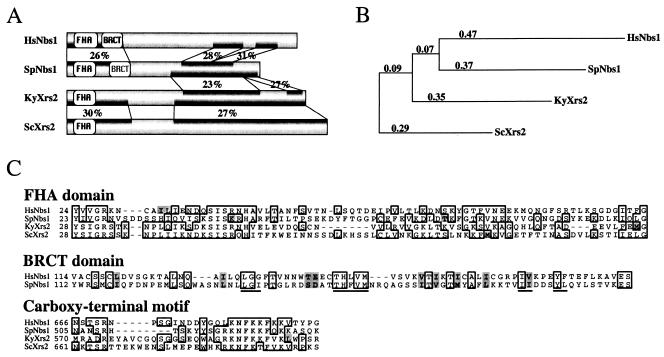
Initial database searches failed to identify any fission yeast proteins with statistically significant similarity to either human Nbs1 or budding yeast Xrs2. Given the lack of similarity between human Nbs1 and budding yeast Xrs2, we reasoned that a fission yeast homolog may have diverged beyond recognition. We therefore adopted a comparative genomics approach with the hope of finding evolutionary intermediates between budding yeast Xrs2 and a putative fission yeast homolog. We began by searching for homologs of Xrs2 in the sequenced genomes of other budding yeasts (31). Among four saccharomycetes (S. bayanus, S. cerevisiae, S. mikatii, and S. paradoxus), Xrs2 is conserved at between 52 and 71% pairwise identity (data not shown). Consistent with the high degree of identity among these proteins, none of the other Xrs2 homologs shares significant similarity with any predicted proteins in the fission yeast genome. We also found an Xrs2 homolog in a more distantly related budding yeast, K. yarowii, whose Xrs2 is 23% identical to S. cerevisiae Xrs2 across the whole protein. Moreover, the region of the K. yarowii genome encoding Xrs2 shows extensive long-range synteny with the corresponding region of the S. cerevisiae genome, confirming that K. yarowii Xrs2 is homologous to S. cerevisiae Xrs2 (M. Kellis, personal communication). In addition, K. yarowii Xrs2 shares a statistically significant 25% identity (P = 0.0019) over 307 residues with a predicted fission yeast protein that we have named Nbs1 (Fig. 1). In an independent approach, we used several short carboxy-terminal motifs that are conserved between S. cerevisiae Xrs2 and Nbs1 from various vertebrate genomes to search the S. pombe protein database (65). Iterative and overlapping searches led to the identification of the same predicted protein. Fission yeast Nbs1, as originally annotated in the fission yeast genome, showed recognizable, but not statistically significant (P > 0.3), identity to human Nbs1 over the this region. However, upon inspection of the fission yeast genomic sequence encoding Nbs1, it was clear that four small 5′ exons encoding the Nbs1 FHA domain had been omitted from the annotated coding sequence. When these exons are included, fission yeast Nbs1 and human Nbs1 are significantly similar (P = 0.0012), with 28% identity over 188 N-terminal residues (the FHA and BRCT domains) and 175 C-terminal residues (Fig. 1). We confirmed the organization of the nbs1 open reading frame by cDNA cloning and sequencing. The fission yeast genome annotation has been updated to include these four exons. Taken together, these results indicate that the fission yeast Nbs1 is evolutionarily related to both human Nbs1 and budding yeast Xrs2. Fission yeast Nbs1 has also identified by Ueno et al. (67a).
FIG. 1.
Alignment of the Nbs1/Xrs2 homologs. (A) Graphic representation of the sequence similarity between the Nbs1/Xrs2 homologs. Percent identity is shown for regions identified as significantly similar by BLASTP. The FHA and BRCT domains recognized by CDD are depicted. The putative S. pombe BRCT domain is in gray because although it is similar to the human BRCT domain, it is not recognized by either the CDD or ProfileScan algorithms. (B) Phylogenetic tree of the Nbs1/Xrs2 homologs. The branch lengths represent relative phylogenetic distances, as determined by ClustalX (28). (C) Alignment of the FHA domain, the BRCT domain, and a conserved carboxy-terminal motif. Residues identical in any two sequences are boxed; residues similar a majority of the sequences are shaded. The signature motifs of a BRCT domain are underlined. The conserved carboxy-terminal motif is contained within a region that has been implicated in binding to Mre11 (65).
Fission yeast Nbs1 is functionally conserved.
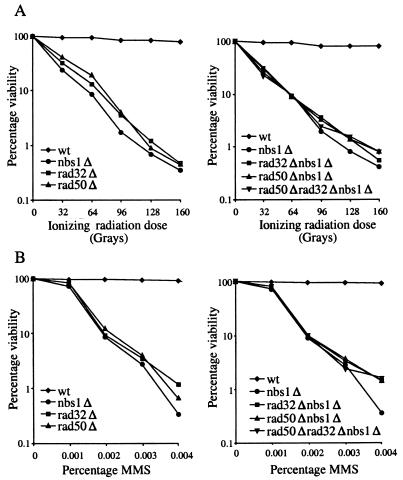
The sequence conservation between fission yeast Nbs1 and human Nbs1 prompted us to test if the DNA damage repair and telomere maintenance functions of Nbs1 are also conserved in fission yeast. We compared the DNA damage sensitivity of nbs1Δ cells to that of wild-type, rad32Δ, rad50Δ, and double- and triple-mutant cells by using MMS, which causes base alkylation damage, and gamma radiation, which causes double-strand breaks (Fig. 2) (21, 66). nbs1Δ cells are equally as sensitive as rad32Δ and rad50Δ cells to both MMS- and gamma radiation-induced DNA damage. Furthermore, cells doubly mutant for nbs1Δ and either rad32Δ or rad50Δ and cells triply mutant for nbs1Δ, rad32Δ, and rad50Δ are no more sensitive than any of the single-mutant cells. Taken together, these results show that fission yeast Nbs1 is required to respond to DNA damage and suggest that Nbs1 is involved in the same DNA damage repair pathway as Rad32 and Rad 50.
FIG. 2.
Nbs1 is required for DNA damage resistance. (A) Sensitivity of nbs1Δ (CC3223), rad32Δ (TMN2799), rad50Δ (NR2841), rad32Δ nbs1Δ (CC3227), rad50Δ nbs1Δ (CC3228), and rad50Δ rad32Δ nbs1Δ (CC3229) cells to ionizing radiation. (B) Sensitivity of nbs1Δ (CC3223), rad32Δ (TMN2799), rad50Δ (NR2841), rad32Δ nbs1Δ (CC3227), rad50Δ nbs1Δ (CC3228), and rad50Δ rad32Δ nbs1Δ (CC3229) cells to MMS.
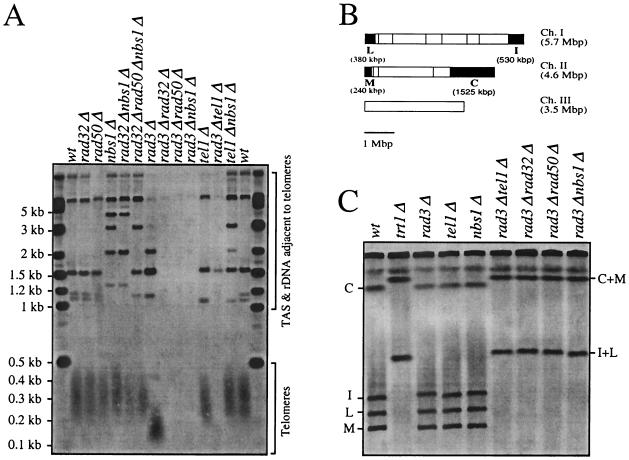
To investigate the role of fission yeast Nbs1 in telomere maintenance, we examined the steady-state lengths of telomeres in nbs1Δ cells. We previously reported that deletion of rad32 or rad50 has no effect on telomere length but that deletion of either of these genes in combination with deletion of rad3 causes catastrophic loss of telomeres, resulting in chromosome fusions (45). nbs1Δ cells share these phenotypes in that nbs1Δ cells, cells doubly mutant for nbs1Δ and either rad32Δ, rad50Δ, or tel1Δ (a rad3 paralog involved in telomere maintenance), and cells triply mutant for nbs1Δ, rad32Δ, and rad50Δ have wild-type telomere lengths (Fig. 3A). However, cells doubly mutant for rad3Δ and either nbs1Δ, rad32Δ, rad50Δ, or tel1Δ show loss of telomere and telomere-associated sequences (Fig. 3A). Fission yeast can survive without telomeres if their chromosome ends fuse to produce circular chromosomes (42, 43). We observed such fusions, which can be detected on Southern blots of NotI-digested genomic DNA, in all of the rad3Δ double-mutant strains (Fig. 3C). These results are consistent with the hypothesis that Nbs1 functions together with Rad32 and Rad 50 in telomere maintenance.
FIG. 3.
Nbs1 is involved in telomere-length maintenance. (A) Telomere length in wild-type (wt) (TMN2665), rad32Δ (TMN2799), rad50Δ (NR2840), nbs1Δ (TMN3224), rad32Δ nbs1Δ (CC3227), rad32Δ rad50Δ nbs1Δ (CC3229), rad3Δ (TMN2937), rad3Δ rad32Δ (TMN2994), rad3Δ rad50Δ (CC3233), rad3Δ nbs1Δ (CC3230), tel1Δ (TMN2967), rad3Δ tel1Δ (TMN3052) and tel1Δ nbs1Δ (CC3231) cells. A Southern blot of ApaI-digested S. pombe chromosomal DNA was hybridized to telomere-specific probes (44). The ApaI site is located in the telomere-associated sequence (TAS) 30 to 40 bp away from telomeric repeat sequences in both ends of chromosomes I and II and at least one end of chromosome III, giving rise to a broad ∼300-bp telomere hybridization signal in the wt strain (Telomeres). Hybridization signals (TAS & rDNA adjacent telomeres) come from cross-hybridization to TAS or hybridization to telomere(s) of chromosome III which contain rDNA repeats directly adjacent to the telomeric repeat sequence and therefore lack the TAS-associated ApaI site directly adjacent to the telomeric repeat sequence. (B) NotI restriction enzyme map of S. pombe chromosomes (vertical lines). The telomeric fragments C, I, L, and M are filled black. Chromosome III lacks a NotI site. (C) Chromosome structure in wt (TMN2665), trt1 (TMN2669), rad32 (TMN2799), tel1 (TMN2967), nbs1 (TMN3224), rad3 tel1 (TMN3052), rad3 rad32 (TMN2994), rad3 rad50 (CC3233), and rad3 nbs1 (CC3230) cells. A pulsed-field gel Southern blot of NotI-digested S. pombe chromosomal DNA was hybridized to C-, I-, L-, and M-specific probes (43). Four telomeric fragments (C, I, L, and M) and fusion products (C+M and I+L) are marked.
In addition to their conserved DNA damage and telomere length phenotypes, nbs1Δ, rad32Δ, and rad50Δ strains share a slow-growth phenotype. This reduction in growth rate is also seen in budding yeast mrx mutant strains and is manifested as lethality in metazoan cells lacking MRN proteins (64). This requirement for MRN in a normal cell cycle presumably reflects a constitutive role for MRN in repairing spontaneous damage generated during replication (9, 52, 64). Cultures of cells with nbs1Δ, rad32Δ, or rad50Δ mutations contain many inviable checkpoint-arrested cells and double in about 4 h at 30°C, compared with 2.5 h for wild-type cultures (data not shown). Double- and triple-mutant combinations of nbs1Δ, rad32Δ, and rad50Δ do not grow more slowly than the single mutants. This slow-growth phenotype can be seen in untreated synchronous controls (see Fig. 5E). The nbs1Δ, rad32Δ, and rad50Δ cells show a variable G2 delay, presumably due to varying amounts of damage remaining after replication. This heterogeneous G2 delay results in a less synchronous entry into mitosis compared to the wild-type culture, with approximately 10% of the cells remaining arrested in G2.
Fission yeast Nbs1 interacts with Rad32.
The structural and functional conservation of fission yeast Nbs1, Rad32, and Rad50 suggests that the three proteins form a stable trimer in fission yeast, as they do in human and budding yeast cells. To test this hypothesis, we investigated the interaction of Nbs1 with Rad32. We tagged the carboxy terminus of Nbs1 with a protein A affinity (TAP) tag and tagged the carboxy terminus of Rad32 with 13 copies of the Myc epitope (2). Both tags were integrated at the genomic locus of the respective gene, and neither interfered with protein function, as judged by the wild-type UV radiation sensitivity of the resulting strains (data not shown). Cells expressing both tagged proteins were lysed, and Nbs1 was affinity precipitated from the soluble lysate with immunoglobulin G (IgG) agarose. After extensive washing, we found that Rad32-Myc was bound to the Nbs1-TAP beads but not to control beads (Fig. 4). Thus, Nbs1 and Rad32 stably interact in cell lysate, suggesting that fission yeast Rad32, Rad50, and Nbs1 form a stable complex, which we will call MRN, in accord with the corresponding human Mre11-Rad50-Nbs1 complex.
FIG. 4.
The Nbs1 and Rad32 associate in vivo. Protein A affinity (TAP)-tagged Nbs1 was precipitated with IgG Sepharose from a soluble nbs1-TAP rad32-13Myc (CC3235) cell lysate. The IgG beads were then washed four times with binding buffer. Protein from the cell lysate, protein from the first and last washes, and protein remaining on the IgG beads was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and probed with anti-Myc antibodies to visualize Rad32-13Myc. Rad32-13Myc coprecipitated with the IgG-bound Nbs1-TAP; insignificant Rad32-13Myc precipitated in the absence of Nbs1-TAP.
The fission yeast MRN proteins are required for the S-phase DNA damage checkpoint but not the G2 DNA damage checkpoint.
In human cells, MRN plays a crucial role in the slowing of replication in response to DNA damage (8, 63). To determine if this role is conserved between humans and fission yeast, we examined the requirement for Rad32, Rad50, and Nbs1 in the fission yeast S-phase DNA damage checkpoint. Cells were synchronized in G1 by using a temperature-sensitive mutation of Cdc10, a protein required for the G1/S transition. These cells were then released into the S phase at the permissive temperature in the presence or absence of the DNA-damaging agent MMS, and the progress of replication was monitored by flow cytometry. As previously shown, cells with wild-type checkpoint response slow replication in response to the DNA damage (Fig. 5A and B) (36, 55). Untreated cells replicate their DNA and progress from 1C DNA content to 2C DNA content between 60 and 90 min after release from G1. In contrast, cells treated with MMS do not complete replication even after 180 min (Fig. 5A and B). This slowing of replication is a checkpoint response, since it is dependent on the checkpoint kinase Rad3 (Fig. 5A and B). This response is also dependent on Rad32, Rad50, and Nbs1, since the replication delay is greatly reduced in their absence (Fig. 5A and B). The minor residual slowing seen in the checkpoint mutants in Fig. 5B is presumably a checkpoint-independent effect that may be due to the physical blocking of replication by the DNA damage.
To confirm the role of the MRN proteins in the S-phase DNA damage checkpoint, we used a synchronization strategy in which the cells are not arrested, but rather size selected from an asynchronous logarithmically growing culture with an elutriating centrifuge. Because such cells are never removed from logarithmic growth conditions, this technique should pose a minimum of synchronization artifacts. However, the organization of the fission yeast cell cycle somewhat complicates the interpretation of the results. Elutriation selects a synchronous population of G2 cells, which have 2C DNA content. As these cells progress through the cell cycle, they execute mitosis and begin cytokinesis. At the same time as cytokinesis, the cells enter the S phase, and by the end of cytokinesis, cells have also finished replication. Thus, the newly divided cells are already 2C, and no 1C peak of G1 cells is ever seen. However, if cells have not finished replication by the time they divide, the newly divided cells will have less than 2C DNA content, as can be seen for the MMS-treated wild-type cells in Fig. 5C. In contrast, no sub-2C cells are seen in the rad32Δ culture, confirming that these cells do not slow replication in response to DNA damage (Fig. 5C). To show that the two cultures divided with similar kinetics, we monitored septation and division of the cells (Fig. 5D). Taken together, our results show that in fission yeast, as in humans, the components of the MRN are required for the S-phase DNA damage checkpoint.
In contrast to its requirement in the S-phase DNA damage checkpoint, fission yeast MRN is not required for the G2 DNA damage checkpoint. Synchronous G2 cultures of wild-type, rad3Δ, rad32Δ, rad50Δ, or nbs1Δ cells were irradiated with 0, 50, or 200 Gy of X-ray radiation. At the lower dose, the rad32Δ, rad50Δ, and nbs1Δ cells show a somewhat lengthened G2 delay, closer to 60 min than to the 20 min seen in wild-type cells; at the higher dose, the rad32Δ, rad50Δ, and nbs1Δ cells show a greatly lengthened G2 delay (Fig. 5E). These lengthened delays are consistent with the DNA damage-repair defect seen in rad32Δ cells and demonstrate that cells lacking MRN can still properly recognize and arrest in response to G2 DNA damage (66).
DISCUSSION
The regulation of replication by the S-phase DNA damage checkpoint is a crucial but poorly understood factor in the maintenance of genomic stability. Genetic studies in humans have identified Nbs1 as a central player in this checkpoint (1, 8, 38, 68). To investigate the role of Nbs1 in the regulation of replication, we have identified and characterized the fission yeast homolog of Nbs1. We have shown that Nbs1 interacts with Rad32 (the fission yeast homolog of Mre11) and presumably Rad50 to form a conserved heterotrimer known as MRN, which is required for the S-phase DNA damage checkpoint. Furthermore, fission yeast MRN shares conserved roles in DNA repair and telomere maintenance with its human counterpart, suggesting that fission yeast will serve as a useful model system for understanding the various roles of MRN in the cell cycle (21, 66).
Nbs1 and Xrs2 constitute a family of homologous proteins.
Nbs1 and Xrs2, the founding members of the Nbs1/Xrs2 gene family, show little sequence similarity (8, 38, 68). In fact, based on the sequence of these two proteins alone, it is not clear that they are homologs. However, using a comparative genomic approach to find other members of the family, we have shown that Nbs1 and Xrs2 are evolutionarily related. Each stepwise comparison from S. cerevisiae Xrs2 to K. yarowii Xrs2 to S. pombe Nbs1 to human Nbs1 shows about 25% identity (P < 0.01). In contrast, comparison between S. cerevisiae Xrs2 and S. pombe Nbs1 or between K. yarowii Xrs2 and human Nbs1 shows no significant similarity (P > 0.5), demonstrating that the members of the family are diverging very rapidly. Nonetheless, it has been noted that there are short regions of recognizable similarity between even S. cerevisiae Xrs2 and human Nbs1 (Fig. 1C) (8, 38, 65). Furthermore, these residues occupy structurally important positions in the amino-terminal FHA domain of both proteins (14). The conservation of these residues, along with the conserved functions of the proteins, is consistent with an evolutionary relationship between Nbs1 and Xrs2. The rapid divergence of the family may have also obscured a functional BRCT domain in fission yeast Nbs1. Although the region of fission yeast Nbs1 corresponding to the human Nbs1 BRCT domain is not recognized as a BRCT domain by the CDD or ProfileScan conserved domain recognition algorithms, several structurally important residues are conserved, suggesting that fission yeast Nbs1 may contain a divergent BRCT domain (Fig. 1C). These residues are not generally conserved in the budding yeast Xrs2s, leaving open the question of whether Xrs2s contain a structurally or functionally conserved BRCT-like domain.
In addition to its structural conservation, Nbs1 is functionally conserved. In humans, budding yeast, and fission yeast, Nbs1/Xrs2 is a member of a heterotrimeric complex along with Mre11/Rad32 and Rad50 (8, 67). The functions of MRN/X in DNA damage repair, meiotic recombination, telomere maintenance, and the S-phase DNA damage checkpoint are generally conserved (20, 64). Why a functionally conserved family of proteins should be diverging so quickly in primary sequence is an interesting question that remains to be answered. Since Nbs1 is thought to be a regulatory subunit of the Mre11-Rad50 complex, this question has important implications for the conservation of the complex's regulation.
The role of Rad32, Rad50, and Nbs1 in the S-phase DNA damage checkpoint.
A hallmark of human Mre11 and Nbs1 is their requirement in the S-phase DNA damage checkpoint (52, 61, 63). We have shown that fission yeast Rad32, Rad50, and Nbs1 are also required for the S-phase DNA damage checkpoint (Fig. 4). It has previously been reported that neither Rad32 nor Rad50 is required for the S-phase DNA damage checkpoint in fission yeast (21, 37). However, in both these studies, cells were synchronized by nitrogen starvation-induced G1 arrest, and this treatment may affect the cellular response to DNA damage. We have found, in fact, that prolonged G1 arrest masks the effect of the S-phase DNA damage checkpoint. Therefore, for all of our checkpoint experiments, we used either logarithmically growing cells or cells arrested only briefly in G1 (see Materials and Methods).
The fact that MRN is required for the S-phase DNA damage checkpoint has been interpreted to mean that MRN is required for the recognition of DNA damage (12). However, there are conflicting results as to whether Nbs1 is required for DNA damage recognition in human cells. One study found that Cds1 phosphorylation and activation are defective in Nbs1 cells, implicating Nbs1 in activation of the checkpoint signaling pathway (7). In contrast, other studies that examined the role of Nbs1 and Mre11 in the activation of Cds1 and ATM found no requirement (16, 35, 71). It is possible that the different results are due to differences in doses of DNA damage used, with Nbs1 being required at low doses (12, 18). The situation appears to be different in budding yeast, where the MRX proteins have been shown to be required for DNA damage signaling (11, 19). Whether the apparent difference in the requirement for MRN/X in DNA damage recognition between humans and budding yeast is due to divergence in function or differences in experimental protocol remains to be determined. In fission yeast, MRN is not required for G2 cell cycle arrest in response to DNA damage (Fig. 5E). This result implies that fission yeast MRN, like human MRN, is not required for general DNA damage recognition.
A model for the S-phase DNA damage checkpoint.
The involvement of MRN in both DNA recombination and the S-phase DNA damage checkpoint suggests a role for recombination in the checkpoint (56). One possible role for recombination during replication would be to allow the replication fork to bypass single-strand damage. By coupling replication and recombination, the undamaged parental stand could be replicated and then used as a template for the synthesis of the second nascent strand, avoiding replication of the damaged sequence (39). The four-way junctions predicted by this model have been detected in damaged DNA isolated from both budding yeast and bacteria; in budding yeast, the formation of the junctions is checkpoint regulated (10, 62). Checkpoint-dependent strand switching is consistent with the observation that cells unable to remove UV-induced lesions can still tolerate low levels of such damage as long as the S-phase DNA damage checkpoint is functional, most likely because strand switching allows the cell to replicate around the damage, which is then diluted out in subsequent generations (5). Another function of recombination could be to restart replication after a replication fork collapses at a lesion and is transformed into a double-strand break (33, 48).
Taken together, these observations suggest a model for the S-phase DNA damage checkpoint: the checkpoint activates recombination when replication forks encounter DNA lesions, and the coupling of recombination and replication slows the overall rate of replication. This model differs from the standard model, in which the checkpoint arrests replication so that DNA can be repaired before it is replicated. Our model suggests that instead of delaying replication and allowing repair as a consequence, the checkpoint activates recombination and delays replication as a consequence. Such a model explains why the checkpoint slows replication but does not halt it, why the extent of slowing increases with the dose of DNA damaging agent, and why damage remains after the completion of replication (17, 56). It also explains the role of MRN in the checkpoint in terms of MRN′s biochemical role in recombination, instead of invoking a new function for the complex (12, 13, 24).
Conclusions. We have identified the fission yeast Nbs1 and have shown that it is an evolutionary intermediate between human Nbs1 and budding yeast Xrs2. Nbs1 associates with Rad32 (the fission yeast homolog of Mre11) and presumably Rad50 to form a functionally conserved MRN heterotrimer. In particular, fission yeast MRN is required for the S-phase DNA damage checkpoint but is not required for general DNA damage recognition. These results suggest a model in which MRN functions in the checkpoint to induce replication-coupled recombination. Although this model is still unproven, it integrates a diverse set of previous data concerning the S-phase DNA damage checkpoint and provides a testable framework for understanding the role of this crucial checkpoint in the maintenance of genomic stability and prevention of cancer in humans.
Acknowledgments
We are grateful to Manolis Kellis and Chad Nussbaum for providing us with the sequences of the budding yeast Xrs2s before publication and for determining the synteny between the XRS2 regions of S. cerevisiae and K. yarowii genomes. We also thank Felicity Watts and Tony Carr for sharing strains with us and Masaru Ueno and Hiroshi Iwasaki for sharing unpublished results.
T.M.N. was supported by a fellowship from the Damon Runyon Cancer Research Foundation. The work in the laboratory of P.R. was funded by the NIH. N.R. was supported by a Leukemia and Lymphoma Society Special Fellowship and a Chestnut Hill Charitable Foundation New Investigator Award.
REFERENCES
- 1.Archives of Disease in Childhood. 2000. Nijmegen breakage syndrome. The International Nijmegen Breakage Syndrome Study Group. Arch. Dis. Child. 82:400-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bähler, J., J. Q. Wu, M. S. Longtine, N. G. Shah, A. McKenzie III, A. B. Steever, A. Wach, P. Philippsen, and J. R. Pringle. 1998. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14:943-951. [DOI] [PubMed] [Google Scholar]
- 3.Bentley, N. J., D. A. Holtzman, G. Flaggs, K. S. Keegan, A. DeMaggio, J. C. Ford, M. Hoekstra, and A. M. Carr. 1996. The Schizosaccharomyces pombe rad3 checkpoint gene. EMBO J. 15:6641-6651. [PMC free article] [PubMed] [Google Scholar]
- 4.Boddy, M. N., B. Furnari, O. Mondesert, and P. Russell. 1998. Replication checkpoint enforced by kinases Cds1 and Chk1. Science 280:909-912. [DOI] [PubMed] [Google Scholar]
- 5.Boddy, M. N., A. Lopez-Girona, P. Shanahan, H. Interthal, W. D. Heyer, and P. Russell. 2000. Damage tolerance protein Mus81 associates with the FHA1 domain of checkpoint kinase Cds1. Mol. Cell. Biol. 20:8758-8766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bressan, D. A., B. K. Baxter, and J. H. Petrini. 1999. The Mre11-Rad50-Xrs2 protein complex facilitates homologous recombination-based double-strand break repair in Saccharomyces cerevisiae. Mol. Cell. Biol. 19:7681-7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buscemi, G., C. Savio, L. Zannini, F. Micciche, D. Masnada, M. Nakanishi, H. Tauchi, K. Komatsu, S. Mizutani, K. Khanna, P. Chen, P. Concannon, L. Chessa, and D. Delia. 2001. Chk2 activation dependence on Nbs1 after DNA damage. Mol. Cell. Biol. 21:5214-5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carney, J. P., R. S. Maser, H. Olivares, E. M. Davis, M. Le Beau, J. R. Yates, L. Hays, W. F. Morgan, and J. H. Petrini. 1998. The hMre11/hRad50 protein complex and Nijmegen breakage syndrome: linkage of double-strand break repair to the cellular DNA damage response. Cell 93:477-486. [DOI] [PubMed] [Google Scholar]
- 9.Costanzo, V., K. Robertson, M. Bibikova, E. Kim, D. Grieco, M. Gottesman, D. Carroll, and J. Gautier. 2001. Mre11 protein complex prevents double-strand break accumulation during chromosomal DNA replication. Mol. Cell 8:137-147. [DOI] [PubMed] [Google Scholar]
- 10.Courcelle, J., J. R. Donaldson, K. H. Chow, and C. T. Courcelle. 2003. DNA damage-induced replication fork regression and processing in Escherichia coli. Science 299:1064-1067. [DOI] [PubMed] [Google Scholar]
- 11.D'Amours, D., and S. P. Jackson. 2001. The yeast Xrs2 complex functions in S phase checkpoint regulation. Genes Dev. 15:2238-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Amours, D., and S. P. Jackson. 2002. The Mre11 complex: at the crossroads of DNA repair and checkpoint signaling. Nat. Rev. Mol. Cell Biol. 3:317-327. [DOI] [PubMed] [Google Scholar]
- 13.de Jager, M., J. van Noort, D. C. van Gent, C. Dekker, R. Kanaar, and C. Wyman. 2001. Hum. Rad50/Mre11 is a flexible complex that can tether DNA ends. Mol. Cell 8:1129-1135. [DOI] [PubMed] [Google Scholar]
- 14.Durocher, D., I. A. Taylor, D. Sarbassova, L. F. Haire, S. L. Westcott, S. P. Jackson, S. J. Smerdon, and M. B. Yaffe. 2000. The molecular basis of FHA domain: phosphopeptide binding specificity and implications for phospho-dependent signaling mechanisms. Mol. Cell 6:1169-1182. [DOI] [PubMed] [Google Scholar]
- 15.Elledge, S. J. 1996. Cell cycle checkpoints: preventing an identity crisis. Science 274:1664-1672. [DOI] [PubMed] [Google Scholar]
- 16.Falck, J., J. H. Petrini, B. R. Williams, J. Lukas, and J. Bartek. 2002. The DNA damage-dependent intra-S phase checkpoint is regulated by parallel pathways. Nat. Genet. 30:290-294. [DOI] [PubMed] [Google Scholar]
- 17.Foiani, M., A. Pellicioli, M. Lopes, C. Lucca, M. Ferrari, G. Liberi, M. Muzi Falconi, and P. Plevani. 2000. DNA damage checkpoints and DNA replication controls in Saccharomyces cerevisiae. Mutat. Res. 451:187-196. [DOI] [PubMed] [Google Scholar]
- 18.Girard, P. M., E. Riballo, A. C. Begg, A. Waugh, and P. A. Jeggo. 2002. Nbs1 promotes ATM dependent phosphorylation events including those required for G1/S arrest. Oncogene 21:4191-4199. [DOI] [PubMed] [Google Scholar]
- 19.Grenon, M., C. Gilbert, and N. F. Lowndes. 2001. Checkpoint activation in response to double-strand breaks requires the Mre11/Rad50/Xrs2 complex. Nat. Cell Biol. 3:844-847. [DOI] [PubMed] [Google Scholar]
- 20.Haber, J. E. 1998. The many interfaces of Mre11. Cell 95:583-586. [DOI] [PubMed] [Google Scholar]
- 21.Hartsuiker, E., E. Vaessen, A. M. Carr, and J. Kohli. 2001. Fission yeast Rad50 stimulates sister chromatid recombination and links cohesion with repair. EMBO J. 20:6660-6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hartwell, L. H., and M. B. Kastan. 1994. Cell cycle control and cancer. Science 266:1821-1828. [DOI] [PubMed] [Google Scholar]
- 23.Hopfner, K. P., A. Karcher, L. Craig, T. T. Woo, J. P. Carney, and J. A. Tainer. 2001. Structural biochemistry and interaction architecture of the DNA double- strand break repair Mre11 nuclease and Rad50-ATPase. Cell 105:473-485. [DOI] [PubMed] [Google Scholar]
- 24.Hopfner, K. P., A. Karcher, D. S. Shin, L. Craig, L. M. Arthur, J. P. Carney, and J. A. Tainer. 2000. Structural biology of Rad50 ATPase: ATP-driven conformational control in DNA double-strand break repair and the ABC-ATPase superfamily. Cell 101:789-800. [DOI] [PubMed] [Google Scholar]
- 25.Hopfner, K. P., L. Craig, G. Moncalian, R. A. Zinkel, T. Usui, B. A. Owen, A. Karcher, B. Henderson, J. L. Bodmer, C. T. McMurray, J. P. Carney, J. H. Petrini, and J. A. Tainer. 2002. The Rad50 zinc-hook is a structure joining Mre11 complexes in DNA recombination and repair. Nature 418:562-566. [DOI] [PubMed] [Google Scholar]
- 26.Ito, A., H. Tauchi, J. Kobayashi, K. Morishima, A. Nakamura, Y. Hirokawa, S. Matsuura, K. Ito, and K. Komatsu. 1999. Expression of full-length NBS1 protein restores normal radiation responses in cells from Nijmegen breakage syndrome patients. Biochem. Biophys. Res. Commun. 265:716-721. [DOI] [PubMed] [Google Scholar]
- 27.Ivanov, E. L., N. Sugawara, C. I. White, F. Fabre, and J. E. Haber. 1994. Mutations in XRS2 and RAD50 delay but do not prevent mating-type switching in Saccharomyces cerevisiae. Mol. Cell. Biol. 14:3414-3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeanmougin, F., J. D. Thompson, M. Gouy, D. G. Higgins, and T. J. Gibson. 1998. Multiple sequence alignment with Clustal X. Trends Biochem. Sci. 23:403-405. [DOI] [PubMed] [Google Scholar]
- 29.Jongmans, W., M. Vuillaume, K. Chrzanowska, D. Smeets, K. Sperling, and J. Hall. 1997. Nijmegen breakage syndrome cells fail to induce the p53-mediated DNA damage response following exposure to ionizing radiation. Mol. Cell. Biol. 17:5016-5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kastan, M. B., and D. S. Lim. 2000. The many substrates and functions of ATM. Nat. Rev. Mol. Cell Biol. 1:179-186. [DOI] [PubMed] [Google Scholar]
- 31.Kellis, M., N. Patterson, M. Endrizzi, B. Birren, and E. S. Lander. 2003. Sequencing and comparison of yeast species to identify genes and regulatory elements. Nature 423:241-254. [DOI] [PubMed] [Google Scholar]
- 32.Kobayashi, J., H. Tauchi, S. Sakamoto, A. Nakamura, K. Morishima, S. Matsuura, T. Kobayashi, K. Tamai, K. Tanimoto, and K. Komatsu. 2002. NBS1 localizes to gamma-H2AX foci through interaction with the FHA/BRCT domain. Curr. Biol. 12:1846-1851. [DOI] [PubMed] [Google Scholar]
- 33.Kowalczykowski, S. C. 2000. Initiation of genetic recombination and recombination-dependent replication. Trends Biochem. Sci. 25:156-165. [DOI] [PubMed] [Google Scholar]
- 34.Lee, S. E., J. K. Moore, A. Holmes, K. Umezu, R. D. Kolodner, and J. E. Haber. 1998. Saccharomyces Ku70, mre11/rad50 and RPA proteins regulate adaptation to G2/M arrest after DNA damage. Cell 94:399-409. [DOI] [PubMed] [Google Scholar]
- 35.Lim, D. S., S. T. Kim, B. Xu, R. S. Maser, J. Lin, J. H. Petrini, and M. B. Kastan. 2000. ATM phosphorylates p95/nbs1 in an S-phase checkpoint pathway. Nature 404:613-617. [DOI] [PubMed] [Google Scholar]
- 36.Lindsay, H. D., D. J. Griffiths, R. J. Edwards, P. U. Christensen, J. M. Murray, F. Osman, N. Walworth, and A. M. Carr. 1998. S-phase-specific activation of Cds1 kinase defines a subpathway of the checkpoint response in Schizosaccharomyces pombe. Genes Dev. 12:382-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marchetti, M. A., S. Kumar, E. Hartsuiker, M. Maftahi, A. M. Carr, G. A. Freyer, W. C. Burhans, and J. A. Huberman. 2002. A single unbranched S-phase DNA damage and replication fork blockage checkpoint pathway. Proc. Natl. Acad. Sci. USA 99:7472-7477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsuura, S., H. Tauchi, A. Nakamura, N. Kondo, S. Sakamoto, S. Endo, D. Smeets, B. Solder, B. H. Belohradsky, V. M. Der Kaloustian, M. Oshimura, M. Isomura, Y. Nakamura, and K. Komatsu. 1998. Positional cloning of the gene for Nijmegen breakage syndrome. Nat. Genet. 19:179-181. [DOI] [PubMed] [Google Scholar]
- 39.McGlynn, P., and R. G. Lloyd. 2001. Rescue of stalled replication forks by RecG: simultaneous translocation on the leading and lagging strand templates supports an active DNA unwinding model of fork reversal and Holliday junction formation. Proc. Natl. Acad. Sci. USA 98:8227-8234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moreno, S., A. Klar, and P. Nurse. 1991. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194:795-823. [DOI] [PubMed] [Google Scholar]
- 41.Myung, K., and R. D. Kolodner. 2002. Suppression of genome instability by redundant S-phase checkpoint pathways in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 99:4500-4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naito, T., A. Matsuura, and F. Ishikawa. 1998. Circular chromosome formation in a fission yeast mutant defective in two ATM homologues. Nat. Genet. 20:203-206. [DOI] [PubMed] [Google Scholar]
- 43.Nakamura, T. M., J. P. Cooper, and T. R. Cech. 1998. Two modes of survival of fission yeast without telomerase. Science 282:493-496. [DOI] [PubMed] [Google Scholar]
- 44.Nakamura, T. M., G. B. Morin, K. B. Chapman, S. L. Weinrich, W. H. Andrews, J. Lingner, C. B. Harley, and T. R. Cech. 1997. Telomerase catalytic subunit homologs from fission yeast and human. Science 277:955-959. [DOI] [PubMed] [Google Scholar]
- 45.Nakamura, T. M., B. A. Moser, and P. Russell. 2002. Telomere binding of checkpoint sensor and DNA repair proteins contributes to maintenance of functional fission yeast telomeres. Genetics 161:1437-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nyberg, K. A., R. J. Michelson, C. W. Putnam, and T. A. Weinert. 2002. Toward maintaining the genome: DNA damage and replication checkpoints. Annu. Rev. Genet. 36:617-656. [DOI] [PubMed] [Google Scholar]
- 47.Painter, R. B., and B. R. Young. 1980. Radiosensitivity in ataxia-telangiectasia: a new explanation. Proc. Natl. Acad. Sci. USA 77:7315-7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paques, F., and J. E. Haber. 1999. Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 63:349-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paull, T. T., and M. Gellert. 1998. The 3′ to 5′ exonuclease activity of Mre11 facilitates repair of DNA double-strand breaks. Mol. Cell 1:969-979. [DOI] [PubMed] [Google Scholar]
- 50.Paull, T. T., and M. Gellert. 1999. Nbs1 potentiates ATP-driven DNA unwinding and endonuclease cleavage by the Mre11/Rad50 complex. Genes Dev. 13:1276-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Petrini, J. H. 1999. The mammalian Mre11-Rad50-Nbs1 protein complex: integration of functions in the cellular DNA-damage response. Am. J. Hum. Genet. 64:1264-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petrini, J. H. 2000. The Mre11 complex and ATM: collaborating to navigate S phase. Curr. Opin. Cell Biol. 12:293-296. [DOI] [PubMed] [Google Scholar]
- 53.Raymond, W. E., and N. Kleckner. 1993. Rad50 protein of S. cerevisiae exhibits ATP-dependent DNA binding. Nucleic Acids Res. 21:3851-3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rhind, N., and P. Russell. 1998. Mitotic DNA damage and replication checkpoints in yeast. Curr. Opin. Cell Biol. 10:749-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rhind, N., and P. Russell. 1998. The Schizosaccharomyces pombe S-phase checkpoint differentiates between different types of DNA damage. Genetics 149:1729-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rhind, N., and P. Russell. 2000. Checkpoints: it takes more than time to heal some wounds. Curr. Biol. 10:R908-R911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rhind, N., and P. Russell. 2000. Chk1 and Cds1: linchpins of the DNA damage and replication checkpoint pathways. J. Cell Sci. 113:3889-3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rhind, N., and P. Russell. 2001. Roles of the mitotic inhibitors Wee1 and Mik1 in the G2 DNA damage and replication checkpoints. Mol. Cell. Biol. 21:1499-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Savitsky, K., A. Bar-Shira, S. Gilad, G. Rotman, Y. Ziv, L. Vanagaite, D. A. Tagle, S. Smith, T. Uziel, S. Sfez, et al. 1995. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science 268:1749-1753. [DOI] [PubMed] [Google Scholar]
- 60.Sharples, G. J., and D. R. Leach. 1995. Structural and functional similarities between the SbcCD proteins of Escherichia coli and the Rad50 and Mre11 (Rad32) recombination and repair proteins of yeast. Mol. Microbiol. 17:1215-1217. [DOI] [PubMed] [Google Scholar]
- 61.Shiloh, Y. 1997. Ataxia-telangiectasia and the Nijmegen breakage syndrome: related disorders but genes apart. Annu. Rev. Genet. 31:635-662. [DOI] [PubMed] [Google Scholar]
- 62.Sogo, J. M., M. Lopes, and M. Foiani. 2002. Fork reversal and ssDNA accumulation at stalled replication forks owing to checkpoint defects. Science 297:599-602. [DOI] [PubMed] [Google Scholar]
- 63.Stewart, G. S., R. S. Maser, T. Stankovic, D. A. Bressan, M. I. Kaplan, N. G. Jaspers, A. Raams, P. J. Byrd, J. H. Petrini, and A. M. Taylor. 1999. The DNA double-strand break repair gene hMRE11 is mutated in individuals with an ataxia-telangiectasia-like disorder. Cell 99:577-587. [DOI] [PubMed] [Google Scholar]
- 64.Symington, L. S. 2002. Role of RAD52 epistasis group genes in homologous recombination and double-strand break repair. Microbiol. Mol. Biol. Rev. 66:630-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tauchi, H., J. Kobayashi, K. Morishima, S. Matsuura, A. Nakamura, T. Shiraishi, E. Ito, D. Masnada, D. Delia, and K. Komatsu. 2001. The forkhead-associated domain of NBS1 is essential for nuclear foci formation after irradiation but not essential for hRAD50-hMRE11-NBS1 complex DNA repair activity. J. Biol. Chem. 276:12-15. [DOI] [PubMed] [Google Scholar]
- 66.Tavassoli, M., M. Shayeghi, A. Nasim, and F. Z. Watts. 1995. Cloning and characterisation of the Schizosaccharomyces pombe rad32 gene: a gene required for repair of double strand breaks and recombination. Nucleic Acids Res. 23:383-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Usui, T., T. Ohta, H. Oshiumi, J. Tomizawa, H. Ogawa, and T. Ogawa. 1998. Complex formation and functional versatility of Mre11 of budding yeast in recombination. Cell 95:705-716. [DOI] [PubMed] [Google Scholar]
- 67a.Ueno, M., T. Nakazaki, Y. Akamatsu, K. Watanabe, K. Tomita, H. D. Lindsay, H. Shinagawa, and H. Iwasaki. 2003. Molecular characterization of the Schizosaccharomyces pombe nbs1 gene, involved in DNA repair and telomere maintenance. Mol. Cell. Biol. 23:6553-6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Varon, R., C. Vissinga, M. Platzer, K. M. Cerosaletti, K. H. Chrzanowska, K. Saar, G. Beckmann, E. Seemanova, P. R. Cooper, N. J. Nowak, M. Stumm, C. M. Weemaes, R. A. Gatti, R. K. Wilson, M. Digweed, A. Rosenthal, K. Sperling, P. Concannon, and A. Reis. 1998. Nibrin, a novel DNA double-strand break repair protein, is mutated in Nijmegen breakage syndrome. Cell 93:467-476. [DOI] [PubMed] [Google Scholar]
- 69.Westphal, C. H. 1997. Cell-cycle signaling: Atm displays its many talents. Curr. Biol. 7:R789-R792. [DOI] [PubMed] [Google Scholar]
- 70.Xu, B., S. Kim, and M. B. Kastan. 2001. Involvement of Brca1 in S-phase and G2-phase checkpoints after ionizing irradiation. Mol. Cell. Biol. 21:3445-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yazdi, P. T., Y. Wang, S. Zhao, N. Patel, E. Y. Lee, and J. Qin. 2002. SMC1 is a downstream effector in the ATM/NBS1 branch of the human S-phase checkpoint. Genes Dev. 16:571-582. [DOI] [PMC free article] [PubMed] [Google Scholar]