Abstract
A synthetic triterpenoid, 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid (CDDO), has been reported to have anti-inflammatory properties and to decrease the interleukin-1 (IL-1)-induced expression of matrix metalloproteinase-1 (MMP-1) and MMP-13. We have shown previously that IL-1 induces expression of the inhibitor of NF-κB (IκB) family member Bcl-3, and that this contributes to MMP-1 expression. To quantify the effects of CDDO on IL-1-induced MMP-1, MMP-13 and Bcl-3 expression, we stimulated the chondrosarcoma cell line SW-1353 and human primary chondrocytes with IL-1, in the presence or absence of CDDO. Harvested RNA was subjected to quantitative real-time reverse-transcriptase polymerase chain reaction. In SW-1353 cells, 300 nM CDDO significantly decreased the induction of MMP-1 and MMP-13 by IL-1. In human primary chondrocytes, 300 nM CDDO inhibited the induction of these genes by IL-1 to an even greater extent. In both cell types, inhibition of MMP-1 required 24 hours of pretreatment with CDDO, whereas MMP-13 could be inhibited when CDDO and IL-1 were added simultaneously to culture. In human primary chondrocytes, IL-1-induced Bcl-3 expression was inhibited when cells were pretreated with CDDO. To determine whether the inhibitory effect of CDDO on MMP worked through inhibition of Bcl-3 gene expression, SW-1353 cells stably transfected with a Bcl-3 expression plasmid were treated with IL-1 and/or CDDO, and MMP gene expression was assayed. Overexpression of Bcl-3 increased MMP-1, but not MMP-13, mRNA levels. Furthermore, overexpressed Bcl-3 could sustain the CDDO-dependent inhibition of IL-1-induced MMP-1 expression. Our data demonstrate that CDDO inhibits IL-1-induced MMP-1 and MMP-13 expression in human chondrocytes. CDDO also inhibits the expression of Bcl-3, an IL-1-responsive gene that preferentially contributes to MMP-1 gene expression.
Keywords: CDDO, chondrocytes, interleukin-1, matrix metalloproteinase, Bcl-3
Introduction
In diarthodial joints, cartilage provides a smooth surface that enables joints to articulate and to withstand compressional and shear stress [1]. Embedded in the cartilage are chondrocytes that respond to biochemical and physical stimuli to maintain this tissue. Cartilage is primarily composed of type II collagen and proteoglycan, which are synthesized by the chondrocytes. In addition, chondrocytes produce enzymes, such as the matrix metalloproteinases (MMPs), that degrade the cartilage [2]. In arthritis, an imbalance of these processes in favour of degradation results in a loss of cartilage. For the patient, this is manifested as a degeneration of joint function, loss of mobility and concomitant increased morbidity and mortality [3,4]. For the clinician, this imbalance identifies a need for therapeutic agents to prevent cartilage loss [5].
In identifying targets for therapeutic intervention, it is important to note that the loss of collagen, rather than proteoglycan, is correlated with disease severity [6]. Furthermore, whereas lost proteoglycan is replaced rapidly, degraded collagen is resynthesized very slowly [7,8]. Consequently, understanding collagen degradation and providing therapeutics to prevent it are valuable aims. Within the MMP family, MMP-1 (collagenase-1) and MMP-13 (collagenase-3) are expressed in arthritic joints, and efficiently degrade type II collagen [9]. MMP-13 is more effective than MMP-1 in cleaving type II collagen, but it is debated which is the principal collagenase in vivo [10,11]. These enzymes have been implicated in the pathology of rheumatoid arthritis (RA) and osteoarthritis (OA), and have long been therapeutic targets [12]. Historically, inhibition of MMP-active sites has been a strategy; however, with wide-ranging side-effects and lack of efficacy, these studies are proving unfruitful in providing an effective drug [13].
Although the etiologies and pathologies of RA and OA differ, it is clear that in both of these diseases pro-inflammatory cytokines are present, resulting in an inflammatory state as well as cartilage degradation [14]. As further evidence for the role of pro-inflammatory cytokines in RA, anti-tumor necrosis factor-α (anti-TNF-α) and anti-interleukin 1 (anti-IL-1) therapies can reduce inflammation and retard the progression of disease as assessed radiographically [15,16]. However, side-effects with these approaches, such as the development of lymphomas in patients using anti-TNF-α therapies, demonstrate that alternative therapies are needed [17].
An alternative approach to the prevention of cartilage degradation is the inhibition of MMPs by targeting either the expression of their genes or the synthesis of the proteins. Triterpenoids are a novel family of steroid-like compounds with weak anti-inflammatory properties [18]. Synthetic triterpenoids have been produced with the aim of achieving increased potency [19,20]. 2-Cyano-3,12-dioxoolean-1,9-dien-28-oic acid (CDDO) is a synthetic triterpenoid that has been shown to inhibit expression of the inflammatory genes inducible nitric oxide synthase and cyclo-oxygenase-2 [20-22]. In a recent report, MMP-1 and MMP-13 expression were induced with IL-1, a known pro-inflammatory mediator in vivo in joint tissues [23]. It was shown that CDDO could inhibit the IL-1-induced expression of these pro-inflammatory MMPs. These findings make CDDO an attractive molecule to study as a potential anti-arthritic agent.
Here we report quantification of the effects of CDDO on gene expression in the human chondrosarcoma cell line SW-1353 with the use of real-time reverse-transcriptase polymerase chain reaction (RT–PCR). To ensure that the chondroprotective effects of CDDO are not limited to this cell line, we studied its effects on human primary chondrocytes. We found that, at concentrations that do not induce apoptosis, CDDO effectively inhibits the induction of both MMP-1 and MMP-13 gene expression by IL-1 in these cells. Whereas the inhibition of MMP-1 by CDDO requires pretreatment, inhibition of MMP-13 does not. We have recently reported the role of the inhibitor of NF-κB (IκB) family member Bcl-3 in IL-1-induced MMP-1 expression [24]. Here we show that overexpression of Bcl-3 can partly abrogate CDDO's inhibitory effects on MMP-1 in SW-1353 cells, suggesting that Bcl-3 might be a target for CDDO.
Materials and methods
Materials, cells and cell culture
CDDO was kindly provided by Dr Tadashi Honda and Dr Gordon Gribble (Dartmouth College, Hanover, NH, USA).
Human SW-1353 chondrosarcoma cells were purchased from the American Type Culture Collection (Rockville, MD, USA). Stable SW-1353 cell lines carrying a pBkRSV vector with a Bcl-3 expression insert were created as described previously [24]. Freshly excised, macroscopically normal cartilage and synovium from OA patients undergoing knee replacement surgery were obtained from a local orthopedic unit (Dartmouth Hitchcock Memorial Hospital, Lebanon, NH, USA). These tissues constitute waste from the operations. Under our current protocol with the Committee for Human Subjects as mandated by HIPPA, we did not collect subject information. We therefore made no selection on the basis of age or sex. Cartilage and synovium were degraded in 4 mg/ml collagenase (Sigma, St Louis, MO, USA) for 16 hours at 37°C, with shaking at 90 r.p.m., and cells were cultured to one passage (chondrocytes) or four passages (synovial fibroblasts).
SW-1353 cells (normal or stably transfected with pBkRSV–Bcl-3), human primary chondrocytes, or synovial fibroblasts were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT, USA), penicillin/streptomycin and L-glutamine (Cellgro; Mediatech, Herndon, VA, USA) before use in experiments.
Assay of CDDO's effects on chondrocytes and fibroblasts
At the beginning of each experiment, chondrocytes or OA synovial fibroblasts were washed three times with Hanks balanced salt solution (Cellgro) to remove traces of serum, and placed in DMEM containing 0.2% lactalbumin hydrolysate (Invitrogen, Carlsbad, CA, USA). Cells were cultured for 24 hours with CDDO as a pretreatment, or CDDO was added at the same time as 10 ng/ml recombinant IL-1β at time 0 (Promega, Madison, WI, USA). After a further 18 hours of culture with IL-1, total RNA was harvested with TRIzol (Invitrogen) and assayed for MMP-1, MMP-13, and Bcl-3 expression by using quantitative real-time RT–PCR. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was assayed to normalize data. Reverse transcription and quantitative real-time PCR methods were as described previously [24]. MMP-13 primers used were as follows: upper, 5'-TGGCATTGCTGACATCATGA-3' ; lower, 3'-GCCAGAGGGCCCATCAA-5'. The product size was 76 base pairs. A plasmid containing MMP-13 cDNA (provided by Dr Carlos Lopez-Otin, Oviedo, Spain) was used to generate an MMP-13 standard curve. Primers and standards for MMP-1, Bcl-3, and GAPDH were as described previously [24]. The generation and use of standard curves were as described previously [24].
Measurement of programmed cell death by staining with Hoechst 33342
SW-1353 and human primary chondrocytes were seeded at 5 × 104 cells per well in 24-well plates (Becton Dickinson, Franklin Lakes, NJ, USA). At 60–90% confluence, cells were treated with CDDO (10 nM to 10 μM) for 24 hours, followed by IL-1 for 18 hours. Hoechst 33342 (Sigma) (1 μg/ml) was then added to each well to detect cells with condensed chromatin, a hallmark of programmed cell death. After 30 minutes of incubation at 37°C, cells were examined under a fluorescent microscope as described previously [24].
Statistical analysis
Analyses of real-time PCR data were performed with unpaired two-tailed Student's t-tests on triplicate wells cultured simultaneously.
Results
Effects of CDDO in SW-1353 cells
Previous work suggested that preincubation with CDDO was required for the effective inhibition of IL-1-induced MMP gene expression in SW-1353 cells [23]. To quantify the effect of altering the period of CDDO preincubation, 300 nM CDDO was added to SW-1353 cells simultaneously with IL-1, or 24 hours before treatment with the cytokine. We found that SW-1353 cells expressed very low basal levels of MMP-1 and MMP-13, and that these were not significantly affected by incubation with CDDO (Fig. 1). IL-1 induced the expression of MMP-1 and MMP-13, and these were significantly inhibited when the cultures were pretreated with CDDO for 24 hours, the respective decreases being 55% for MMP-1 (P < 0.1) and 66% for MMP-13 (P < 0.01). In addition, when added simultaneously with the cytokine, CDDO significantly inhibited the IL-1-induced expression of MMP-13, but not that of MMP-1. Under these circumstances, MMP-13 expression was decreased by 41% (P < 0.05). The different profiles for MMP-1 and MMP-13 inhibition when CDDO was added simultaneously with the cytokine suggest a difference in the action of the inhibitor on the expression of each enzyme.
Figure 1.
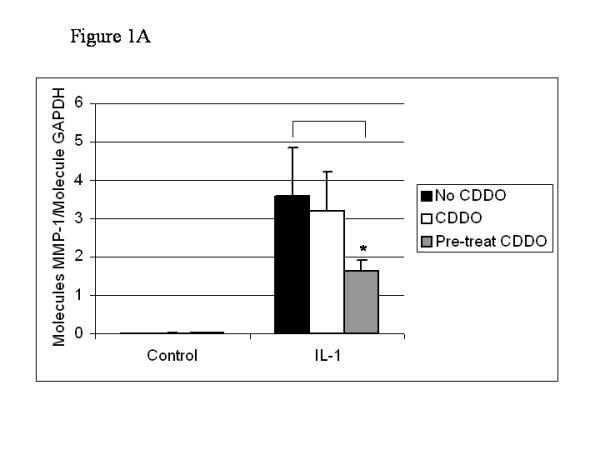
IL-1-induced MMP-1 and MMP-13 expression is inhibited in SW-1353 cells preincubated with CDDO for 24 hours. SW-1353 cells were treated for 24 hours with 300 nM CDDO before the addition of 10 ng/ml IL-1β. Alternatively, CDDO was added simultaneously with the cytokine. After a further 18 hours of culture, RNA was harvested, reverse transcribed and subjected to quantitative real-time PCR for MMP-1 (a) and MMP-13 (b). Results were normalized to GAPDH, and are means of culture triplicates. They are expressed as molecules of MMP per molecule of GAPDH. Two-tailed Student's t-tests were performed on the data, and significance is indicated as follows: *P < 0.1; **0.01 <P < 0.05; ***P < 0.01.
Previously, with the use of Northern blotting, dose–response assays in SW-1353 cells showed that 300 nM was the lowest dose of CDDO sufficient to decrease the IL-1-induced expression of MMP-1 and MMP-13 [23]. This was confirmed by using quantitative real-time PCR. Doses below 300 nM did not decrease MMP expression, whereas those above 1 μM resulted in poor RNA harvests and low expression of the MMPs (data not shown). This effect at higher doses might be due to the reported apoptotic effects of CDDO [25,26]. To examine for such effects, SW-1353 cells were treated for 24 hours with CDDO under serum-free conditions, and then IL-1 was added for a further 18 hours. Cells were stained with Hoechst 33342 and examined under a fluorescent microscope. Doses of 300 nM and 1 μM CDDO, with or without IL-1, did not increase apoptosis compared with control (medium alone) or IL-1 (data not shown). However, a dose of 5 μM caused apoptosis in SW-1353 cells, as seen in lower cell numbers, changed morphology and an increased number of fluorescing cells. These data demonstrate that at 300 nM and 1 μM, doses that inhibit MMP gene expression, CDDO does not cause programmed cell death.
CDDO time-course in human primary chondrocytes
As described in the Introduction, the aim of studying the triterpenoids is to develop a therapeutic agent for the treatment of arthritis. We were therefore interested in studying the effects of CDDO in human primary cells. Quantitative real-time RT–PCR revealed that human chondrocytes expressed low levels of MMP-1 and MMP-13 in the absence of IL-1, and increased levels with addition of the cytokine (Figs 2a,2b). Pretreatment of cultures with 300 nM CDDO for 24 hours resulted in a decreased expression of IL-1-induced MMP-1 and MMP-13. MMP-1 expression was decreased by 99% (P < 0.01) and MMP-13 by 96% (P < 0.01). As was observed with SW-1353 cells, MMP-13 expression was significantly decreased when CDDO was added simultaneously with the cytokine: we observed a decrease of 67% (P < 0.1). MMP-1 expression was not significantly inhibited by CDDO added simultaneously with IL-1.
Figure 2.
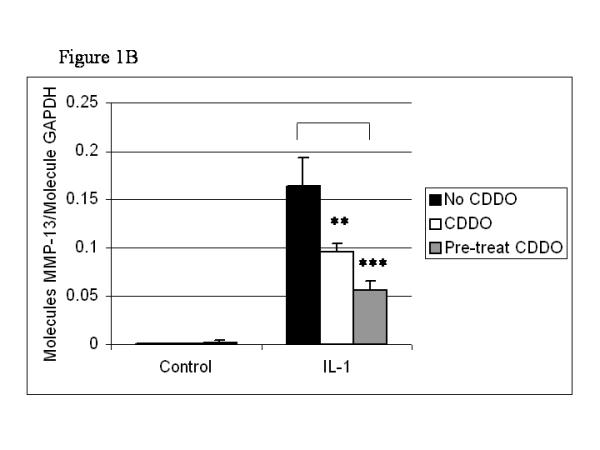
Expression of MMP-1, MMP-13, and Bcl-3 is inhibited in human chondrocytes pretreated with CDDO for 24 hours. Human primary chondrocytes (passage 1) were treated for 24 hours with 300 nM CDDO before the addition of 10 ng/ml IL-1. Alternatively, CDDO was added simultaneously with the cytokine. After a further 18 hours of culture, RNA was harvested, reverse transcribed and subjected to quantitative real-time PCR for MMP-1 (a), MMP-13 (b), and Bcl-3 (c). Results were normalized to GAPDH, and are means of culture triplicates. They are expressed as molecules per molecule of GAPDH. Two-tailed Student's t-tests were performed on the data, and significance is indicated as follows: *P < 0.1; **0.01 <P < 0.05; ***P < 0.01.
Effects of CDDO on expression of Bcl-3 in human primary chondrocytes
In the same time-course experiment, we assayed for the presence of Bcl-3, an IκB family member that we have previously shown to have a role in MMP-1 expression. We found that Bcl-3 was inducible with IL-1 in human primary chondrocytes, and that CDDO significantly inhibited this expression (Fig. 2c). In a similar manner to MMP-1 inhibition, the decreased expression was observed only after preincubation of the cells with CDDO for 24 hours (Figs 2a,2c). This was in contrast to MMP-13, whose inhibition was possible without pretreatment with CDDO (Fig. 2b). These results present further evidence of a link in MMP-1 and Bcl-3 expression, and indicate that both molecules are similarly affected by CDDO.
CDDO dose–response study in human primary chondrocytes
To examine the effects of increasing doses of CDDO in human primary chondrocytes, cells were preincubated for 24 hours with CDDO (300 nM to 5 μM), or with medium alone, before the addition of IL-1 for 18 hours. Figure 3 shows that human primary chondrocytes have low basal expression of MMP-1, MMP-13 and Bcl-3, and that they are increased in the presence of IL-1. Increasing doses of CDDO resulted in a decreased expression of each gene. For example, MMP-1 expression was inhibited by 36% (P < 0.1) with 300 nM CDDO, by 49% (P < 0.01) with 1 μM CDDO, and by 83% (P < 0.01) by 5 μM CDDO. Similar patterns emerged for MMP-13 (63%, 81%, and 99%) and Bcl-3 (33%, 61%, and 86%) in response to the respective doses of CDDO, all results being significant. Using Hoechst 33342, we assayed for potential toxicity of CDDO in human primary chondrocytes: we found decreased cell numbers and increased apoptosis at or above 5 μM CDDO (data not shown). Lower concentrations did not result in apoptosis.
Figure 3.

Increasing doses of CDDO inhibit the expression of MMP-1, MMP-13, and Bcl-3 in human primary chondrocytes. Human primary chondrocytes (passage 1) were treated for 24 hours with 300 nM to 5 μM CDDO before the addition of 10 ng/ml IL-1. After a further 18 hours of culture, RNA was harvested, reverse transcribed and subjected to quantitative real-time PCR for MMP-1 (a), MMP-13 (b), and Bcl-3 (c). Results were normalized to GAPDH, and are means of culture triplicates. They are expressed as molecules per molecule of GAPDH. Two-tailed Student's t-tests were performed on the data, and significance is indicated as follows: *P < 0.1; **0.01 <P < 0.05; ***P < 0.01.
Inhibition of MMP-1, MMP-13, and Bcl-3 in OA synovial fibroblasts
In parallel with the study on human primary chondrocytes, we examined the effects of 300 nM CDDO on synovial fibroblasts excised from OA synovium. There were low basal expressions of MMP-1, MMP-13, and Bcl-3, which were increased in the presence of IL-1 and inhibited by CDDO, following the trends observed in SW-1353 and human primary chondrocytes (data not shown).
Effect of overexpression of Bcl-3 on the inhibition of MMP-1 by CDDO
To examine further the role of Bcl-3 in IL-1-induced MMP-1 and MMP-13 expression, we used SW-1353 cells stably transfected with a Bcl-3 expression construct. These cells were pretreated with 300 nM CDDO for 24 hours and then cultured with IL-1 for 18 hours. In the presence of empty vector, IL-1 induced both MMP-1 and MMP-13, and their expression was decreased by CDDO by 48% and 46%, respectively (Fig. 4). In cells stably transfected with the Bcl-3 expression plasmid, IL-1 induced MMP-1 expression to levels 3.4-fold higher than in the cells transfected with empty vector (Fig. 4a). In comparison, MMP-13 expression was not significantly altered by the overexpression of Bcl-3 (Fig. 4b). Furthermore, the presence of exogenous Bcl-3 sustained MMP-1 expression in the presence of CDDO in comparison with cells expressing empty vector. When cultured in the presence of IL-1 and CDDO, cells transfected with Bcl-3 expressed 3.6-fold more MMP-1 than did cells transfected with the empty vector (Fig. 4a). In contrast, Bcl-3 did not sustain MMP-13 expression in CDDO-treated cells (Fig. 4b). Thus, Bcl-3, which is repressed by CDDO, preferentially contributes to MMP-1 gene expression.
Figure 4.
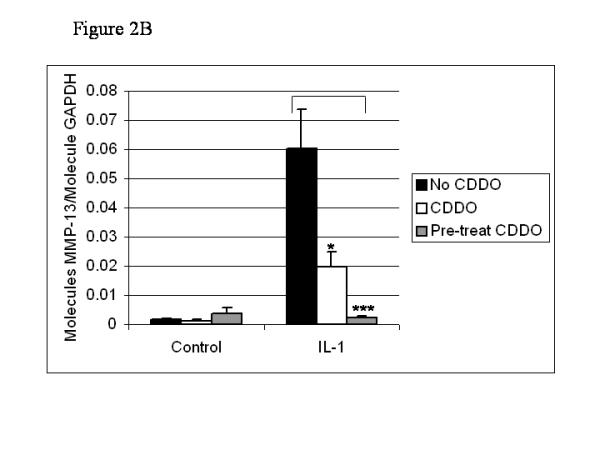
Overexpression of Bcl-3 sustains MMP-1 expression against inhibition by CDDO in SW-1353 cells. SW-1353 cells, stably transfected with a pBkRSV vector with or without a Bcl-3 insert, were incubated for 24 hours with 300 nM CDDO before the addition of 10 ng/ml IL-1. After a further 18 hours of culture, RNA was harvested, reverse transcribed and subjected to quantitative real-time PCR for MMP-1 (a) and MMP-13 (b). Results were normalized to GAPDH, and are means of culture triplicates. They are expressed as molecules per molecule of GAPDH. Two-tailed Student's t-tests were performed on the data, and significance is indicated as follows: *P < 0.1; **0.01 <P < 0.05; ***P < 0.01.
Discussion
In a previous study, Northern blot analysis showed that treating SW-1353 cells with 300 nM CDDO for 24 hours before treatment with IL-1 inhibited the expression of MMP-1 and MMP-13 [23]. In the present study we used quantitative real-time RT–PCR to examine the action of CDDO on the expression of MMP-1 and MMP-13 in SW-1353 cells and in two primary cultures: human primary chondrocytes and OA synovial fibroblasts. We have shown in a quantitative manner that in all three cell types, MMP-1 and MMP-13 are inducible with IL-1, and that CDDO inhibits the expression of these collagenases, with optimal inhibition being observed after 24 hours of pretreatment with CDDO. Differing effects were found on the two enzymes: MMP-13 was inhibited more than MMP-1. In addition, significant inhibition of MMP-13 did not require the pretreatment of cells with CDDO. In addition, the observation that synovial fibroblasts respond to CDDO in a similar manner to chondrocytes suggests that the inhibitor can act in all cells of the joint to decrease the expression of MMP-1 and MMP-13.
In the human primary chondrocytes and OA synovial fibroblasts, we assayed for CDDO's effects on Bcl-3 expression. We have recently described the role of Bcl-3, an IκB family member, in the signaling pathways of IL-1 leading to MMP-1 expression in SW-1353 cells and rabbit synovial fibroblasts [24]. We found that IL-1 could induce the expression of Bcl-3, and that Bcl-3 had a direct effect on MMP-1 expression through cooperation with NF-κB1/p50. In the present study we found that Bcl-3 expression was inducible by IL-1 in human primary chondrocytes and in OA synovial fibroblasts. In addition, we found that pretreatment with CDDO decreased the IL-1-induced expression of Bcl-3 in these cells. Furthermore, with the use of SW-1353 cells stably transfected with a Bcl-3 expression plasmid, Bcl-3 could sustain the expression of MMP-1, but not that of MMP-13, against inhibition by CDDO. These data show that Bcl-3 gene expression is a target of CDDO in chondrocytes and suggest that this might contribute to decreased MMP-1 expression. Consistent with this model is the finding that pretreatment with CDDO for 24 hours is required for the inhibition of MMP-1, unlike that for MMP-13. We speculate that, during this period, the inhibitor is entering the cell and altering the expression of components of the IL-1 signalling pathway. Bcl-3 is decreased by CDDO during this preincubation period, and it might be one of several proteins involved in MMP expression whose own expression is lowered by CDDO.
In all cell types, CDDO inhibited the IL-1-induced expression of MMP-13 mRNA more effectively than that of MMP-1. This might be due to the much lower MMP-13 expression in all tissues examined. There is disagreement over which collagenase predominates in cartilage degradation in vivo [10,11]. It is therefore of interest to find the levels of MMP-1 expressed compared with that of MMP-13 in SW-1353 and human primary chondrocytes. For example, in human primary chondrocytes (Figs 3a,3b), IL-1 induced MMP-1 to 14 molecules per molecule of GAPDH, whereas MMP-13 was expressed at 0.06 molecules per molecule of GAPDH, a more than 200-fold difference. These results suggest that MMP-1 expression might be a dominant target for IL-1 in chondrocytes.
It is interesting to note that there was significant variation of both MMP-1 and MMP-13 expression in the primary human chondrocyte experiments. In excising chondrocytes from the extracted knees of OA patients, great care was taken to remove only tissue that seemed macroscopically normal, with the aim of excluding cells of diseased tissue. When these cells were cultured with medium alone, they expressed low levels of MMP-1 and MMP-13, similar to those found in unstimulated SW-1353 cells. These results support the contention that the cells were in a basal state, unaffected by the environment of the diseased knee from which they had been extracted. Furthermore, both enzymes were consistently increased by IL-1 and inhibited by CDDO. Thus, the observed variation of MMP expression is probably due to genetic variability and not to experimental artifacts.
Naturally occurring triterpenoids, such as ursolic acid, have been found to have mild anti-inflammatory effects [18,27]. These have been improved with the development of synthetic triterpenoids such as CDDO, offering a potential therapeutic tool for the treatment of arthritis and other diseases [19-21]. Furthermore, it has been reported that CDDO at high doses (5–10 μM) can have pro-apoptotic effects, ideal for the treatment of leukemia but of concern with regard to chondrocyte cell death [25,26]. However, we found that CDDO, at concentrations that decrease MMP-1 and MMP-13 expression (namely 300 nM and 1 μM), did not cause cell death. The inhibitory effect of CDDO on MMP-1 and MMP-13 expression, as well as that of Bcl-3, is very encouraging for future applications of triterpenoids in arthritic disease. CDDO is an early-generation synthetic triterpenoid, and further modifications continue to be made. Further analysis of these compounds will provide additional information about their mechanisms and potential as therapeutic agents.
Conclusion
Inhibition of collagen loss is crucial to minimizing cartilage destruction in the arthritides. MMP-1 and MMP-13 have been identified as the significant enzymes involved in this process in vivo. We examined a novel, synthetic triterpenoid, CDDO, as a potential inhibitor of collagenase expression. We found that CDDO inhibited the expression of these enzymes in a chondrocyte cell line, human primary chondrocytes and synovial fibroblasts. Furthermore, CDDO decreased the expression of the pro-inflammatory mediator Bcl-3, suggesting one possible mechanism of action. CDDO is an early-generation therapeutic agent with great potential for the treatment of the arthritides and other inflammatory diseases.
Competing interests
None declared.
Abbreviations
CDDO = 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid; DMEM = Dulbecco's modified Eagle's medium; GAPDH = glyceraldehyde-3-phosphate dehydrogenase; IκB = inhibitor of NF-κB; IL = interleukin; MMP = matrix metalloproteinase; OA = osteoarthritis; RA = rheumatoid arthritis; RT–PCR = reverse-transcriptase polymerase chain reaction; TNF-α = tumor necrosis factor-α.
Acknowledgments
Acknowledgements
We thank Dr Nicholas Johnston and Ms Brenda Petrella for critical reading of this manuscript. This study was supported by grants awarded to MPV by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01-AR46977 and K01-AR02024), and by grants to MBS by the National Cancer Institute (R01 78814) and by the National Foundation for Cancer Research.
References
- Muir H. The chondrocyte, architect of cartilage. Biomechanics, structure, function and molecular biology of cartilage matrix macromolecules. BioEssays. 1995;17:1039–1048. doi: 10.1002/bies.950171208. [DOI] [PubMed] [Google Scholar]
- Mengshol JA, Mix KS, Brinckerhoff CE. Matrix metalloproteinases as therapeutic targets in arthritic diseases: bull's-eye or missing the mark? Arthritis Rheum. 2002;46:13–20. doi: 10.1002/1529-0131(200201)46:1<13::AID-ART497>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Doran MF, Crowson CS, Pond GR, O'Fallon WM, Gabriel SE. Predictors of infection in rheumatoid arthritis. Arthritis Rheum. 2002;46:2294–2300. doi: 10.1002/art.10529. [DOI] [PubMed] [Google Scholar]
- Doran MF, Crowson CS, Pond GR, O'Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46:2287–2293. doi: 10.1002/art.10524. [DOI] [PubMed] [Google Scholar]
- Kobelt G, Jonsson L, Lindgren P, Young A, Eberhardt K. Modeling the progression of rheumatoid arthritis: a two-country model to estimate costs and consequences of rheumatoid arthritis. Arthritis Rheum. 2002;46:2310–2319. doi: 10.1002/art.10471. [DOI] [PubMed] [Google Scholar]
- Pelletier JP, Martel-Pelletier J, Howell DS, Ghandur-Mnaymneh L, Enis JE, Woessner JF., Jr Collagenase and collagenolytic activity in human osteoarthritic cartilage. Arthritis Rheum. 1983;26:63–68. doi: 10.1002/art.1780260110. [DOI] [PubMed] [Google Scholar]
- Jubb RW, Fell HB. The breakdown of collagen by chondrocytes. J Pathol. 1980;130:159–167. doi: 10.1002/path.1711300304. [DOI] [PubMed] [Google Scholar]
- Page Thomas DP, King B, Stephens T, Dingle JT. In vivo studies of cartilage regeneration after damage induced by catabolin/interleukin-1. Ann Rheum Dis. 1991;50:75–80. doi: 10.1136/ard.50.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlopov BV, Lie WR, Mainardi CL, Cole AA, Chubinskaya S, Hasty KA. Osteoarthritic lesions: involvement of three different collagenases. Arthritis Rheum. 1997;40:2065–2074. doi: 10.1002/art.1780401120. [DOI] [PubMed] [Google Scholar]
- Bau B, Gebhard PM, Haag J, Knorr T, Bartnik E, Aigner T. Relative messenger RNA expression profiling of collagenases and aggrecanases in human articular chondrocytes in vivo and in vitro. Arthritis Rheum. 2002;46:2648–2657. doi: 10.1002/art.10531. [DOI] [PubMed] [Google Scholar]
- Wu W, Billinghurst RC, Pidoux I, Antoniou J, Zukor D, Tanzer M, Poole AR. Sites of collagenase cleavage and denaturation of type II collagen in aging and osteoarthritic articular cartilage and their relationship to the distribution of matrix metalloproteinase 1 and matrix metalloproteinase 13. Arthritis Rheum. 2002;46:2087–2094. doi: 10.1002/art.10428. [DOI] [PubMed] [Google Scholar]
- Vincenti MP, Clark IM, Brinckerhoff CE. Using inhibitors of metalloproteinases to treat arthritis. Easier said than done? Arthritis Rheum. 1994;37:1115–1126. doi: 10.1002/art.1780370802. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- van den Berg WB. The role of cytokines and growth factors in cartilage destruction in osteoarthritis and rheumatoid arthritis. Z Rheumatol. 1999;58:136–141. doi: 10.1007/s003930050163. [DOI] [PubMed] [Google Scholar]
- Feldmann M, Maini RN. Anti-TNF alpha therapy of rheumatoid arthritis: what have we learned? Annu Rev Immunol. 2001;19:163–196. doi: 10.1146/annurev.immunol.19.1.163. [DOI] [PubMed] [Google Scholar]
- Dayer JM, Bresnihan B. Targeting interleukin-1 in the treatment of rheumatoid arthritis. Arthritis Rheum. 2002;46:574–578. doi: 10.1002/art.10168. [DOI] [PubMed] [Google Scholar]
- Kent PD, Davis JM, 3rd, Davis MD, Matteson EL. Bullous skin lesions following infliximab infusion in a patient with rheumatoid arthritis. Arthritis Rheum. 2002;46:2257–2258. doi: 10.1002/art.10348. [DOI] [PubMed] [Google Scholar]
- Liu J. Pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacol. 1995;49:57–68. doi: 10.1016/0378-8741(95)01310-5. [DOI] [PubMed] [Google Scholar]
- Suh N, Honda T, Finlay HJ, Barchowsky A, Williams C, Benoit NE, Xie QW, Nathan C, Gribble GW, Sporn MB. Novel triterpenoids suppress inducible nitric oxide synthase (iNOS) and inducible cyclooxygenase (COX-2) in mouse macrophages. Cancer Res. 1998;58:717–723. [PubMed] [Google Scholar]
- Honda T, Rounds BV, Gribble GW, Suh N, Wang Y, Sporn MB. Design and synthesis of 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid, a novel and highly active inhibitor of nitric oxide production in mouse macrophages. Bioorg Med Chem Lett. 1998;8:2711–2714. doi: 10.1016/S0960-894X(98)00479-X. [DOI] [PubMed] [Google Scholar]
- Suh N, Wang Y, Honda T, Gribble GW, Dmitrovsky E, Hickey WF, Maue RA, Place AE, Porter DM, Spinella MJ, Williams CR, Wu G, Dannenberg AJ, Flanders KC, Letterio JJ, Mangelsdorf DJ, Nathan CF, Nguyen L, Porter WW, Ren RF, Roberts AB, Roche NS, Subbaramaiah K, Sporn MB. A novel synthetic oleanane triterpenoid, 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid, with potent differentiating, antiproliferative, and anti-inflammatory activity. Cancer Res. 1999;59:336–341. [PubMed] [Google Scholar]
- Honda T, Rounds BV, Bore L, Finlay HJ, Favaloro FG, Jr, Suh N, Wang Y, Sporn MB, Gribble GW. Synthetic oleanane and ursane triterpenoids with modified rings A and C: a series of highly active inhibitors of nitric oxide production in mouse macrophages. J Med Chem. 2000;43:4233–4246. doi: 10.1021/jm0002230. [DOI] [PubMed] [Google Scholar]
- Mix KS, Mengshol JA, Benbow U, Vincenti MP, Sporn MB, Brinckerhoff CE. A synthetic triterpenoid selectively inhibits the induction of matrix metalloproteinases 1 and 13 by inflammatory cytokines. Arthritis Rheum. 2001;44:1096–1104. doi: 10.1002/1529-0131(200105)44:5<1096::AID-ANR190>3.3.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Elliott SF, Coon CI, Hays E, Stadheim TA, Vincenti MP. Bcl-3 is an interleukin-1-responsive gene in chondrocytes and synovial fibroblasts that activates transcription of the matrix metalloproteinase 1 gene. Arthritis Rheum. 2002;46:3230–3239. doi: 10.1002/art.10675. [DOI] [PubMed] [Google Scholar]
- Ito Y, Pandey P, Sporn MB, Datta R, Kharbanda S, Kufe D. The novel triterpenoid CDDO induces apoptosis and differentiation of human osteosarcoma cells by a caspase-8 dependent mechanism. Mol Pharmacol. 2001;59:1094–1099. doi: 10.1124/mol.59.5.1094. [DOI] [PubMed] [Google Scholar]
- Stadheim TA, Suh N, Ganju N, Sporn MB, Eastman A. The novel triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid (CDDO) potently enhances apoptosis induced by tumor necrosis factor in human leukemia cells. J Biol Chem. 2002;277:16448–16455. doi: 10.1074/jbc.M108974200. [DOI] [PubMed] [Google Scholar]
- Subbaramaiah K, Michaluart P, Sporn MB, Dannenberg AJ. Ursolic acid inhibits cyclooxygenase-2 transcription in human mammary epithelial cells. Cancer Res. 2000;60:2399–2404. [PubMed] [Google Scholar]


