Abstract
Malignant tumors are characterized by excessive growth, immortalization, and metastatic spread, whereas benign tumors do not express gene products that mediate invasion. The molecular basis for this difference is incompletely understood. We have screened signal transduction molecules associated with the epidermal growth factor (EGF) receptor and have identified constitutive phosphorylation, indicative of activation, of Akt kinase in MT2994 breast cancer cells. In contrast, cells of the benign breast epithelial cell lines Comma-D and FSK-7 are immortalized through pathways that are independent of the EGF-phosphatidylinositol 3-kinase-Akt kinase cascade, but this is not associated with invasiveness. Transfection of constitutively active Akt kinase causes accelerated cell division and osteopontin expression. Conversely, dominant-negative Akt kinase slows cell cycle progression and suppresses osteopontin expression. The manipulation of osteopontin expression in this setting by transfection of the gene or its antisense does not affect the growth rate of the cells but alters cell motility and anchorage independence. Therefore, Akt kinase activates two distinct genetic programs: the program of growth and survival, which is independent of osteopontin expression, and the program of invasiveness and anchorage independence, which is mediated by osteopontin. These studies define Akt kinase as a molecular bridge between cell cycle progression and dissemination.
The defining characteristics of benign and malignant tumors are excessive growth and immortalization. In contrast, only malignant tumors express gene products that mediate invasiveness. Uncontrolled proliferation is a consequence of gain-of-function mutations of proto-oncogenes or loss-of-function mutations of tumor suppressor genes. Metastatic dissemination is a consequence of aberrant expression or splicing of stress response genes (53). The consistent topology of metastasis formation by specific cancers, such as the high frequency of colony formation in bone and brain by malignant breast tumors, implies that metastasis gene expression is an inevitable consequence of gain of function by specific oncogenes. This raises the following question: what molecular mechanisms connect the signal transduction pathways associated with dysregulated growth to the expression of metastasis genes in malignant but not in benign tumors?
Gain-of-function mutations in the epidermal growth factor (EGF) family of receptors and their associated pathways of signal transduction often underlie the transformation of breast tissue, as is evidenced by the cases of breast cancers that overexpress the EGF family receptor Her-2/neu. This dysregulation is also prominent in steroid hormone-independent breast cancer, where excessive activation of EGF receptor pathways may be the only driving force for cell cycle progression (5). The intracellular signal transduction associated with members of the EGF receptor family is mediated by multiple proto-oncogene products, including protein kinase C, phosphatidylinositol 3-kinase (PI 3-kinase), and Akt kinase (31, 35). Their constitutive activation occurs as a consequence of overexpression of Her-2/neu (6, 24, 33, 41, 56) and may be sufficient to cause transformation.
Expression of the cytokine osteopontin is necessary and may be sufficient for the formation of metastases by breast cancer. High levels of osteopontin in the disease are an adverse prognostic factor (42, 45). Multiple metastatic breast cancer cell lines express osteopontin, and transfection of the osteopontin gene into weakly tumorigenic human breast tumor cell lines confers invasive behavior (47, 50, 51). Increasing the expression of osteopontin or transfection of osteopontin-encoding cDNA into a previously benign cell line is sufficient to produce a metastatic phenotype in a rat mammary model (38). Short regulatory DNAs exist in human cancer cells that can be transferred into model rat mammary cell lines and can induce metastatic spread. These noncoding fragments of DNA act via the common effector gene osteopontin (4, 11, 19, 20).
Receptor ligation by EGF can induce osteopontin gene expression (2, 34) through signal transduction that proceeds via protein kinase C and tyrosine kinases (8). This implies that gain-of-function mutations in the EGF receptor pathway in breast cancer, causing dysregulated growth, may also mediate the overexpression of osteopontin, leading to dissemination. We find osteopontin to be constitutively expressed in malignant but not in benign transformed breast cells. Here, we trace the cause for this to constitutive activation of Akt kinase, an enzyme that is part of the EGF signaling pathway.
MATERIALS AND METHODS
Cells.
We used three murine BALB/c breast tumor cell lines with various levels of malignancy (3, 26, 29, 39). Comma-D cells are derived from culture of midpregnancy mammary glands and develop hyperplasia when injected into mice. FSK-7 cells were obtained from primary breast cell culture. MT2994 cells were selected from mammary tumors that had been induced by dimethylbenz[a]anthracene. Among these cells, only MT2994 is malignant in vivo. Even though these cell lines were derived from various mice at various time points, their shared inbred genomic background (the BALB/c strain) allows them to be viewed as a progression series of breast tumor cell lines. The cells were grown in MECL medium (Dulbecco's modified Eagle medium-Ham F-12 medium [1:1] with 2% adult bovine serum, 5 ng of EGF/ml, and 10 μg of insulin/ml). For growth factor deprivation, the medium was made without serum, EGF, and insulin.
Induction of growth factor signaling.
Cells were plated at 5 × 105 per well in 6-well plates. After 7 h, cultures were washed twice with phosphate-buffered saline and maintained in growth factor-deprived medium (containing 0.05% bovine serum albumin) for 14 h. The cells were then stimulated with the indicated amounts of EGF (obtained from Sigma, St. Louis, Mo.) for the indicated time frames before harvesting and analysis.
To assess candidate signal transduction molecules involved in the induction of osteopontin gene expression, specific inhibitors were added to the cells. The PI 3-kinase inhibitor wortmannin and the phospholipase C (PLC) inhibitor U73122 were purchased from Calbiochem (San Diego, Calif.). The cells were pretreated with the respective inhibitors or vehicle (dimethyl sulfoxide) alone for 30 min and then cotreated with EGF for an additional 8 h. Preliminary dose-response experiments had established that the concentrations of 100 nM wortmannin and 500 nM U73122 were effective and nontoxic.
For the investigation of cell growth rates, each cell line was plated at 5,000 cells/well in 24-well plates. Daily, five wells per group were harvested by trypsinization, and the cell numbers were determined in a Coulter Z-Series Counter. At each time point, the cell numbers from the five wells of the various groups of transfectants were analyzed for statistically significant differences by the Wilcoxon-Mann-Whitney test, by accepting a probability of error of <5%. The cell viability was always higher than 95%.
RNase protection assay.
Osteopontin expression was analyzed by an RNase protection assay on total RNA by using a 400-bp probe from the 3′ region of the transcript and controlling for loading with a probe for β-actin. Total RNA was isolated using the RNeasy Mini Kit (QIAGEN GmbH, Hilden, Germany), and its amount and purity were confirmed by spectrophotometry. The RNase protection assay was performed with a commercial kit (Ambion). Briefly, [α-32P]UTP-labeled probes were mixed with sample RNA and coprecipitated. Hybridization proceeded for 10 min at 68°C, followed by digestion of the unprotected RNA with RNase A-T1 for 30 min at 37°C and separation of the hybridized RNA on 5% acrylamide-8 M urea gels. The gels were dried on filter paper in a gel dryer (Bio-Rad) and exposed to X-ray film. Autoradiographs were scanned using an AlphaImager 2200 spot densitometer (Alpha Innotech Corporation), and the integrated densities of areas were recorded.
Northern blotting.
Total RNA was isolated by using the RNeasy Mini Kit (QIAGEN GmbH) according to the manufacturer's instructions. Purity and yield were confirmed by spectrophotometry at 260 and 280 nm. Ten micrograms of RNA was denatured and separated on formaldehyde-containing 1.2% agarose gels by electrophoresis, blotted onto nylon membranes (Amersham), and probed with a 32P-labeled osteopontin cDNA probe (generated by PCR using forward primer 5′-GCGAATTCTGGCTTATGGACTGAGGTC-3′ and reverse primer 5′-ATGGATCCGGAACTGTGTTTTTGCCTC-3′) or a 36B4 cDNA probe (generously provided by Goberdhan Dimri). Hybridization of the probe to the RNA was allowed to proceed overnight, and the membranes were washed and exposed to X-ray film at −80°C before development.
Immunoblot assay.
For analysis of secreted osteopontin, the growth factor-deprived cell culture supernatant was collected from each treatment group. Forty microliters of supernatant per sample was electrophoresed on sodium dodecyl sulfate (SDS)-10% polyacrylamide minigels with nonreducing sample buffer. For analysis of intracellular proteins, cells were lysed in radioimmunoprecipitation assay buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS) for the osteopontin blot or Akt kinase blot or in NP-40 buffer (150 mM NaCl, 1 mM EDTA, 20 mM Tris-HCl [pH 8.0], 0.5% NP-40) for the phospho-Akt blot. Cell lysates at equal amounts of protein (10 to 20 μg/lane) were electrophoresed on reducing SDS-10% polyacrylamide gels. The separated proteins were transferred to Pure Nitrocellulose membranes (Osmonics Inc.) and probed with antibodies to osteopontin (R&D Systems), Akt, and Akt phosphorylated at Ser473 (New England Biolabs, Beverly, Mass.). Detection was accomplished with the ECL chemiluminescence reagent.
Reporter assays.
Comma-D cells stably transfected with vector, wild-type Akt, or constitutively active Akt were plated at 5 × 105 cells per 60-mm-diameter petri dish and grown for 24 h before transfection with Fugene. Various reporter constructs containing the luciferase reporter gene under the control of truncation mutants of the osteopontin promoter were used at 2 μg per transfection. The common internal transfection standard pRL-SV40 (10 ng per transfection) served as a control for transfection efficiency. Twenty-four hours after transfection, the cells were harvested in 0.4 ml of reporter lysis buffer (Promega), and dual luciferase reporter assays were performed by following the protocol provided by the manufacturer. Forty microliters of lysate was used for measurement in a luminometer (Turner Designs TD-20/20). Three separate experiments were performed, and results were calculated as means ± standard deviations. The lengths of the osteopontin promoter fragments tested were −88 to +79, −258 to +79, −472 to +79, −600 to +79, −777 to +79, and −882 to +79 (23).
DNA constructs and infection.
The constructs of Akt kinase, constitutively active Akt kinase, and dominant-negative Akt kinase (K179M) in the pCMV-6 vector or in the retrovirus vector pLNCX were generously provided by Thomas Franke (12, 32) (the constitutively active Akt kinase was recloned into pLNCX in our laboratory). The construct for expression of the mouse osteopontin coding sequence was obtained by reverse transcription-PCR from osteosarcoma cells by using the sense primer 5′-ATGGATCCCGACCATGAGATTGGCAG-3′ and the antisense primer 5′-GCGAATTCGGACTGTGTTTTTGCCTC-3′. The resultant BamHI-EcoRI cDNA fragment was ligated into the retrovirus vector pBabe. The construct for expression of osteopontin antisense, a full-length murine osteopontin cDNA generated by PCR, was ligated in the antisense orientation into the EcoRI and BamHI sites of pBabe. The integrity of all constructs was confirmed by DNA sequencing.
The packaging cell line tsa54 was used to generate high-titer retrovirus (16) for infection of all breast epithelial cell lines. Ten micrograms of each retrovirus construct in conjunction with 10 μg of the pIK packaging vector was transfected into tsa54 cells by the calcium phosphate coprecipitation method, and the culture supernatant was collected on the day following transfection. In order to generate stable Akt transfectants, the murine breast tumor cell lines were infected with virus-containing supernatants in the presence of 8 μg of Polybrene (Sigma)/ml. Infected cells were selected in MECL medium with G418 (200 to 500 μg/ml). For double infection, cells expressing constitutively active Akt or mutant Akt were infected with plasmids encoding sense or antisense osteopontin. Forty-eight hours later, selection with puromycin (2 to 4 μg/ml) commenced for 5 days, followed by analysis of RNA and protein expression. Several clones were selected and characterized for all single infectants, while the doubly transduced cells were selected and analyzed in bulk.
Cell motility.
The cell motility experiment is often referred to as an in vitro wounding assay (21, 27) because a cell monolayer is disrupted and the rate of repopulation is assessed. Breast epithelial cells were plated at 2.5 × 105 per 100-mm-diameter petri dish. After 2 days, they were grown in growth factor-deprived medium for 24 h. The cultured cells were disrupted by scratching with a pipet aid equipped with a 1,000-μl tip. Repopulation of the cell-free area was examined under an inverted microscope after 22 h. In this time frame, cell division is negligible in growth factor-deprived medium.
Soft-agar growth.
Cells (5 × 105 per 60-mm-diameter dish) were plated in triplicate with a top layer of 0.3% agar (Bacto Agar; Difco, Detroit, Mich.) and a bottom layer of 0.5% agar (both in MECL medium). Every other day, 0.3 ml of MECL medium was supplemented and the plates were examined microscopically for growth. After 3 weeks, the number of cell clusters per microscopic field and the number of cells per cluster were counted in five views per plate (one central, four peripheral), and cell clusters were photographed. Soft-agar assay conditions have not been standardized because they depend on the specific cell lines under study. The size of the cell clusters obtained differs widely with the cell lines used; cell clusters may comprise fewer than 10 cells (7, 25) or reach microscopically (36) or macroscopically (43) visible diameters. For Comma-D cells transfected with Akt, an incubation time of 3 weeks before enumeration has been found suitable (40). We chose to determine the frequency of clones formed and the clone size (numbers of cells per cluster). At the plating density and within the time frame employed here, this quantitation could be performed accurately, and the resulting histograms of cluster size and frequency were considered to be more informative than the simple enumeration of clusters above a randomly chosen size. The resulting histograms are characteristic of the individual cell lines and were analyzed for statistical differences in variance by the F-test, by accepting a probability of error of 1, 5, or 10% with a one-sided paired comparison.
In vivo tumorigenesis.
MT2994 cells or FSK-7 cells were harvested by trypsinization. A total of 0.5 × 106 cells in 0.5 ml of phosphate-buffered saline were injected orthotopically into the left inguinal mammary fat pads of female BALB/c mice (age, 8 to 10 weeks). After 21 days, the animals were sacrificed and necropsy was performed for the detection of primary tumors or hyperplasia and metastases. To ascertain that the FSK-7 cells were free of murine pathogens, serum samples from injected animals were tested for Sendai virus, pneumonia virus of mice, mouse hepatitis virus, minute virus of mice, Theiler's murine encephalomyelitis virus, reovirus, Mycoplasma pulmonis, mouse parvovirus, epizootic diarrhea of infant mice virus, lymphocytic choriomeningitis virus, mouse adenovirus, ectromelia (mousepox virus), K virus, polyomavirus, mouse thymic virus, mouse cytomegalovirus, hantavirus, Encephalitozoon cuniculi, and cilium-associated respiratory bacillus. Results of serological testing were negative in all instances. The in vivo experiments were performed under a protocol approved by the institutional animal care and use committee at Tufts-New England Medical Center.
RESULTS
The malignant phenotype is associated with constitutive osteopontin expression in breast tumor cells.
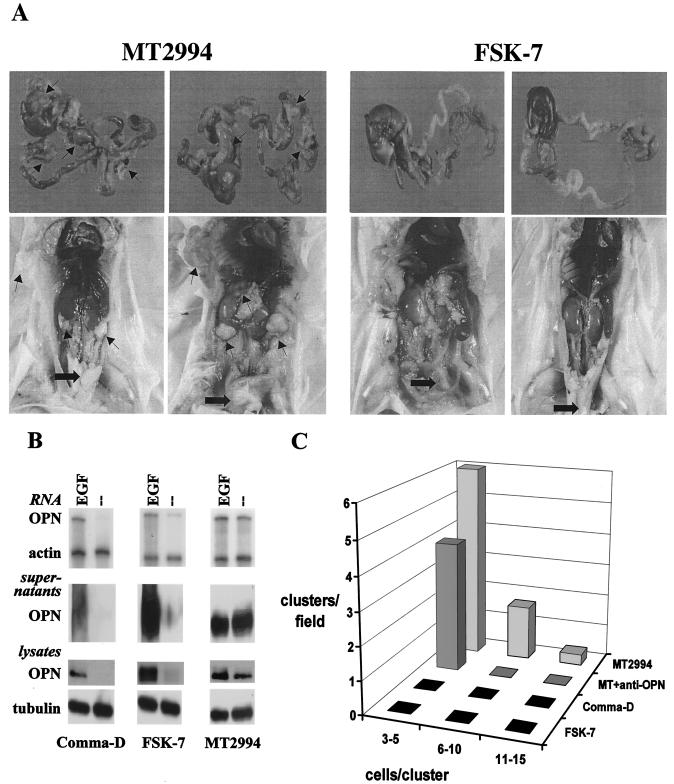
MT2994 cells formed disseminating tumors after orthotopic injection, whereas FSK-7 cells showed modest local growth, diagnosed as hyperplasia, that did not develop into tumors and did not spread beyond the site of injection (Fig. 1A). Comma-D cells and FSK-7 cells secreted osteopontin in EGF-containing medium. Under growth factor-deprived conditions, no osteopontin RNA or protein expression was detectable. In contrast, MT2994 cells had high constitutive levels of osteopontin, regardless of the presence or absence of growth factors (Fig. 1B). Soft-agar colony formation measures the ability of cells to grow independently of anchorage and correlates well with the capacity of tumor cells for invasiveness in vivo (55). We hypothesized that the importance of osteopontin for the dissemination of breast cancer cells would also be reflected in an essential contribution by this molecule to soft-agar colony formation. In initial experiments, we asked whether malignant cells form clones in soft agar in an osteopontin-dependent fashion. As expected, the benign Comma-D and FSK-7 cells did not form clones under these conditions. MT2994 cells formed clones in soft agar in a manner that was specifically inhibitable by addition of an antiosteopontin antibody (Fig. 1C). Hence, we set out to analyze the molecular mechanisms of osteopontin induction in breast cancer cells.
FIG. 1.
Constitutive osteopontin expression contributes to a malignant phenotype in breast tumors. (A) MT2994 cells or FSK-7 cells (0.5 × 106) were injected orthotopically into the left inguinal mammary fat pads of BALB/c mice. After 21 days, the mice were analyzed for tumor formation and metastasis. The upper panel shows the livers and gastrointestinal tracts; the lower panel depicts the opened abdomens. Arrows point to metastases. Thick arrows indicate the primary tumors (in MT2994 cells) or hyperplasias (in FSK-7 cells). These results are representative for 10 mice in each group. (B) Cells were maintained under growth factor-deprived conditions in the presence or absence of EGF for 14 h. Supernatants were collected, and cells were lysed. Osteopontin (OPN) expression was assessed on the RNA level by an RNase protection assay (using β-actin as a loading control) and on the protein level by Western blotting (using tubulin as a loading control for the cell lysates). (C) MT2994 cells (5 × 105 cells/60-mm-diameter petri dish) were grown in soft agar in the absence of antibodies (back row, MT2994) or in the presence of an anti-osteopontin antibody (third row from front, MT + anti-OPN) for 21 days. The frequency of clone formation and clone size (number of cells per cluster) were enumerated in five random microscopic fields. While control immunoglobulin had no effect (data not shown), the anti-osteopontin antibody suppressed clone formation. Comma-D cells and FSK-7 cells do not constitutively secrete osteopontin and do not form clones in soft agar when grown under identical conditions (front rows, FSK-7 and Comma-D).
Osteopontin gene expression is linked to the activation of growth factor signaling.
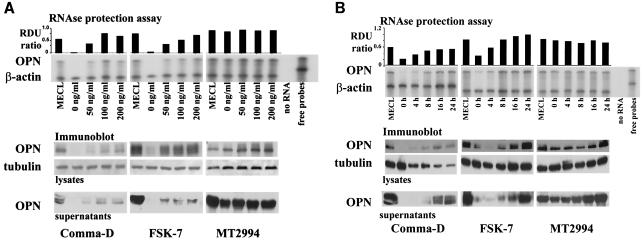
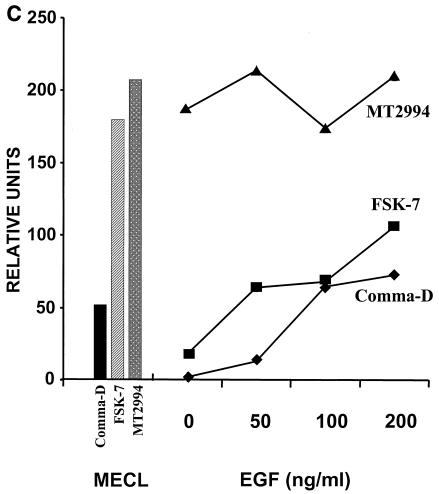
We asked whether osteopontin gene expression by breast tumor cells is secondary to activation of growth factor signaling pathways. The elimination of growth factors from the medium reduced the expression of osteopontin in the benign breast epithelial cell lines Comma-D and FSK-7 but not in the malignant breast tumor line MT2994. Conversely, stimulation of the cells with EGF induced osteopontin expression in Comma-D cells and FSK-7 cells in a time- and dose-dependent manner but did not affect osteopontin expression in MT2994 cells. (Fig. 2A and B). To quantitate this observation, we performed densitometry of osteopontin on the Western blots of cell lysates and supernatants and calculated the total amounts of osteopontin protein per cell culture dish (5 × 105 cells) consecutive to the titration of EGF. At 200 ng of EGF/ml over 8 h, the levels of osteopontin expression in Comma-D cells and FSK-7 cells are about one-half of those in MT2994 cells. In the latter cell line, the osteopontin expression levels are not further induced by EGF (Fig. 2C).
FIG. 2.
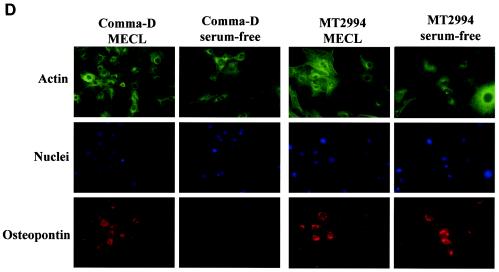
EGF-mediated signaling induces osteopontin in benign but not in malignant breast epithelial cells. A total of 5 × 105 cells were plated in 6-well plates for 7 h. Following serum starvation for 14 h, the transformed breast epithelial cells were stimulated dose dependently at 24 h (A) or time dependently at 100 ng/ml (B) with EGF. MECL, normal growth medium containing 2% adult bovine serum, EGF, and insulin. Osteopontin (OPN) expression was analyzed by an RNase protection assay on total RNA. The results were confirmed by analysis on the protein level by immunoblotting of cell lysates and supernatants. Tubulin and β-actin served as loading controls, respectively. RDU ratio, relative density units of osteopontin RNA divided by relative density units of actin RNA. (C) Quantitation of total osteopontin in culture 24 h after treatment with increasing amounts of EGF. (D) Comma-D cells and MT2994 cells were grown on coverslips in the presence or absence of growth factors. The cells were stained with fluorescently labeled antibodies to actin (FITC; green) or osteopontin (phycoerythrin; red). Nuclei were stained with DAPI (blue). No nonspecific signal was detected after incubation with streptavidin-phycoerythrin in the absence of the antiosteopontin antibody. Likewise, a nonspecific FITC-labeled antibody did not visibly stain the cells (data not shown).
Differences in osteopontin expression between the benign and malignant cell lines were also confirmed by fluorescent microscopy with a biotinylated anti-osteopontin antibody (R&D Systems) and streptavidin-phycoerythrin. The nuclei were stained with 4′,6′-diamidino-2-phenylindole (DAPI), and the cytoplasm was stained with fluorescein isothiocyanate (FITC)-conjugated anti-β-actin. The signal for osteopontin was constitutive in MT2994 cells in growth factor-deprived medium and was not further enhanced by the addition of exogenous EGF. In contrast, Comma-D cells and FSK-7 cells (data not shown) depended on the addition of growth factors in the medium for significant osteopontin staining (Fig. 2D). The location of the osteopontin signal was predominantly perinuclear, which is characteristic of secreted proteins (15).
The constitutive osteopontin expression in malignant breast epithelial cells correlates with a gain of function in the PI 3-kinase pathway.
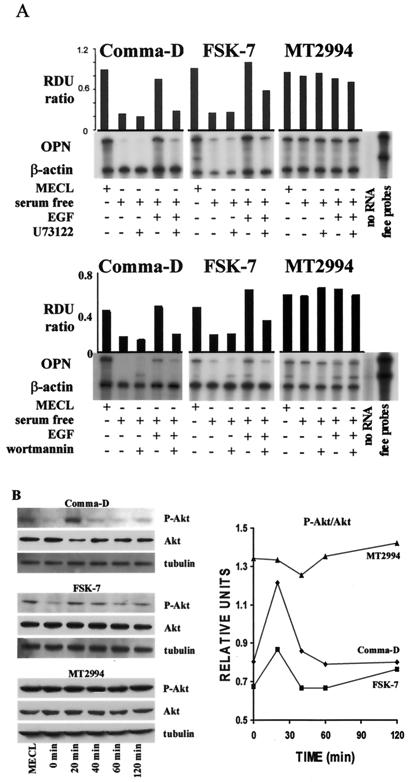
We analyzed the involvement of signal transduction molecules associated with the EGF receptor in the induction of osteopontin gene expression. Addition of the PLC inhibitor U73122 or the PI 3-kinase inhibitor wortmannin reversed the EGF-mediated increase in osteopontin mRNA levels in Comma-D and FSK-7 cells. In contrast, the high abundance of osteopontin mRNA in MT2994 cells remained unaffected by either inhibitor (Fig. 3A).
FIG. 3.
Osteopontin expression depends on the PI 3-kinase pathway in breast epithelial cells. (A) The PLC inhibitor U73122 or the PI 3-kinase inhibitor wortmannin was used in growth factor-deprived medium to assess EGF-dependent signal transduction leading to osteopontin gene expression. Wortmannin (100 nM) was added to the cells before incubation in the presence or absence of 100 ng of EGF/ml for 8 h. Similarly, U73122 was added to the cells at a final concentration of 500 nM. Osteopontin gene expression was analyzed by an RNase protection assay on total RNA and standardized by comparison to β-actin. (B) Phosphorylation of Akt kinase in breast epithelial cells. A total of 5 × 105 cells were seeded in 6-well plates for 7 h. Following serum starvation for 14 h, they were incubated for the indicated times in growth factor-deprived medium with 100 ng of EGF/ml. The cells were harvested and lysed, and 20 μg of lysate was probed by immunoblotting with antitubulin, anti-Akt, and anti-phospho-Akt antibodies. (Left panel) Immunoblots; (right panel) spot densitometry (integrated density values) for phospho-Akt (P-Akt)/Akt.
Because wortmannin could not reverse the constitutive osteopontin gene expression in MT2994 cells, the defect leading to its up-regulation was likely to be downstream of PI 3-kinase. PI 3-kinase can phosphorylate and activate Akt kinase. In malignant cells, Akt kinase activity may be elevated due to phosphorylation on serine 473 and threonine 308. We therefore tested Akt phosphorylation in the breast tumor cells on Western blots with a phospho-Akt-specific antibody. Stripping and reprobing for total Akt kinase and for tubulin served as controls for Akt expression levels and for equal loading of the gels, respectively. Akt was constitutively phosphorylated in the malignant cell line MT2994 but not in Comma-D or FSK-7 cells, where Akt kinase phosphorylation was EGF induced and transient. This is not accounted for by overexpression of the kinase, because levels of total Akt were not higher in MT2994 cells than in Comma-D or FSK-7 cells (Fig. 3B).
Overexpression of Akt kinase induces osteopontin gene expression.
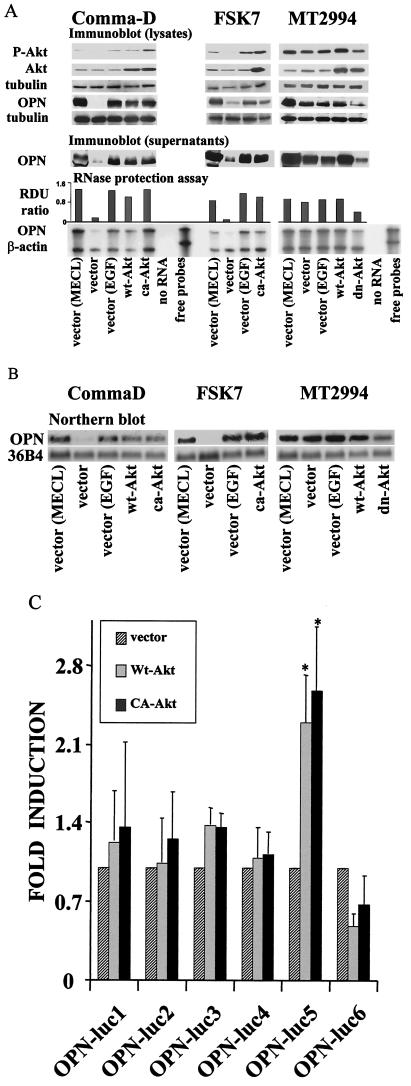
To gain further mechanistic insight into the contributions by Akt kinase to osteopontin gene expression, we stably transduced the breast epithelial cells with various Akt kinase constructs. Comma-D cells were transfected with vector, wild-type Akt, or constitutively active Akt. FSK-7 cells were transfected with vector or constitutively active Akt. MT2994 cells were transfected with vector, wild-type Akt, or a dominant-negative Akt mutant. The expression levels of the transfected genes were measured on the RNA and protein levels. As expected, osteopontin was constitutively expressed in Akt kinase-transduced Comma-D cells and FSK-7 cells, while the constitutive osteopontin gene expression in MT2994 cells was suppressed by the dominant-negative Akt kinase mutant, as shown by an RNase protection assay and Western blotting (Fig. 4A). We confirmed the osteopontin message levels by Northern blotting (with 36B4 as a loading control) and found this measurement to be in good correlation with the RNase protection assay results (Fig. 4B). The levels of osteopontin induced by wild-type Akt and constitutively active Akt are comparable, likely reflecting the substantial overexpression of Akt kinase. At these amounts, the baseline activity of wild-type Akt kinase is sufficient to transduce a signal. It should be noted that the reduction of osteopontin gene expression by dominant-negative Akt kinase is partial. Only moderate levels of overexpression can be achieved for dominant-negative Akt kinase, because this mutant also slows down cell division (compare Fig. 5B below), consistent with the hypothesis that the constitutive activation of Akt kinase in MT2994 cells is causative for the transformation of these cells.
FIG.4.
Akt kinase activity induces osteopontin gene expression. (A) Following serum starvation for 14 h, the transfected breast epithelial cells were grown under growth factor-deprived conditions, stimulated by EGF, or kept in normal growth medium containing 2% adult bovine serum, EGF, and insulin (MECL). Osteopontin (OPN) expression was analyzed by an RNase protection assay, and a probe for β-actin was used to control for loading. RDU ratio, relative density units of osteopontin RNA divided by those of actin RNA. The results were confirmed by analysis on the protein level by immunoblotting of cell culture supernatants. Overexpression of the transfected Akt constructs was confirmed by Western blotting using cell lysates. Lysates from transiently transfected 293T cells confirmed the integrity of the transfected DNA constructs (data not shown). The levels of resulting Akt phosphorylation were tested with a phospho-Akt (P-Akt)-specific antibody. Tubulin served as a loading control. wt-Akt, wild-type Akt; ca-Akt, constitutively active Akt; dn-Akt, dominant-negative Akt. (B) The results from the RNA protection assay were confirmed by Northern blotting on total RNA from cells transfected with various Akt constructs (ca-Akt, wt-Akt, and dn-Akt). The cells had been cultured in complete medium (MECL) or in serum-free medium with or without EGF. The blot was probed for osteopontin and for 36B4 (loading control). Similar results were obtained with additional clones of ca-Akt-transfected FSK-7 cells and dn-Akt-transfected MT2994 cells (data not shown). (C) Reporter assays with osteopontin promoter constructs. Comma-D cells stably expressing wild type Akt kinase (Wt-Akt), constitutively active Akt kinase (CA-Akt), or the vector control were transiently transfected with luciferase (luc) reporter constructs under the control of various fragments of the osteopontin promoter. The lengths of the osteopontin promoter truncation mutants tested were −88 to +79 (OPN-luc1), −258 to +79 (OPN-luc2), −472 to +79 (OPN-luc3), −600 to +79 (OPN-luc4), −777 to +79 (OPN-luc5), and −882 to +79 (OPN-luc6). With OPN-luc5, the data for Wt-Akt and for CA-Akt are significantly different from the data for the vector control (P < 0.05 [asterisked] according to the t test for paired samples, after testing for normal distribution and equal variance). Luciferase activity is expressed in relative units, with the activity of the vector-transduced Comma-D cells set at 1. Data reflect averages of three independent experiments. Error bars, standard deviations.
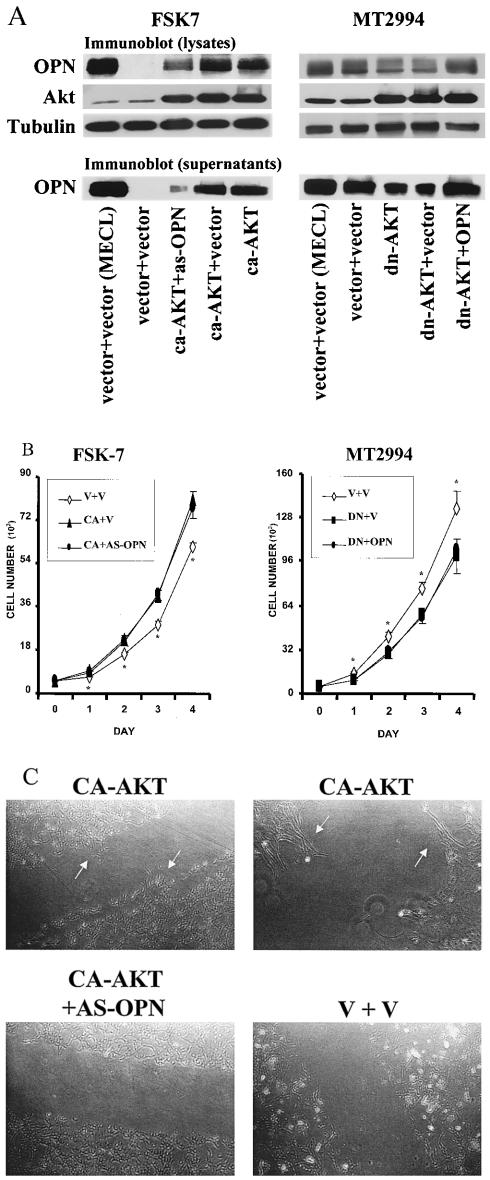
FIG. 5.
Osteopontin is a downstream effector of Akt kinase for migration but not for growth. (A) After serum starvation for 14 h, transfected breast epithelial cells were grown under growth factor-deprived conditions or kept in normal growth medium (MECL). Overexpression of the transfected constructs was confirmed by Western blotting using cell lysates and cell culture supernatants from transduced FSK-7 or MT2994 cells. Expression of the transfected Aktconstructs was analyzed by Western blotting with antihemagglutinin or anti-Akt kinase. Tubulin served as a loading control. Osteopontin expression levels were measured by probing blots of cell lysates and supernatants with a specific antiosteopontin antibody. ca-Akt, constitutively active Akt kinase; dn-Akt, dominant-negative Akt kinase; OPN, osteopontin; as-OPN, osteopontin antisense. (B) Akt kinase induces cell growth independently of osteopontin. Cells were plated at 5 × 103 per well in 24-well plates. Cell numbers per well were determined daily using a Coulter Z Series cell counter. Each data point represents the mean ± standard deviation of five measurements. Data points labeled with an asterisk are significantly different (P < 0.05) from the other data sets. V, vector transfectant; CA, transfectant of constitutively active Akt kinase; DN, transfectant of dominant-negative Akt kinase; OPN, transfectant of osteopontin; AS-OPN, transfectant of osteopontin antisense). (C) Constitutive osteopontin expression is necessary for cell motility. Transduced FSK-7 cells were plated at 2.5 × 105 per 100-mm-diameter dish. After 2 days, they were grown in growth factor-deprived medium for 24 h. The cultured cells were disrupted by scratching with a pipet aid equipped with a 1,000-μl tip. Repopulation of the cell-free area was examined under an inverted microscope after 22 h. Transfectants with constitutively active Akt (CA-AKT) sprout into the depleted area (arrows, top left). A higher magnification shows the reorientation of the cells (arrows, top right). No substantial changes are seen in cells transduced with constitutively active Akt plus antisense osteopontin (CA-AKT + AS-OPN) (bottom left) or vector controls (V + V) (bottom right). Similar results were obtained in three additional experiments.
Previous studies have shown that the induction of osteopontin in renal epithelial cells by transforming growth factor β or EGF is caused by increased transcription (34). Akt kinase activity has previously been associated with activation of the AP-1 transcription factor and Ets family transcription factors (17, 18, 28), both of which are known to bind to the osteopontin promoter (reviewed in reference 52). We set out to further map the molecular connections between Akt kinase activity and osteopontin transcription in breast epithelial cells. For this purpose, we took advantage of the breast epithelial cell lines constitutively expressing various Akt constructs that we had generated. We used Comma-D cells stably expressing wild-type Akt kinase, constitutively active Akt kinase, or vector in luciferase reporter assays under the control of full-length or truncated osteopontin promoter sequences. As expected, Akt kinase activity can induce the transactivation of the osteopontin promoter (Fig. 4C). The Akt-responsive promoter domain was mapped to a region between base −600 and base −777. Consistent with a previous report (23), a far-distal promoter element (between bases −777 and −882) appeared to contain a repressor.
Akt kinase enhances the growth rate through osteopontin-independent pathways and mediates migration through osteopontin.
In order to differentiate between osteopontin-dependent and osteopontin-independent consequences of Akt kinase overexpression, we generated doubly transduced lines. FSK-7 cells that stably expressed constitutively active Akt kinase were infected with a retrovirus containing osteopontin antisense, yielding FSK-7 vector plus vector, FSK-7 constitutively active Akt plus vector, and FSK-7 constitutively active Akt plus antisense osteopontin cells. Similarly, we generated MT2994 vector plus vector, MT2994 dominant-negative Akt plus vector, and MT2994 dominant-negative Akt plus osteopontin cells. As before, the expression levels of the modulated genes were measured by Western blotting. The elevated osteopontin expression in transfectants of constitutively active Akt kinase could be reversed by cotransfection of antisense osteopontin, and the suppression of osteopontin by dominant-negative Akt was reversed by cotransfection with the osteopontin gene (Fig. 5A).
We tested the growth rates of doubly transduced cells by plating 5,000 cells per well in 24-well plates, followed by daily cell counts in quintuplicate. Remarkably, within only 1 day, transfection of constitutively active Akt kinase into FSK-7 cells significantly induced the growth rate, while transfection of dominant-negative Akt kinase into MT2994 cells significantly reduced it. The differences remained significant throughout the 4 days of measurement. In contrast, the stable transfection of osteopontin into cells overexpressing dominant-negative Akt kinase or of osteopontin antisense into cells overexpressing constitutively active Akt kinase had no effect on the growth rates (Fig. 5B). Identical results were obtained with singly transduced FSK-7 cells expressing constitutively active Akt kinase and MT2994 cells expressing dominant-negative Akt kinase (data not shown).
We also analyzed cell motility in vitro. Doubly transduced FSK-7 cells were plated at 2.5 × 105 per 100-mm-diameter dish and grown for 2 days. They were starved for 24 h in growth factor-deprived medium. The geometry of the cell culture was disturbed by scratching across the diameter of the cell culture dish. After 22 h, FSK-7 cells transfected with constitutively active Akt displayed reorientation and migration into the space between the continuous layers. Cotransfected osteopontin antisense completely reversed this effect, and like cells transfected with the vector control, these cells did not move (Fig. 5C). For a more quantitative analysis of cell motility, we performed a transwell chamber chemokinesis assay with coating of both sides of the membrane with 10 μg of fibronectin/ml. A total of 2.5 × 104 cells were placed in the upper chamber, and serum- and growth factor-free medium was placed in both compartments. After 4 h, the numbers of cells per optical field on the lower side of the membrane were 9 ± 2 for FSK-7 vector plus vector, 19 ± 6 for FSK-7 vector plus antisense osteopontin, 51 ± 2 for FSK-7 constitutively active Akt plus vector, and 32 ± 4 for FSK-7 constitutively active Akt plus antisense osteopontin. These results are consistent with osteopontin-independent roles of Akt as a growth-promoting gene product and osteopontin-dependent roles of Akt as a migration-promoting gene product.
Osteopontin-dependent and -independent pathways contribute to generating a malignant phenotype.
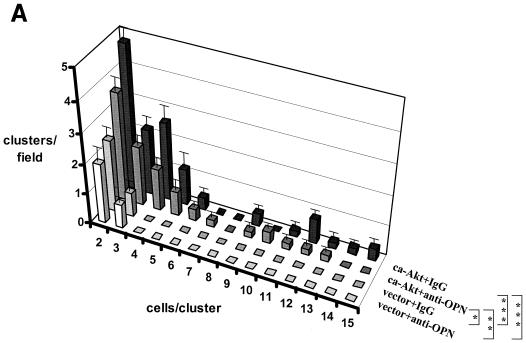
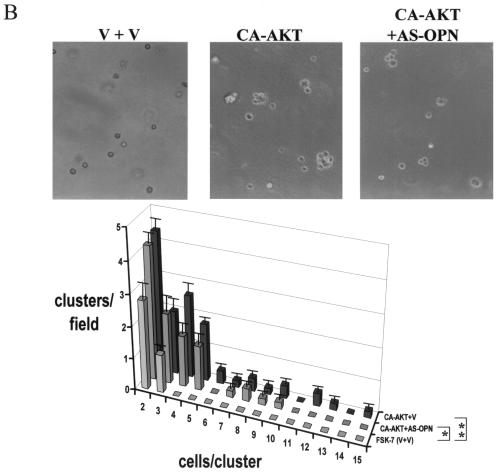
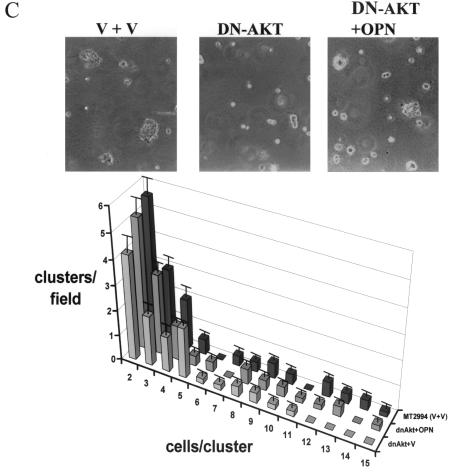
Because osteopontin is necessary for soft-agar colony formation (see Fig. 1C) and Akt kinase induces osteopontin expression, we asked whether the constitutive overexpression of Akt kinase in FSK-7 cells was sufficient to confer anchorage-independent growth. As expected, Akt-transfected FSK-7 cells displayed clone formation in soft agar, whereas vector-transfected FSK-7 cells did not. The repeated addition of an anti-osteopontin antibody to the cell culture reduced the numbers and sizes of clones formed. In contrast, a control immunoglobulin had no effect (Fig. 6A). As in the preceding experiment (compare Fig. 1C), the inhibition of clone formation by an antiosteopontin antibody was partial, a finding which may reflect limitations in the neutralization by the antibody. We therefore analyzed the doubly transduced cells for colony formation in soft agar. Coexpression of osteopontin antisense in FSK-7 cells expressing constitutively active Akt led to a partial reduction in colony formation. Consistent with the earlier results, FSK-7 cells transduced with vector constructs did not form clones in soft agar (Fig. 6B). In a complementary approach, expression of dominant-negative Akt in MT2994 cells caused a partial reduction in the numbers and sizes of clones formed (a shift of the histogram to the left), consistent with the partial suppression of Akt kinase activity by the dominant-negative construct. Coexpression of osteopontin virtually completely reversed this inhibition (Fig. 6C). The differences among the transduced MT2994 cells did not reach statistical significance, which is likely due to the incomplete suppression of endogenous Akt activity by the dominant-negative construct. Dominant-negative variants of essential growth-promoting gene products can typically be overexpressed only at moderate levels (1). Residual Akt activity has previously been observed after transfection with the Akt (K179M) construct (12, 32).
FIG. 6.
Invasive growth depends on Akt kinase and osteopontin. (A) FSK-7 cells stably transfected with vector or with constitutively active Akt were plated in soft agar with repeated additions of either an antiosteopontin antibody or a control immunoglobulin. After 21 days, the frequency of clone formation and the clone size (numbers of cells per cluster) were enumerated in five random microscopic fields. The number of clusters per field is plotted against the number of cells per cluster for each cell line and treatment. ca-Akt, constitutively active Akt; IgG, immunoglobulin; OPN, osteopontin. (B) FSK-7 cells were transfected with either vector plus vector (V + V), constitutively active Akt plus vector, or constitutively active Akt plus antisense to osteopontin. Cells (5 × 105 per 60-mm-diameter dish) were grown in soft agar for 21 days. Micrographs were taken at a high magnification. Clone formation and clone size were enumerated in five random microscopic fields. (C) MT2994 cells transfected with either vector plus vector, dominant-negative Akt plus vector, or dominant-negative Akt plus osteopontin. CA-AKT, constitutively active Akt; dnAkt, dominant-negative Akt; V, vector; OPN, osteopontin; AS-OPN, antisense osteopontin. Error bars, standard errors. *, P < 0.1; **, P < 0.05; ***, P < 0.01.
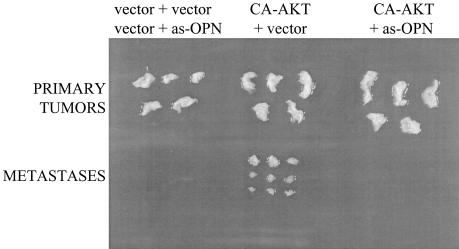
We tested in vivo tumorigenesis and dissemination by using doubly transduced FSK-7 cells. As expected, cells transduced with vector plus vector or with vector plus antisense osteopontin generated small hyperplastic lesions. In contrast, cells expressing constitutively active Akt generated larger tumors. Only the FSK-7 cells containing Akt plus vector, not the FSK-7 cells expressing Akt plus antisense osteopontin, formed distal lesions (Fig. 7). These experiments were extended to 30 days (at that time point, equal numbers of MT2994 cells are lethal), and the distal foci formed were smaller than those observed with MT2994, suggesting that additional factors may contribute to MT2994 malignancy. However, the observations are consistent with a critical role of Akt kinase in tumor growth and dissemination, the latter being mediated by high constitutive levels of osteopontin.
FIG. 7.
In vivo growth and dissemination are mediated by Akt kinase and osteopontin. FSK-7 cells were doubly transduced, and 106 cells were injected orthotopically into the left inguinal mammary fat pads of BALB/c mice. One group of five mice received either FSK-7 cells transduced with vector plus vector or FSK-7 cells transduced with vector plus antisense osteopontin (as-OPN), five mice were injected with FSK-7 cells transduced with constitutionally activated Akt (CA-AKT) plus vector, and five mice were given FSK-7 cells transduced with constitutionally activated Akt plus antisense osteopontin. After 30 days, the mice were analyzed for tumor formation and metastasis. Primary tumors are shown at the top, and metastases detected during necropsy are shown at the bottom.
DISCUSSION
The molecular processes that lead to dysregulated growth of cancer cells can be distinguished from the molecular mechanisms that mediate dissemination. Osteopontin mRNA expression and secretion by ras-transformed NIH 3T3 cells correlate with these cells' levels of ras expression and their metastatic ability (9, 10). In contrast, LTA cells transfected with ras or with ras plus v-myc decrease the expression of osteopontin and remain tumorigenic but nonmetastatic (13, 14, 37). Osteopontin is the physiologic ligand of CD44v6, which also mediates dissemination without altering tumor growth. Expression of the CD44 splice form containing the variant exon 6 on various tumor cells is necessary and sufficient to mediate metastasis formation. Overexpression of CD44v6 in nonmetastasizing pancreatic tumor cells was sufficient to establish full metastatic behavior (22). In a complementary approach, a monoclonal antibody to the metastasis-specific exon of CD44 retarded lymph node and lung colonization by a metastatic pancreas tumor line (44). In mice that are susceptible to carcinogenesis due to p53 mutation, the absence of the CD44 gene leads to abolishment of osteosarcoma metastasis but not to changes in tumor incidence or growth (54). Based on the phenotypes of mice with knockouts of various confirmed metastasis genes, including osteopontin and CD44, the genetic basis of metastasis formation has been identified as aberrant expression or splicing of a unique set of developmentally nonessential genes that physiologically mediate the homing of immune system cells (53).
Metastasis formation arises with consistent patterns of organ preference. It has not been clear how the primary defect of growth dysregulation is linked to the expression of specific metastasis genes. We have screened signal transduction molecules associated with the EGF receptor and have identified constitutive phosphorylation, indicative of activation, of Akt kinase in malignant, but not in benign growth-dysregulated, immortal breast epithelial cells. Akt kinase activity supports tumor growth as well as the induction of osteopontin, a metastasis gene that is essential for breast cancer dissemination to bone. Our results may provide a molecular explanation for the previous observation that transfection of Akt1 into immortalized, not fully transformed Comma-1D cells mediates, mutually independently, the transformation and invasion of breast epithelial cells (40). Human breast carcinogenesis is associated with a loss of cellular retinal-binding protein (CRBP) expression in about one-fourth of all cases. The inhibition of the PI 3-kinase/Akt kinase pathway by CRBP suppresses breast cancer cell survival and anchorage-independent growth (30). The latter is likely due to the inhibition of osteopontin gene expression. Elevated Akt kinase activity is also seen in metastasizing, but not in nonmetastasizing, liver tumor cells (36). Akt kinase has also been associated with the invasiveness of pancreas cancer, but here the pro-metastatic gene induced by Akt is the IGF-I receptor (48).
The cause for the constitutive activation of Akt kinase in MT2994 cells remains unknown. Activating mutations in Akt itself seem unlikely, because mutants with known activating amino acid changes, such as E40K, still respond to inhibition by wortmanin. The loss of negative regulators of Akt kinase may desensitize the enzyme and generate the appearance of PI 3-kinase independence. We have sequenced the mRNA of PTEN, the most prominent negative regulator of Akt, in MT2994 cells and FSK-7 cells (data not shown). No mutations were found in either case. Reduced expression levels of PTEN or a lack of other negative regulators are likely causes.
Our studies of murine transformed breast epithelial cells have indicated that constitutive activation of Akt kinase, independent of PI 3-kinase, causes a malignant phenotype through continuing cell cycle progression and expression of osteopontin (MT2994 cells). In contrast, breast epithelial cells may be immortalized through signal transduction that is independent of the EGF/PLC/PI 3-kinase/Akt kinase pathway (Comma-D cells and FSK-7 cells), but this transformation is not associated with osteopontin gene expression or with metastasis. Dysregulation of Akt kinase activity also plays a prominent role in human breast tumor cells. There, the constitutive activation of Akt kinase is typically secondary to overexpression of ErbB on the cell surface and is dependent on PI 3-kinase. Estrogen stimulates Akt activation, which is reflected in phosphorylation at serine 473 of the oncoprotein, in estrogen receptor-negative breast cancer cells. Activation of Akt by estrogen in these cells is time and dose dependent and can be blocked by inhibitors of PI 3-kinase and Src kinase but not by estrogen antagonists (49). The interdependent regulation between the estrogen receptor α and PI 3-kinase/Akt pathways may play an important role in human breast carcinogenesis and could contribute to ligand-independent breast cancer cell growth (46). Here we describe a single molecular defect responsible for both growth dysregulation and induction of metastatic potential in breast cancer cells. It enhances our molecular insights into carcinogenesis and defines candidate targets for therapeutic intervention.
Acknowledgments
This study was supported by National Institutes of Health research grant CA76176 and U.S. Army breast cancer grant BC011270 to G.F.W.
We are grateful to Joseph Jerry for kindly providing the cell lines. We thank Thomas Franke for generously sharing the Akt kinase constructs and David Denhardt for supplying the osteopontin reporter constructs.
REFERENCES
- 1.Adler, B., G. F. Weber, and H. Cantor. 1998. Activation of T-cells by superantigen: cytokine production but not apoptosis depends on MEK-1 activity. Eur. J. Immunol. 28:3749-3754. [DOI] [PubMed] [Google Scholar]
- 2.Atkins, K. B., R. U. Simpson, and M. J. Somerman. 1997. Stimulation of osteopontin mRNA expression in HL-60 cells is independent of differentiation. Arch. Biochem. Biophys. 343:157-163. [DOI] [PubMed] [Google Scholar]
- 3.Barcellos-Hoff, M. H., and S. A. Ravani. 2000. Irradiated mammary gland stroma promotes the expression of tumorigenic potential by unirradiated epithelial cells. Cancer Res. 60:1254-1260. [PubMed] [Google Scholar]
- 4.Barraclough, R., H. J. Chen, B. R. Davies, M. P. Davies, Y. Ke, B. H. Lloyd, A. Oates, and P. S. Rudland. 1998. Use of DNA transfer in the induction of metastasis in experimental mammary systems. Biochem. Soc. Symp. 63:273-294. [PubMed] [Google Scholar]
- 5.Biswas, D. K., A. P. Cruz, E. Gansberger, and A. B. Pardee. 2000. Epidermal growth factor-induced nuclear factor κB activation: a major pathway of cell-cycle progression in estrogen-receptor negative breast cancer cells. Proc. Natl. Acad. Sci. USA 97:8542-8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brockhoff, G., P. Heiss, J. Schlegel, F. Hofstaedter, and R. Knuechel. 2001. Epidermal growth factor receptor, c-erbB2 and c-erbB3 receptor interaction, and related cell cycle kinetics of SK-BR-3 and BT474 breast carcinoma cells. Cytometry 44:338-348. [DOI] [PubMed] [Google Scholar]
- 7.Carles-Kinch, K., K. E. Kilpatrick, J. C. Stewart, and M. S. Kinch. 2002. Antibody targeting of the EphA2 tyrosine kinase inhibits malignant cell behavior. Cancer Res. 62:2840-2847. [PubMed] [Google Scholar]
- 8.Chackalaparampil, I., A. Peri, M. Nemir, M. D. Mckee, P. H. Lin, B. B. Mukherjee, and A. B. Mukherjee. 1996. Cells in vivo and in vitro from osteopetrotic mice homozygous for c-src disruption show suppression of synthesis of osteopontin, a multifunctional extracellular matrix protein. Oncogene 12:1457-1467. [PubMed] [Google Scholar]
- 9.Chambers, A. F., E. I. Behrend, S. M. Wilson, and D. T. Denhardt. 1992. Induction of expression of osteopontin (OPN; secreted phosphoprotein) in metastatic, ras-transformed NIH 3T3 cells. Anticancer Res. 12:43-48. [PubMed] [Google Scholar]
- 10.Chambers, A. F., and A. B. Tuck. 1993. Ras-responsive genes and tumor metastasis. Crit. Rev. Oncog. 4:95-114. [PubMed] [Google Scholar]
- 11.Chen, H., Y. Ke, A. J. Oates, R. Barraclough, and P. S. Rudland. 1997. Isolation of and effector for metastasis-inducing DNAs from a human metastatic carcinoma cell line. Oncogene 14:1581-1588. [DOI] [PubMed] [Google Scholar]
- 12.Cheng, H.-L., M. Steinway, C. L. Delaney, T. F. Franke, and E. L. Feldman. 2000. IGF-I promotes Schwann cell motility and survival via action of Akt. Mol. Cell. Endocrinol. 170:211-215. [DOI] [PubMed] [Google Scholar]
- 13.Craig, A. M., G. T. Bowden, A. F. Chambers, M. A. Spearman, A. H. Greenberg, J. A. Wright, M. McLeod, and D. T. Denhardt. 1990. Secreted phosphoprotein mRNA is induced during multi-stage carcinogenesis in mouse skin and correlates with the metastatic potential of murine fibroblasts. Int. J. Cancer 46:133-137. [DOI] [PubMed] [Google Scholar]
- 14.Craig, A. M., M. Nemir, B. B. Mukherjee, A. F. Chambers, and D. T. Denhardt. 1988. Identification of the major phosphoprotein secreted by many rodent cell lines as 2ar/osteopontin: enhanced expression in H-ras-transformed 3T3 cells. Biochem. Biophys. Res. Commun. 157:166-173. [DOI] [PubMed] [Google Scholar]
- 15.Denhardt, D. T., C. M. Giachelli, and S. R. Rittling. 2001. Role of osteopontin in cellular signaling and toxicant injury. Annu. Rev. Pharmacol. Toxicol. 41:723-749. [DOI] [PubMed] [Google Scholar]
- 16.Dimri, G. P., K. Ithana, M. Acosta, and J. Campisi. 2000. Regulation of a senescence checkpoint response by the E2F1 transcription factor and p14ARF tumor suppressor. Mol. Cell. Biol. 20:273-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunn, S. E., J. V. Torres, J. S. Oh, D. M. Cykert, and J. C. Barrett. 2001. Up-regulation of urokinase-type plasminogen activator by insulin-like growth factor-I depends upon phosphatidylinositol-3 kinase and mitogen-activated protein kinase kinase. Cancer Res. 61:1367-1374. [PubMed] [Google Scholar]
- 18.Eder, A. M., L. Dominguez, T. F. Franke, and J. D. Ashwell. 1998. Phosphoinositide 3-kinase regulation of T cell receptor-mediated interleukin-2 gene expression in normal T cells. J. Biol. Chem. 273:28025-28031. [DOI] [PubMed] [Google Scholar]
- 19.El-Tanani, M., R. Barraclough, M. C. Wilkinson, and P. S. Rudland. 2001. Metastasis-inducing DNA regulates the expression of the osteopontin gene by binding the transcription factor Tcf-4. Cancer Res. 61:5619-5629. [DOI] [PubMed] [Google Scholar]
- 20.El-Tanani, M. K., R. Barraclough, M. C. Wilkinson, and P. S. Rudland. 2001. Regulatory region of metastasis-inducing DNA is the binding site for T cell factor-4. Oncogene 20:1793-1797. [DOI] [PubMed] [Google Scholar]
- 21.Giap, A. Q., A. Tarnawski, N. T. Hoa, V. Akotia, and Y. Ma. 2002. NSAID inhibition of RGM1 gastric monolayer wound re-epithelialization: comparison of selective Cox-2 versus non-selective Cox inhibitors. Life Sci. 70:3029-3037. [DOI] [PubMed] [Google Scholar]
- 22.Gunthert, U., M. Hofmann, W. Rudy, S. Reber, M. Zoller, I. Haussmann, S. Matzku, A. Wenzel, H. Ponta, and P. Herrlich. 1991. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell 65:13-24. [DOI] [PubMed] [Google Scholar]
- 23.Guo, X., Y. P. Zhang, D. A. Mitchell, D. T. Denhardt, and A. F. Chambers. 1995. Identification of a ras-activated enhancer in the mouse osteopontin promoter and its interaction with a putative ETS-related transcription factor whose activity correlates with the metastatic potential of the cell. Mol. Cell. Biol. 15:476-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hermanto, U., C. S. Zong, and L. H. Wang. 2001. ErbB2-overexpressing human mammary carcinoma cells display an increased requirement for the phosphatidylinositol 3-kinase signaling pathway in anchorage-independent growth. Oncogene 20:7551-7562. [DOI] [PubMed] [Google Scholar]
- 25.James, N. H., J. Ashby, and R. A. Roberts. 1995. Enhanced hepatocyte colony growth in soft agar after in vivo treatment with a genotoxic carcinogen: a potential assay for hepatocarcinogens? Cancer Lett. 93:121-128. [DOI] [PubMed] [Google Scholar]
- 26.Jerry, D. J., M. A. Ozbun, F. S. Kittrell, D. P. Lane, D. Medina, and J. S. Butel. 1993. Mutations in p53 are frequent in the preneoplastic stage of mouse mammary tumor development. Cancer Res. 53:3374-3381. [PubMed] [Google Scholar]
- 27.Katz, R. W., S. Y. Teng, S. Thomas, and R. Landesberg. 2002. Paracrine activation of extracellular signal-regulated kinase in a simple in vitro model of wounded osteoblasts. Bone 31:288-295. [DOI] [PubMed] [Google Scholar]
- 28.Kitaura, J., K. Asai, M. Maeda-Yamamoto, Y. Kawakami, U. Kikkawa, and T. Kawakami. 2000. Akt-dependent cytokine production in mast cells. J. Exp. Med. 192:729-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kittrell, F. S., C. J. Oborn, and D. Medina. 1992. Development of mammary preneoplasias in vivo from mouse mammary epithelial cell lines in vitro. Cancer Res. 52:1924-1932. [PubMed] [Google Scholar]
- 30.Kuppumbatti, Y. S., B. Rexer, S. Nakajo, K. Nakaya, and R. Mira-y-Lopez. 2001. CRBP suppresses breast cancer cell survival and anchorage-independent growth. Oncogene 20:7413-7419. [DOI] [PubMed] [Google Scholar]
- 31.Lenferink, A. E., D. Busse, W. M. Flanagan, F. M. Yakes, and C. L. Arteaga. 2001. ErbB2/neu kinase modulates cellular p27Kip1 and cyclin D1 through multiple signaling pathways. Cancer Res. 61:6583-6591. [PubMed] [Google Scholar]
- 32.Li, W., J. Zhang, L. Flechner, T. Hyun, A. Yam, T. F. Franke, and J. H. Pierce. 1999. Protein kinase C-α overexpression stimulates Akt activity and suppresses apoptosis induced by interleukin 3 withdrawal. Oncogene 18:6564-6572. [DOI] [PubMed] [Google Scholar]
- 33.Lim, S. J., G. Lopez-Berestein, M. C. Hung, R. Lupu, and A. M. Tari. 2000. Grb2 downregulation leads to Akt inactivation in heregulin-stimulated and ErbB2-overexpressing breast cancer cells. Oncogene 19:6271-6276. [DOI] [PubMed] [Google Scholar]
- 34.Malyankar, U. M., M. Almeida, R. J. Johnson, R. H. Pichler, and C. M. Giachelli. 1997. Osteopontin regulation in cultured rat renal epithelial cells. Kidney Int. 51:1766-1773. [DOI] [PubMed] [Google Scholar]
- 35.Moasser, M. M., A. Basso, S. D. Averbuch, and N. Rosen. 2001. The tyrosine kinase inhibitor ZD1839 (“Iressa”) inhibits HER2-driven signaling and suppresses the growth of HER2-overexpressing tumor cells. Cancer Res. 61:7184-7188. [PubMed] [Google Scholar]
- 36.Nakanishi, K., M. Sakamoto, J. Yasuda, M. Takamura, N. Fujita, T. Tsuruo, S. Todo, and S. Hirohashi. 2002. Critical involvement of the phosphatidylinositol 3-kinase/Akt pathway in anchorage-independent growth and hematogeneous intrahepatic metastasis of liver cancer. Cancer Res. 62:2971-2975. [PubMed] [Google Scholar]
- 37.Nose, K., H. Saito, and T. Kuroki. 1990. Isolation of a gene sequence induced later by tumor-promoting 12-O-tetradecanoylphorbol-13-acetate in mouse osteoblastic cells (MC3T3-E1) and expressed constitutively in ras-transformed cells. Cell Growth Differ. 1:511-518. [PubMed] [Google Scholar]
- 38.Oates, A. J., R. Barraclough, and P. S. Rudland. 1996. The identification of osteopontin as a metastasis-related gene product in a rodent mammary tumour model. Oncogene 13:97-104. [PubMed] [Google Scholar]
- 39.Ozbun, M. A., D. J. Jerry, F. S. Kittrell, D. Medina, and J. S. Butel. 1993. p53 mutations selected in vivo when mouse mammary epithelial cells form hyperplastic outgrowths are not necessary for establishment of mammary cell lines in vitro. Cancer Res. 53:1646-1652. [PubMed] [Google Scholar]
- 40.Park, B.-K., X. Zeng, and R. Glazer. 2001. Akt1 induces extracellular matrix invasion and matrix metalloproteinase-2 activity in mouse mammary epithelial cells. Cancer Res. 61:7647-7653. [PubMed] [Google Scholar]
- 41.Pianetti, S., M. Arsura, R. Romieu-Mourez, R. J. Coffey, and G. E. Sonenshein. 2001. Her-2/neu overexpression induces NF-κB via a PI3-kinase/Akt pathway involving calpain-mediated degradation of IκB-α that can be inhibited by the tumor suppressor PTEN. Oncogene 20:1287-1299. [DOI] [PubMed] [Google Scholar]
- 42.Rudland, P. S., A. Platt-Higgins, M. El-Tanani, S. De Silva Rudland, R. Barraclough, J. H. Winstanley, R. Howitt, and C. R. West. 2002. Prognostic significance of the metastasis-associated protein osteopontin in human breast cancer. Cancer Res. 62:3417-3427. [PubMed] [Google Scholar]
- 43.Ryo, A., Y. C. Liou, G. Wulf, M. Nakamura, S. W. Lee, and K. P. Lu. 2002. PIN1 is an E2F target gene essential for Neu/Ras-induced transformation of mammary epithelial cells. Mol. Cell. Biol. 22:5281-5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seiter, S., R. Arch, S. Reber, D. Komitowski, M. Hofmann, H. Ponta, P. Herrlich, S. Matzku, and M. Zoller. 1993. Prevention of tumor metastasis formation by anti-variant CD44. J. Exp. Med. 177:443-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singhal, H., D. S. Bautista, K. S. Tonkin, F. P. O'Malley, A. B. Tuck, A. F. Chambers, and J. F. Harris. 1997. Elevated plasma osteopontin in metastatic breast cancer associated with increased tumor burden and decreased survival. Clin. Cancer Res. 3:605-611. [PubMed] [Google Scholar]
- 46.Sun, M., J. E. Paciga, R. I. Feldman, A. Yuan, D. Coppola, Y. Y. Lu, S. A. Shelley, S. V. Nicosia, and J. Q. Cheng. 2001. Phosphatidylinositol-3-OH kinase (PI3K)/AKT2, activated in breast cancer, regulates and is induced by estrogen receptor α (ERα) via interaction between ERα and PI3K. Cancer Res. 61:5985-5991. [PubMed] [Google Scholar]
- 47.Sung, V., C. Gilles, A. Murray, R. Clarke, A. D. Aaron, N. Azumi, and E. W. Thompson. 1998. The LCC15-MB human breast cancer cell line expresses osteopontin and exhibits an invasive and metastatic phenotype. Exp. Cell Res. 241:273-284. [DOI] [PubMed] [Google Scholar]
- 48.Tanno, S., S. Tanno, Y. Mitsuuchi, A. Altomare, G.-H. Xiao, and J. R. Testa. 2001. AKT activation up-regulates insulin-like growth factor I receptor expression and promotes invasiveness of human pancreatic cancer cells. Cancer Res. 61:589-593. [PubMed] [Google Scholar]
- 49.Tsai, E. M., S. C. Wang, J. N. Lee, and M. C. Hung. 2001. Akt activation by estrogen in estrogen receptor-negative breast cancer cells. Cancer Res. 61:8390-8392. [PubMed] [Google Scholar]
- 50.Tuck, A. B., D. M. Arsenault, F. P. O'Malley, C. Hota, M. C. Ling, S. M. Wilson, and A. F. Chambers. 1999. Osteopontin induces increased invasiveness and plasminogen activator expression of human mammary epithelial cells. Oncogene 18:4237-4246. [DOI] [PubMed] [Google Scholar]
- 51.Urquidi, V., D. Sloan, K. Kawai, D. Agarwal, A. C. Woodman, D. Tarin, and S. Goodison. 2002. Contrasting expression of thrombospondin-1 and osteopontin correlates with absence or presence of metastatic phenotype in an isogenic model of spontaneous human breast cancer metastasis. Clin. Cancer Res. 8:61-74. [PubMed] [Google Scholar]
- 52.Weber, G. F. 2001. The metastasis gene osteopontin: a candidate target for cancer therapy. Biochim. Biophys. Acta 1552:61-85. [DOI] [PubMed]
- 53.Weber, G. F., and S. Ashkar. 2000. Stress response genes—the genes that make cancer metastasize. J. Mol. Med. 78:404-408. [DOI] [PubMed] [Google Scholar]
- 54.Weber, G. F., R. Bronson, J. Ilagan, H. Cantor, R. Schmitz, and T. W. Mak. 2002. Absence of CD44 prevents sarcoma metastasis. Cancer Res. 62:2281-2286. [PubMed] [Google Scholar]
- 55.Zhang, R. D., I. J. Fidler, and J. E. Price. 1991. Relative malignant potential of human breast carcinoma cell lines established from pleural effusions and a brain metastasis. Invasion Metastasis 11:204-215. [PubMed] [Google Scholar]
- 56.Zhou, B. P., Y. Liao, W. Xia, B. Spohn, M. H. Lee, and M. C. Hung. 2001. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat. Cell Biol. 3:245-252. [DOI] [PubMed] [Google Scholar]